Abstract
Background
Overgowns are widely used in newborn nurseries and neonatal intensive care units. It is thought that gowns may help to prevent the spread of nosocomial infection and serve as a reminder to staff and visitors to wash their hands before contact with the infant.
Objectives
The objective of this review is to assess the effects of the wearing of an overgown by attendants and visitors on the incidence of infection and death in infants in newborn nurseries.
Search methods
The standard methods of the Cochrane Collaboration and its Neonatal Review Group were used. We searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 1, 2009), MEDLINE (1950 ‐ January 2009), Embase (1950 ‐ January 2009) and CINAHL (1982 ‐ January 2009).
This search was updated in December 2010.
Selection criteria
The review includes all published trials using random or quasi‐random patient allocation, in which overgowns worn by attendants or visitors were compared with no overgowns worn by attendants or visitors.
Data collection and analysis
The standard methods of the Cochrane Collaboration and its Neonatal Review Group were used. Data extraction and study quality were independently assessed by the two review authors. Missing information was sought from three authors, but only one responded. Results are expressed as relative risk or mean difference with 95% confidence intervals .
Main results
Eight trials were included, reporting outcomes for 3,811 infants. Trial quality varied, with only two assessed as being of good quality. Not wearing overgowns was associated with a trend to reduction in the death rate (typical RR 0.84, 95% CI 0.70 to 1.02) compared to wearing overgowns, but these results did not reach statistical significance. There was no statistically significant effect of gowning policy on incidence of systemic nosocomial infection, (typical RR 1.24, 95% CI 0.90 to 1.71). The overall analysis showed no significant effects of gowning policy on the incidence of colonisation, length of hospital stay or handwashing frequency. No trials of visitor gowning were found.
Authors' conclusions
There is no evidence from this systematic review and meta‐analysis to demonstrate that overgowns are effective in limiting death, infection or bacterial colonisation in infants admitted to newborn nurseries.
Plain language summary
Gowning by attendants and visitors in newborn nurseries for prevention of neonatal morbidity and mortality
Newborn nurseries and neonatal intensive care units often require staff and visitors to wear overgowns with the intention of preventing the spread of infection. It has also been thought that putting on an overgown will remind people to wash their hands, which is of proven importance in preventing infection. A review of the medical literature identified eight clinical trials on gowning in these settings, involving 3811 newborns. Infection rates, death rates, or the length of stay of infants were not significantly affected by wearing gowns. Only two of the trials were considered to be of good quality, and there was variation between trials regarding gowning policies. Gowning did not increase the rate of handwashing. There is no evidence to support the use of gowning by staff to prevent the spread of infection. Based on these studies, gowning may not be a cost effective policy.
Background
Newborn infants, particularly those admitted to neonatal intensive care units, are at risk for a variety of bacterial, viral and fungal infections (Gaynes 1996). Neonatal infection carries a high risk of morbidity and mortality, especially among very low birth weight infants (Barton 1999). Reasons for higher rates of infection amongst newborns includes their lowered ability to resist disease agents (Levy 1999), exposure to endemic nursery pathogens (Foca 2000; Webster 1994), prolonged use of central venous catheters (Chathas 1990) and exposure to intrauterine infections (Seaward 1998).
Organisms introduced into the nursery may be transmitted to other infants by a variety of routes making cross infection a particular problem (Baltimore 1998). A colonised or infected infant has the potential to impact on the colonisation or infection rates in particular time periods. Handwashing is recognised as the single most effective method of reducing the transmission of microorganisms between patients (Larson 1999) and is an integral part of hospital infection control programs. Other practices, such as various barrier methods, are also used to control cross infection in hospitals. Gowning is one barrier method of infection control frequently used to restrict the transmission of infection (Cloney 1986). It is common practice for attendants and visitors to wear overgowns in some neonatal intensive care nurseries. For attendants, this is to prevent patient‐to‐patient transmission of microorganisms and infection; for visitors, it is to protect newborns from organisms which they may carry. Although wearing overgowns is believed to increase compliance with hand washing, one non‐controlled study has not demonstrated this effect (Donowitz 1987). In recent times, cost considerations have led some institutions to abandon the use of overgowns in newborn nurseries (Thigpen 1991).
Although many centres use overgowns for attendants and visitors as a means of infection control in newborn nurseries and neonatal intensive care units, the benefits and risks of gowning remain unclear.
Objectives
Primary: To determine the effects of wearing overgowns compared to no gowns by attendants and visitors to newborn infants admitted to a Neonatal Intensive Care Unit or a Newborn Nursery on hospital acquired infection and death. Secondary: To determine the effects of wearing overgowns for subgroups of newborn infants by gestational age, by nursery type and by visitors and attendants.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials in which the unit of allocation is either the individual or a cluster (such as randomisation by physician or hospital or time period).
Types of participants
Attendants of infants admitted to a neonatal intensive care or newborn nursery.
Visitors to infants admitted to a neonatal intensive care or newborn nursery.
The unit of randomisation will either be the individual infant or the entire unit/service.
Types of interventions
Use of overgowns compared with no gowns by attendants and visitors in the care of newborn infants.
Types of outcome measures
Primary outcomes
Death (before discharge from nursery).
Systemic nosocomial infection (any systemic infection identified > 48 hours after admission to the nursery).
Localised nosocomial infection (any localised infection identified > 48 hours after admission to the nursery) (Garner 1996).
Secondary outcomes
Nosocomial colonisation (bacterial colonisation of any site cultured, identified > 48 hours after admissions to the nursery).
Cost (directly related to laundering and replacement of gowns and time taken to 'gown').
Handwashing (frequency).
Length of stay (days).
In addition for preterm infants:
Duration of mechanical ventilation (days).
Duration of neonatal intensive care nursery stay (days).
Antibiotic use.
Search methods for identification of studies
The standard search strategy of the Cochrane Neonatal Group was used. See: Cochrane Neonatal Group search strategy.
Electronic searches
The review authors conducted searches of the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 1, 2009), MEDLINE (1950 ‐ January 2009), Embase (1950 ‐ January 2009), and CINAHL (1982‐ January 2009), which were published in the English language, using MeSH terms infant‐ preterm, infant‐newborn, cross infection‐prevention, cross infection‐control, protective clothing and text words neonat*, intensive care unit, nurser* hospital, postpartum, gown*, overgown, covergown, infection*, and colonis*, handwash*. The Oxford Data Base of Perinatal Trials was searched for unpublished trials.
In December, 2010, we updated the search as follows: MEDLINE (search via PubMed), CINAHL, EMBASE and CENTRAL (The Cochrane Library) were searched from 2008 to Dec 2010. Search terms: (cross infection‐prevention OR cross infection‐control OR protective clothing OR postpartum OR gown* OR overgown OR covergown OR infection* OR colonis* OR handwash*) AND (intensive care unit OR nurser* OR hospital OR nursery OR ER) AND ((infant, newborn[MeSH] OR newborn OR neon* OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])). No language restrictions were applied. In addition, clinicaltrials.gov and controlled‐trials.com were searched for relevant studies.
Data collection and analysis
The standard methods of the Cochrane Collaboration and its Neonatal Review Group were used.
Selection of studies
All randomised and quasi‐randomised controlled trials fulfilling the selection criteria described in the previous section were included. Review authors independently assessed whether studies met the inclusion criteria. Results were compared and discrepancies resolved by discussion.
Data extraction and management
Review authors independently extracted data. Results were compared and discrepancies resolved by consensus or referral to a third party.
Assessment of risk of bias in included studies
The methodological quality of each trial was independently reviewed by each review author taking account of blinding at randomisation, intervention and outcome measurement and completeness of follow up. Additional information was sought from three trial authors. Any disagreement was resolved by discussion. This information was added to the Characteristics of Included Studies table.
In addition, for the update in 2011, the following issues were evaluated and entered into the Risk of Bias table:
1) Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated? For each included study, we categorized the method used to generate the allocation sequence as:
‐ adequate (any truly random process e.g. random number table; computer random number generator);
‐ inadequate (any non random process e.g. odd or even date of birth; hospital or clinic record number);
‐ unclear.
(2) Allocation concealment (checking for possible selection bias). Was allocation adequately concealed? For each included study, we categorized the method used to conceal the allocation sequence as:
‐ adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
‐ inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
‐ unclear.
(3) Blinding (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment? For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods as:
‐ adequate, inadequate or unclear for participants;
‐ adequate, inadequate or unclear for personnel;
‐ adequate, inadequate or unclear for outcome assessors.
In some situations there may be partial blinding e.g. where outcomes are self‐reported by unblinded participants but they are recorded by blinded personnel without knowledge of group assignment. Where needed “partial” was added to the list of options for assessing quality of blinding.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed? For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as:
‐ adequate (< 20% missing data);
‐ inadequate (≥ 20% missing data):
‐ unclear.
(5) Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting? For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
‐ adequate (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
‐ inadequate (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
‐ unclear.
(6) Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias? For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
‐ yes; no; or unclear.
If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Measures of treatment effect
The standard methods of the Neonatal Review Group were used. Statistical analyses were performed using Review Manager software. Categorical data were analysed using relative risk (RR), risk difference (RD) and the number needed to treat (NNT). Continuous data were analysed using weighted mean difference (WMD). The 95% Confidence interval (CI) was reported on all estimates.
Unit of analysis issues
Trials which allocated clusters of patients to each intervention were not analysed using the number of clusters as the unit of analysis, as intended in the protocol, but analysed as if the allocation was by individual. This was necessary because none of the authors of these trials used the cluster as the unit of analysis. Analysing cluster trials in this way has the potential to over‐estimate the effect of treatment (Mollison 2000). Consequently, for each outcome there is a meta‐analysis of all trials and also of two subgroups where appropriate, one which includes the trials which randomised the individual participant and one which includes the cluster allocated trials.
Assessment of heterogeneity
We assessed heterogeneity between results using the I2 statistic (Higgins 2009). This examined the percentage of total variation across studies due to heterogeneity rather than chance. We used a random effects model where the values of I2 were over 50%, indicating a high level of heterogeneity. For all other meta analyses, we used a fixed effect model.
Data synthesis
The meta‐analysis was performed using Review Manager software (RevMan 5) supplied by the Cochrane Collaboration. For estimates of typical relative risk and risk difference, we used the Mantel‐Haenszel method. For measured quantities, we used the inverse variance method. We used a random effects model where the values of I2 were over 50%, indicating a high level of heterogeneity. For all other meta analyses, we used a fixed effect model.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned on the basis of nursery type (well baby nursery and intensive care nursery), gestational age at birth (37 or more completed weeks, < 37 to 30 completed weeks and < 30 weeks) and by visitors and attendants.
Results
Description of studies
Twelve studies were identified. Four were excluded for the reasons given in the table, Characteristics of Excluded Studies. Eight studies were considered eligible for inclusion in the review. See Table, Characteristics of Included Studies.
Three of the studies randomised by individual infants. Each of these was conducted in a well baby, full‐term nursery (Birenbaum 1990; Forfar 1958; Rush 1990). In two of these studies, rooming in was practiced and infants spent only short periods of time in the nursery (Birenbaum 1990; Rush 1990). Methods used in the three studies were similar with staff and visitors in the control arm using gowns and those in the experimental arm not wearing gowns. In both groups, infection control precautions such as handwashing before entering the nursery and before and after handling infants were observed.
Five studies used cluster allocation by alternating blocks of time for the gown and no‐gown periods, in either neonatal intensive care or special care nurseries (Agbayani 1981; Evans 1971; Pelke 1994; Silverman 1967; Tan 1995). In the gowning time periods, gowns were worn by all staff and visitors on entering the nursery and for all infant contacts. In the no‐gown time periods, there were between study variations in how 'gowning' was defined. In the earlier studies (Agbayani 1981; Evans 1971; Silverman 1967), gowns were not worn when entering the nursery but they were worn if the incubator hood was opened or when an infant was being held. In the two later studies (Pelke 1994; Tan 1995), gowns were not used at all during the no‐gown periods. In the Tan 1995 trial, gowns were defined as a plastic apron.
Alternate time periods used in each study were two or three‐month blocks and the length of studies varied between eight to 25 months. One study attempted to eliminate exposure effects from one time period to another by excluding infants who were admitted in the last 10 days of each two month interval (Agbayani 1981). Adjustment for seasonal biases was made in a further study where the gowning period was extended for one month at the end of the first 12 months to ensure a different distribution of gowning periods in the second year (Silverman 1967). One study provided evidence of community follow‐up to establish if any infections had occurred after hospital discharge (Birenbaum 1990).
Each infant was allocated to the gowning and no‐gowning groups according to the gowning policy in place during the month the infant was admitted and outcomes for that infant were attributed to the gowning policy as allocated on admission.
Evans 1971 and Agbayani 1981 reported nasal and umbilical colonisation rates by day of life for the gown and no‐gown groups (Evans 1971 on days 2, 4, 6, 8, 10, 14 and 21 and Agbayani 1981 on days 1, 2, 4, 7, 10, 21 and 28). We chose day four results for the meta‐analysis to ensure that colonisation was hospital acquired (i.e. acquired more than 48 hours after admission) and to maximise the number included in the sample (i.e. it was not until day 10 when similar days were again used for reporting and by this time, many of the infants had been discharged). Groin swabs were also analysed using day four results (Agbayani 1981).
Effect by visitors and attendants None of the studies reported on independent effects of wearing gowns by either visitors or attendants. All of the included studies focused on comparisons when both the visitors and attendants wore or did not wear gowns.
Risk of bias in included studies
See: table, Characteristics of Included Studies.
Concealment of allocation There was adequate concealment of allocation in two of the trials (Birenbaum 1990; Rush 1990), each randomising the individual patient. One used shuffled sealed envelopes (Birenbaum 1990) and the second used consecutively numbered sealed envelopes that contained a folded card with the group allocation (Rush 1990). No information was provided for the allocation technique used by Forfar 1958, but it is stated that infants were randomly assigned to one of two full term nurseries. None of the trials using cluster allocation used randomly allocated periods for the intervention; all use pre‐determined two or three month blocks.
Blinding Blinding of the intervention was not possible. Blinding of outcome assessment was reported in only one study (Rush 1990).
Completeness of follow up In the Birenbaum 1990 study, there was no indication of how many infants were randomised on admission to either the gown or no‐gown groups. Infants were excluded if they did not have nose and umbilical cultures taken within six hours of delivery or if they did not have cultures taken before discharge. This makes the possibility of attrition bias likely. Rush 1990 enrolled 234 infants in the no‐gown group and 239 infants in the gown group. Length of stay was the only outcome calculated using these numbers. Infection and colonisation data were reported on 222 infants and 230 infants respectively. In the Forfar 1958 trial, follow‐up data are complete for infection but not for colonisation. There were also incomplete colonisation data in the Agbayani 1981, Evans 1971 and Pelke 1994 trials.
Effects of interventions
See: List of comparisons
Eight studies met the inclusion criteria and reported on 3,811 infants who were cared for by attendants who wore or did not wear gowns. PRIMARY OUTCOMES Death before discharge (Outcome 1.1): The death rate was reported in all the cluster allocation trials, each conducted in intensive care settings (Silverman 1967; Evans 1971; Agbayani 1981; Tan 1995; Pelke 1994). None of the trials found a statistically significant effect on death. The meta‐analysis was confined to four trials (Silverman 1967; Evans 1971; Agbayani 1981; Tan 1995). Overall, not wearing a gown was associated with a trend towards reduction in death rate (typical RR 0.84, 95% CI 0.70 to 1.02; typical RD ‐0.03, 95% CI ‐0.05 to 0.00), but these results did not reach statistical significance. The death rate as reported by Pelke 1994 was similar between groups (0.44 per 100 patient days in the no gown periods and 0.51 per 100 patient days in the gown periods). Due to the way in which they were reported, these data could not be included in the meta‐analysis. Systemic nosocomial infection (Outcome 1.2): Five cluster allocation trials reported information on systemic infection (septicaemia, meningitis, necrotizing enterocolitis, pneumonia) (Agbayani 1981; Evans 1971; Pelke 1994; Silverman 1967; Tan 1995). One of these (Silverman 1967) reported only meningitis or septicaemia confirmed by postmortem examination. None of the trials found a statistically significant effect on the incidence of systemic nosocomial infection. The meta‐analysis, confined to four trials not including Pelke 1994, found no significant effect on systemic nosocomial infection (typical RR 0.95, 95% CI 0.40 to 2.23)]. Substantial heterogeneity was found in this comparison (I2= 57.1%) so a random effects model was used for the meta‐analysis. Pelke 1994 also provided data for systemic infections (no gowning period 1.38 infections per 100 patient days; gowning period 1.21 infections per 100 patient days); the difference was not statistically significant. Due to the way in which they were reported, the data of Pelke 1994 could not be included in the meta‐analysis. Localised nosocomial infection (Outcome 1.3): Four studies were identified that evaluated localised nosocomial infection. Two were trials that randomised the individual patient (Forfar 1958; Rush 1990). These showed no statistically significant effect (typical RR 1.17, 95% CI 0.74 to 1.86). Two were cluster allocation trials Agbayani 1981; Evans 1971). These also showed no significant effect on localised nosocomial infection (typical RR 1.29, 95% CI 0.84 to 2.00). The overall estimate for the four studies showed no significant effect (typical RR 1.24, 95% CI 0.90 to 1.71).
SECONDARY OUTCOMES Colonisation: The methods used to collect and process swabs were similar, but the days on which swabs were taken varied between studies. Two of the trials limited their investigation to staphylococcal carriage (Forfar 1958; Rush 1990) and one to methicillin resistant Staphylococcus aureus carriage (MRSA) (Tan 1995). In the Tan 1995 study, the site of colonisation was not noted but carriage rates were similar between groups (no‐gown group 4/1002 MRSA positive swabs, gown group 6/904 MRSA positive swabs).
Nasal colonisation (Outcome 1.4): Nasal colonisation data was compared in six of the eight included studies. Three trials that randomised the individual patient (Birenbaum 1990; Forfar 1958; Rush 1990) found no significant differences in nasal colonisation rates (typical RR 1.02, 95% CI 0.89 to 1.18). There was also no significant effect seen in the two cluster trials (Agbayani 1981; Evans 1971) (typical RR 0.91, 95% CI 0.77 to 1.07). When the results of all five trials were combined in an overall meta‐analysis, there was no significant effect (typical RR 0.98, 95% CI 0.88, 1.09). In the Pelke 1994 trial the number of swabs taken was used as the denominator with no indication of how many infants were swabbed. There were no significant differences in the rate of positive cultures between the no‐gowning and gowning periods (no‐gown group 179/375 positive swabs; gown group 208/351 positive swabs).
Umbilical colonisation (Outcome 1.5): Six trials provided data on umbilical colonisation. Those randomising by individual (Birenbaum 1990; Forfar 1958; Rush 1990) showed no significant effect on this outcome (typical RR 1.03, 95% CI 0.93, 1.14). Results from two of the cluster allocation trials (Agbayani 1981; Evans 1971) also showed no significant difference on this outcome (typical RR 0.96, 95% CI 0.82 to 1.12). When results from the five trials were combined, the result was not significant (typical RR 1.01, 95% CI 0.93 to 1.10). The other cluster allocation trial (Pelke 1994) reported similar proportions of positive cultures among the total cultures taken (no‐gown group 92/213 positive swabs; gown group 86/167 positive swabs). Eye colonisation (Outcome 1.6): One study using random allocation by individual (Forfar 1958) collected data on eye colonisation. No significant difference was found between the no‐gowned and gowned groups (RR 0.97, 95% CI 0.90 to 1.05).
Groin colonisation (Outcome 01.07): One of the trials that randomised by individual, reported collected data on groin colonisation (Birenbaum 1990). Gowning policy did not significantly effect this outcome (RR 1.05, 95% CI 0.69 to 1.57).
Stool colonisation: In one study (Pelke 1994) there was a significant difference in the rate of stool colonisation between the no‐gown (84/372) and the gown groups (48/346). A total of 718 cultures were taken from 230 infants, so it is unknown how many repeat cultures were taken from each infant with a positive culture result.
Cost: The cost of wearing gowns was estimated in three of the trials. Forfar 1958 included an estimate of the annual cost of gowning (nursing time, cost of gown laundering and maintenance) and calculated that the cost of time alone was equivalent to employing more than one full time equivalent nurse for one year. Tan 1995 compared the cost of gowns used in the no‐gowning period with those used in the gowning period. During the gowning period, the average number of gowns used was 312 per day compared with 177 per day in the no‐gowning periods. Gowns were defined as plastic aprons and cost Singapore $0.05 each. This resulted in a cost difference of S$1,696 per annum. Rush 1990 concluded that the projected annual cost savings associated with discontinuing gowns would be approximately $US 8,000 per annum.
Handwashing: One cluster allocation trial compared handwashing frequency between the no‐gowning and gowning time periods (Pelke 1994). Direct observation at an infant's bedside three times weekly for 30 minutes was used to collect data. A sample of 87 contacts were observed in the no‐gowning period and 34 infant contacts during the gowning period. The rate of hand wash compliance was similar in the two groups (no gowning 60%, gowning 62%, p = 0.84).
Length of hospital stay (Outcome 1.8): Length of hospital stay in a well baby nursery was measured in three trials randomising the individual (Birenbaum 1990; Forfar 1958; Rush 1990). In the Rush 1990 study, hospital stay was similar in both groups, (MD 0.40 days, 95% CI ‐5.82 to 6.62). The number of in‐patient days did not differ significantly in either the Forfar 1958 trial (no gown 9.0 days, gown 8.5 days) or the Birenbaum 1990 trial (no gown 2.81 days, gown 2.84 days). Standard deviations were unavailable for these two studies, preventing inclusion of these data in the outcome table.
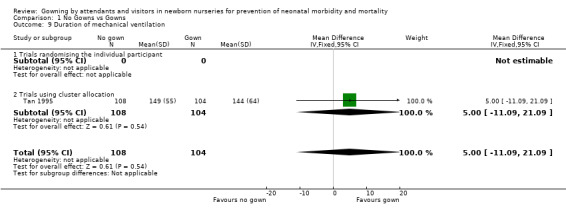
ADDITIONAL OUTCOMES FOR PRETERM INFANTS: Duration of mechanical ventilation (Outcome 1.9): One cluster allocation trial included results on the duration of mechanical ventilation (Tan 1995). The number of ventilator days was similar for infants admitted during the no‐gowning and gowning time periods (MD 5.00 days, 95% CI ‐11.09 to 21.09).
Duration of neonatal intensive care nursery stay: Pelke 1994 measured length of stay in a neonatal nursery environment. The mean length of stay between the no‐gown and gown groups was not statistically different (no‐gowning periods: mean number of days = 15; gowning periods: mean number of days = 20).
Antibiotic use in preterm infants: None of the trials provided data for this outcome
OTHER OUTCOMES Nursery traffic: In a cluster allocation trial, Pelke 1994 used two 15‐minute observation periods to monitor the number of people entering the nursery. The patterns of traffic were identical during the no‐gown and gown periods with an average of 10 entries during each 15‐minute observation period.
Post discharge follow‐up: In the Birenbaum 1990 study, 83 from the no‐gown group and 81 infants in the gown group were able to be followed up four weeks after discharge. Within this time, one infant from the no‐gowning group was treated for conjunctivitis and one infant from the gowning group required hospitalisation for a viral infection.
SUBGROUP ANALYSIS:
Effect by nursery type: All of the trials that randomised the individual patient were conducted in well‐baby nurseries and all of the cluster allocation trials were undertaken in neonatal intensive care units. Thus, the analyses of sub‐categories for trials randomising the individual are synonymous with well‐baby nurseries and sub‐categories for cluster allocation trials are synonymous with neonatal intensive care units.
Effect by gestational age at birth: We intended to investigate the effects of wearing gowns for subgroups of newborn infants by gestational age; however, none of the trials reported outcomes specifically by gestational age so this analysis could not be done. Effect by visitors and attendants: There were no eligible studies reporting the independent effect of visitors or attendants wearing gowns on the study outcomes.
Discussion
Since overgowns are widely used in neonatal units, it was surprising that the evidence supporting their efficacy was limited. Of the eight studies meeting our inclusion criteria, three used the individual as the unit of allocation, but one of these did not describe the method used for allocation concealment. The nature of the study prevented blinding of the intervention and there was limited reporting of blinding of outcome assessment. Five of the studies had incomplete follow‐up data on one or more of the outcomes (Agbayani 1981; Evans 1971; Forfar 1958; Pelke 1994; Rush 1990) and there was evidence of post‐randomisation exclusions in one of the trials (Birenbaum 1990). Sample size calculations were absent in all but one study (Rush 1990).
Among the five cluster allocation trials there were a number of methodological variations that made comparisons difficult. In one study, colonisation rates were reported as outcomes per swab rather than per infant, leading to non‐independence of multiple measures of the same outcome in the same patient. Similarly, the day on which swabs were routinely taken varied between studies. Rates of colonisation tend to increase with length of hospital stay, so comparing data on this outcome was not feasible unless swabs had been collected on the same postnatal day. Other data were reported as a rate per 100 days making it impossible to combine these results with other outcome data to estimate an overall effect. Although techniques are now available for analysing cluster allocated studies, results were all analysed as if allocation was by individual, ignoring the cluster design and creating a potential to over‐estimate the intervention effect. However, based on the consistency of findings between studies, the method of analysis is unlikely to have changed the primary results of this review.
There was little evidence in this review of either harm or benefit of overgown use when outcomes such as systemic infection, localised infection or colonisation were compared. The only important outcome that showed a strong trend in either direction was death before discharge, where the trend was towards a lower death rate among infants nursed in the non‐gowning periods. The two studies contributing to the trend were conducted over 30 years ago when death rates in neonatal intensive care units were very high (Evans 1971; Silverman 1967). Both of the studies used a cluster design and analysed results as though allocation was by individual, which may have tended to overestimate treatment effect. In addition, overgowns were worn by attendants and visitors whenever incubator lids were open or if the infant was removed from the cot, making it unlikely that gowning could account for the observed differences. In the most recent and largest trial, no deaths were reported in either the gowning or no‐gowning periods (Tan 1995). The one result that showed a significant difference when overgowns were worn or not worn by visitors and attendants was stool colonisation, with a reduction during gown periods. This result was flawed by the study methodology, where there was evidence of repeat measures on the same infant.
Other outcomes such as handwashing frequency, length of hospital stay, duration of mechanical ventilation and traffic in and out of the nursery were not significantly affected by overgown use. Based on these results and considering the costs associated with gowning, hospital personnel may wish to review their policies.
Heterogeneity effected one comparison, systemic infection. This may be explained by some of the issues outlined above, or because there was some variation in outcome when the older studies were compared with more recent investigations.
All the NICU studies included in this review used cluster allocation rather than allocating individual patients to the experimental and control groups. Allocation by cluster might be seen as a strength of study design for this question. It mirrors the way the intervention is offered in practice and minimises contamination of the experimental and control groups. Secondary cases (of colonisation, infection, death) are included in the measure of effect. If a favoured policy is identified in such a study, the application of that result in practice would be to use the favoured policy in all babies, thus mimicking a cluster allocation design. However, future trials which use cluster allocation should use truly random methods for allocating by cluster and should analyse the data taking into account the clustering of allocation.
Authors' conclusions
Implications for practice.
This systematic review does not provide evidence that overgowns are effective in limiting infant colonisation, infection or death in newborn nurseries. Nor does gowning appear to impact on handwashing frequency. The costs associated with gowning are considerable.
Implications for research.
In light of changes in hospital practices (such as rooming in, shortened length of stay and widespread discontinuation of overgown use) since many of the included studies were conducted, further investigations of the effect of overgowns on infection or colonisation rates in well‐baby newborn nurseries appear to be unwarranted as their results would not be applicable to current practice.
The question of gowning in neonatal intensive care settings has not been tested using a randomised controlled design. Future investigations in this area should focus on important outcomes such as death and systemic infection using high quality randomised controlled designs of sufficient size to yield a conclusive result. Future studies that use cluster allocation should use truly random rather than quasi‐random methods for allocating by cluster, and should analyse the data using methods which take into account the cluster design.
What's new
| Date | Event | Description |
|---|---|---|
| 25 February 2013 | Amended | Contact details updated. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 11 February 2011 | New search has been performed | This review updates the existing review "Gowning by attendants and visitors in newborn nurseries for prevention of neonatal morbidity and mortality" published in the Cochrane Database of Systematic Reviews (Webster 2009). Updated search found no new trials. No changes to conclusions. |
| 6 February 2009 | New search has been performed | This review updates the existing review "Gowning by attendants and visitors in newborn nurseries for prevention of neonatal morbidity and mortality" published in The Cochrane Library Issue 3, 2006 (Webster 2006). No new trials were identified. The conclusions of the review are unchanged. |
| 5 April 2006 | New search has been performed | This review updates the existing review of "Gowning by attendants and visitors in newborn nurseries for prevention of neonatal morbidity and mortality" which was published in The Cochrane Library Issue 2, 2003 (Webster 2003). No new trials were identified as a result of this updated search. The conclusions of the review are unchanged. |
| 31 January 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Data and analyses
Comparison 1. No Gowns vs Gowns.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death before discharge | 4 | 2285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.70, 1.02] |
| 1.1 Trials randomising the individual participant | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Trials using cluster allocation | 4 | 2285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.70, 1.02] |
| 2 Systemic nosocomial infection | 4 | 3979 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.40, 2.23] |
| 2.1 Trials randomising the individual participant | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Trials using cluster allocation | 4 | 3979 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.40, 2.23] |
| 3 Localised nosocomial infection | 4 | 1947 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.90, 1.71] |
| 3.1 Trials randomising the individual participant | 2 | 619 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.74, 1.86] |
| 3.2 Trials using cluster allocation | 2 | 1328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.84, 2.00] |
| 4 Nasal colonisation | 5 | 1122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.88, 1.09] |
| 4.1 Trials randomising the individual participant | 3 | 787 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.18] |
| 4.2 Trials using cluster allocation | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.77, 1.07] |
| 5 Umbilical colonisation | 5 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.10] |
| 5.1 Trials randomising the individual participant | 3 | 781 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.93, 1.14] |
| 5.2 Trials using cluster allocation | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.82, 1.12] |
| 6 Eye colonisation | 1 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.90, 1.05] |
| 6.1 Trials randomising the individual participant | 1 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.90, 1.05] |
| 6.2 Trials using cluster allocation | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Groin colonisation | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.69, 1.57] |
| 7.1 Trials randomising the individual participant | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.69, 1.57] |
| 7.2 Trials using cluster allocation | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Length of hospital stay | 1 | 473 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐5.82, 6.62] |
| 8.1 Trials randomising the individual participant | 1 | 473 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐5.82, 6.62] |
| 8.2 Trials using cluster allocation | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Duration of mechanical ventilation | 1 | 212 | Mean Difference (IV, Fixed, 95% CI) | 5.00 [‐11.09, 21.09] |
| 9.1 Trials randomising the individual participant | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Trials using cluster allocation | 1 | 212 | Mean Difference (IV, Fixed, 95% CI) | 5.00 [‐11.09, 21.09] |
1.1. Analysis.
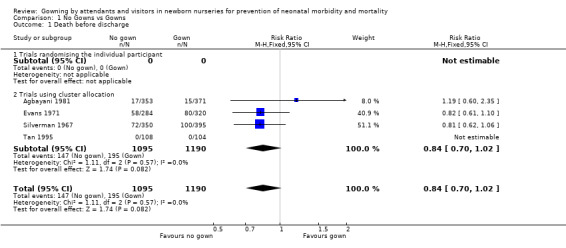
Comparison 1 No Gowns vs Gowns, Outcome 1 Death before discharge.
1.2. Analysis.
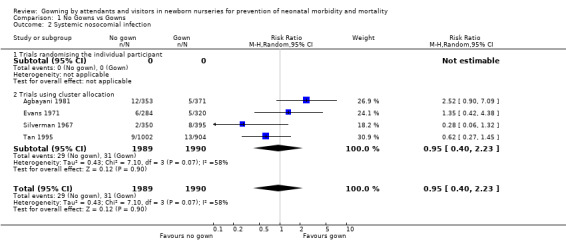
Comparison 1 No Gowns vs Gowns, Outcome 2 Systemic nosocomial infection.
1.3. Analysis.
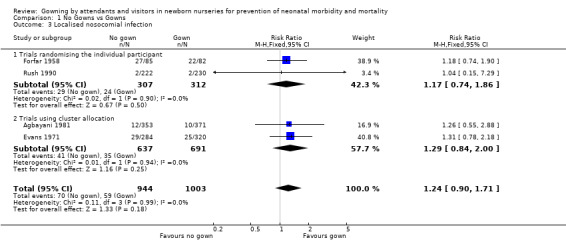
Comparison 1 No Gowns vs Gowns, Outcome 3 Localised nosocomial infection.
1.4. Analysis.
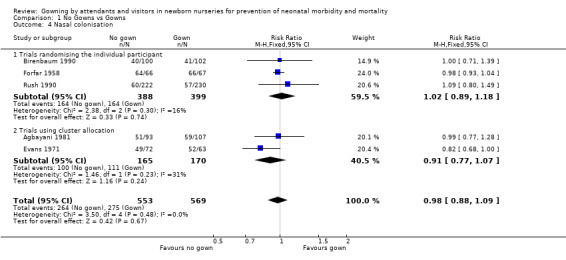
Comparison 1 No Gowns vs Gowns, Outcome 4 Nasal colonisation.
1.5. Analysis.
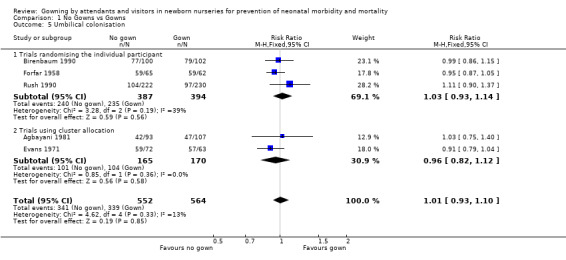
Comparison 1 No Gowns vs Gowns, Outcome 5 Umbilical colonisation.
1.6. Analysis.
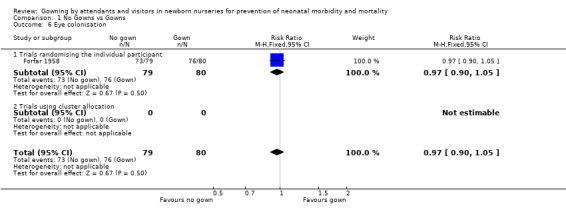
Comparison 1 No Gowns vs Gowns, Outcome 6 Eye colonisation.
1.7. Analysis.
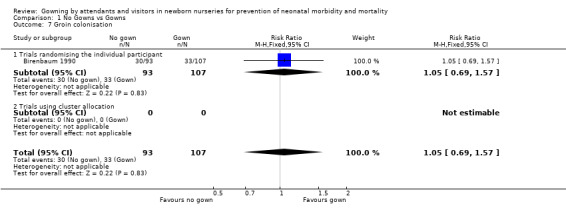
Comparison 1 No Gowns vs Gowns, Outcome 7 Groin colonisation.
1.8. Analysis.
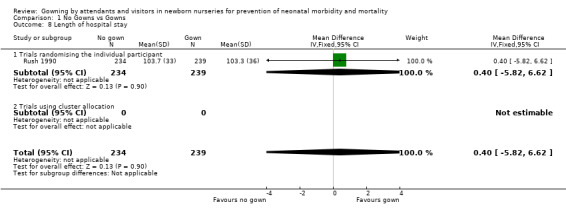
Comparison 1 No Gowns vs Gowns, Outcome 8 Length of hospital stay.
1.9. Analysis.
Comparison 1 No Gowns vs Gowns, Outcome 9 Duration of mechanical ventilation.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agbayani 1981.
| Methods | Single centre cluster‐allocation trial. Blinding of randomisation: No. Allocation occurred using a pre‐established list of 6 alternate 2 month blocks of gowning and modified gowning over a 12‐month period. Blinding of intervention: No. Blinding of outcome assessment: Unknown. Completeness of follow‐up: Complete for primary outcomes. Incomplete for subgroup of 273 infants. |
|
| Participants | A total of 724 outborn (123) and inborn (601) term and preterm infants. A subgroup of 273 newborns who met the following inclusion criteria were swabbed for the presence of colonising bacteria. Inclusion criteria: admitted to the NICU between Monday and Thursday who were less than 12 hours old and who had negative blood cultures on admission. Infants who were enrolled in the last 10 days of each two month interval were excluded. | |
| Interventions | No gown: Hands and forearms were washed with povidone‐iodine for two minutes. Jewelry was removed from wrists and fingers. Nurses wore scrub gowns. Street clothes were worn by physicians, other staff and visitors. Gown: As for no gown periods but gowns were donned before entering the nursery. In both gown and no gown groups, gowns were worn by anyone holding an infant. Anterior nares, umbilicus and groin were swabbed on admission to the nursery and on days 2,4,7,10,14,21 and 28 only among the subgroup of infants. | |
| Outcomes | 1) Death before discharge 2) Systemic nosocomial infection, defined by documented sepsis, meningitis and necrotising enterocolitis. 3) Localised nosocomial infection, defined as conjunctivitis, pustules and abscesses. 4) Colonisation (prevalence of bacteria from the nares, umbilicus and groin) amongst the subgroup at 7 different time points from day 1 to day 28. | |
| Notes | This trail was analysed as if allocation was by individual. It was unclear how all infections were diagnosed. Pathology results were available for systemic infections but not for localised infections. Death, systemic and localised infection was reported for the whole sample. Colonisation data was available for a subgroup of 273 infants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Single centre cluster‐allocation trial Allocation occurred using a pre‐established list of 6 alternate 2 month blocks of gowning and modified gowning over a 12‐month period |
| Allocation concealment? | High risk | Blinding of randomisation: No |
| Blinding? All outcomes | Unclear risk | Blinding of intervention: No Blinding of outcome assessment: Unknown |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Complete for primary outcomes. Incomplete for subgroup of 273 infants. |
Birenbaum 1990.
| Methods | Single centre randomised controlled trial. Blinding of randomisation: Yes. Group assignment from shuffled sealed envelopes that designated the gowning or no gowning group. Blinding of intervention: No Blinding of outcome assessment : Unknown. Completeness of follow‐up: Unknown. |
|
| Participants | Drawn from 1218 deliveries with no indication of how many were randomised. Study outcomes were reported for 202 infants. Inclusion criteria: (for 202 infants) Admission to a combination of newborn nursery and rooming in care. Exclusion criteria: Infants with mothers determined to be clinically unwell (e.g. defined by fever chorioamnionitis and premature or prolonged rupture of membranes), infant requiring intensive or intermediate care, infants for whom admission cultures were not obtained within 6 hours of delivery, and infants who did not have all admission and discharge cultures performed. | |
| Interventions | No gown: Attendants and visitors washed their hands before entering the nursery or mothers room. Gowns were not worn when handling the infant. Gown: As for no gowns except a gown was worn for all infant related procedures. Routines in the nursery remained unchanged. Four swabs were taken from infants, two within 6 hours of admission (nose and umbilicus) and two on discharge. | |
| Outcomes | 1) Nasal colonisation on admission and on discharge 2) Umbilical colonisation on admission and on discharge Any organic growth was considered to be a positive nose or umbilical culture. | |
| Notes | Strong possibility of post‐randomised exclusions (infants who did not have initial cultures within 6 hours of delivery and those who did not have 4 cultures performed). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Single centre randomised controlled trial. Group assignment from shuffled sealed envelopes that designated the gowning or no gowning group. |
| Allocation concealment? | Low risk | Blinding of randomisation: Yes |
| Blinding? All outcomes | High risk | Blinding of intervention: No Blinding of outcome assessment : Unknown |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Completeness of follow‐up: Unknown. |
Evans 1971.
| Methods | Single centre cluster‐allocation trial. Blinding of randomisation: No. Allocation was by alternating 2 or 3 month periods (5 separate gowning periods totaling 11 months and 4 separate non‐gowning periods totaling 10 months). One month was excluded from the study Blinding of intervention: No Blinding of outcome assessment: Unknown Completeness of follow‐up: unknown |
|
| Participants | 604 preterm infants admitted to the premature nursery. Inclusions: Infants nursed in incubators Exclusions: Infants who were severely ill (no definition provided). | |
| Interventions | No gown: Visitors and attendants did not cover their outer clothing and nor did they wash their hands before entering the room. Nurses wore the white uniforms used to travel to the hospital. Those handling newborn infants through ports did not wear gowns but scrubbed for 3 minutes with an antiseptic soap. When infants were removed from an isolette, or when a hood was opened, all persons in the room wore a gown. Gown: Attendants and visitors removed their outer jackets, washed their hands for 3 minutes and donned a gown before entering the room. Nurses changed into scrub gowns at the beginning of their shift. Both anterior nares and the umbilicus were swabbed 4 to 5 mornings weekly until the infant was transferred from an incubator to an open crib. | |
| Outcomes | 1) Death 2) Systemic infection (pneumonia, meningitis, sepsis) 3) Localised infection (pyodermia, conjunctivitis and diarrhoea) 4) Colonisation of the nares and umbilicus were reported at 7 time points from day 1 to 21 but were tabulated by day of life acquired and by species. No overall prevalence by group was reported. | |
| Notes | This trial was analysed as if allocation was by individual. The study was interrupted in September, during a non‐gowning period, because of transfer of the nursery to a new building. It was unclear how all infections were diagnosed. Pathology results were available for systemic infections but not for localised infections. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Single centre cluster‐allocation trial. Allocation was by alternating 2 or 3 month periods (5 separate gowning periods totaling 11 months and 4 separate non‐gowning periods totaling 10 months). One month was excluded from the study |
| Allocation concealment? | High risk | Blinding of randomisation: No |
| Blinding? All outcomes | Unclear risk | Blinding of intervention: No Blinding of outcome assessment: Unknown |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Completeness of follow‐up: unknown |
Forfar 1958.
| Methods | Single centre randomised controlled trial. Blinding of randomisation: Unknown. Infants were allocated at random to one of two nurseries. No description of the process of random allocation was documented. Blinding of intervention: No Blinding of outcome assessment: Unknown. Completeness of follow‐up: Localised infection , yes. Colonisation, no |
|
| Participants | 167 infants admitted to either of two newborn nurseries without rooming in facilities. Inclusion criteria: none documented. Exclusion criteria: none documented. | |
| Interventions | No gowns: No gowns or masks were worn by attendants or visitors. Gown: Attendants and visitors observed a strict gowning and masking regime before entering the nursery. In addition, a 'personalised' gown, one for each baby was donned over the first gown, when handling that infant. Gowns were changed every 24 hours or when soiled. Staff were common to both nurseries. For each baby, an eye swab was taken on the fourth day, a nasal swab on the eighth day and an umbilical swab at the time of separation of the cord. Swabs were taken from infected lesions if possible. Microbiological examination was limited to staphylococcal positive species. | |
| Outcomes | 1) Localised nosocomial infection, diagnosed clinically . 2) Nasal colonisation 3) Umbilical colonisation 4) Eye colonisation 5) Length of stay 6) Nursing time 7) Cost | |
| Notes | Infections were assessed clinically. If possible, a swab was taken from an infected lesion but pathology results were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Single centre randomised controlled trial. Infants were allocated at random to one of two nurseries. No description of the process of random allocation was documented. |
| Allocation concealment? | Unclear risk | Blinding of randomisation: Unknown. Infants were allocated at random to one of two nurseries. No description of the process of random allocation was documented. |
| Blinding? All outcomes | High risk | Blinding of intervention: No Blinding of outcome assessment: Unknown |
| Incomplete outcome data addressed? All outcomes | Low risk | Completeness of follow‐up: Localised infection , yes. Colonisation, no |
Pelke 1994.
| Methods | Single centre cluster‐allocation trial: Blinding of randomisation: No. Allocation was by alternate 2‐month gowning and no gowning cycles (4 cycles over a period of 8 months). One entire 4 month period was repeated to eliminate the potential for seasonal variables and outbreaks. Blinding of intervention: No Blinding of outcome assessment: Unknown Completeness of follow‐up: Unclear. The number of cultures exceeded the number of infants but it was unclear if all infants were swabbed. |
|
| Participants | 313 term and preterm infants admitted to the Neonatal Intensive Care Unit
Inclusion/exclusion criteria:
None documented A subgroup of 230 infants (those who had cultures taken) were studied. |
|
| Interventions | No gown: Nursing staff wore scrub suits, which were home ‐laundered and worn to the hospital from home. Other visitors and staff wore their street clothes when entering the NICU. Residents were the only group who continued to wear hospital‐laundered scrubs and they wore an over‐gown when leaving the area. Gowns were available for parents to use when holding their infants but these were not used. Gown: Nursery staff changed into scrub dresses or suits and covered these with a gown if they left the area. Other visitors or staff wore gowns over their street clothes when entering the NICU. Infants had nasopharyngeal (or tracheal aspirate if intubated), umbilical and rectal or stool swab taken weekly. Nursery traffic was monitored by tallying the number of people who entered the NICU during two 15‐minute periods per day on two days per week. Handwashing compliance was studied by 30 minutes observation by one infants bedside three times weekly. Bedside areas were rotated each week. | |
| Outcomes | 1) Neonatal mortality 2) Nasopharyngeal colonisation 3) Umbilical colonisation 3) Stool colonisation 4) RSV 5) NEC 6) Length of stay 7) Traffic flow 8) Handwashing compliance | |
| Notes | This trial was analysed as if allocation was by individual. Infection rates and mortality were reported as 'rate per 100 days'. Information about the numerator and denominator were requested but the author could not provide these details. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Single centre cluster‐allocation trial: Blinding of randomisation: No. Allocation was by alternate 2 month gowning and no gowning cycles (4 cycles over a period of 8 months). One entire 4 month period was repeated to eliminate the potential for seasonal variables and outbreaks. |
| Allocation concealment? | High risk | Blinding of randomisation: No. Allocation was by alternate 2 month gowning and no gowning cycles (4 cycles over a period of 8 months). One entire 4 month period was repeated to eliminate the potential for seasonal variables and outbreaks. |
| Blinding? All outcomes | High risk | Blinding of intervention: No Blinding of outcome assessment: Unknown |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Completeness of follow‐up: Unclear. The number of cultures exceeded the number of infants but it was unclear if all infants were swabbed. |
Rush 1990.
| Methods | Single centre randomised controlled trial: Blinding of randomisation: Yes, by sealed envelope. Blinding of Intervention: No Blinding of outcome: yes Complete follow‐ up: No, due to culture reports missing or research staff unavailable to abstract data |
|
| Participants | 473 infants. Sample drawn from 1130 infants consecutively admitted to a newborn nursery. Inclusions: >2500 grams, at least 37 weeks gestation and Apgar at 5 minutes > 7 at 5 minutes. Exclusions: infants initially admitted to the NICU. | |
| Interventions | No gown: No cover gowns were worn by staff or visitors during any infant contact. Gown: Staff and visitors wore cover gowns for all infant contact. In both groups, staff members, parents and visitors continued to be advised to wash their hands carefully before providing patient care. Nasal & umbilical swabs were taken by nursing staff on the 3rd postnatal day or before discharge, whichever was the sooner. | |
| Outcomes | 1) Nasal colonisation 2) Umbilical colonisation 3) Colonisation of nose and umbilicus Clarification was requested and received for whether (i) all staff followed the protocol (ii) how infections were diagnosed and when, (iv) clarification of Table 2 and (v) how cost of gowns was estimated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Single centre randomised controlled trial |
| Allocation concealment? | Low risk | Blinding of randomisation: Yes, by sealed envelope |
| Blinding? All outcomes | High risk | Blinding of Intervention: No Blinding of outcome: Yes |
| Incomplete outcome data addressed? All outcomes | High risk | Complete follow‐up: No, due to culture reports missing or research staff unavailable to abstract data |
Silverman 1967.
| Methods | Single centre cluster‐allocation trial: Blinding of randomisation: No. Allocation was by 12 alternate 2 month periods over a 25 month time frame. At the end of the first year, the standard gowning period was extended for one month to ensure a different distribution of gowning periods in the second year. Blinding of Intervention: No Blinding of outcome: Unknown Complete follow‐ up: Yes |
|
| Participants | 745 high risk infants admitted to the special care nursery. Inclusion criteria: birthweight < 2kg, and others with major disorders. Exclusion criteria: infants with diarrhoea. | |
| Interventions | No gown: Outer coats were not removed, nor were hands washed before entering the patients room. Outer coats were removed and hands washed before and after infant contact. In addition, gowns were worn if the incubator hood was open. Gown: Outer jackets were removed, hands were washed and a gown donned before entering the room. Hands were washed before and after any infant contact. | |
| Outcomes | 1) Death 2) Systemic infection (included only infants who had died and had a confirmed diagnosis at postmortem of either meningitis or septicaemia. | |
| Notes | This trial was analysed as if allocation was by individual. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Single centre cluster‐allocation trial: Allocation was by 12 alternate 2 month periods over a 25 month time frame. At the end of the first year, the standard gowning period was extended for one month to ensure a different distribution of gowning periods in the second year |
| Allocation concealment? | High risk | Blinding of randomisation: No |
| Blinding? All outcomes | High risk | Blinding of Intervention: No Blinding of outcome: Unknown |
| Incomplete outcome data addressed? All outcomes | Low risk | Complete follow‐up: Yes |
Tan 1995.
| Methods | Single centre cluster‐allocation trial. Blinding of randomisation: No. Allocation was by alternate 2 month periods (6 periods over 12 months). Blinding of Intervention: No Blinding of outcome: Unknown Complete follow‐ up: Yes |
|
| Participants | 1906 infants admitted to a neonatal intensive care (212) or special care nursery (1694). Exclusion criteria: infants who required strict isolation. | |
| Interventions | No gown: Hands were washed but no gowning was required before entering the nursery. Aprons were worn by staff during both time periods if soiling was anticipated when infants were being handled. Gown: Health care professionals & visitors washed their hands and donned a plastic apron before entering the nursery. Twice weekly endotracheal aspirates were obtained from intubated infants. Nasal swabs (for MRSA only) were obtained on admission then weekly from day three. | |
| Outcomes | 1) Death
2) Systemic infection
3) Localised infection
3) MRSA colonisation
4) Cost of gowns
5) Device related infections NB. Outcomes were reported separately by special care or intensive care unit |
|
| Notes | This trial was analysed as if allocation was by individual. Clarification sought from author about systemic and localised infections but no information received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Single centre cluster‐allocation trial. Allocation was by alternate 2 month periods (6 periods over 12 months) |
| Allocation concealment? | High risk | Blinding of randomisation: No |
| Blinding? All outcomes | High risk | Blinding of Intervention: No Blinding of outcome: Unknown |
| Incomplete outcome data addressed? All outcomes | Low risk | Complete follow‐up: Yes |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Altimier 1996 | Comparison with historical controls. |
| Haque 1989 | No randomisation. |
| Renaud 1983 | Comparison with historical controls. |
| Williams 1969 | Comparison with historical controls. |
Contributions of authors
Joan Webster (JW) conceived the idea for the review and wrote the protocol. JW and Margo Pritchard (MP) conducted searches independently and agreed on inclusions. Data was extracted independently by the two review authors. JW and MP wrote the review. JW has conducted the updates.
The February 2011 update was conducted centrally by the Cochrane Neonatal Review Group staff (Yolanda Montagne, Diane Haughton, and Roger Soll). This update was reviewed and approved by JW.
Sources of support
Internal sources
No sources of support supplied
External sources
Centre for Clinical Studies ‐ Women's and Children's Health, Mater Hospital, Sth Brisbane, Queensland, Australia.
Department of Health and Ageing, Commonwealth Government, Canberra ACT, Australia.
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Agbayani 1981 {published data only}
- Agbayani M, Rosenfeld W, Evans H, Salazar D, Jhaveri R, Braun J. Evaluation of modified gowning procedures in a neonatal intensive care unit. American Journal of Diseases of Children 1981;135:650‐2. [DOI] [PubMed] [Google Scholar]
Birenbaum 1990 {published data only}
- Birenbaum HJ, Glorioso L, Rosenberger C, Arshad C, Edwards K. Gowning on a postpartum ward fails to decrease colonization in the newborn infant. American Journal of Diseases of Children 1990;144:1031‐3. [DOI] [PubMed] [Google Scholar]
Evans 1971 {published data only}
- Evans HE, Akpata SO, Baki A. Bacteriologic and clinical evaluation of gowning in a premature nursery. Journal of Pediatrics 1971;78:883‐6. [DOI] [PubMed] [Google Scholar]
Forfar 1958 {published data only}
- Forfar JO, MacCabe AF. Masking and gowning in nurseries for the newborn infant. Effect on staphylococcal carriage and infection. British Medical Journal 1958;1:76‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pelke 1994 {published data only}
- Pelke S, Ching D, Easa D, Melish ME. Gowning does not affect colonization or infection rates in the neonatal intensive care nursery. Archives of Pediatric and Adolescent Medicine 1994;148:1016‐20. [DOI] [PubMed] [Google Scholar]
Rush 1990 {published data only}
- Rush J, Fiorina‐Chiovitti R, Kaufman K, Mitchell A. A randomized controlled trial of a nursery ritual: wearing cover gowns to care for healthy newborns. Birth 1990;17:25‐30. [DOI] [PubMed] [Google Scholar]
Silverman 1967 {published data only}
- Silverman WA, Sinclair JC. Evaluation of precautions before entering a neonatal unit. Pediatrics 1967;40:900‐1. [PubMed] [Google Scholar]
Tan 1995 {published data only}
- Tan SG, Lim SH, Malathi I. Does routine gowning reduce nosocomial infection and mortality rates in a neonatal nursery? A Singapore experience. International Journal of Nursing Practice 1995;1:52‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Altimier 1996 {published data only}
- Altimier LB, Lott JW, McCain G. Research utilization: evaluating the use of cover gowns in a neonatal intensive care unit. Neonatal Intensive Care 1996;Nov/Dec:52‐8. [Google Scholar]
Haque 1989 {published data only}
- Haque KN, Chagla AH. Do gowns prevent infection in neonatal intensive care units. Journal of Hospital Infection 1989;14:159‐62. [DOI] [PubMed] [Google Scholar]
Renaud 1983 {published data only}
- Renaud M. Effects of discontinuing cover gowns on a postpartal ward upon cord colonization of the newborn. Journal of Obstetric, Gynecologic, and Neonatal Nursing 1983;12:399‐401. [DOI] [PubMed] [Google Scholar]
Williams 1969 {published data only}
- Williams CPS, Oliver TK. Nursery routines and staphylococcal colonization of the newborn. Pediatrics 1969;44:640‐6. [PubMed] [Google Scholar]
Additional references
Baltimore 1998
- Baltimore RS. Neonatal nosocomial infections. Seminars in Perinatology 1998;22:25‐32. [DOI] [PubMed] [Google Scholar]
Barton 1999
- Barton L, Hodgman JE, Pavlova Z. Causes of death in the extremely low birth weight infant. Pediatrics 1999;103:446‐51. [DOI] [PubMed] [Google Scholar]
Chathas 1990
- Chathas MK, Paton JB, Fisher DE. Percutaneous central venous catheterization. Three years' experience in a neonatal intensive care unit. American Journal of Diseases of Children 1990;144:1246‐50. [DOI] [PubMed] [Google Scholar]
Cloney 1986
- Cloney DL, Donowitz LG. Overgown use for infection control in nurseries and neonatal intensive care units. American Journal of Diseases of Children 1986;140:680‐3. [DOI] [PubMed] [Google Scholar]
Donowitz 1987
- Donowitz LG. Handwashing technique in a pediatric intensive care unit. American Journal of Diseases of Children 1987;141:683‐5. [DOI] [PubMed] [Google Scholar]
Foca 2000
- Foca M, Jacob K, Whittier S, Della Latta P, Factor S, Rubenstein D, et al. Endemic pseudomonas aeruginosa infection in a neonatal intensive care unit. New England Journal of Medicine 2000;343:695‐700. [DOI] [PubMed] [Google Scholar]
Garner 1996
- Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. APIC Infection Control and Applied Epidemiology: Principles and Practice. St Louis: Mosby, 1996. [Google Scholar]
Gaynes 1996
- Gaynes RP, Edwards JR, Jarvis WR, Culver DH, Tolson JS, Martone WJ. Nosocomial infections among neonates in high‐risk nurseries in the United States. National Nosocomial Infection Surveillance System. Pediatrics 1996;98:357‐61. [PubMed] [Google Scholar]
Larson 1999
- Larson E. Skin hygiene and infection prevention: more of the same or different approaches?. Clinical Infectious Diseases 1999;29:1287‐94. [DOI] [PubMed] [Google Scholar]
Levy 1999
- Levy O, Martin S, Eichenwald E, Ganz T, Valore E, Carroll SF, et al. Impaired innate immunity in the newborn: newborn neutrophils are deficient in bactericidal/permeability increased protein. Pediatrics 1999;104:1327‐33. [DOI] [PubMed] [Google Scholar]
Mollison 2000
- Mollison J, Simpson JA, Campbell MK, Grimshaw JM. Comparison of analytical methods for cluster randomised trials: an example from a primary care setting. Journal of Epidemiology and Biostatistics 2000;5:339‐48. [PubMed] [Google Scholar]
Seaward 1998
- Seaward PG, Hannah ME, Myhr TL, Farine D, Ohlsson A, Wang EE, et al. International multicentre PROM study: evaluation of predictors of neonatal infection in infants born to patients with premature rupture of membranes at term. Premature Rupture of the Membranes. American Journal of Obstetrics and Gynecology 1998;179:635‐9. [DOI] [PubMed] [Google Scholar]
Thigpen 1991
- Thigpen JL. Responding to research: realistic use of scrub clothes and cover gowns. Neonatal Network 1991;9:41‐4. [PubMed] [Google Scholar]
Webster 1994
- Webster J, Faoagali JL, Cartwright D. Elimination of methicillin‐resistant Staphylococcus aureus from a neonatal intensive care nursery after handwashing with triclosan. Journal of Paediatrics and Child Health 1994;30:59‐64. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Webster 2003
- Webster J, Pritchard MA. Gowning by attendants and visitors in newborn nurseries for prevention of neonatal morbidity and mortality. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003670] [DOI] [PMC free article] [PubMed] [Google Scholar]
Webster 2006
- Webster J, Pritchard MA. Gowning by attendants and visitors in newborn nurseries for prevention of neonatal morbidity and mortality. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD003670] [DOI] [PMC free article] [PubMed] [Google Scholar]
Webster 2009
- Webster J, Pritchard MA. Gowning by attendants and visitors in newborn nurseries for prevention of neonatal morbidity and mortality. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD003670] [DOI] [PMC free article] [PubMed] [Google Scholar]


