Abstract
Despite significant advances in therapies for pediatric type 1 diabetes, achievement of glycemic targets remains elusive, and management remains burdensome for patients and their families. This article identifies common challenges in diabetes management at the patient-provider and health care system levels and proposes practical approaches to overcoming therapeutic inertia to enhance health outcomes for youth with type 1 diabetes.
Effective management of type 1 diabetes requires frequent monitoring of blood glucose levels, calculation, and administration of insulin doses to match food intake while adjusting for a dynamic context of activity levels and physiologic requirements. For children with type 1 diabetes, these tasks must be coordinated between themselves and their caregivers. Over time, as children developmentally mature, it is reasonable for them to take on more responsibility for self-management in partnership with adult caregivers. Throughout all phases, children with diabetes benefit from the support of trained secondary caregivers such as school nurses, family members, and friends with appropriate diabetes education.
The impact of glycemic levels on long-term outcomes for individuals with type 1 diabetes is well established (1–4). Yet, globally, despite advances in diabetes pharmacology, technology, and evidence to guide practice, achievement of glycemic targets as measured by A1C remains elusive, and management remains burdensome for patients and their families. In the United States–based T1D Exchange Clinic Registry, a minority of youth (17%) attain an A1C <7.5% across all pediatric age ranges, and mean A1C levels have risen from the period 2010–2012 to 2016–2018 in teens and emerging adults (5).
Challenges of meeting glycemic targets are manifold and due in part to the imperfect nature of treatment strategies coupled with the complexity of psychosocial influences (e.g., family dynamics, social support, financial constraints, self-efficacy, distress, and depression) on effective self-management but also reflect limitations of provider-patient interactions in our current type 1 diabetes health care delivery system. In the context of such challenges, it may be tempting to succumb to a collective tolerance for suboptimal results accepted as “good enough.”
The term “clinical inertia” (6) is often applied to the care of adults with type 2 diabetes to describe a failure by health care professionals (HCPs) to initiate or intensify treatment in a timely manner (7); in the pediatric diabetes context, this inertia may take the form of underdiagnosed and untreated comorbidities (8).
The more expansive term “therapeutic inertia” recognizes missed opportunities for action to optimize clinical outcomes that can involve interactions between patients, caregivers, and HCPs. This article identifies some recognized common challenges in diabetes management at the patient-provider and health care system levels and proposes pragmatic approaches to overcoming acceptance of the status quo and to continually seeking means of overcoming therapeutic inertia to enhance health outcomes for youth with type 1 diabetes (Table 1).
TABLE 1.
Moving From Therapeutic Inertia to Therapeutic Action: Challenges, Strategies, and Opportunities
| Common Challenge | Strategies | Opportunities |
|---|---|---|
| Inadequate glucose data | Collect collateral data (e.g., from caregiver verbal reports and school nurse). | Discuss existing challenges to monitoring (e.g., lack of supplies, discomfort, forgetting, or not wanting to stop activities) and engage in shared decision-making about monitoring options such as CGM if the patient is not already using it. |
| Use available data (i.e., growth pattern, A1C level, and prescribed insulin units/kg) to ascertain if current insulin doses are appropriate. If evidence suggests that patient is receiving inadequate insulin, judicious dose adjustments can be made with plan for close follow-up. | ||
| Overwhelming and perplexing glucose data | Follow a standard method of reviewing glucose and insulin delivery data (similar to learning how to read an electrocardiogram). | Seek opportunities to limit variability to gain new insights into treatment (e.g., basal rate testing on weekends). |
| For insulin pump users, consider limiting the number of different settings used until a more consistent pattern emerges. | Adopt advanced technology solutions for data interpretation (e.g., DreaMed Advisor Pro). | |
| Persistent elevated A1C; “not doing anything” | Ask about how often long-acting (basal) insulin is given and what circumstances help the patient to remember these doses. | Review treatment options for insulin delivery (injections, pump therapy, or untethered pump therapy with long-acting/basal insulin in background to account for prolonged periods when the pump is disconnected). |
| Ask about the patient's activities and goals outside of diabetes management. | Consider alternative basal insulin with more forgiving pharmacokinetic profile (e.g., degludec) if doses are given at different times of day. | |
| Use a supportive tone while acknowledging that diabetes management is difficult. | ||
| Static diabetes management | Teach families a dynamic approach to diabetes management (e.g., how to incorporate CGM trend arrows into real-time management decisions). | Provide patient-centered education modules (e.g., 30–60–90 Rule) to encourage dynamic diabetes management. |
| Fear of hypoglycemia | Acknowledge the concerns of patients and parents/caregivers. | Educate patient's family on the signs, symptoms, and treatment of mild, moderate, and severe hypoglycemia. |
| Determine whether the patient retains hypoglycemia awareness symptoms. | Offer technology options (e.g., CGM, integrated insulin pumps with low glucose suspend features, and automated insulin delivery systems). | |
| Address dose adjustment needs with a priority to reduce patterns of recurrent hypoglycemia. | ||
| Diabetes burnout | Screen adolescents with type 1 diabetes routinely for diabetes distress and depression symptoms. | Discuss roles with patients and parents/caregivers. Negotiate some responsibilities parents/caregivers can take on for a few weeks to alleviate patients' burden (e.g., ordering supplies, giving injections, overseeing pattern management, or packing lunches that include carbohydrate counts). |
| Acknowledge the challenges of diabetes management, and ask about the patient's activities and goals outside of diabetes management. | Encourage engagement with peer support groups in the diabetes community. | |
| Refer patients and families to mental health services if needed. | ||
| Satisfaction with A1C “close enough” to target | Acknowledge successful efforts and accomplishments to date, but resist complacency. | Shift the treatment paradigm to focus on time in range rather than A1C or mean blood glucose level. |
| Review patients' ambulatory glucose profile with actionable, individualized goal-setting to increase time spent in the target glycemic range (i.e., 70–180 mg/dL) and reduce time spent in hyperglycemia (i.e., >180 or >250 mg/dL). | ||
| Limited time in clinic | Schedule more frequent clinic visits for higher-risk patients. | Explore telehealth opportunities, patient portal communication, community outreach, and other mechanisms to promote touch points between clinic visits. |
Therapeutic Inertia at the Patient-Provider Level in Pediatric Diabetes
Not Enough Data
There are notable limitations to clinical decision-making in the context of clinic visits. For example, patients may present with a paucity of blood glucose data due to lack of checking, forgetting to bring their glucose meter for data downloading, or limited sensor glucose data if sensors are worn inconsistently. Incomplete data may lead to the diabetes HCP feeling that dose adjustments cannot be made. Instead, this could be viewed as an opportunity to motivate and encourage the patient. Motivational interviewing (9–12) may help to inspire behavioral change through discrete, achievable goals. For example, if a patient has several days with no blood glucose results on a meter download, setting an attainable goal of checking once each morning under the supervision of caregivers may be more realistic than setting a goal of checking four to six times daily.
An additional strategy that clinicians may consider in clinical encounters with limited blood glucose data is to seek more information from school nurses or rely on verbal reports by the patient and caregivers to attain blood glucose trend data so the diabetes management plan can be optimized. In cases in which patients have not been checking blood glucose, an A1C value provides a representation of average blood glucose, and insulin doses may be adjusted based on the age, weight, BMI, and basal-bolus insulin profile of the patient.
Too Much Data
On the opposite end of the spectrum from having not enough data, the amount of glucose data offered by continuous glucose monitoring (CGM) systems and insulin delivery data from pumps may cause providers to feel overwhelmed or confused, leading to information overload paralysis. Youth with diabetes often have very wide glycemic variability, and discerning a clear pattern in glucose trends may be difficult, even for experienced clinicians.
To prevent information overload paralysis, clinicians should adopt a standard method of reviewing glucose and insulin delivery data (similar to a cardiologist’s approach to reading an electrocardiogram) focusing on glycemic metrics such as time in range and pattern recognition on daily view graphs. For insulin pump users, consider limiting the number of settings until a more consistent pattern emerges.
Advanced technology solutions for data interpretation use machine learning or artificial intelligence (AI) for automated glycemic pattern analysis and decision support. For example, DreaMed Advisor Pro (dreamed-diabetes.com) is a U.S. Food and Drug Administration–approved automated decision support system that aggregates data from diabetes devices to enable clinicians to make personalized therapeutic decisions to optimize clinical outcomes for patients with diabetes. Similarly, there are patient-facing tools that also use machine learning and AI to assist patients with dose optimization between clinic visits (e.g., the TypeZero inControl platform [typezero.com] and IBM Watson/Medtronic [www.ibm.com/case-studies/Medtronic]).
Fear of Hypoglycemia
Fear of hypoglycemia among providers, patients, and caregivers is an additional factor that may lead to therapeutic inertia. Providers practice medicine with the mantra of “First, do no harm,” and some may perceive the risk of acute, severe hypoglycemia as the most pressing concern for a child with diabetes. This perception may lead to permissive hyperglycemia to avoid the risk of low blood glucose episodes. Allowing blood glucose to run high out of fear of lows is not a neutral option and may lead to long-term adverse outcomes such as micro- and macrovascular complications or even impaired neurocognitive development (13,14).
With the availability of basal-bolus insulin regimens, insulin pumps, and CGM devices, there should be a paradigm shift away from simply preventing hypoglycemia and toward optimizing time in range (i.e., time spent maintaining blood glucose in the range of 70–180 mg/dL). Real-time CGM systems provide low, high, and rate-of-change alerts with adjustable thresholds to allow timely intervention to reduce or prevent hypoglycemia. Remote monitoring of CGM data by caregivers and loved ones provides an added layer of support. In recent years, advances in sensor-augmented pump therapy systems such as low threshold suspend (15) and predictive low glucose suspend (16) have helped to minimize hypoglycemia risk. Hybrid closed-loop systems (17) with automated basal rate attenuation may help to reduce hypoglycemia as well as hyperglycemia to optimize time in range outcomes. Looking to the future, a bihormonal closed-loop system (18) that can provide stable, soluble glucagon as well as insulin could provide an additional layer of safety and confidence in diabetes management to help overcome therapeutic inertia.
Inadequate Attention to Psychosocial and Social Determinants of Health
Notwithstanding the time constraints of a clinical encounter, it is worth pausing to acknowledge that integrating the complex requirements of diabetes management into the competing priorities of daily life is often challenging for patients and families and may present barriers to care (19). Psychosocial factors that affect well-being and overall health include social, emotional, behavioral, and environmental considerations that extend beyond physical examination and laboratory findings.
HCPs are encouraged to inquire about patients’ interests, activities, support systems, and personal goals beyond the scope of diabetes management in an effort to treat the whole person rather than a data set of numbers. Person-centered care (20) (in contrast to illness-focused care) encompasses specific communication strategies that promote mutual exchange of information and collaborative goal-setting. Language that focuses on strengths over deficits and that places emphasis on the person rather than the condition (21) can enhance patients’ motivation and the therapeutic alliance with HCPs. Recent pilot studies of strengths-based clinical interventions (22) may help advance this approach in practice.
Many youths with type 1 diabetes experience diabetes distress, burnout, and depression, which are often linked to poor motivation for self-management and subsequent decline in glycemic stability (23–25). Current national and international guidelines recommend an interdisciplinary team approach with psychosocial evaluation included as a component of comprehensive medical care (26,27). Routine depression screening is an example of a brief, clinically relevant assessment that can be reliably integrated into standard visits (28).
Interdisciplinary teams, including diabetes educators, social workers, and psychologists, play an invaluable role in identifying social determinants and psychosocial factors that affect diabetes care. Equally important, connecting to online and offline community resources outside of the clinic, such as peer support, mentoring, camps, and conferences, can contribute to a more robust support system for patients and families as part of a person-centered diabetes care approach.
Static Diabetes Management
The majority of diabetes care transpires between visits, outside of clinical encounters. For children living an active life with diabetes, a dynamic approach to diabetes management is required to optimize glycemic outcomes. However, a standard approach to diabetes management tends to be static and staccato: “Check the blood glucose before each meal and dose insulin based on the current glucose and carbohydrates to be consumed.” Data are then reviewed at 3-month intervals during quarterly clinic visits.
There are many opportunities for diabetes clinics to adopt education strategies to better instruct families to take a dynamic, proactive role in diabetes self-management. As an example, understanding and incorporating CGM trend arrows into real-time management decisions (29–34) may help patients prevent hypo- and hyperglycemia and optimize their time in range. Steven Ponder recommends a dynamic diabetes management approach in his book, Sugar Surfing: How to Manage Type 1 Diabetes in a Modern World (35), which encourages being aware of all variables affecting blood glucose and continuously looping through a four-step cycle of 1) monitoring, 2) being “in the moment,” 3) analyzing, and 4) execution.
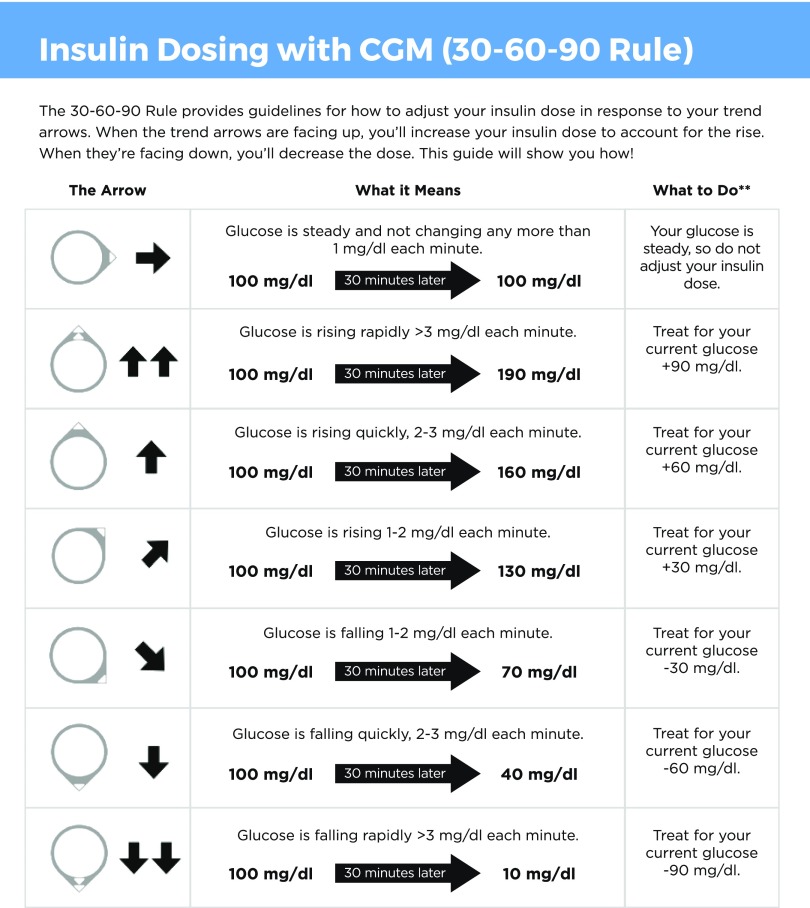
In our clinical practice, we adapted existing published resources and developed the 30–60–90 Rule for optimizing bolus insulin doses based on glucose trend arrows and associated predicted rates of glucose change for pediatric patients using a Dexcom CGM system (Figure 1). Based on predicted rates of glucose change on Dexcom sensors, the 30–60–90 Rule allows users to incorporate glucose trend arrows (rising or falling) in anticipation of the expected change in blood glucose 30 min in the future. Users can add 30, 60, or 90 mg/dL to the current glucose value for a diagonal arrow up, single arrow up, or double arrow up, respectively, or subtract 30, 60, or 90 mg/dL from the current glucose value for a diagonal arrow down, a single arrow down, or a double arrow down, respectively. For example, if the sensor glucose is 200 mg/dL with a double arrow up, the patient would use 290 mg/dL when calculating the correction insulin dose; by contrast, if the sensor glucose is 200 mg/dL with a double arrow down, the patient would use 110 mg/dL when calculating the correction dose.
FIGURE 1.
Insulin dosing using the 30–60–90 Rule to incorporate glucose trend arrows on Dexcom CGM devices in anticipation of the expected change in blood glucose 30 minutes in the future. Graphic content: Daniel DeSalvo. Graphic design: Joyce Lee and Jake Dwyer.
Finally, the selection of an insulin therapy plan is influenced by a variety of factors, including cost, the patient’s lifestyle, prescribing patterns, and pragmatic considerations. Matching a treatment plan to a pediatric patient should be a fluid process, with periodic reassessments of patient/caregiver satisfaction with the plan over time as children grow and develop, technologies evolve, and preferences may change.
Therapeutic Inertia at the Health Care System Level in Pediatric Diabetes
Inattention to Metrics Beyond A1C
Current guidelines from the American Diabetes Association recommend treating pediatric patients with type 1 diabetes to a target A1C of <7.5% (36), and the International Society for Pediatric and Adolescent Diabetes recommends a target of <7% (37). Although A1C has been a gold-standard metric in diabetes, there is widespread recognition of the limitations of the measure (38). Adjunctive glycemic outcomes such as time in range (70–180 mg/dL), severe and clinically important hypoglycemia events, percentage of time in hyperglycemia, and glycemic variability metrics (38) are gaining traction for both clinical and research applications. As CGM uptake continues, there is concurrent research to better understand relationships between A1C and values from CGM metrics (39,40), as well as validate glycemic outcomes beyond A1C to predict acute and long-term complications (41). In addition, integration of patient-reported outcomes into clinical care (38,42–44) and evaluation of patient experience with newer technologies (45) are recognized as important complements to existing and evolving glycemic measures.
Ineffective Medical Nutrition Therapy
The advent of flexible basal-bolus insulin regimens has resulted in liberalized dietary recommendations for youth with type 1 diabetes. Often, the recommendation is to “just eat what you want and cover the carbohydrates with insulin.” Although youth with type 1 diabetes appreciate the flexibility of modern insulin therapy and the greater dietary choices permitted (46,47), the loosening of recommendations about carbohydrate consumption may have contributed to weight gain and diminished glycemic stability in this population. Indeed, more than one in three children in the T1D Exchange Clinic Registry are overweight or obese, including 34% of non-Hispanic white, 43% of black, and 43% of Hispanic children (48), placing them at even higher risk for future cardiovascular complications.
For people with type 1 diabetes, eating fewer refined carbohydrates in favor of more vegetables, unsaturated fat, and protein can reduce hyperglycemia and glycemic variability. In his book Bright Spots and Landmines: The Diabetes Guide I Wish Someone Had Handed Me (49), Adam Brown of the diaTribe Foundation writes, “Eating fewer carbs is the single most important decision I’ve ever made for keeping my blood glucose in a tight range, taking insulin safely, reducing my diabetes burden and stress, and improving my quality of life and overall health.” As professionals in the field of diabetes care, we must enhance medical nutrition therapy for youth with type 1 diabetes to emphasize strategies for “eating for time in range” while concurrently providing flexibility in dietary choices.
Limited Tools
In the past decade, there has been a proliferation of novel drug treatments approved for adults with type 2 diabetes; however, only insulin and pramlinitide are approved for people with type 1 diabetes. This may leave providers feeling that they have limited tools for treating pediatric diabetes. To overcome the challenge of achieving glycemic targets and avoiding weight gain, use of off-label antihyperglycemic agents (oral or injectable) as adjunctive therapy to insulin may be considered (50). However, rates of adjunctive therapy remain low; only 5.3% of all T1D Exchange registrants use any agent other than insulin, and the lowest rates are in those who are <18 years of age (51).
Perhaps the most promising study results evaluating adjunctive therapy options for type 1 diabetes have involved sodium–glucose cotransporter 2 inhibitors, which act through an insulin-independent mechanism to promote urinary glucose excretion and in adults with type 1 diabetes have been shown to reduce A1C, insulin dose, and weight (52–54). However, there are concerns about side effects (55), including fungal and urinary tract infections, acute kidney injury, lower-limb amputation, and euglycemic diabetic ketoacidosis (variably defined as blood glucose <200–300 mg/dL [56]). Strategies to mitigate the ketoacidosis risk have been proposed (57,58). To overcome the therapeutic inertia that exists in the treatment of pediatric type 1 diabetes, adjunctive therapy may be considered in light of the clinical benefit versus risk profile.
Antiquated Clinic Model With Limited Time and Touch Points
In the current type 1 diabetes practice model, patient and family expertise is an underused resource. Existing models of diabetes care delivery that rely on quarterly visits with A1C checks neglect the reality of life with diabetes that is continuous. The vast majority of care tasks and decisions are made outside of the clinic setting, with patients and families poised to acquire the most relevant information about their diabetes management. Therefore, it behooves the care system to support care between clinic visits with infrastructure equal to that used when care is delivered within the confines of a brick-and-mortar clinic encounter.
Effective self-management involves a combination of knowledge, skills, and behaviors to integrate complex requirements of diabetes care into the daily routine (59). Despite the widespread recognition of the essential nature of diabetes self-management, an antiquated gatekeeping model for decision-making (e.g., the perception that families are required to contact the diabetes center for insulin dose changes) persists, particularly in pediatrics. Clinics are encouraged to develop strategies that expand the concept of patient-centered care to patient-empowered care. Engaging in shared decision-making (60,61) about dose adjustments that acknowledges patients’ expertise in diabetes care while still providing input and professional guidance provides an opportunity to nurture autonomy in a supported context. As patients and families build confidence and competence, clinics are encouraged to offer assistance when needed, while simultaneously deputizing patients to practice more proactive self-management between visits (9,62).
Summary and Call to Action
As a diabetes community of HCPs and patient and family experts, we must remain vigilant against the temptation to succumb to complacency in the form of therapeutic inertia. There are opportunities to move beyond therapeutic inertia toward therapeutic alliance at both the patient-provider and health care system levels.
As clinicians, we can bring positive change to the lives of our patients, affecting not just clinical outcomes, but also the quality of life of children with diabetes. Instead of viewing patients who are struggling with diabetes management as burdensome, we can choose to recognize an opportunity to provide support and motivation and facilitate a meaningful therapeutic alliance that can improve health outcomes. We must recognize our own internal biases and ensure that all youth with diabetes are provided comprehensive medical and psychosocial care and an opportunity to progress in their diabetes self-management. Interdisciplinary diabetes care teams must practice a person- and family-centered approach that incorporates the physical, emotional, behavioral, and social needs of patients.
Health system interventions include developing national and international registries and learning networks (63). Quality improvement science offers a methodology to identify an aim, evaluate complex contributors to the goal, and test potential interventions to achieve outcomes of interest. Networks of clinical sites working in collaboration with quality improvement methods provide an evolving mechanism for dissemination and widespread application of best practices (64–67). Persistent advocacy for innovative and affordable treatment options is necessary in the current health care climate, with disruptive do-it-yourself solutions in the diabetes community often acting as an accelerator. In pediatric diabetes care, we need to adopt a sense of urgency and focus to change the tide of suboptimal health outcomes by embracing a strategy of therapeutic action.
Therapeutic inertia in pediatric diabetes has impeded our ability to deliver optimal clinical outcomes at both the individual patient and population health levels. Clearly, there is no “silver bullet” solution or single issue to address, but rather, innovative and disruptive multifactorial solutions are required to transform outcomes in pediatric diabetes.
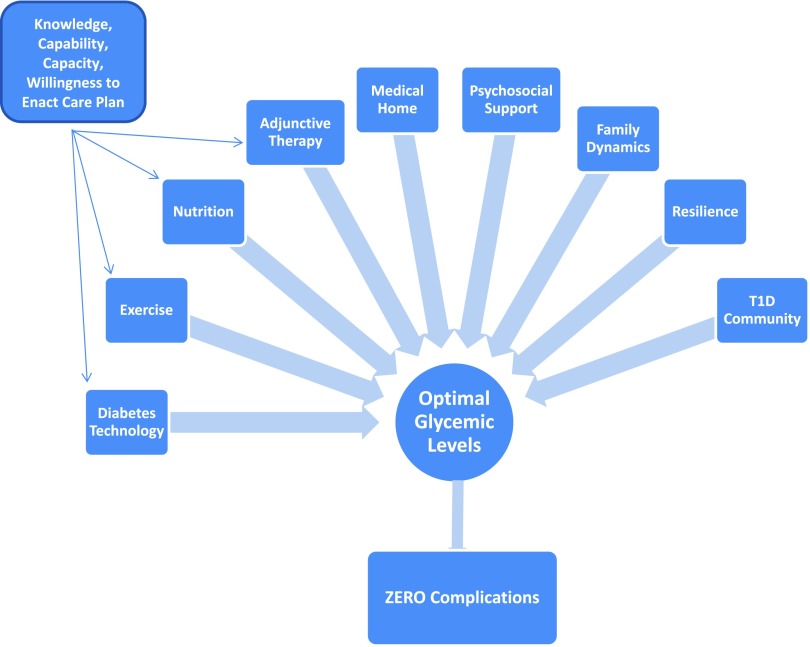
In traffic safety culture, Vision Zero (visionzeronetwork.org) is a visionary target to eliminate all traffic fatalities and severe injuries, based on the ethical belief that everyone has the right to move safely in their communities. We propose an aspiration for a pediatric diabetes Vision Zero (Figure 2), in which the health care system assumes responsibility to collectively overcome therapeutic inertia by continuously adapting to better account for the needs, vulnerabilities, and limitations of patients and current treatment paradigms. Understanding the life circumstances and individual needs of patients and families will be essential to shared medical decision-making in optimizing diabetes self-management. As we move past therapeutic inertia and toward therapeutic alliance and action, we envision a potential for children with diabetes to be empowered to live long, healthy, active lives with zero complications.
FIGURE 2.
Proposed Vision Zero–style aspiration for pediatric diabetes, in which the health care system collectively adapts to overcome therapeutic inertia and facilitate therapeutic alliance, empowering children with diabetes to live long, healthy, active lives without complications.
Acknowledgments
The authors acknowledge the contributions of patients, families, diabetes care teams, and collaborators within the TID Exchange quality improvement collaborative to continually seek to improve care and outcomes for people with diabetes.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
Both authors researched data, wrote, and edited the manuscript. Together they are the guarantors of this work and take responsibility for the integrity of the data and ideas presented and the accuracy of the data analysis.
References
- 1.DCCT Research Group Diabetes Control and Complications Trial (DCCT). Update. Diabetes Care 1990;13:427–433 [DOI] [PubMed] [Google Scholar]
- 2.Lachin JM, White NH, Hainsworth DP, Sun W, Cleary PA, Nathan DM; Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group . Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes 2015;64:631–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gosmanov AR, Gosmanova EO. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the DCCT/EDIC cohort [Letter]. Arch Intern Med 2011;171:1596–; author reply 1597. [DOI] [PubMed] [Google Scholar]
- 4.Genuth SM, Backlund JY, Bayless M, et al. ; DCCT/EDIC Research Group . Effects of prior intensive versus conventional therapy and history of glycemia on cardiac function in type 1 diabetes in the DCCT/EDIC. Diabetes 2013;62:3561–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster NC, Beck RW, Miller KM, et al. . State of type 1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips LS, Branch WT, Cook CB, et al. . Clinical inertia. Ann Intern Med 2001;135:825–834 [DOI] [PubMed] [Google Scholar]
- 7.Avignon A, Attali C, Sultan A, Ferrat E, Le Breton J.. Clinical inertia: viewpoints of general practitioners and diabetologists. Diabetes Metab 2012;38(Suppl. 3):S53–S58 [DOI] [PubMed] [Google Scholar]
- 8.Nambam B, DuBose SN, Nathan BM, et al. ; T1D Exchange Clinic Network . Therapeutic inertia: underdiagnosed and undertreated hypertension in children participating in the T1D Exchange Clinic Registry. Pediatr Diabetes 2016;17:15–20 [DOI] [PubMed] [Google Scholar]
- 9.Powell PW, Corathers SD, Raymond J, Streisand R. New approaches to providing individualized diabetes care in the 21st century. Curr Diabetes Rev 2015;11:222–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gayes LA, Steele RG. A meta-analysis of motivational interviewing interventions for pediatric health behavior change. J Consult Clin Psychol 2014;82:521–535 [DOI] [PubMed] [Google Scholar]
- 11.Powell PW, Hilliard ME, Anderson BJ. Motivational interviewing to promote adherence behaviors in pediatric type 1 diabetes. Curr Diab Rep 2014;14:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Channon SJ, Huws-Thomas MV, Rollnick S, et al. . A multicenter randomized controlled trial of motivational interviewing in teenagers with diabetes. Diabetes Care 2007;30:1390–1395 [DOI] [PubMed] [Google Scholar]
- 13.Marzelli MJ, Mazaika PK, Barnea-Goraly N, et al. ; Diabetes Research in Children Network (DirecNet) . Neuroanatomical correlates of dysglycemia in young children with type 1 diabetes. Diabetes 2014;63:343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mauras N, Mazaika P, Buckingham B, et al. ; Diabetes Research in Children Network (DirecNet) . Longitudinal assessment of neuroanatomical and cognitive differences in young children with type 1 diabetes: association with hyperglycemia. Diabetes 2015;64:1770–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergenstal RM, Klonoff DC, Garg SK, et al. ; ASPIRE In-Home Study Group . Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013;369:224–232 [DOI] [PubMed] [Google Scholar]
- 16.Forlenza GP, Li Z, Buckingham BA, et al. . Predictive low-glucose suspend reduces hypoglycemia in adults, adolescents, and children with type 1 diabetes in an at-home randomized crossover study: results of the PROLOG Trial. Diabetes Care 2018;41:2155–2161 [DOI] [PubMed] [Google Scholar]
- 17.Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol 2017;5:501–512 [DOI] [PubMed] [Google Scholar]
- 18.El-Khatib FH, Balliro C, Hillard MA, et al. . Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Lancet 2017;389:369–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valenzuela JM, Seid M, Waitzfelder B, et al. ; SEARCH for Diabetes in Youth Study Group . Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. J Pediatr 2014;164:1369–1375.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz DD, Stewart SD, Aikens JE, Bussell JK, Osborn CY, Safford MM. Seeing the person, not the illness: promoting diabetes medication adherence through patient-centered collaboration. Clin Diabetes 2017;35:35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickinson JK, Guzman SJ, Maryniuk MD, et al. . The use of language in diabetes care and education. Diabetes Educ 2017;43:551–564 [DOI] [PubMed] [Google Scholar]
- 22.Hilliard ME, Eshtehardi SS, Minard CG, et al. . Strengths-based, clinic-integrated nonrandomized pilot intervention to promote type 1 diabetes adherence and well-being. J Pediatr Psychol 2019;44:5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGrady ME, Laffel L, Drotar D, Repaske D, Hood KK. Depressive symptoms and glycemic control in adolescents with type 1 diabetes: mediational role of blood glucose monitoring. Diabetes Care 2009;32:804–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hood KK, Rausch JR, Dolan LM. Depressive symptoms predict change in glycemic control in adolescents with type 1 diabetes: rates, magnitude, and moderators of change. Pediatr Diabetes 2011;12:718–723 [DOI] [PubMed] [Google Scholar]
- 25.Hilliard ME, Herzer M, Dolan LM, Hood KK. Psychological screening in adolescents with type 1 diabetes predicts outcomes one year later. Diabetes Res Clin Pract 2011;94:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young-Hyman D, de Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016;39:2126–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delamater AM, de Wit M, McDarby V, et al. . ISPAD Clinical Practice Consensus Guidelines 2018: Psychological care of children and adolescents with type 1 diabetes. Pediatr Diabetes 2018;19(Suppl. 27):237–249 [DOI] [PubMed] [Google Scholar]
- 28.Corathers S, Mara CA, Chundi PK, Kichler JC. Depression screening of adolescents with diabetes: 5-years of implementation and outcomes. J Am Acad Child Adolesc Psychiatry 2019;58:628–632 [DOI] [PubMed] [Google Scholar]
- 29.Aleppo G, Laffel LM, Ahmann AJ, et al. . A practical approach to using trend arrows on the Dexcom G5 CGM system for the management of adults with diabetes. J Endocr Soc 2017;1:1445–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laffel LM, Aleppo G, Buckingham BA, et al. . A practical approach to using trend arrows on the Dexcom G5 CGM system to manage children and adolescents with diabetes. J Endocr Soc 2017;1:1461–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kudva YC, Ahmann AJ, Bergenstal RM, et al. . Approach to using trend arrows in the FreeStyle Libre flash glucose monitoring systems in adults. J Endocr Soc 2018;2:1320–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diabetes Research In Children Network (DirecNet) Study Group; Buckingham B, Xing D, Weinzimer S, et al. . Use of the DirecNet Applied Treatment Algorithm (DATA) for diabetes management with a real-time continuous glucose monitor (the FreeStyle Navigator). Pediatr Diabetes 2008;9:142–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pettus J, Edelman SV. Recommendations for using real-time continuous glucose monitoring (rtCGM) data for insulin adjustments in type 1 diabetes. J Diabetes Sci Technol 2017;11:138–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klonoff DC, Ahn D, Drincic A. Continuous glucose monitoring: a review of the technology and clinical use. Diabetes Res Clin Pract 2017;133:178–192 [DOI] [PubMed] [Google Scholar]
- 35.Ponder SW, McMahon KL. Sugar Surfing: How to Manage Type 1 Diabetes in a Modern World. Sausalito, CA, Mediself Press, 2015 [Google Scholar]
- 36.Chiang JL, Kirkman MS, Laffel LM, Peters AL; Type 1 Diabetes Sourcebook Authors . Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 2014;37:2034–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiMeglio LA, Acerini CL, Codner E, et al. . ISPAD Clinical Practice Consensus Guidelines 2018: Glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes 2018;19(Suppl. 27):105–114 [DOI] [PubMed] [Google Scholar]
- 38.Agiostratidou G, Anhalt H, Ball D, et al. . Standardizing clinically meaningful outcome measures beyond HbA1c for type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care 2017;40:1622–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergenstal RM, Beck RW, Close KL, et al. . Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care 2018;41:2275–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beck RW, Bergenstal RM, Cheng P, et al. . The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol 2019;13:614–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beck RW, Bergenstal RM, Riddlesworth TD, et al. . Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 2019;42:400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Wit M, Delemarre-van de Waal HA, Bokma JA, et al. . Monitoring and discussing health-related quality of life in adolescents with type 1 diabetes improve psychosocial well-being: a randomized controlled trial. Diabetes Care 2008;31:1521–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boogerd EA, Damhuis AM, van Alfen-van der Velden JA, et al. . Assessment of psychosocial problems in children with type 1 diabetes and their families: the added value of using standardised questionnaires in addition to clinical estimations of nurses and paediatricians. J Clin Nurs 2015;24:2143–2151 [DOI] [PubMed] [Google Scholar]
- 44.Corathers SD, Mara CA, Chundi PK, Kichler JC. Psychosocial patient-reported outcomes in pediatric and adolescent diabetes: a review and case example. Curr Diab Rep 2017;17:45. [DOI] [PubMed] [Google Scholar]
- 45.Weissberg-Benchell J, Hood K, Laffel L, et al. . Toward development of psychosocial measures for automated insulin delivery. J Diabetes Sci Technol 2016;10:799–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehta SN, Haynie DL, Higgins LA, et al. . Emphasis on carbohydrates may negatively influence dietary patterns in youth with type 1 diabetes. Diabetes Care 2009;32:2174–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rankin D, Cooke DD, Clark M, Heller S, Elliott J, Lawton J; UK NIHR DAFNE Study Group . How and why do patients with type 1 diabetes sustain their use of flexible intensive insulin therapy? A qualitative longitudinal investigation of patients’ self-management practices following attendance at a Dose Adjustment for Normal Eating (DAFNE) course. Diabet Med 2011;28:532–538 [DOI] [PubMed] [Google Scholar]
- 48.Willi SM, Miller KM, DiMeglio LA, et al. ; T1D Exchange Clinic Network . Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics 2015;135:424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown A. Bright Spots and Landmines: The Diabetes Guide I Wish Someone Had Handed Me. San Francisco, CA, The diaTribe Foundation, 2017 [Google Scholar]
- 50.Nathan DM. Adjunctive treatments for type 1 diabetes. N Engl J Med 2017;377:2390–2391 [DOI] [PubMed] [Google Scholar]
- 51.Lyons SK, Hermann JM, Miller KM, et al. . Use of adjuvant pharmacotherapy in type 1 diabetes: international comparison of 49,996 individuals in the Prospective Diabetes Follow-up and T1D Exchange registries. Diabetes Care 2017;40:e139–e140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and safety of canagliflozin, a sodium–glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care 2015;38:2258–2265 [DOI] [PubMed] [Google Scholar]
- 53.Dandona P, Mathieu C, Phillip M, et al. ; DEPICT-1 Investigators . Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT-1 52-week study. Diabetes Care 2018;41:2552–2559 [DOI] [PubMed] [Google Scholar]
- 54.Rosenstock J, Marquard J, Laffel LM, et al. . Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care 2018;41:2560–2569 [DOI] [PubMed] [Google Scholar]
- 55.Ueda P, Svanström H, Melbye M, et al. . Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: nationwide register based cohort study. BMJ 2018;363:k4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peters AL, Henry RR, Thakkar P, Tong C, Alba M. Diabetic ketoacidosis with canagliflozin, a sodium–glucose cotransporter 2 inhibitor, in patients with type 1 diabetes. Diabetes Care 2016;39:532–538 [DOI] [PubMed] [Google Scholar]
- 57.Garg SK, Peters AL, Buse JB, Danne T. Strategy for mitigating DKA risk in patients with type 1 diabetes on adjunctive treatment with SGLT inhibitors: a STICH protocol. Diabetes Technol Ther 2018;20:571–575 [DOI] [PubMed] [Google Scholar]
- 58.Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care 2015;38:1638–1642 [DOI] [PubMed] [Google Scholar]
- 59.Modi AC, Pai AL, Hommel KA, et al. . Pediatric self-management: a framework for research, practice, and policy. Pediatrics 2012;129:e473–e485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiley J, Westbrook M, Greenfield JR, Day RO, Braithwaite J. Shared decision-making: the perspectives of young adults with type 1 diabetes mellitus. Patient Prefer Adherence 2014;8:423–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valenzuela JM, Smith LB, Stafford JM, et al. . Shared decision-making among caregivers and health care providers of youth with type 1 diabetes. J Clin Psychol Med Settings 2014;21:234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iannotti RJ, Schneider S, Nansel TR, et al. . Self-efficacy, outcome expectations, and diabetes self-management in adolescents with type 1 diabetes. J Dev Behav Pediatr 2006;27:98–105 [DOI] [PubMed] [Google Scholar]
- 63.Corathers SD, Schoettker PJ, Clements MA, et al. . Health-system-based interventions to improve care in pediatric and adolescent type 1 diabetes. Curr Diab Rep 2015;15:91. [DOI] [PubMed] [Google Scholar]
- 64.Kilo CM. A framework for collaborative improvement: lessons from the Institute for Healthcare Improvement’s Breakthrough Series. Qual Manag Health Care 1998;6:1–13 [DOI] [PubMed] [Google Scholar]
- 65.Peterson A, Hanberger L, Akesson K, Bojestig M, Andersson Gäre B, Samuelsson U. Improved results in paediatric diabetes care using a quality registry in an improvement collaborative: a case study in Sweden. PLoS One 2014;9:e97875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lyren A, Brilli RJ, Zieker K, Marino M, Muething S, Sharek PJ. Children’s hospitals’ solutions for patient safety collaborative impact on hospital-acquired harm. Pediatrics 2017;140:e20163494. [DOI] [PubMed] [Google Scholar]
- 67.McLinden D, Myers S, Seid M, Busch M, Davis D, Murphy J. The Learning Exchange, a community knowledge commons for learning networks: qualitative evaluation to test acceptability, feasibility, and utility. JMIR Form Res 2019;3:e9858. [DOI] [PMC free article] [PubMed] [Google Scholar]