Abstract
The incidence of colonic diseases (e.g., inflammatory bowel diseases and colon cancer) is rapidly rising. Nanotherapeutic has been considered as a promising strategy in the treatment of colonic diseases. Silk fibroin (SF) has been widely used as a drug-carrier matrix. Interestingly, SF-based nanoparticles (SFNPs) have intrinsic anti-inflammatory activity, wound healing capacity and lysosomal environment-responsive drug-release property. With further investigations, the sequences of SF molecules could be precisely modified through chemical reactions or transgenic techniques to greatly improve the properties of SFNPs. Here, we review recent advances in the application of SFNPs toward the treatment of colonic diseases. We also discuss future developments that might improve the anti-inflammatory and anti-colon cancer activities of SF-based nanotherapeutics.
Keywords: : colon cancer, inflammatory bowel disease, nanoparticle, silk fibroin, treatment
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal tract (GIT) that mainly comprises ulcerative colitis (UC) and Crohn’s disease (CD) [1]. Millions of patients in the US and Europe currently suffer from IBD, and the prevalence rate of IBD is rapidly rising in developing countries [2]. More seriously, IBD is apt to develop into colon cancer if the associated inflammation is not effectively relieved [3]. The medications for clinical treatment of colonic diseases are limited by their low therapeutic efficacies and serious adverse effects [4]. In recent years, nanotherapeutics have attracted increasing attention for colonic disease therapy owing to their capacities for controlled drug-release, passive targeting to disease sites via the enhanced permeability and retention (EPR) effect or epithelial EPR (eEPR) effect and reduced systematic toxicities [5–7]. However, extensive applications of these nanotherapeutics are seriously stymied by the lack of appropriate carrier materials [8].
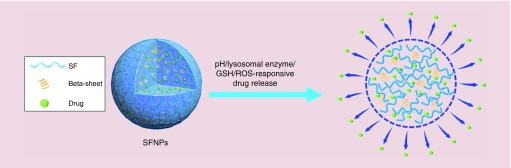
Silk fibroin (SF), a natural protein from the cocoons of the Bombyx mori silkworm, consists of a heavy chain unit, a light chain unit and a P25 glycoprotein unit [9,10]. Its heavy chain contains highly conserved repeats of the sequence GAGAGS and less conserved repeats of GAGAGX (X = V or Y) [11]. These repeats tend to self-assemble into antiparallel β-sheet structures that crosslink the molecular chains of SF molecules through inter- and intra-molecular hydrogen bonds and van der Waals interactions [12]. SF-based nanoparticles (SFNPs) have recently been proposed as promising drug carriers because of their numerous advantageous features, including biodegradability, low immunogenicity, easily controllable sequences and low cost [13]; large number of reactive groups, such as disulfide bonds, imidazolyl groups, amino groups and carboxyl groups [14]; ease of genetic improvement through transgenic technologies [15]; suitability for fabrication under mild conditions [16]; intrinsic anti-inflammatory activity [17]; high drug-loading capacity and encapsulation efficiency [18] and size controllability over a range from 60 to 1000 nm, which contributes to the penetration and accumulation of SFNPs into inflamed tissues and cancerous tissues [19]. Importantly, recent studies have highlighted the clear pH/lysosomal enzyme/glutathione (GSH)/reactive oxygen species (ROS) responsiveness of SFNPs (Figure 1), which would facilitate the on-demand release of drugs in cytoplasm [20,21].
Figure 1. . Schematic illustration of the multi-responsive drug release capacity of silk fibroin-based nanoparticles.
GSH: Glutathione; ROS: Reactive oxygen species; SFNP: Silk fibroin-based nanoparticle.
SFNPs for IBD treatment
IBD patients show exacerbated inflammatory responses to enteric microorganisms and unknown antigens in the GIT. These patients also exhibit intestinal epithelial barrier disruptions, which facilitate the penetration of luminal antigens into the lamina propria and further stimulate inflammatory responses [22]. Therefore, anti-inflammation and mucosal healing are the two goals for clinical IBD therapy [23].
Interestingly, SF has intrinsic anti-inflammatory properties. Kim and her colleagues have reported that SF molecules showed anti-inflammatory effects in a mouse model of edema. This beneficial effect was attributed to the suppression of the major pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, IL-1β, IL-6 and cyclooxygenase-2 [17]. The same research group further demonstrated that the anti-inflammatory activity of SF molecules was comparable to that of the immunophilin derivative, PEP-1-FK506BP [24].
Rodriguez-Nogales et al. [25] recently established a rat model of IBD induced by 2,4,6-trinitrobenzenesulfonic acid (TNBS), and further evaluated the therapeutic outcomes of blank SFNPs. They found that the treatment with 2,4,6-trinitrobenzenesulfonic acid remarkably increased the mRNA expression levels of the major pro-inflammatory cytokines (e.g., IL-1β, IL-6, IL-12 and IL-17), and decreased the levels of the anti-inflammatory factor IL-10. Further experiments indicated that intrarectal administration of blank SFNPs significantly downregulated the expression levels of these pro-inflammatory cytokines and promoted the expression levels of IL-10 mRNA. Subsequent investigations by this group revealed that the mRNA expression levels of important biomarkers of epithelial protection and colonic mucus constituents, namely villin, trefoil factor-3 and mucins (MUC-2 and MUC-3), were significantly increased after treatment with blank SFNPs, demonstrating that these NPs had the capacity to improve the integrity of epithelial layers.
It is known that NPs preferentially penetrate disrupted epithelial layers and accumulate in inflamed colon tissues on the basis of the epithelial EPR effect, which is attributable to the impairment of mucosal barriers and the accumulation of immune cells in colitis tissues [26]. Although NPs can passively deliver drugs to inflamed sites through this effect, their drug delivery efficiencies are still unsatisfactory. Accordingly, active targeting strategies have been employed to improve the cell specificity of NPs [5]. Integrin, an important glycoprotein in the pathogenesis of IBD and a specific receptor for arginine-glycine-aspartic acid (RGD), is highly overexpressed in the GIT of IBD patients [27]. Thus, one such targeting strategy is conjugation of the integrin ligand, RGD, onto the surface of SFNPs. Intrarectal administration of the resultant RGD-SFNPs in an animal model of IBD clearly mitigated the damage to colonic epithelial layers, reduced the infiltration of immune cells and decreased the oxidative status of colon. An evaluation of the histological appearance of colon tissues and mRNA expression levels of pro-inflammatory cytokines showed that RGD-SFNPs exerted much stronger anti-IBD activities than nonfunctionalized SFNPs [28], consistent with the improved targeting capacity.
More recently, Lozano-Perez et al. [29] encapsulated resveratrol into SFNPs (RL-SFNPs), and further investigated their potential as nanotherapeutics for IBD. RL-SFNPs showed immunomodulatory properties upon incubation with macrophages under basal conditions and inhibited the activation of macrophages following treatment with lipopolysaccharide. Moreover, in vivo experiments clearly indicated that RL-SFNPs showed much better therapeutic effects than blank SFNPs and free RL in terms of downregulation of inflammatory reactions and improved recovery of injured mucosal layer, suggesting the synergistic therapeutic effects of SFNPs and RL. Additionally, these researchers found that the curative effect of RL-SFNPs was similar to that of dexamethasone, which has been widely utilized in human IBD therapy.
The above results demonstrate that it is not the case that SFNPs are only recognized as inert nanocarriers in the treatment of IBD, as they exerted strong intrinsic anti-inflammatory activity and mucosal healing capacity [30,31].
SFNPs for treatment of colon cancer
Although chemotherapeutic drugs can inhibit the growth of tumor cells, their clinical utility is seriously limited by their hydrophobicity, random systemic distribution and unwanted adverse effects [32,33]. Thus, NPs have been introduced to overcome these limitations in cancer treatment. Over the past 20 years, a number of nanotherapeutics (e.g., doxil, abraxane) have been approved for clinical cancer treatment, either by the US FDA or the EMA. SFNPs can encapsulate various anticancer drugs (e.g., hydrophilic small molecules, hydrophobic small molecules, protein drugs and nucleic acids) with high encapsulation efficiencies [34,35], and thus, have been considered as promising nanocarriers for cancer therapy.
Zhang et al. [36] was the first to report that SFNPs could be fabricated from regenerated SF (RSF) using water-miscible organic solvents, showing that RSF molecules were loosely aggregated to form globular structures in aqueous solution. After the addition of excess organic solvent, the loosely dispersed RSF molecules were rapidly dehydrated, leading to chain–chain interactions and the formation of hydrophobic β-sheet domains. These researchers further speculated that drugs could be efficiently loaded into SFNPs through the conformational transition from unordered coil and α-helix to β-sheet, which was confirmed by our recent report [21].
To ascertain the drug-delivery potential of SFNPs, Kundu and colleagues [37] prepared SFNPs using dimethyl sulfoxide as a desolvating agent and VEGF as a model protein drug. These NPs had a particle size ranging from 150 to 170 nm, a narrow size distribution and a negatively charged surface. Interestingly, almost all the VEGF molecules were encapsulated into SFNPs and their release profile showed an initial linear release without a burst effect, followed by a sustained slow release. It was further confirmed that these NPs could be efficiently internalized by cancer cells, suggesting that SFNPs were liable to be applied in cancer therapy.
Green fabrication of NPs is critical for their medical translation, as toxic organic solvents, surfactants and harsh fabrication conditions can pose serious safety issues. In this context, Wu et al. [16] reported that the anticancer drug paclitaxel (PTX) could be facilely loaded into SFNPs in the presence of ethanol without the involvement of organic solvents or toxic components. The results from cell experiments clearly revealed that the obtained NPs could be efficiently taken up by cancer cells, and PTX maintained its anticancer activity and pro-apoptotic property after incorporation into SFNPs. Furthermore, in vivo experiments demonstrated that locoregional delivery of PTX-SFNPs resulted in excellent therapeutic efficacy against subcutaneous tumors by inhibiting their growth.
A variety of synthetic polymers have been used as carrier materials for lysosomotropic drug delivery. Nevertheless, few natural polymer-based NPs have been reported to show intrinsic on-demand drug release in response to physiological pH. Kaplan’s group [18] was the first to demonstrate that doxorubicin (Dox)-loaded SFNPs showed a clear pH-dependent drug release profile. The Dox-SFNPs in this study were fabricated based on a desolvation method and the resulting NPs had a uniform particle size of approximately 98 nm and a negatively charged surface. Further investigations of drug release profiles of Dox-SFNPs in buffers with various pH values (7.4, 6.0 and 4.5) showed that the Dox release rate of SFNPs in buffer (pH 4.5) was significantly higher than that in other buffers, which was ascribed to a reduction in the interaction forces between SFNPs and Dox in an acidic environment. In addition, Totten et al. [20] mimicked the lysosome environment (e.g., acidity, enzymes), and found that the release profile of Dox from Dox-SFNPs was further accelerated by the addition of lysosomal enzymes in acidic solution; thereby, providing direct evidence for the on-demand drug release properties of SFNPs in lysosomes. Moreover, our recent study discovered the GSH/ROS-responsible capacities of SFNPs [21]. We found that their GSH sensitivity was attributed to the cleavage of disulfide bonds between heavy chains and light chains of SF molecules, resulting in the exposure of β-sheet regions to the external environment. Their ROS sensitivity was due to the damage of the β-sheet domains of NPs, leading to the acceleration of drug release. Overall, these results demonstrated that SFNPs had intrinsic pH/lysosomal enzyme/GSH/ROS responsibility.
Transgenic protein-based polymers with advanced functions can be introduced into NPs for drug delivery. To combine the unique mechanical and biological features of SF and elastin, Xia et al. [38] produced a series of silk-elastin like protein polymers (SE8Y, S2E8Y and S4E8Y) using transgenic technologies. They found that SE8Y-based NPs had the highest drug-loading capacity among these three kinds of NPs. Furthermore, these SE8Y-based Dox-loaded NPs were taken up by cancer cells through endocytosis, and showed 1.8-fold greater anticancer activity than the free Dox.
Because SFNPs have a number of attractive attributes, including desirable particle size, high drug-encapsulation efficiency and intrinsic on-demand drug-release capacity, they were recently applied in the treatment of colon cancer. In these studies, Xie et al. [16] produced curcumin (CUR)-loaded SFNPs using a technique termed solution-enhanced dispersion by supercritical CO2, which had the merits of a mild fabrication process, no organic solvent residue and environmentally friendly production. The resultant curcumin SFNPs, with a particle size less than 100 nm, showed a time-dependent cellular uptake profile and a strong anticolon cancer effect. The underlying anticancer mechanism could be attributed to cell-cycle arrest in G0/G1 and G2/M phases and activation of apoptotic processes.
Conclusion
SFNPs, designed and produced for the treatment of IBD and colon cancer, have attracted increasing research attention owing to their biocompatibility, controlled degradability, facile self-assembly and intrinsic bioactivities, including anti-inflammatory activity, mucosal healing and bioresponsive capacity. The application of SFNPs in drug delivery required adjusting the extraction protocol of SF molecules, NP fabrication processes, crystallinity in SFNPs and interactions between SF molecules and drugs. It will also be important to improve their targeted drug delivery capacity and other properties through chemical or biological technologies. These adjustments, improvements and standardization of techniques will further expand the application of SFNPs in the treatment of colonic diseases.
Future perspective
Although SF has become an attractive biomaterial for drug delivery, several challenges remain to be overcome. Inconsistencies in the molecular weight of SF after degumming may cause difficulties in quality control of SFNPs. However, transgenic SF proteins, produced by heterogeneous expression, have the potential to overcome this problem. Lack of control of particle size, variable shape and use of organic solvents during preparation processes are also challenges for loading bioactive drugs – especially protein drugs – in SFNPs.
SF-based medical devices could conceivably be envisioned for various tissue repair applications, precise drug release, specific targeting and responsiveness to multiple stimuli. SF molecules can be designed and modified through chemical reactions and transgenic technologies, and subsequently processed to SFNPs that meet all the requirements for drug delivery. Additionally, hybrid SFNPs could combine the advantages of various materials and become an increasingly attractive option.
Executive summary.
Silk fibroin (SF) has a number of advantageous features, including biocompatibility, biodegradability, nonimmunogenicity, self-assembly and amenability to large-scale production.
Blank SF-based nanoparticles (SFNPs) have intrinsic anti-inflammatory activity and mucosal healing properties, and are thus bioactive nanotherapeutics in their own right.
SFNPs show on-demand drug release profiles in response to the physiological environment (e.g., acidity, enzyme) of lysosomes, providing a benefit for drug delivery.
Chemical modifications and transgenic technologies can endow SF molecules, and resulting SFNPs, with specific functions that may be helpful in improving therapeutic outcomes against colonic diseases.
Footnotes
Financial & competing interests disclosure
This work was supported by the National Institutes of Health of Diabetes and Digestive and Kidney (RO1-DK-107739 & 116306 to D Merlin), the National Natural Science Foundation of China (51503172 & 81571807 to B Xiao), the Department of Veterans Affairs (Merit Award BX002526 to D Merlin), the Young Core Teacher Program of the Municipal Higher Educational Institution of Chongqing, the Venture & Innovation Support Program for Chongqing Overseas Returnees (cx2018029) and the Chinese Scholarship Council. D Merlin is a recipient of a Senior Research Career Scientist Award from the Department of Veteran Affairs (BX004476). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Zhang X, Deeke SA, Ning ZB. et al. Metaproteomics reveals associations between microbiome and intestinal extracellular vesicle proteins in pediatric inflammatory bowel disease. Nat. Commun. 9(1), 2873–2887 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 152(2), 313–321 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Long MD, Sands BE. When do you start and when do you stop screening for colon cancer in inflammatory bowel disease? Clin. Gastroenterol. Hepatol. 16(5), 621–623 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Si XY, Merlin D, Xiao B. Recent advances in orally administered cell-specific nanotherapeutics for inflammatory bowel disease. World J. Gastroenterol. 22(34), 7718–7726 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao B, Chen QB, Zhang Z. et al. TNF alpha gene silencing mediated by orally targeted nanoparticles combined with interleukin-22 for synergistic combination therapy of ulcerative colitis. J. Control. Rel. 287, 235–246 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kedmi R, Veiga N, Ramishetti S. et al. A modular platform for targeted RNAi therapeutics. Nat. Nanotechnol. 13(3), 214–219 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science 319(5863), 627–630 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q, Gou S, Ma P. et al. Oral administration of colitis tissue-accumulating porous nanoparticles for ulcerative colitis therapy. Int. J. Pharm. 557, 135–144 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Qi Y, Wang H, Wei K. et al. A review of structure construction of silk fibroin biomaterials from single structures to multi-level structures. Int. J. Mol. Sci. 18(3), 237–258 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathur AB, Gupta V. Silk fibroin-derived nanoparticles for biomedical applications. Nanomedicine 5(5), 807–820 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Mottaghitalab F, Farokhi M, Shokrgozar MA, Atyabi F, Hosseinkhani H. Silk fibroin nanoparticle as a novel drug delivery system. J. Control. Rel. 206, 161–176 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Seib FP. Silk nanoparticles-an emerging anticancer nanomedicine. Aims Bioeng. 4(2), 239–258 (2017). [Google Scholar]
- 13.Crivelli B, Perteghella S, Bari E. et al. Silk nanoparticles: from inert supports to bioactive natural carriers for drug delivery. Soft Matter 14(4), 546–557 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Chen JM, Venkatesan H, Hu JL. Chemically modified silk proteins. Adv. Eng. Mater. 20(7), 1700961–1700975 (2018). [Google Scholar]
- 15.Xia XX, Xu QB, Hu X, Qin GK, Kaplan DL. Tunable self-assembly of genetically engineered silk-elastin-like protein polymers. Biomacromolecules 12(11), 3844–3850 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Introduces the potential of transgenic silk fibroin as nanocarriers for drug delivery.
- 16.Xie MB, Fan DJ, Li Y. et al. Supercritical carbon dioxide-developed silk fibroin nanoplatform for smart colon cancer therapy. Int. J. Nanomed. 12, 7751–7761 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DW, Hwang HS, Kim DS. et al. Effect of silk fibroin peptide derived from silkworm Bombyx mori on the anti-inflammatory effect of Tat-SOD in a mice edema model. BMB Rep. 44(12), 787–792 (2011). [DOI] [PubMed] [Google Scholar]; •• Describes the pH-responsible property of silk fibroin-based nanoparticles.
- 18.Seib FP, Jones GT, Rnjak-Kovacina J, Lin YN, Kaplan DL. pH-dependent anticancer drug release from silk nanoparticles. Adv. Healthc. Mater. 2(12), 1606–1611 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uddin MJ, Werfel TA, Crews BC. et al. Fluorocoxib A loaded nanoparticles enable targeted visualization of cyclooxygenase-2 in inflammation and cancer. Biomaterials 92, 71–80 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Totten JD, Wongpinyochit T, Seib FP. Silk nanoparticles: proof of lysosomotropic anticancer drug delivery at single-cell resolution. J. Drug Target. 25(10), 865–872 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Gou S, Huang Y, Wan Y. et al. Multi-bioresponsive silk fibroin-based nanoparticles with on-demand cytoplasmic drug release capacity for CD44-targeted alleviation of ulcerative colitis. Biomaterials 212, 39–54 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Martens EC, Neumann M, Desai MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat. Rev. Microbiol. 16, 457–470 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Xiao B, Zhang Z, Viennois E. et al. Combination therapy for ulcerative colitis: orally targeted nanoparticles prevent mucosal damage and relieve inflammation. Theranostics 6(12), 2250–2266 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SY, Sohn EJ, Kim DW. et al. Transduced PEP-1-FK506BP ameliorates atopic dermatitis in NC/Nga Mice. J. Invest. Dermatol. 131(7), 1477–1485 (2011). [DOI] [PubMed] [Google Scholar]; •• Insightful study showing that blank silk fibroin-based nanoparticles can alleviate inflammatory bowel disease (IBD).
- 25.Rodriguez-Nogales A, Lozano-Perez AA, Aznar-Cervantes SD. et al. Effect of aqueous and particulate silk fibroin in a rat model of experimental colitis. Int. J. Pharmaceut. 511(1), 1–9 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Xiao B, Xu ZG, Viennois E. et al. Orally Targeted delivery of tripeptide KPV via hyaluronic acid-functionalized nanoparticles efficiently alleviates ulcerative colitis. Mol. Ther. 25(7), 1628–1640 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nielsen OH, Seidelin JB, Munck LK, Rogler G. Use of biological molecules in the treatment of inflammatory bowel disease. J. Intern. Med. 270(1), 15–28 (2011). [DOI] [PubMed] [Google Scholar]; • Describes the therapeutic outcomes of blank targeted silk fibroin-based nanoparticles against IBD.
- 28.Rodriguez-Nogales A, Algieri F, De Matteis L. et al. Intestinal anti-inflammatory effects of RGD-functionalized silk fibroin nanoparticles in trinitrobenzenesulfonic acid-induced experimental colitis in rats. Int. J. Nanomed. 11, 5945–5958 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lozano-Perez AA, Rodriguez-Nogales A, Ortiz-Cullera V. et al. Silk fibroin nanoparticles constitute a vector for controlled release of resveratrol in an experimental model of inflammatory bowel disease in rats. Int. J. Nanomed. 9(1), 4507–4520 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez-Mora C, Mrowiec A, Garcia-Vizcaino EM, Alcaraz A, Cenis JL, Nicolas FJ. Fibroin and sericin from bombyx mori silk stimulate cell migration through upregulation and phosphorylation of c-Jun. PLoS ONE 7(7), e44271 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim DW, Hwang HS, Kim DS. et al. Enhancement of anti-inflammatory activity of PEP-1-FK506 binding protein by silk fibroin peptide. J. Microbiol. Biotechn. 22(4), 494–500 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Falke LL, van Vuuren SH, Kazazi-Hyseni F. et al. Local therapeutic efficacy with reduced systemic side effects by rapamycin-loaded subcapsular microspheres. Biomaterials 42, 151–160 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Ma LJ, Chen QB, Ma PP. et al. iRGD-functionalized PEGylated nanoparticles for enhanced colon tumor accumulation and targeted drug delivery. Nanomedicine 12(16), 1991–2006 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werner V, Meinel L. From silk spinning in insects and spiders to advanced silk fibroin drug delivery systems. Eur. J. Pharm. Biopharm. 97, 392–399 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, You RC, Liu GY. et al. Antheraea pernyi silk fibroin-coated PEI/DNA complexes for targeted gene delivery in HEK 293 and HCT 116 Cells. Int. J. Mol. Sci. 15(5), 7049–7063 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang YQ, Shen WD, Xiang RL, Zhuge LJ, Gao WJ, Wang WB. Formation of silk fibroin nanoparticles in water-miscible organic solvent and their characterization. J. Nanopart. Res. 9, 885–900 (2007). [Google Scholar]
- 37.Kundu J, Chung YI, Kim YH, Taeb G, Kundu SC. Silk fibroin nanoparticles for cellular uptake and control release. Int. J. Pharmaceut. 388(2), 242–250 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Xia XX, Wang M, Lin YA, Xu QB, Kaplan DL. Hydrophobic drug-triggered self-assembly of nanoparticles from silk-elastin-like protein polymers for drug delivery. Biomacromolecules 15(3), 908–914 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]