Abstract
Background and objective
Exercise is a cornerstone of management for type 2 diabetes; however, little is known about the cardiovascular (CV) response to submaximal functional exercise in people with type 2 diabetes. The aim of this study was to compare performance and CV response during a 6-minute walk test (6MWT) between people with type 2 diabetes and matched control subjects.
Methods
CV response and distance walked during the 6MWT were assessed in 30 people with type 2 diabetes, matched for age, body composition, physical activity, and estimated aerobic capacity with 34 control subjects (type 2 diabetes group: 16 men, 59.8 ± 8.8 years of age, 33.3 ± 10.9% body fat, physical activity of 7,968 ± 3,236 steps·day−1, estimated aerobic capacity 31.9 ± 11.1 mLO2·kg−1·min−1; control group: 19 men, 59.3 ± 8.8 years of age, 32.7 ± 8.5% body fat, physical activity 8,228 ± 2,941 steps·day−1, estimated aerobic capacity 34.9 ± 15.4 mLO2·kg−1·min−1).
Results
People with type 2 diabetes walked a similar distance (590 ± 75 vs. 605 ± 69 m; P = 0.458) compared with control subjects during the 6MWT and had similar ratings of perceived exertion (RPE) after the 6MWT (4.19 ± 1.56 vs. 3.65 ± 1.54, P = 0.147). However, at the end of the 6MWT, people with type 2 diabetes had a higher heart rate (108 ± 23 vs. 95 ± 18 beats·min−1; P = 0.048), systolic blood pressure (169 ± 26 vs. 147 ± 22 mmHg, P = 0.003), and rate-pressure product (18,762 ± 5,936 vs. 14,252 ± 4,330, P = 0.009) than control subjects.
Conclusion
Although people with type 2 diabetes had similar performance and RPE during the 6MWT compared with control subjects, the CV response was greater for people with type 2 diabetes, indicating greater cardiac effort for similar perceived effort and performance of 6MWT. These data suggest that observation and prescription of exercise intensity should include both perceived effort and CV response.
Submaximal exercise is a cornerstone of management for type 2 diabetes, and along with diet and weight loss is a first line of medical management (1). Moderate to vigorous aerobic exercise training, such as brisk walking, 3–7 days per week for ∼30 minutes per day, is the primary exercise recommendation for people with type 2 diabetes (1). However, rates of exercise intolerance are high in people with type 2 diabetes, thought to be caused by impairments in peripheral blood flow and oxygen diffusion to the exercising muscle, as recently reviewed (2). For example, among a small cohort (n = 20) of sedentary people with type 2 diabetes and age- and activity-matched control subjects, those with type 2 diabetes had ∼24% lower performance and a lower relative rate of oxygen consumption (mLO2·kg−1·min−1) during maximal walking exercise and lower oxygen uptake at similar submaximal absolute loads (speed and gradient in treadmill walking), likely indicative of impaired muscle oxygen delivery (3). Additionally, a recent review provided evidence of reduced maximal heart rate response during exercise and blunted cardiac output in people with type 2 diabetes compared with control subjects (4); however, the studies included in the review compared untrained people with type 2 diabetes with low maximal rates of oxygen consumption to control subjects with higher maximal rates of oxygen consumption. Any level of cardiovascular (CV) impairment is therefore difficult to quantify in these studies because those with type 2 diabetes were less fit than the control subjects. Thus, it is important to determine the CV response to brisk walking in active people with type 2 diabetes compared with control subjects appropriately matched for fitness; however, this has not been studied to date.
Therefore, the primary purpose of this study was to compare 6-minute walk test (6MWT) performance and CV response between people with type 2 diabetes and control subjects without diabetes matched for age, body composition, measured physical activity, and estimated aerobic capacity. The hypotheses were that people with type 2 diabetes would demonstrate impaired performance and exhibit greater CV response to the 6MWT compared with healthy control subjects. The novelty of this investigation lies in examining the CV response to walking in people with type 2 diabetes who were closely matched to healthy control subjects on body composition, physical activity, and fitness parameters, hence minimizing potential confounders. The secondary purpose of this study, per recommendations by the National Institutes of Health (NIH) for all clinical research (NIH notice number NOT-OD-15–102), was to test sufficient men and women in each cohort to determine potential sex-related differences in the performance of and CV response to the 6MWT.
Methods
Thirty people with type 2 diabetes (16 men, 14 women) and 34 control subjects without diabetes (19 men, 15 women) matched for age (59.8 ± 8.8 vs. 59.3 ± 8.8 years, P = 0.858), relative body fat (33.3 ± 10.9 vs. 32.7 ± 8.5%, P = 0.846), total lean muscle mass (53.1 ± 12.1 vs. 51.5 ± 12.2 kg, P = 0.194), daily physical activity (7,968 ± 3,236 vs. 8,228 ± 2,941 steps·day−1, P = 0.905), and estimated aerobic capacity (31.9 ± 11.1 vs. 34.9 ± 15.4 mLO2·kg−1·min−1, P = 0.393) completed a 6MWT. As expected, people with type 2 diabetes had higher A1C (P <0.001), fasting plasma glucose (P <0.001), fasting plasma insulin (P = 0.004), and homeostatic model assessment of insulin resistance (HOMA-IR) values (P = 0.001) compared with control subjects, and control subjects did not evidence insulin resistance or impaired glycemic control. Participant demographics can be found in Table 1.
TABLE 1.
Participant Characteristics, Diabetes Markers, and Medications
| Type 2 Diabetes (n = 30) | Control (n = 34) | |
|---|---|---|
| Age, years | 59.8 ± 8.8 | 59.3 ± 8.8 |
| Height, cm | 172.5 ± 8.5 | 171.5 ± 9.0* |
| Weight, kg | 88.6 ± 24.7 | 81.4 ± 16.2* |
| BMI, kg·m−2 | 29.6 ± 7.0 | 27.5 ± 4.2 |
| Body fat, % | 33.3 ± 10.9 | 32.7 ± 8.5* |
| Lean mass, kg | 53.1 ± 12.1 | 51.5 ± 12.2* |
| Daily physical activity, steps·day−1 | 7,970 ± 3,240 | 8,230 ± 2,940 |
| eVO2, mL·kg−1·min−1 | 31.9 ± 11.1 | 35.4 ± 15.3* |
| Diabetes duration, years | 7.1 ± 6.3 | —† |
| A1C, mmol·mol−1 | 53.1 ± 11.0 | 36.7 ± 3.6† |
| A1C, % | 7.01 ± 1.00 | 5.51 ± 0.32† |
| Fasting plasma glucose, mg·dL−1 | 123.5 ± 31.7 | 89.2 ± 13.3† |
| Fasting plasma insulin, pmol·L−1 | 60.2 ± 41.9 | 33.7 ± 22.2† |
| HOMA-IR, AU | 2.91 ± 1.69 | 1.39 ± 1.10† |
| Metformin, n | 30 | — |
| Metformin dosage, mg·day−1 | 1,060 ± 531 | — |
| Duration of metformin prescription, years | 4.42 ± 4.60 | — |
| Statin, n | 19 | 3 |
| Statin dose equivalent, mg·day−1‡ | 23.3 ± 26.7 | 11.7 ± 7.6 |
| Duration of statin prescription, years | 6.50 ± 5.52 | 3.00 ± 4.36 |
| ACE inhibitor, n | 7 | 2 |
| ACE inhibitor dose equivalent, mg·day−1§ | 27.0 ± 14.5 | 6.3 ± 5.3 |
| Duration of ACE inhibitor prescription, years | 7.1 ± 5.2 | 4.3 ± 5.3 |
Values are displayed as mean ± SD.
Sex difference (male vs. female), P <0.05. †Group differences (type 2 diabetes vs. control), P <0.05. ‡Statin data are reported using an atorvastatin equivalent dose per published drug information.
ACE inhibitor data are reported using a lisinopril equivalent dose per published drug information. AU, arbitrary unit.
To minimize heterogeneity within and between the type 2 diabetes and control groups, exclusion criteria included coronary artery disease, vascular disease, kidney disease, hormone replacement therapy, A1C >10%, prescribed insulin or insulin secretagogue, diabetic peripheral or autonomic neuropathy, peripheral edema, severe obesity (BMI >45 kg·m−2), thyroid dysfunction, epilepsy, anxiety, depression, current smoking, possibility of pregnancy, and any neurological, CV, or musculoskeletal disease that precluded exercise testing. Additionally, all women enrolled self-reported a postmenopausal status, with their final menstrual period 3–11 years before study enrollment. All participants with type 2 diabetes were prescribed metformin; 19 from the type 2 diabetes group and 3 from the control group were prescribed a statin. Also, seven participants with type 2 diabetes and two control subjects were prescribed an ACE inhibitor. There were no additional prescribed medications other than metformin, statins, and ACE inhibitors.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants included in the study. The protocol was approved by the Marquette University institutional review board (HR-2402).
People with type 2 diabetes were screened for diabetic polyneuropathy using a 10-g monofilament test (12 pedal sites), 128-Hz vibration sensation test (malleoli and first metatarsal head), and Achilles reflex test. Potential participants who evidenced signs of neuropathy were excluded. Participants were screened for cardiac autonomic neuropathy via measurement of blood pressure response to standing and exercise electrocardiogram (EKG). Estimated maximal aerobic capacity (eVO2) was determined using a submaximal, three-stage (increments within 40–70% heart rate reserve), graded exercise cycle ergometer (VIAsprint 150P; CareFusion, San Diego, CA) test with 12-lead EKG monitoring (CASE; General Electrics, Madison, WI). Linear regression analysis was performed between submaximal workload and steady-state heart rate response according to validated YMCA standards (5); and eVO2 was based on individual regressions. Body fat and lean (muscle) mass were assessed using dual X-ray absorptiometry (Lunar Prodigy full-body scanner; GE, Madison, WI). A1C and fasting plasma glucose concentration were determined by assay using certified point-of-care instruments (DCA 2000+, Siemens Healthcare Diagnostics, Malvern, PA, and Alere Cholestech LDX System, Alere Inc., Waltham, MA, respectively), which were calibrated monthly. Plasma insulin was quantitatively assayed in duplicate per manufacturer instructions using an enzyme-linked immunoassay kit (Quantikine Human Insulin Immunoassay, R&D Systems, Minneapolis, MN). HOMA-IR was calculated using fasting plasma insulin (FPI, mU·L−1) and fasting plasma glucose (FPG, mmol·L−1) concentrations: HOMA-IR = (FPI × FPG) · 22.5−1. After potential participants completed the informed consent and screening tests described above, daily physical activity was assessed via accelerometry using a triaxial accelerometer (Actigraph GT3×; ActiGraph, Pensacola, FL) worn for at least 3 days for at least 9 hours (540 minutes) per day, from which step counts were recorded and analyzed (ActiLife v4; ActiGraph, Pensacola, FL). Potential participants were excluded if they were classified as sedentary (<5,000 steps·day−1, n = 2) or highly active (>12,500 steps·day−1, n = 2) (6) to minimize heterogeneity.
The 6MWT was performed on an indoor course, and participants were instructed (using standardized encouragement) to walk as quickly as possible for 6 minutes. The distance walked was measured to the closest meter. The 6MWT is highly correlated with the NIH Toolbox measure of functional endurance (the 2-minute walk test) (7), and performance on the 6MWT is predictive of morbidity and mortality (8). Predicted performance was estimated using standard equations based on participant characteristics (i.e., height, weight, age, and biological sex) (9), and relative performance (percentage of predicted distance) was calculated as follows: (measured walk distance)·(predicted walk distance)−1.
Immediately before and after the 6MWT, while participants were seated, blood pressure was manually auscultated using a standard aneroid sphygmomanometer and a stethoscope placed over the antecubital fossa. Simultaneously, heart rate was manually palpated at the radial artery on the opposite wrist. The rate-pressure product (systolic blood pressure [mmHg]·heart rate [beats·min−1]) was calculated for both time points. Participants were seated for at least 5 minutes before the baseline assessments of blood pressure and heart rate before the 6MWT. After completion of the 6MWT, participants were immediately seated on the same chair used for baseline assessments, and assessments of blood pressure and heart rate were performed again. Values are reported as mean ± SD in the text and tables and displayed as mean ± SE in the figure.
The statistical approach and sample size were determined a priori. Univariate ANOVA with group (type 2 diabetes, control) and sex (male, female) as between-subject factors were used to compare participant characteristics (Table 1) and 6MWT performance. Repeated-measures ANOVA, with time as a within-subject factor (pre- vs. post-6MWT) and group and sex as between-subject factors, was used to determine CV response to the 6MWT. Ratings of perceived exertion (RPE) were not normally distributed for people with type 2 diabetes or control subjects (Shapiro-Wilks test of normality, P = 0.048 and P <0.001, respectively); thus, a Mann-Whitney U test was used to compare RPE after the 6MWT between groups. All other data passed assumptions of parametric statistical tests. Significance was accepted at P <0.05, and analyses were performed using a statistical package (SPSS, version 24; IBM, Armonk, NY). The effect size (η2) appropriate for ANOVA is reported for statistically significant results, interpreted as small (0.01 ≤ η2 < 0.06), medium (0.06 ≤ η2 < 0.14), and large (0.14 ≤ η2) (10).
Results
For all variables, the main effect of sex or the interactions of sex with time or group are only reported if significant (P <0.05); otherwise, these statistics are not reported.
Under resting conditions, heart rate (group, P = 0.098), diastolic blood pressure (group, P = 0.212), and mean arterial pressure (group, P = 0.081) did not differ between people with type 2 diabetes compared with control subjects without diabetes. However, people with type 2 diabetes had greater systolic blood pressure (group, P = 0.036, η2 = 0.071) and rate-pressure product (group, P = 0.018, η2 = 0.104) than control subjects.
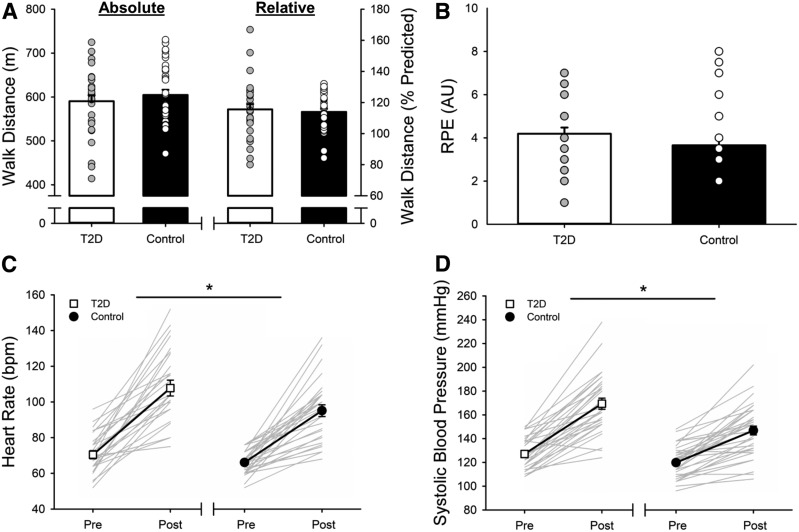
The 6MWT distance achieved (group, P = 0.458) did not differ between people with type 2 diabetes and control subjects; therefore, walking speed was not different between groups; relative performance (percentage of predicted distance) was also not different (group, P = 0.678) (Figure 1A). However, 6MWT distance was greater for men than for women (615 ± 74 vs. 578 ± 64 m; sex, P = 0.048, η2 = 0.064), although women had better relative performance (119 ± 9 vs. 111 ± 18%; sex, P = 0.026, η2 = 0.080). The increase in RPE (from zero) at rest to the end of the 6MWT was not different for people with type 2 diabetes compared with control subjects (time, P <0.001; time × group, P = 0.273; Figure 1B). However, people with type 2 diabetes had greater increases in heart rate (time, P <0.001, η2 = 0.756; time × group, P = 0.048, η2 = 0.052; Figure 1C), systolic blood pressure (time, P <0.001, η2 = 0.774; time × group, P = 0.003, η2 = 0.137; Figure 1D), and rate-pressure product (post-6MWT: 18,762 ± 5,936 vs. 14,251 ± 4,330; time, P <0.001, η2 = 0.751; time × group, P = 0.009, η2 = 0.124) compared with control subjects. Diastolic blood pressure did not change during the 6MWT for either group (time, P = 0.488; time × group, P = 0.150), and the increase in mean arterial pressure did not differ between groups (time, P <0.001, η2 = 0.722; time × group, P = 0.160) (Table 2).
FIGURE 1.
6MWT performance (A) and the increase in RPE (B), heart rate (C), and systolic blood pressure (D) for people with type 2 diabetes and control subjects without diabetes. Values are displayed as mean ± SE, superimposed on top of individual data. A: The 6MWT distance and relative performance (percentage of predicted distance) did not differ for people with type 2 diabetes and control subjects; individual data are represented as circles. B: The increase in RPE (from zero) did not differ between people with type 2 diabetes and control subjects; individual data are represented as circles. C: The increase in heart rate was greater for people type 2 diabetes than for control subjects (*P <0.05); individual data are represented as gray lines. D: The increase in systolic blood pressure was greater for people type 2 diabetes than for control subjects (*P <0.05); individual data are represented as gray lines.
TABLE 2.
CV Variables at Rest Before and Immediately After a 6MWT
| Type 2 Diabetes (n = 30) | Control (n = 34) | |
|---|---|---|
| Before 6MWT | ||
| Heart rate, beats·min−1 | 70.4 ± 11.8 | 66.1 ± 6.7 |
| Systolic blood pressure, mmHg | 127.1 ± 13.2 | 119.8 ± 13.4* |
| Diastolic blood pressure, mmHg | 76.7 ± 9.4 | 73.8 ± 8.3 |
| Mean arterial pressure, mmHg | 93.5 ± 9.6 | 89.1 ± 9.5 |
| Rate-pressure production, mmHg·beats·min−1 | 9,007 ± 19,858 | 7,927 ± 1,139* |
| After 6MWT | ||
| Heart rate, beats·min−1 | 107.8 ± 23.1 | 95.1 ± 17.6*† |
| Systolic blood pressure, mmHg | 169.4 ± 25.6 | 146.8 ± 21.6*† |
| Diastolic blood pressure, mmHg | 75.5 ± 11.5 | 75.9 ± 9.0 |
| Mean arterial pressure, mmHg | 106.8 ± 11.5 | 99.5 ± 11.5 |
| Rate-pressure product, mmHg·beats·min−1 | 18,762 ± 5,936 | 14,252 ± 4,330*† |
Values are displayed as mean ± SD.
Group differences (type 2 diabetes vs. control), P <0.05.
Time × group interaction (type 2 diabetes vs. control), P <0.05.
Discussion
People with type 2 diabetes exhibited similar absolute and relative performance during the 6MWT compared with control subjects without diabetes who were matched for age, body fat, lean mass, daily physical activity, and estimated aerobic fitness. Both groups were physically active (>7,500 steps·day−1) (11) and performed ∼20% better than predicted on the 6MWT. Additionally, people with type 2 diabetes had similar increases in RPE after a 6MWT compared with healthy control subjects. However, despite similar levels of performance and perceived effort and no clinical evidence of cardiac autonomic neuropathy, people with type 2 diabetes evidenced greater cardiac effort (heart rate, systolic blood pressure, and rate-pressure product indicating greater myocardial oxygen demand) during a 6MWT than matched control subjects without diabetes. Despite sex-related differences in 6MWT performance, RPE and CV responses to a 6MWT did not differ between men and women.
Previous research has demonstrated a blunted heart rate (∼2–3% lower) during maximal exercise in people with type 2 diabetes compared with control subjects (4,12); however, this blunted heart rate is not ubiquitously observed, and a higher exercise heart rate has also been observed among women with type 2 diabetes during submaximal exercise compared with control women (13).
Previous research has also demonstrated impaired maximal performance and reduced maximal oxygen consumption during brisk walking (graded treadmill exercise to exhaustion) in sedentary people with type 2 diabetes compared with control subjects matched for age, sex, and body mass, as well as lower submaximal oxygen consumption at the same treadmill speed and grade (work rate) (3). Lower oxygen consumption at submaximal and maximal work rates is interpreted as reflecting impaired peripheral oxygen delivery in people with type 2 diabetes (2), suggesting that impaired oxygen delivery limited exercise performance in the cohort of people with uncomplicated, non–insulin-dependent type 2 diabetes in the previous study (3). This impaired peripheral oxygen delivery may elicit a compensatory increase in heart rate and could account for the exaggerated heart rate responses in previous investigations (13) and in our study.
Additionally, there is evidence of a blunted increase in cardiac output due to impaired left ventricular filling in men and women with type 2 diabetes and no overt CV disease compared with control subjects during submaximal bicycle exercise at the same relative workload (14). However, in that study, although groups were matched for self-reported fitness, resting heart rate was higher and peak oxygen consumption and peak workload were lower in the group with type 2 diabetes (14).
In another study, postmenopausal women with type 2 diabetes had increased plasma lactate concentrations and heart rate responses during low- to moderate-intensity cycling (both at absolute [30 W] and relative [35% VO2peak] work rates) compared with women without diabetes who were matched for age and BMI; however, the women with type 2 diabetes in that study also had lower relative aerobic fitness than control subjects (15.4 vs. 17.8 mL·kg−1·min−1) (13). Despite the differences in lactate and heart rate, women with type 2 diabetes and control subjects exhibited similar RPE during low- to moderate-intensity cycling, suggesting a blunted RPE response in women with type 2 diabetes.
These results from previous studies support an altered relationship between CV response during exercise and perceived effort and performance among less active and less fit people with type 2 diabetes compared with control subjects. Our data demonstrate that this effect is also evident during walking among relatively active and fit people with type 2 diabetes who were matched for fitness (among other factors) with control subjects. Future research could investigate CV responses and RPE at a number of steady-state workloads to help identify threshold levels of differentiation between those with and without diabetes and among people with diabetes with poorer metabolic control.
Conclusion
Our findings support previous reports of a blunted association between CV response and perceived effort in people with type 2 diabetes (i.e., higher cardiac work for the same RPE). However, for the first time, we demonstrate that this altered relationship in people with type 2 diabetes was not because of differences in fitness, physical activity levels, or body composition and instead was the result of effects of type 2 diabetes per se. Although we cannot exclude subclinical effects of diabetes on the heart, it is likely that impaired peripheral oxygen kinetics (reduced muscle blood flow and oxygen delivery) in people with type 2 diabetes compared with control subjects at the same relative workload during submaximal exercise may partially explain our findings. We showed that, although RPE increased with exercise heart rate during brisk walking, people with type 2 diabetes had lesser increases in perceived effort (i.e., blunted RPE) for a given increase in exercising heart rate. Consequently, we suggest that clinical observation/prescription of exercise intensity in people with type 2 diabetes should include both perceived effort (RPE) and CV (heart rate and mean arterial pressure) measures rather than one or the other alone to better reflect cardiac effort during exercise.
Acknowledgments
Acknowledgments
The authors thank Dr. Mehdi Maadooliat for assistance with statistical analyses and oversight, Bonnie Schlinder-Delap for assistance with scheduling participants, and Michael Danduran for assistance with administration and interpretation of EKG recordings during submaximal exercise testing. They also thank the research participants for volunteering for this study.
Funding
This work was supported by a Marquette University Way Klingler Fellowship Award to S.K.H.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
J.W.S., A.R.H., and S.K.H. contributed to the study design. J.W.S., S.E.D., and A.R.H. contributed to data acquisition. J.W.S. and S.E.D. wrote the manuscript and researched data. All authors contributed to the discussion, reviewed/edited the manuscript, and approved the final version of the manuscript. J.W.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016;39:2065–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poitras VJ, Hudson RW, Tschakovsky ME. Exercise intolerance in type 2 diabetes: is there a cardiovascular contribution? J Appl Physiol (1985) 2018;124:1117–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Regensteiner JG, Sippel J, McFarling ET, Wolfel EE, Hiatt WR. Effects of non-insulin-dependent diabetes on oxygen consumption during treadmill exercise. Med Sci Sports Exerc 1995;27:875–881 [PubMed] [Google Scholar]
- 4.Green S, Egaña M, Baldi JC, Lamberts R, Regensteiner JG. Cardiovascular control during exercise in type 2 diabetes mellitus. J Diabetes Res 2015;2015:654204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beekley MD, Brechue WF, deHoyos DV, Garzarella L, Werber-Zion G, Pollock ML. Cross-validation of the YMCA submaximal cycle ergometer test to predict VO2max. Res Q Exerc Sport 2004;75:337–342 [DOI] [PubMed] [Google Scholar]
- 6.Tudor-Locke C, Bassett DR Jr. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med 2004;34:1–8 [DOI] [PubMed] [Google Scholar]
- 7.Bohannon RW, Bubela D, Magasi S, et al. Comparison of walking performance over the first 2 minutes and the full 6 minutes of the Six-Minute Walk Test. BMC Res Notes 2014;7:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman AB, Haggerty CL, Kritchevsky SB, Nevitt MC, Simonsick EM; Health ABC Collaborative Research Group . Walking performance and cardiovascular response: associations with age and morbidity—the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci 2003;58:715–720 [DOI] [PubMed] [Google Scholar]
- 9.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 1998;158:1384–1387 [DOI] [PubMed] [Google Scholar]
- 10.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ, Lawrence Earlbaum Associates, 1988 [Google Scholar]
- 11.Tudor-Locke C, Craig CL, Aoyagi Y, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act 2011;8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts TJ, Burns AT, MacIsaac RJ, MacIsaac AI, Prior DL, La Gerche A. Diagnosis and significance of pulmonary microvascular disease in diabetes. Diabetes Care 2018;41:854–861 [DOI] [PubMed] [Google Scholar]
- 13.Huebschmann AG, Kohrt WM, Herlache L, et al. Type 2 diabetes exaggerates exercise effort and impairs exercise performance in older women. BMJ Open Diabetes Res Care 2015;3:e000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson GA, Wilkins GT, Cotter JD, Lamberts RR, Lal S, Baldi JC. Impaired ventricular filling limits cardiac reserve during submaximal exercise in people with type 2 diabetes. Cardiovasc Diabetol 2017;16:160. [DOI] [PMC free article] [PubMed] [Google Scholar]