Abstract
Background and aims:
Abdominal aortic calcification (AAC) and low ankle-brachial index (ABI) are markers of multisite atherosclerosis. We sought to estimate their associations in older men with health care costs and utilization adjusted for each other, and after accounting for CVD risk factors and prevalent CVD diagnoses.
Methods:
This was an observational cohort study of 2393 community-dwelling men (mean age 73.6 years) enrolled in the Osteoporotic Fractures in Men (MrOS) study and U.S. Medicare Fee for Service (FFS). AAC was scored on baseline lateral lumbar spine x-rays using a 24-point scale. ABI was measured as the lowest ratio of arm to right or left ankle blood pressure. Health care costs, hospital stays, and SNF stays were identified from Medicare FFS claims over 36 months following the baseline visit.
Results:
Men with AAC score ≥9 (n=519 [21.7% of analytic cohort]) had higher annualized total health care costs of $1473 (95% C.I. 293, 2654, 2017 U.S. dollars) compared to those with AAC score 0-1, after multivariable adjustment. Men with ABI <0.90 (n=154 [6.4% of analytic cohort]) had higher annualized total health care costs of $2705 (95% CI 634, 4776) compared to men with normal ABI (≥0.9 and <1.4), after multivariable adjustment.
Conclusions:
High levels of AAC and low ABI in older men are associated with higher subsequent health care costs, after accounting for clinical CVD risk factors, prevalent CVD diagnoses, and each other. Further investigations of whether preventing progression of peripheral vascular disease and calcification reduces subsequent health care costs are warranted.
Keywords: Health care costs, abdominal aortic calcification, ankle-brachial index, peripheral arterial disease, atherosclerosis
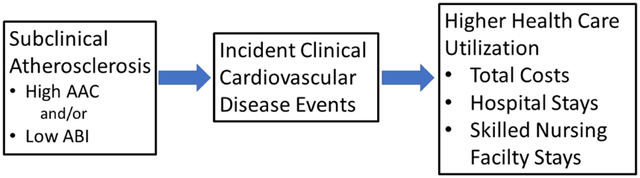
Graphical Abstract
INTRODUCTION
Abdominal aortic calcification (AAC) and ankle-brachial index (ABI) are both markers of subclinical multisite cardiovascular disease (CVD), and have consistently been shown to predict incident CVD events,1-9 including myocardial infarction,7 stroke,6 congestive heart failure10, CVD hospitalization,11 CVD mortality,1, 8, 9 and all-cause mortality.8, 9, 12, 13 Individuals with symptomatic peripheral arterial disease have higher health care costs and utilization,14-16 but no studies have estimated the direct association of low ABI (the majority of whom are asymptomatic17) or high AAC with total health care costs. In particular, it is unclear if AAC and/or ABI predict subsequent total health care costs and utilization after accounting for CVD risk factors, previous clinical cardiovascular disease diagnoses, and each other.
ABI can be assessed during clinical encounters, and AAC is often seen coincidentally on lateral lumbar spine x-rays,4, 7 abdominal CT scans18 and lateral spine imaging obtained for other reasons, including densitometric lateral spine images obtained for vertebral fracture assessment (VFA) at the time of bone densitometry.19, 20 If the presence of high AAC and/or low ABI predict subsequent higher health care costs and utilization, that would further heighten the clinical relevance of assessment of AAC and ABI to patients, health care delivery systems, and health care insurers.
Our primary objective was to estimate the associations of AAC (scored semi-quantitatively on lateral spine radiographs) and ABI with total health care costs, adjusted for each other, clinical CVD risk factors, and known prevalent clinical CVD. Our secondary objective was to estimate with the associations of AAC and ABI with all-cause and CVD hospitalizations and skilled nursing facility (SNF) stays.
MATERIALS AND METHODS
Between 2000 and 2002, the Osteoporotic Fractures in Men (MrOS) study enrolled 5994 community-dwelling ambulatory men age 65 years and older at six geographic sites in the United States, as described in previous publications21, 22, after Institutional Review Board (IRB) approval at each of the six study sites. Eligible participants were age 65 years or older, community dwelling, able to walk without assistance, and did not have bilateral hip arthroplasties. All participants signed informed consent documents. The Centers for Medicare and Medicare Services approved the linkage to MrOS participants and successful matches to Medicare were achieved for 5876 (98%) of the men in the cohort.
For these retrospective analyses, men were included if they had baseline lateral lumbar spine radiographs interpretable for AAC, a valid ABI measurement at the right or left ankle and were enrolled in Medicare Fee-for-Service parts A and B for three years from the date of their baseline MrOS visit (or until death within this 3-year period). Those enrolled in Medicare Advantage (2813 men) were excluded because their healthcare costs and utilization are not fully observable in available Medicare Claims Data. The final analytic cohort consisted of 2393 men (Supplemental Figure 1). Those excluded because of Medicare Advantage enrollment had slightly lower ABI, higher systolic blood pressure, higher LDL cholesterol, were slightly younger, and slightly less likely to have had a prior myocardial infarction or stroke, but the magnitude of these differences was very small and unlikely to be clinically relevant (Supplemental Table 1).
Measurement of Abdominal Aortic Calcification (AAC) and Ankle-Brachial Index (ABI)
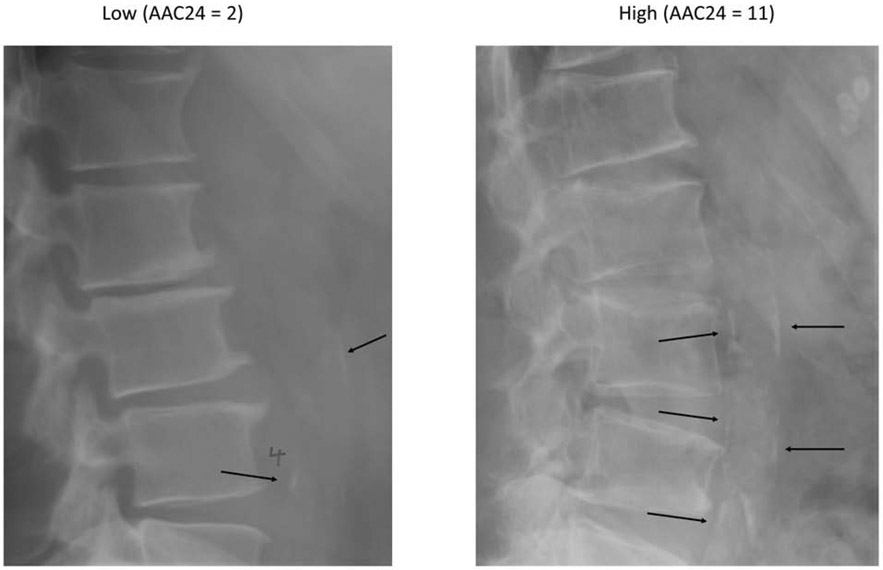
Lumbar lateral spine digitized radiographs were obtained at the baseline MrOS visit for all 5994 study participants and could be accurately scored for AAC in 5400 men (90%). AAC was scored using the Framingham 24-point semi-quantitative scale by an expert reader (PS) (Figure 1). A second reader (JTS) read 40 lateral spine radiographs to assess inter-rater reliability. Intra-rater and inter-rater reliability were both very high (intraclass correlation coefficients of 0.98 [95% CI 0.88-0.97] and 0.94 [95% CI 0.88-0.97]).23
Figure 1:
Examples of AAC on lateral spine radiographs (black arrows)
ABI was measured as described in a prior publication.24 Systolic blood pressure was measured in each arm once, and the average of the 2 measurements was obtained. Systolic blood pressure was measured twice in each foot, and an average was taken of the 2 readings from each foot. From this, an ABI was obtained for each leg, and we used the lowest of these measurements.
Other covariates
Height was measured with a Harpenden stadiometer, weight recorded with a balance beam or electronic scale, and body mass index calculated as weight (kg) divided by height squared (m2) at the MrOS baseline visit. Participants were asked about smoking status, current medication use including aspirin, statin and antihypertensive medications, and whether they had been diagnosed previously by a physician with angina, a myocardial infarction, stroke, atrial fibrillation, congestive heart failure, and/or diabetes mellitus. Systolic blood pressure was measured in the right arm twice with the participant sitting and averaged. Serum creatinine, HDL and LDL cholesterol were measured using Roche COBAS Integra 800 automated analyzer (Roche Diagnostics Corporation, Indianapolis, IN), with coefficients of variation, respectively, of 5.3%, 2.4% and 3.2%. Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI formula.25
Health care costs and utilization after baseline visit
Our primary outcome variable was annualized total health care costs for the 36 months after the baseline MrOS visit (or until death for the 127 men [5.3%] who died before the three-year anniversary of the baseline exam). Total health care costs, a measure of overall health burden, were calculated as the sum of costs for hospital stays, SNF stays paid under Medicare part A, inpatient rehabilitation facility (IRF) stays, outpatient care (including diagnostic and therapeutic procedures), and home health care. All hospital stays, SNF stays, and IRF stays during that year were identified in the Medical Provider Analysis and Review (MedPAR) file. CVD-related hospitalizations were those with a primary or secondary discharge diagnosis of coronary heart disease (ICD-9 codes 414.xx), congestive heart failure (ICD-9 codes 398.91, 428.0), myocardial infarction (ICD-9 codes 410.xx, 412, 429.7x) or cerebrovascular accident (stroke) (ICD-9 codes 433.xx, 434.xx). Standardized costs for hospital stays, SNF stays, and IRF stays were estimated using previously published and validated methods.26-28 Health care costs were adjusted for health care cost inflation to U.S. 2017 dollars, using previously published methods.26
Statistical analysis
AAC was strongly right skewed and categorized as a four-level ordinal variable (AAC 24-point score 0-1; 2-4; 5-8; and ≥9) as described in a previous publication, with AAC 0 to 1 being the reference category.23 We modeled ABI as a categorical variable, defining the lowest category of ABI as <0.90 (154/2393, 6.4%), an established ABI cut point defining hemodynamically significant peripheral arterial disease for epidemiological studies.17, 24, 29, 30 We defined the highest category of ABI as ≥1.40, since ABI values at or above this threshold also are associated with higher subsequent mortality.8, 9 ABI ≥0.9 and <1.4 was the normal reference category.
We used generalized linear models to estimate the associations of AAC with annualized total health care costs. Based on Modified Park31 and Pregibon link32 tests, we chose a log link and gamma distribution for these regression models in order to account for the right skewed distribution of health care costs and assure well-specified models. Logistic models were used to estimate the associations of AAC with risks of all-cause hospitalization, CVD hospitalization, and SNF stays (one or more episodes) during the three years following the baseline MrOS visit.
Base models were adjusted for age and study enrollment site. A second set of models also adjusted for race, education, systolic blood pressure, HDL cholesterol, LDL cholesterol, triglycerides, creatinine clearance, smoking status, angina, prior myocardial infarction, prior stroke, congestive heart failure, diabetes mellitus, and chronic kidney disease (CKD, eGFR< 60 ml/min).
All these models were performed again with ABI as the primary predictor variable in place of AAC (reference category ABI ≥0.9 and <1.4). To assess if the associations of AAC and ABI with health care costs and utilization were independent of each other, final multivariable models for all three outcomes (total health care costs, acute hospital stays, and SNF stays) included both AAC and ABI as predictors. Secondary analyses were done estimating the associations of AAC and ABI with total health care costs, hospital stays, and SNF stays excluding the 653 men who reported clinical cardiovascular disease (angina, prior myocardial infarction, prior stroke, prior congestive heart failure) at baseline. Sensitivity analyses were also run excluding the 127 men who died during the three-year follow-up period.
RESULTS
At baseline, men with higher levels of AAC were more likely to be older, have higher systolic blood pressure, lower HDL cholesterol and higher triglycerides, lower ABI values, to have a creatinine clearance <60 ml/min, and to have had previous diagnoses of angina, myocardial infarction, stroke, congestive heart failure, or diabetes mellitus. (Table 1). Similar differences in baseline characteristics were also noted across levels of ABI (Supplemental Table 2). Five hundred nineteen (21.9%) had an AAC level of 9 or higher and 154 (6.4%) had an ABI< 0.9, with modest overlap between them; 46.8% of men with an ABI < 0.9 had an AAC score of 9 or higher, and conversely 13.9% of men with an AAC score of 9 or higher also had an ABI < 0.9. A higher proportion of men died before the end of the three-year follow-up period among those with an AAC ≥9 (8.1%) compared to those with an AAC score of 0-1 (3.4%, p-value <0.001). Similarly, a higher proportion of men died before end of the follow-up period among those with an ABI <0.9 (13.0%) and those with an ABI ≥1.4 (10.1%) compared to those with ABI ≥0.9 and < 1.4 (4.5%), p-value <0.001). Over the three-year follow-up period, 955 men (39.9%) had a hospital stay for any cause, 490 (20.5%) had a hospital stay for a CVD diagnosis, and 135 men (5.6%) had a SNF stay.
Table 1:
Patient baseline characteristics (N=2393), stratified by AAC level
| Characteristic | AAC 0-1 N=682 |
AAC 2-4 N=649 |
AAC 5-8 N=543 |
AAC ≥9 N=519 |
p-valuea |
|---|---|---|---|---|---|
| Age, years, mean (SD) | 71.3 (5.1) | 73.2 (5.4) | 74.7 (6.0) | 76.0 (5.9) | <0.001 |
| Race | <0.001 | ||||
| Non-White | 87 (12.8%) | 59 (9.1%) | 34 (6.3%) | 27 (5.2%) | |
| White | 595 (87.2%) | 590 (90.9%) | 509 (93.7%) | 492 (94.8%) | |
| Education | 0.11 | ||||
| Less than high school | 35 (5.1%) | 32 (4.9%) | 38 (7.0%) | 39 (7.5%) | |
| High school | 94 (13.8%) | 126 (19.4%) | 96 (17.7%) | 94 (18.1%) | |
| Some college | 152 (22.3%) | 138 (21.3%) | 117 (21.5%) | 109 (21.0%) | |
| ≥4 years college | 401 (58.8%) | 353 (54.4%) | 292 (53.8%) | 277 (53.4%) | |
| Systolic blood pressure, mmHg, mean (SD) | 136.5 (16.7) | 139.6 (19.0) | 142.7 (20.9) | 143.7 (19.9) | <0.001 |
| LDL cholesterol, mg/dl, mean (SD) | 114.4 (29.1) | 112.3 (29.9) | 114.1 (31.0) | 110.1 (30.0) | 0.07 |
| HDL cholesterol, mg/dl, mean (SD) | 50.1 (15.5) | 49.0 (13.5) | 47.5 (14.1) | 49.1 (14.9) | 0.03 |
| Triglycerides, mg/dl, median | 118.5 | 124.0 | 131.0 | 132.0 | <0.001 |
| (IQR) | (84.0, 164.5) | (91.0, 176.0) | (97.0, 189.0) | (90.0, 193.5) | |
| Current smoking (yes/no) | 15 (2.2%) | 20 (3.1%) | 21 (3.9%) | 21 (4.0%) | 0.24 |
| Angina (yes/no) | 54 (7.9%) | 80 (12.3%) | 77 (14.2%) | 108 (20.6%) | <0.001 |
| Prior MI (yes/no) | 42 (6.3%) | 88 (13.9%) | 99 (18.5%) | 111 (22.2%) | <0.001 |
| Prior stroke (yes/no) | 18 (2.6%) | 42 (6.5%) | 32 (5.9%) | 49 (9.4%) | <0.001 |
| Congestive heart failure (yes/no) | 20 (2.9%) | 30 (4.6%) | 29 (5.3%) | 46 (8.9%) | <0.001 |
| Diabetes mellitus (yes/ no) | 57 (8.4%) | 66 (10.2%) | 61 (11.2%) | 76 (14.6%) | 0.006 |
| Lowest ABI, mean (SD) | 1.22 (0.15) | 1.19 (0.15) | 1.17 (0.18) | 1.11 (0.20) | <0.001 |
| Chronic kidney disease (eGFR<60 ml/min) | 79 (12.5%) | 88 (14.4%) | 87 (17.6%) | 96 (19.7%) | 0.005 |
ANOVA (or non-parametric Kruskal-Wallis test) for continuous variables, chi-square for categorical variables
AAC, abdominal aortic calcification; MI, myocardial infarction; ABI, ankle-brachial index; eGFR, estimated glomerular filtration rate
Associations of AAC level with health care costs and utilization
Unadjusted for other covariates, annualized median total health care costs in the 36 months after baseline increased in a graded manner with higher AAC from $2199 (interquartile range [IQR] 961 to 6017) for those with low or minimal AAC (24-point scale score 0-1) to $4796 (IQR 1675 to 11466) for those with a very high burden of AAC (24-point scale score 9 or higher, Table 2). Similarly, the proportions with one or more hospital stays and with one or more post-acute SNF stays over the three-year follow-up period all rose with increasing level of AAC.
Table 2:
Health care utilization after baseline according to AAC level and ABI level
| Annualized total health care costs Median (IQR) |
≥1 acute hospital stay N (%)a |
≥1 SNF stay N (%)a |
||
|---|---|---|---|---|
| P-Valueb | ||||
| AAC Level | <0.001 | |||
| 0-1 | $2199 (961, 6017) | 206 (30.2) | 19 (2.8) | |
| 2-4 | $2955 (1115, 8060) | 266 (41.0) | 29 (4.5) | |
| 5-8 | $3239 (1360, 8953) | 227 (41.8) | 28 (5.2) | |
| ≥9 | $4796 (167, 11466) | 256 (49.3) | 59 (11.4) | |
| ABI level | <0.001 | |||
| ≥ 1.4 | $3126 (145, 9216) | 35 (35.4) | 6 (6.1) | |
| 0.90 - <1.4 | $2908 (1132, 7879) | 828 (38.7) | 105 (4.9) | |
| <0.90 | $6917 (2240, 17294) | 92 (59.7) | 24 (15.6) | |
Over the 3-year follow-up period.
p-value (by Kruskal-Wallis test) for trend of difference in total health care costs across all categories of predictor.
AAC, abdominal aortic calcification; ABI, ankle-brachial index; IQR, interquartile range
Adjusted for age and study enrollment site, an AAC score level of 9 or higher was associated with significantly greater total health care costs compared to an AAC score level of only 0-1 (incremental cost $2745, 95% CI 1484 to 4006, Table 3). This association was attenuated but remained significant (incremental cost $1473 (95% C.I. 293, 2654) after additional adjustment for CVD clinical risk factors, known prevalent CVD disease, presence of CKD, and ABI level. After full multivariable adjustment, an AAC score of 9 or higher was also associated with increased odds ratios (OR) of all-cause hospital stays (OR 1.47, 95% CI 1.12 to 1.94), hospital stays for CVD (OR 2.06, 95% CI 1.45 to 2.93), and post-acute SNF stays (OR 2.44, 95% CI 1.29 to 4.62) over the three-year follow-up period. Excluding the 127 men who died within the three-year follow-up period did not alter these results (data not shown). The strength of associations of an AAC score of 9 or higher with all four health care utilization outcomes were similar to those seen with prevalent angina, congestive heart failure, diabetes mellitus, and chronic kidney disease (Supplemental Table e3).
Table 3:
Association of AAC with health care utilization over 36 months
| AAC Level |
Incremental total health care costs (95% CI) |
p-valued | All-cause hospital stays OR (95% CI) |
CVD hospital stays OR (95% CI) |
SNF stays OR (95% CI) |
|---|---|---|---|---|---|
| Base | |||||
| Modela | <0.0001 | ||||
| 0-1 | Reference | Reference | Reference | Reference | |
| 2-4 | $293 (−628, 1190) | 1.46 (1.16, 1.84) | 1.67 (1.22, 2.28) | 1.28 (0.70, 2.33) | |
| 5-8 | $1030 (−12, 2071) | 1.41 (1.10, 1.80) | 1.84 (1.33, 2.53) | 1.13 (0.61, 2.10) | |
| ≥9 | $2745 (1484, 4006) | 1.80 (1.40-2.31) | 2.95 (2.16, 4.03) | 2.39 (1.37, 4.18) | |
| Model 2b | 0.03 | ||||
| 0-1 | Reference | Reference | Reference | Reference | |
| 2-4 | $120 (−793,1033) | 1.33 (1.04, 1.70) | 1.43 (1.02, 2.02) | 1.42 (0.73, 2.77) | |
| 5-8 | $461 (−563, 1485) | 1.26 (0.97, 1.65) | 1.50 (1.05, 2.15) | 1.26 (0.63, 2.51) | |
| ≥9 | $1651 (458, 2844) | 1.54 (1.17, 2.02) | 2.20 (1.55, 3.12) | 2.56 (1.36, 4.82) | |
| Model 3c | 0.05 | ||||
| 0-1 | Reference | Reference | Reference | Reference | |
| 2-4 | $71 (−841, 982) | 1.33 (1.04, 1.70) | 1.44 (1.02, 2.03) | 1.44 (0.74, 2.80) | |
| 5-8 | $421 (−598, 1440) | 1.25 (0.96, 1.63) | 1.47 (1.02, 2.11) | 1.24 (0.62, 2.49) | |
| ≥9 | $1473 (293, 2654) | 1.47 (1.12, 1.94) | 2.06 (1.45, 2.93) | 2.44 (1.29, 4.62) |
Adjusted for age and clinical site
Adjusted for age, clinical site, race, education, systolic blood pressure, HDL cholesterol, LDL cholesterol, triglycerides, smoking status, angina, prior myocardial infarction, prior stroke, congestive heart failure, diabetes mellitus and CKD (eGFR < 60 ml/min)
Adjusted for Model 2 covariates plus ABI
p-value for trend of change across predictor variable categories
AAC, abdominal aortic calcification; SNF, skilled nursing facility; OR, odds ratio
When analyses were limited to the 1740 men with no known clinical cardiovascular disease at baseline, the fully adjusted association of AAC score ≥9 with total health care costs was very similar, but of borderline significance. The associations of AAC score ≥9 with all-cause and CVD hospital stays, and SNF stays remained strong and statistically significant (Supplemental Table e4). However, when analyses were limited to the 1526 men with neither a prevalent CVD diagnosis at baseline nor an incident CVD hospital stay during the three-year follow-up period, the associations of AAC with all four health care utilization outcomes were substantially attenuated and no longer significant (Supplemental Table e5).
Associations of ABI level with health care costs and utilization
Unadjusted for other covariates, annualized total health care costs were lowest for those with an ABI ≥ 0.9 and <1.4 (median $2908, IQR 1132 to 7879), slightly higher for those with ABI ≥1.4 (median $3126, IQR 1456 to 9216), and highest for those with ABI <0.9 (median $6917, IQR 2240 to 17294). Compared to those with an ABI ≥0.9 and <1.4, the proportions of those with one or more hospitalizations and with one or more post-acute SNF stays were similar for those ABI ≥1.4, but were substantially higher for those with an ABI <0.9 (Table 2).
Adjusted for age and study enrollment site, an ABI less than 0.9 was associated with a higher total health care costs (incremental cost $4318, 95% CI 1811 to 6826, Table 4), compared with the reference category (ABI ≥ 0.9 and <1.4). This association remained significant (incremental cost $2705, 95% CI 634 to 4776) after additional adjustment for clinical CVD risk factors, prevalent CVD diagnoses, presence of CKD, and AAC score category. Compared with the reference category, after full multivariable adjustment, ABI <0.90 was also associated with all-cause hospital stays (OR 1.65, 95% CI 1.14 to 2.40), CVD hospital stays (OR 2.24, 95% CI 1.51 to 3.32), and post-acute SNF stays (OR 1.86, 95% CI 1.07 to 3.22). In contrast, an ABI ≥1.4 was not associated with total health care costs, hospital stays, or SNF stays. Excluding the 127 men who died within the three-year follow-up period did not alter these results (data not shown). The strength of associations of ABI<0.9 with all four health care utilization outcomes were similar to those seen with prior myocardial infarction and prior stroke, and greater than those seen with prevalent angina, congestive heart failure, diabetes mellitus, and chronic kidney disease (Supplemental Table e3).
Table 4:
Association of ABI with health care utilization over 36 months
| ABI Level | Incremental total health care Cost (95% CI) |
p-valued | All-cause hospital stays OR (95% CI) |
CVD hospital stays OR (95% CI) |
SNF stays OR (95% CI) |
|---|---|---|---|---|---|
| Base Modela | 0.0001 | ||||
| Level 3 (≥1.4) | $823 (−1265, 2911) | 0.89 (0.58, 1.37) | 0.93 (0.54, 1.61) | 1.28 (0.52, 3.12) | |
| Level 2 (0.90- <1.4) | Reference | Reference | Reference | Reference | |
| Level 1 (<0.90) | $4318 (1811, 6826) | 1.87 (1.33, 2.63) | 2.68(1.89, 3.78) | 2.37 (1.43, 3.92) | |
| Model 2b | 0.01 | ||||
| Level 3 (≥1.4) | $515 (−1418, 2448) | 0.79 (0.50, 1.26) | 0.70(0.37, 1.31) | 1.40 (0.55, 3.55) | |
| Level 2 (0.90- <1.4) | Reference | Reference | Reference | Reference | |
| Level 1 (<0.90) | $2972 (831, 5113) | 1.72 (1.19, 2.49) | 2.45(1.65, 3.63) | 2.06 (1.19, 3.54) | |
| Model 3c | 0.01 | ||||
| Level 3 (≥1.4) | $635 (−1322, 2591) | 0.80 (0.50, 1.29) | 0.70(0.37, 1.34) | 1.41 (0.55, 3.60) | |
| Level 2 (0.90- <1.4) | Reference | Reference | Reference | Reference | |
| Level 1 (<0.90) | $2705 (634, 4776) | 1.65 (1.14, 2.40) | 2.24(1.51, 3.32) | 1.86 (1.07, 3.22) |
Adjusted for age and clinical site
Adjusted for age, clinical site, race, education, systolic blood pressure, HDL cholesterol, LDL cholesterol, triglycerides, smoking status, angina, prior myocardial infarction, prior stroke, congestive heart failure, diabetes mellitus and CKD (eGFR < 60 ml/min)
Adjusted for Model 2 covariates and AAC score level
p-value for trend of change across predictor variable categories
ABI, ankle-brachial index; SNF, skilled nursing facility; OR, odds ratio
When analyses were limited to the 1740 with no known clinical cardiovascular disease at baseline, the fully adjusted association of ABI <0.9 with total health care costs was very similar to what was found in the full sample (Supplemental Table e6). The associations of ABI <0.9 with all-cause and CVD hospital stays, and SNF stays remained strong and statistically significant. When analyses were limited to the 1526 men with neither a prevalent CVD diagnosis at baseline nor an incident CVD hospital stay during the three-year follow-up period, the associations of low ABI with all four health care utilization outcomes were substantially attenuated and no longer significant (Supplemental Table e7).
DISCUSSION
In this cohort of older men, a high level of AAC and a low ABI level were each associated with higher subsequent total health care costs, increased risks of all-cause and CVD hospitalization, and higher risk of SNF stays, even after adjustment for clinical CVD risk factors, known prevalent CVD, and each other. The increased risk of subsequent CVD hospitalization associated with both factors remained when excluding those with previously diagnosed CVD.
These findings suggest that ABI and AAC can could be used to identify different (albeit overlapping) subsets of those with an increased burden of atherosclerosis that leads to overt clinically evident CVD events, and higher health care costs and utilization. In fact, high AAC and low ABI were no longer significantly associated with all four health care utilization outcomes when those men who had an incident CVD hospitalization over the three-year follow-up period were excluded. Hence, it appears that the higher costs and utilization associated with high AAC and low ABI are largely attributable to subsequent CVD events that might be preventable through more aggressive treatment of modifiable CVD risk factors.
Our findings are consistent with previous studies that have shown that high AAC and low ABI predict incident CVD events (that would incur health care costs and utilization) after accounting for clinical CVD risk factors and known prevalent CVD. Both AAC and ABI also predict non-CVD mortality33, 34 and other non-CVD morbidity associated with higher health care costs such as hip and other fractures.23, 35, 36
A high ABI ≥1.4 has also been associated with subsequent all-cause and CVD mortality.8, 9 We did not find any association of high ABI with total health care costs, all-cause or CVD hospitalizations, or SNF utilization, but our power to find significant associations of high ABI with health care costs and utilization was limited.
Previous cost-effectiveness analyses have suggested that measurement of ABI in asymptomatic individuals is cost effective37, 38, particularly when screening those with additional risk factors such as smoking.39 To date, no modeling studies of the cost-effectiveness of screening for AAC have been published. Screening for ABI has the advantage over screening for AAC that an imaging study is not required. However, imaging studies obtained in clinical practice obtained for other reasons, including abdominal CT scans, lateral spine radiographs, and lateral spine images obtained as part of bone density tests40 (which are indicated at least once in all women age 65 or older,41 and in some men age 70 and older42) afford opportunistic detection of AAC in many older individuals in clinical practice. While no study has directly compared how well AAC scored on one of these imaging modalities predict CVD events compared to the others, the strengths of association of AAC scored on lateral spine radiographs1, 4, 6, 7 and lateral spine bone density images19, 33, 43 with incident CVD events are similar. Moreover, AAC may predict incident stroke and myocardial infarction as well as carotid intimal medial thickness and ABI.6, 7 Additional modeling studies to examine the cost-effectiveness of expanding use of imaging to screen for AAC are also warranted.
AAC and ABI are not as strongly predictive specifically of coronary heart disease events as is coronary artery calcification (CAC).34, 44 However, measurement of ABI can be done in clinical practice inexpensively without the need for additional imaging. AAC predicted CVD and all-cause mortality in one study as well or better than CAC.34 AAC can be assessed conveniently at the same time as a bone density test with minimal radiation exposure and expense (one seventh that of a coronary calcium score, according to the U.S. Medicare Fee for Service 2019 Fee schedule)45. Lateral spine radiography to assess AAC is also much less expensive than measuring CAC.
There is a paucity of evidence that interventions in populations specifically identified on the basis of asymptomatic low ABI or high AAC reduce incident CVD events.46 While current American Heart Association guidelines support screening asymptomatic individuals age ≥65 years using ABI,47 the U.S. Preventive Services Task Force concluded that there is insufficient evidence to recommend ABI screening,48 and the Society for Vascular Surgery recommends against screening asymptomatic individuals.49 There are no guidelines that currently support screening for AAC. Hence, randomized controlled trials of screening regimens for low ABI, high AAC, or both compared to usual practice ultimately may be required to prove the utility of these screening tools prior to adoption into the clinical practice setting.
Our study has several strengths. We used a large cohort representative of the broader population of older community dwelling U.S. men whose phenotypic characteristics have been extensively described. We used rigorous methods to estimate health care costs and utilization using U.S. Medicare Fee-for-Service claims data that reflect the true resource costs required for the health care of these men. We accounted for confounding by CVD clinical risk factors, prevalent CVD diagnoses, and tested whether or not AAC and/or ABI predict subsequent health care costs after accounting for each other. To our knowledge, this is the first study to estimate the joint associations of AAC and ABI with total health care costs and utilization.
However, our study also has a number of limitations. MrOS enrollees are 90% Caucasian, and our results may not be generalizable to older women or other ethnic groups. We excluded those enrolled in Medicare Advantage, although this is mitigated by close similarity of the characteristics of MrOS men enrolled in Medicare Fee-for-Service vs Medicare Advantage. We identified prevalent CVD diagnoses by participant self-report, which is susceptible to recall bias. We used Medicare claims data to identify incident CVD hospitalizations, but this is mitigated by the high positive predictive value of claims for incident CVD events, and the fact that associations of CVD risk factors with incident CVD events in claims data are quite similar to these associations when incident CVD events are detected with gold standard cohort study methods.50 We did not employ statistical techniques to remove distortions in health care cost estimates from individuals censored due to death or other reasons,51 but these distortions are highly likely to be minimal since the number of men who were censored due to death or other reasons were very low. Moreover, our results were unchanged when analyses were limited to men who were uncensored through the entire 3-year follow-up period.
In conclusion, among men age 65 and older, a high level of AAC and a low ABI are both independently associated with higher total health care costs, acute all-cause and CVD hospital stays, and SNF utilization after accounting for age, clinical CVD risk factors, prevalent CVD diagnoses, and each other. Additional studies to investigate the clinical utility and cost-effectiveness of screening programs of older men and women for high AAC and/or low ABI are warranted.
Supplementary Material
Abdominal aortic calcification (AAC) and low ankle-brachial index (ABI) are markers of multisite atherosclerosis
We estimated their associations subsequent health care costs and utilization with after accounting for CVD risk factors, prevalent CVD diagnoses, and each other, in an observational cohort of 2393 older men
Both an AAC 24-point scale score of ≥ 9 and an ABI value of <0.9 were associated with significantly higher subsequent annualized total health care costs, after adjustment for each other and multiple other variables.
ABI and AAC identify different (albeit overlapping) subsets of those with subclinical CVD who are at risk for subsequent high health care costs and utilization
Acknowledgments
Financial support
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128.
The salary of Dr. Lewis is supported by a National Health and Medical Research Council (Australia) Career Development Fellowship (ID: 1107474).
This manuscript is the result of work supported with resources and use of facilities of the Minneapolis VA Health Care System. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
Conflict of Interest Disclosures
Dr. Adabag has received research grant support from the American Heart Association and Medtronic Inc. for work unrelated to this manuscript. All of the other authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Wilson PW, Kauppila LI, O'Donnell CJ, et al. , Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality, Circulation, 2001;103:1529–1534. [DOI] [PubMed] [Google Scholar]
- [2].Schousboe JT, Taylor BC, Kiel DP, et al. , Abdominal aortic calcification detected on lateral spine images from a bone densitometer predicts incident myocardial infarction or stroke in older women, Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research, 2008;23:409–416. [DOI] [PubMed] [Google Scholar]
- [3].Wong ND, Lopez VA, Allison M, et al. , Abdominal aortic calcium and multi-site atherosclerosis: the Multiethnic Study of Atherosclerosis, Atherosclerosis, 2011;214:436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Levitzky YS, Cupples LA, Murabito JM, et al. , Prediction of intermittent claudication, ischemic stroke, and other cardiovascular disease by detection of abdominal aortic calcific deposits by plain lumbar radiographs, Am J Cardiol, 2008;101:326–331. [DOI] [PubMed] [Google Scholar]
- [5].Tatami Y, Yasuda Y, Suzuki S, et al. , Impact of abdominal aortic calcification on long-term cardiovascular outcomes in patients with chronic kidney disease, Atherosclerosis, 2015;243:349–355. [DOI] [PubMed] [Google Scholar]
- [6].Hollander M, Hak AE, Koudstaal PJ, et al. , Comparison between measures of atherosclerosis and risk of stroke: the Rotterdam Study, Stroke, 2003;34:2367–2372. [DOI] [PubMed] [Google Scholar]
- [7].van der Meer IM, Bots ML, Hofman A, et al. , Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam Study, Circulation, 2004;109:1089–1094. [DOI] [PubMed] [Google Scholar]
- [8].Resnick HE, Lindsay RS, McDermott MM, et al. , Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study, Circulation, 2004;109:733–739. [DOI] [PubMed] [Google Scholar]
- [9].Ankle Brachial Index C, Fowkes FG, Murray GD, et al. , Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis, JAMA, 2008;300:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Walsh CR, Cupples LA, Levy D, et al. , Abdominal aortic calcific deposits are associated with increased risk for congestive heart failure: the Framingham Heart Study, Am Heart J, 2002;144:733–739. [DOI] [PubMed] [Google Scholar]
- [11].Lewis JR, Schousboe JT, Lim WH, et al. , Long-Term Atherosclerotic Vascular Disease Risk and Prognosis in Elderly Women With Abdominal Aortic Calcification on Lateral Spine Images Captured During Bone Density Testing: A Prospective Study, Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research, 2018;33:1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cox AJ, Hsu FC, Agarwal S, et al. , Prediction of mortality using a multi-bed vascular calcification score in the Diabetes Heart Study, Cardiovasc Diabetol, 2014;13:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Criqui MH, Denenberg JO, McClelland RL, et al. , Abdominal Aortic Calcium, Coronary Artery Calcium, and Cardiovascular Morbidity and Mortality in the Multi-Ethnic Study of Atherosclerosis, Arteriosclerosis Thrombosis and Vascular Biology, 2014;34:1574-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bura Riviere A, Bouee S, Laurendeau C, et al. , Outcomes and management costs of peripheral arterial disease in France, J Vasc Surg, 2018;67:1834–1843. [DOI] [PubMed] [Google Scholar]
- [15].Luengo-Fernandez R, Howard DPJ, Nichol KG, et al. , Hospital and Institutionalisation Care Costs after Limb and Visceral Ischaemia Benchmarked Against Stroke: Long-Term Results of a Population Based Cohort Study, Eur J Vasc Endovasc Surg, 2018;56:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Scully RE, Arnaoutakis DJ, DeBord Smith A, et al. , Estimated annual health care expenditures in individuals with peripheral arterial disease, J Vasc Surg, 2018;67:558–567. [DOI] [PubMed] [Google Scholar]
- [17].Sigvant B, Wiberg-Hedman K, Bergqvist D, et al. , A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences, J Vasc Surg, 2007;45:1185–1191. [DOI] [PubMed] [Google Scholar]
- [18].Isgum I, van Ginneken B and Olree M, Automatic detection of calcifications in the aorta from CT scans of the abdomen. 3D computer-aided diagnosis, Acad Radiol, 2004;11:247–257. [DOI] [PubMed] [Google Scholar]
- [19].Bolland MJ, Wang TK, van Pelt NC, et al. , Abdominal aortic calcification on vertebral morphometry images predicts incident myocardial infarction, J Bone Miner Res, 2010;25:505–512. [DOI] [PubMed] [Google Scholar]
- [20].Schousboe JT, Wilson KE and Hangartner TN, Detection of aortic calcification during vertebral fracture assessment (VFA) compared to digital radiography, PLoS ONE, 2007;2:e715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Orwoll E, Blank JB, Barrett-Connor E, et al. , Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men, Contemp Clin Trials, 2005;26:569–585. [DOI] [PubMed] [Google Scholar]
- [22].Blank JB, Cawthon PM, Carrion-Petersen ML, et al. , Overview of recruitment for the osteoporotic fractures in men study (MrOS), Contemp Clin Trials, 2005;26:557–568. [DOI] [PubMed] [Google Scholar]
- [23].Szulc P, Blackwell T, Schousboe JT, et al. , High hip fracture risk in men with severe aortic calcification: MrOS study, Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research, 2014;29:968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Collins TC, Ewing SK, Diem SJ, et al. , Peripheral arterial disease is associated with higher rates of hip bone loss and increased fracture risk in older men, Circulation, 2009;119:2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Levey AS, Stevens LA, Schmid CH, et al. , A new equation to estimate glomerular filtration rate, Ann Intern Med, 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schousboe JT, Paudel ML, Taylor BC, et al. , Estimation of standardized hospital costs from Medicare claims that reflect resource requirements for care: impact for cohort studies linked to Medicare claims, Health services research, 2014;49:929–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schousboe JT, Paudel ML, Taylor BC, et al. , Pre-fracture individual characteristics associated with high total health care costs after hip fracture, Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schousboe JT, Paudel ML, Taylor BC, et al. , Estimating True Resource Costs of Outpatient Care for Medicare Beneficiaries: Standardized Costs versus Medicare Payments and Charges, Health services research, 2016;51:205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fowkes FG, The measurement of atherosclerotic peripheral arterial disease in epidemiological surveys, Int J Epidemiol, 1988;17:248–254. [DOI] [PubMed] [Google Scholar]
- [30].Norgren L, Hiatt WR, Dormandy JA, et al. , Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II), Eur J Vasc Endovasc Surg, 2007;33 Suppl 1:S1–75. [DOI] [PubMed] [Google Scholar]
- [31].Manning WG and Mullahy J, Estimating log models: to transform or not to transform?, J Health Econ, 2001;20:461–494. [DOI] [PubMed] [Google Scholar]
- [32].Pregibon D, Goodness of link tests for generalized linear models, Applied Statistics, 1980;29:15–24. [Google Scholar]
- [33].Lewis JR, Schousboe JT, Lim WH, et al. , Long-Term Atherosclerotic Vascular Disease Risk and Prognosis in Elderly Women With Abdominal Aortic Calcification on Lateral Spine Images Captured During Bone Density Testing: A Prospective Study, J Bone Miner Res, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Criqui MH, Denenberg JO, McClelland RL, et al. , Abdominal aortic calcium, coronary artery calcium, and cardiovascular morbidity and mortality in the Multi-Ethnic Study of Atherosclerosis, Arterioscler Thromb Vasc Biol, 2014;34:1574–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wei D, Zheng G, Gao Y, et al. , Abdominal aortic calcification and the risk of bone fractures: a meta-analysis of prospective cohort studies, J Bone Miner Metab, 2018;36:439–446. [DOI] [PubMed] [Google Scholar]
- [36].Szulc P, Blackwell T, Kiel DP, et al. , Abdominal aortic calcification and risk of fracture among older women - The SOF study, Bone, 2015;81:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vaidya A, Joore MA, Ten Cate-Hoek AJ, et al. , Screen or not to screen for peripheral arterial disease: guidance from a decision model, BMC Public Health, 2014;14:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sigvant B, Henriksson M, Lundin F, et al. , Asymptomatic peripheral arterial disease: is pharmacological prevention of cardiovascular risk cost-effective?, Eur J Cardiovasc Prev Rehabil, 2011;18:254–261. [DOI] [PubMed] [Google Scholar]
- [39].Itoga NK, Minami HR, Chelvakumar M, et al. , Cost-effectiveness analysis of asymptomatic peripheral artery disease screening with the ABI test, Vasc Med, 2018;23:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Schousboe JT, Richter SA and Beran MS, Potential Clinical Impact of Abdominal Aortic Calcification on Bone Density Lateral Spine Images, Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry, 2016;19:436–443. [DOI] [PubMed] [Google Scholar]
- [41].Preventive US Services Task Force, Screening for Osteoporosis: U.S. Preventive Services Task Force Recommendation Statement, Ann Intern Med, 2011;154:356–364. [DOI] [PubMed] [Google Scholar]
- [42].Camacho PM, Petak SM, Binkley N, et al. , American Association of Clincial Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the diagnosis and treatment of postmenopausal osteoporosis -2016-Executive Summary. , Endocrine Practice, 2016;22:1111–1118. [DOI] [PubMed] [Google Scholar]
- [43].Golestani R, Tio R, Zeebregts CJ, et al. , Abdominal aortic calcification detected by dual X-ray absorptiometry: A strong predictor for cardiovascular events, Ann Med, 2010;42:539–545. [DOI] [PubMed] [Google Scholar]
- [44].Hoffmann U, Massaro JM, D'Agostino RB Sr., et al. , Cardiovascular Event Prediction and Risk Reclassification by Coronary, Aortic, and Valvular Calcification in the Framingham Heart Study, Journal of the American Heart Association, 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Centers for Medicare and Medicaid Services, Physician Fee Schedule Look-Up Tool, In, 2019.
- [46].Shah S, Antoniou GA and Torella F, Evidence-based analysis of peripheral arterial disease screening based on the WHO criteria, International Angiology, 2015;36:299–305. [DOI] [PubMed] [Google Scholar]
- [47].Rooke TW, Hirsch AT, Misra S, et al. , 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, J Am Coll Cardiol, 2011;58:2020–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Force, USPST, Curry SJ, Krist AH, et al. , Screening for Peripheral Artery Disease and Cardiovascular Disease Risk Assessment With the Ankle-Brachial Index: US Preventive Services Task Force Recommendation Statement, JAMA, 2018;320:177–183. [DOI] [PubMed] [Google Scholar]
- [49].Society for Vascular Surgery Lower Extremity Guidelines Writing, G, Conte MS, Pomposelli FB, et al. , Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication, J Vasc Surg, 2015;61:2S–41S. [DOI] [PubMed] [Google Scholar]
- [50].Psaty BM, Delaney JA, Arnold AM, et al. , Study of Cardiovascular Health Outcomes in the Era of Claims Data: The Cardiovascular Health Study, Circulation, 2016;133:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Basu A and Manning WG, Estimating lifetime or episode-of-illness costs under censoring, Health Econ, 2010;19:1010–1028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.