Abstract
With a recent randomized prospective trial revealing that thermal ablative therapy as local tumor control improved overall survival (OS) in patients with unresectable colorectal cancer liver metastases (CRLM), thermal ablation continues to remain as an important treatment option in this patient population. Our aim of this article is to review the current role of the ablative therapy in the management of CRLM patients. Main indications for thermal ablation include (I) unresectable liver lesions; (II) in combination with hepatectomy; (III) in patients with significant medical comorbidities or poor performance status (PS); (IV) a small (<3 cm) solitary lesion, which would otherwise necessitate a major liver resection; and (V) patient preference. There are several approaches and modalities for ablative therapy, including open, percutaneous, and laparoscopic approaches, as well as radiofrequency ablation (RFA) and microwave ablation (MWA). Each approach and ablation modality have its own pros and cons. Percutaneous and laparoscopic approaches are preferred due to minimally invasive nature, yet laparoscopic approach has more benefits from thorough intraoperative ultrasound (US) exam as well as complete peritoneal staging with laparoscopy. Similarly, whereas high local tumor failure rate has been a major concern with RFA, MWA or microwave thermosphere ablation (MTA) have demonstrated significantly improved local tumor control due to homogenous tissue heating, ability to reach higher tissue temperatures, and less susceptible to the “heat-sink” effect. Although liver resection is the standard of care for CRLM, there have been some retrospective studies demonstrating similar oncological outcome between ablative therapy and surgical resection in very selected populations with small (<3 cm) solitary CRLM. Lastly, ablative therapy and liver resection should not be mutually exclusive, especially in the management of bilobar liver metastases. Concomitant ablative therapy with hepatectomy may spare the patients from having two-stage hepatectomy with less morbidity. The role of the thermal ablation will continue to evolve in patients with resectable and ablatable lesions owing to newly emerging technology, in addition to new systemic treatment options, including immunotherapy for metastatic colorectal cancer (CRC).
Keywords: Thermal ablation, radiofrequency ablation (RFA), microwave ablation (MWA), colorectal cancer liver metastasis (CRLM)
Background
Approximately 50% of patients with colorectal cancer (CRC) develop liver metastases in their lifetime and liver metastases contribute to two-thirds of mortality of CRC (1,2). Despite liver resection being “gold standard” for colorectal cancer liver metastases (CRLM), the majority of the patients are not candidates for resection due to unresectable disease, presence of extrahepatic disease, or patients’ comorbidities (1,3). Among the loco-regional treatment modalities for unresectable hepatic disease, such as cryotherapy and radiofrequency ablation (RFA), RFA had been increasingly recognized since early 1990’s and accepted as an effective therapy by early 2000’s, given its efficacy and relatively low morbidity (3-7). As the RFA technology advancement became stagnant, microwave ablation (MWA) technology gradually gained its popularity in CRLM management owing to its multiple advantages, especially after the newer microwave thermosphere ablation (MTA) device was approved by FDA in 2014 (8-12).
A recent multicenter randomized prospective trial revealed that thermal ablative therapy as local tumor control improved overall survival (OS) compared to palliative chemotherapy alone in patients with unresectable CRLM (13). With this finding, thermal ablative therapy has the potential to be an important treatment option in patients with unresectable CRLM.
Our aim of this article is to review the current role of the ablative therapy in the management of CRLM patients.
Indications
Unless data are available from ongoing trials, ablation for resectable CRLM should not be used in lieu of liver resection (14). We recommend the readers to channel the patients as much as possible to multidisciplinary tumor boards to determine the best option in a given patient. On the other hand, followings are the scenarios that have evolved in our experience as indications for liver tumor ablation:
Unresectable liver lesions, less than 8 in number with involvement of less than 20% of liver volume and the largest lesion less than 4 cm (13);
In combination with hepatectomy to expand the pool of resectable patients;
In patients who are unfit to undergo resection due to their medical comorbidities or performance status (PS);
A small (<3 cm) solitary lesion, which would otherwise necessitate a major liver resection, after discussion of pros and cons;
Patient preference: having already performed their own literature research, some patients present with a preference for ablation over resection. These patients need to be presented the pros and cons of resection versus ablation before commitment to ablation.
These indications are not necessarily limited to the initial treatment. Ablative therapy has a great role in patients with limited recurrent diseases after ablative therapy or liver resection, owing to less morbidity and favorable recovery (15). Indications for ablative therapy for CRLM should be determined by multidisciplinary evaluation. Ablative therapy, in general, is not effective in larger tumors ≥5 cm given its higher incomplete ablation rate and subsequent ablation site recurrence (ASR) rate. The most common cut-off size in the literature is 3 cm (5). With percutaneous approach, four lesions or less are typically suggested in a single session (5). While it is possible to be more aggressive with the laparoscopic or open approach, the median tumor size was 2.2 cm (range, 0.3–5.6 cm) and tumor number was 2 (range, 1–13) for CRLM in our series, the majority of which were performed laparoscopically (16).
Contraindications of ablative therapy include the followings (5,17,18):
Uncorrectable coagulopathy;
Contraindications for general anesthesia for laparoscopic or open approach;
Diffuse metastatic liver disease;
Tumor location abutting either the liver hilum or the right/left hepatic ducts;
Biliary dilatation;
Untreatable extrahepatic disease.
Approaches and techniques
Approaches
There are three approaches to perform ablation, including open, laparoscopic, or percutaneous approaches. In our practice, we prefer to perform laparoscopic approach in lieu of percutaneous approach given several advantages with laparoscopy (4,19).
Open
Open approach is the most invasive approach, which can be performed alone. However, the majority of open ablation is performed in combination with concomitant liver resection or other simultaneous open procedures. Hof et al. recently reported that severe complications were significantly less in the simultaneous ablation group compared to the simultaneous partial hepatectomy group in the synchronous CRLM management, which highlights the significant benefit of lower morbidity associated with ablative therapy (20). Whereas the benefit includes optimal staging by detecting of occult hepatic or peritoneal disease and better mobilization of the liver, the open approach carries the morbidity of a laparotomy.
Percutaneous
Percutaneous approach is the least invasive approach that may be performed without general anesthesia. Several imaging modalities, including ultrasound (US), computational tomography (CT), and magnetic resonance imaging (MRI) are used to guide probe placement (5). Despite its minimally invasive feature, this approach has several limitations, including potential higher ASR rate compared to open or laparoscopic approach, inferior tumor staging for the peritoneal cavity or even additional liver tumors, and limitations to treat superficial lesions close proximity to other organs (4,21-23).
Laparoscopy
Laparoscopic approach has the benefits of both open and percutaneous approaches. Although it requires general anesthesia and an expertise with advanced laparoscopy and US, the associated morbidity is much less than open approach (5,24). Additionally, it has been shown to be superior in terms of local tumor control secondary to precise targeting with laparoscopy and more aggressive ablation with intraoperative US monitoring (19,21). Moreover, thorough exam with laparoscopic US enables the identification of additional liver tumors that are not seen on preoperative imaging studies (25). Finally, similar to the open approach, laparoscopic approach allows better staging for occult peritoneal or hepatic diseases, and treat multiple lesions in the liver with minimal risk of surrounding organ injuries (4,9,26-28).
Techniques
There are two major thermal ablation therapy options: RFA and MWA. Both modalities apply a form of thermal energy in order to destruct the tumor cell with surrounding adjacent liver parenchyma as a matter of local control.
RFA
RFA uses a form of alternating electrical current at a frequency of 400 MHz to generate thermal energy. The generator applies a high-frequency alternating electrical current, causing ionic agitation that heats spherical volume of the tissue in the area of the applicator tip (Figure 1A,B,C). There are multiple systems available that use catheters that can produce up to 5 cm of ablation zones with a single stick that either use temperature or impedance regulation, with or without saline infusion (Figure 2A,B). Although RFA has filled a gap in the treatment of patients with unresectable disease, it has some limitations. The ablation process may be lengthy and may take up to 25–30 minutes when creating 5-cm treatment zones. It is susceptible to the heat-sink effect, causing difficulties to treat lesions in close proximity to large vessels (29). Furthermore, except for the initial improvements that resulted in more powerful (200 versus 50 W) generators that could be used with catheters that could produce larger ablations zones (5 versus 2 cm) with a single deployment, technology was not advanced significantly over the recent years. Lastly, the higher local control failure rate (up to 20–40%) has been a major concern for RFA (Figure 2C) (17,21,30-32).
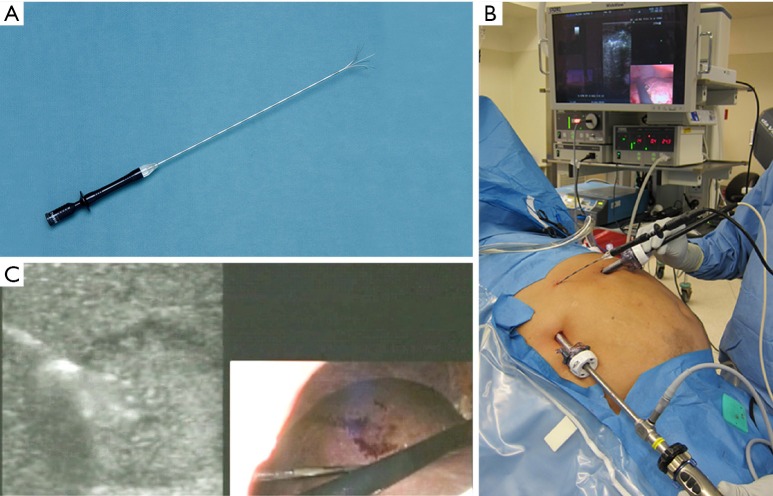
Figure 1.
Radiofrequency ablation (RFA) needle (A) used in our institution. The picture is 15 Gauge, 5 cm catheter. An intraoperative photo demonstrating the performance of RFA guided by intraoperative ultrasound (B). In our practice, two 12 mm trocars were placed in the right upper quadrant, one for the laparoscope and the other for the ultrasound probe. The RFA needle was placed percutaneously under direct visualization. An intraoperative photo demonstrating a laparoscopy/ultrasound picture in picture during the RFA (C).
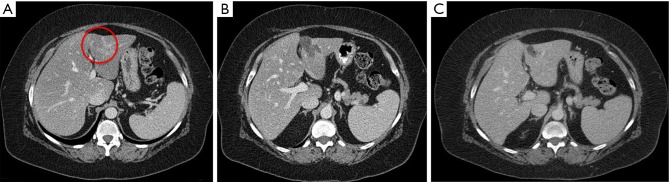
Figure 2.
An example for radiofrequency ablation (RFA). The patient is an 83-year-old female with metachronous liver metastasis from sigmoid colon cancer. During the surveillance 2 years after sigmoidectomy, the patient was found to have a lesion in the segment III. (A) Preoperative lesion (red circle), 1.39 cm × 1.30 cm on the segment III; (B) 3-month postoperative lesion; and (C) local recurrence medial to the previous ablation site in 15-month follow-up imaging.
MWA and MTA
MWA uses electromagnetic waves to agitate water molecules and generate heat for tumor destruction using devices with frequencies greater than 900 MHz (8). MWA has demonstrated significant advantages over RFA, such as more homogenous tissue heating, ability to reach higher tissue temperatures, and less susceptible to the “heat-sink” effect compared to RFA (33,34). However, there were a few major limitations with MWA, such as antenna design and the inability to create predictable, spherical ablation zones due to changing tissue physical properties (10,11).
Nevertheless, in 2014, FDA approved an advanced microwave system that can perform ablation by keeping tissue properties and field shape constant with a cooled-tip antenna with saline irrigation channels. This new MTA technology successfully creates predictable and reproducible spherical ablation zones (35). With a 2.45 GHz generator and 14-G microwave antenna equipped with a saline infusion cooled-tip (Emprint, Covidien, Boulder, CO, USA) (Figure 3A,B), the generator was run at 100 W for 2.5 min for a 3-cm ablation, 10 min for a 4-cm ablation, and 15 min for a 5-cm ablation, which is 100% faster than the RFA system (Figure 4A,B) (11,12,16). Other newer generation microwave systems (Neuwave, Angiodynamic) are also available in the market that can create non-spherical ablation zones in similar ablation times. Since the newer generation microwave system, including MTA, demonstrates multiple advantages over RFA system, RFA system in CRLM management might be replaced in a few years. In fact, owing to these significant benefits with MTA, we changed our practice from RFA to MTA in 2014.
Figure 3.
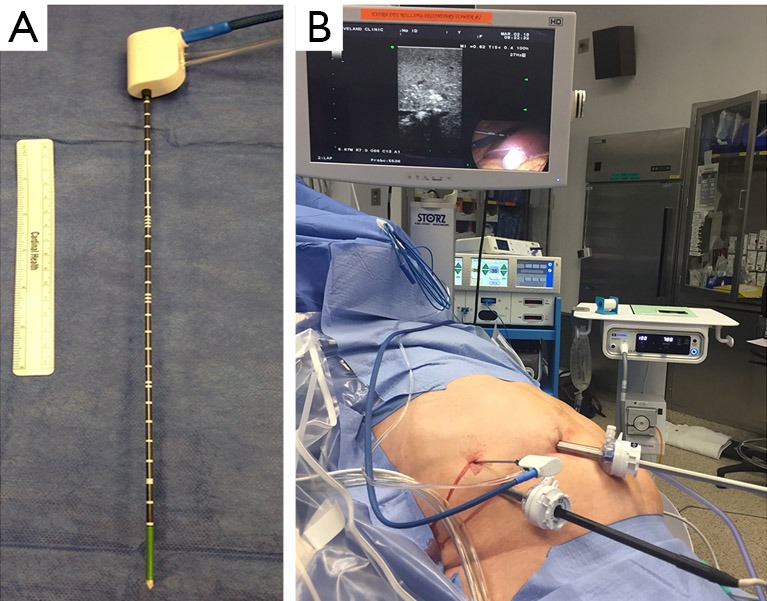
Microwave thermosphere ablation (MTA) needle (A) used in our institution. The needle is 14 Gauge and 30 cm with saline circulation to cool off the antenna tip. An intraoperative photo shows the performance of MTA guided by intraoperative ultrasound (B).
Figure 4.
An example for microwave thermosphere ablation (MTA). The patient is a 75-year-old male with synchronous liver metastasis from right side colon cancer. The patient underwent simultaneous laparoscopic MTA and right hemicolectomy. Green circle represents the targeted tumor, and red circle represents the anticipated ablation size (A). Preoperative lesion using the simulation software (B) 1-week follow-up image after MTA.
Technical description for laparoscopic MTA
The following is a brief description of the technique for laparoscopic MTA (11,12,36). All laparoscopic ablations need to be performed under general anesthesia. Two 12 mm trocars are placed in the right upper quadrant, one for the laparoscope and the other for the US probe. Intraoperative US is performed for complete staging of the liver. A microwave antenna is inserted through an additional 3 mm trocar in the right upper quadrant. The surgeon controls the generator by a foot pedal. Under US guidance, the tumors are targeted with a “free hand” technique. Under real-time US monitoring, surgeons should aim for at least 1cm margin around the tumor (Figure 5). Additional cycles or overlapping ablations can be performed as needed based on the real-time US monitoring. Track ablations also should be performed for deep lesions while pulling back the antenna and ablating every centimeter of the needle track for 2–3 seconds in order to control the bleeding as well as prevent tumor seeding.
Figure 5.
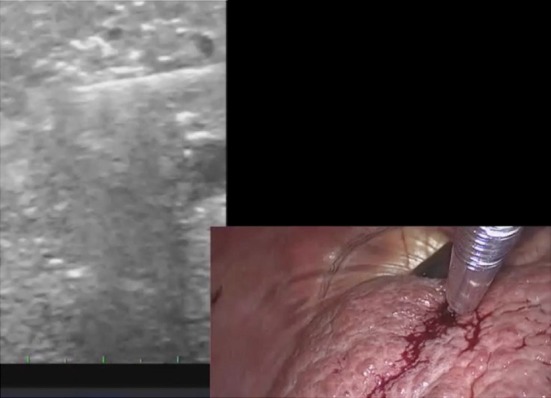
The trocar placement is similar to that in radiofrequency ablation (RFA) except for the use of an additional 3 mm trocar for the microwave thermosphere ablation (MTA) antenna, which is less rigid than the RFA needle. An intraoperative photo demonstrating a laparoscopy/ultrasound picture in picture during the MTA.
Complications
Compared to liver resection, ablative therapy in any approach is generally considered as a relatively safe and less invasive. Morbidity rate has been reported approximately 4–9% (5,17,22,24,37) and mortality rate approximately 0–2.0% in the literature (5,17,37). The common complications with ablative therapy include postoperative bleeding, infectious complications such as wound infection or liver abscesses, biliary tract injury including bile duct stricture or biloma, surrounding visceral organs injury, liver failure, and cardiopulmonary complications, with bleeding and infectious complications being common approximately 1% each (24,37). Complication rates do not significantly change between RFA and MWA (36,38). Additionally, as patients referred to ablative therapy for CRLM often have multiple underlying comorbidities and poor PS, there might be a potential selection bias in higher cardiopulmonary complications in the ablation cohort compared to liver resection in the literature.
Follow up
An early scan (triphasic liver CT or MRI) within 2 weeks of the surgical procedure is important to assess the ablation zones for completeness and also to rule out any missed lesions (Figure 4B). Incomplete ablation and missed lesions are extremely rare nowadays owing to the improved modern imaging quality as well as the use of intraoperative US during laparoscopic ablation. However, in case incomplete ablation or insufficient margin ablation are identified in the immediate imaging studies, it is feasible to consider re-ablation on these lesions to prevent ASR.
For long term follow up after ablative therapy, we recommend repeating the imaging studies and carcinoembryonic antigen (CEA) levels quarterly for the first 2 years and then biannually afterwards (Figure 2C).
Outcomes
Patient and tumor characteristics for clinical outcomes
After successful curative intent treatment for CRLM regardless of ablative therapy or liver resection, tumor biology is likely to dictate eventual clinical outcomes. Clinical characteristics suggesting worse tumor biology include high preoperative CEA values, node-positive primary diseases, short disease-free interval, number of CRLM lesions, larger size of CRLMs, and genetic mutations including RAS or BRAF (15,39-41). Although liver directed therapy, including ablative therapy or liver resection, might not be enough for those with multiple risk factors, it is beyond our scope in this review to discuss further treatment options.
Local tumor control
Local tumor recurrence rate after RFA has been reported to range between 4–40% (Figure 5) (27,30,42-44). In our experience, ASR happened in 26.0% (121/466) of the lesions and 42.7% (76/178) of the patients with CRLM. Predictors of ASR after RFA include tumor size larger or equal to 3 cm [hazard ratio (HR) =2.64], and ablation margin smaller than 0.5 cm (HR =1.6) (30). Additionally, the proximity to larger vessels has often been reported as one of risk factors for ASR as well (18,22). Ablation margin is the only parameter that the surgeons can control; hence, we suggest aiming for 1 cm margin for each colorectal liver metastasis. Similarly, ASR after MWA ranges between 6–10% in the literature, which is significantly reduced compared to RFA (9,16,22,42). In our experience with MTA, ASR happened in 12.0% (12/100) of the lesions and 23.4% (11/47) of the patients with CRLM. Predictors of local control failure include tumor size larger or equal to 3 cm (HR =3.8) and ablation margin smaller than 0.5 cm (HR =3.7) (36). Interestingly, while proximity to larger vessels was often a risk factor for ASR in RFA (17,19), it did not become significant in multivariable analysis in the MTA or MWA studies (16,36,38). This is likely due to its higher energy and less susceptibility to heat-sink effect (33,34).
Having used both technologies, we compared the ASR rate after RFA versus MTA. In this study, ASR rate was significantly lower in the MTA group (20% vs. 10%, P=0.020), and the ablation modality (HR =2.52) and tumor size (HR =2.34) were independent risk factors for local recurrence after the ablative therapy for CRLM. Furthermore, we found that MTA significantly reduced the total ablation time (37±3 vs. 19±3 min, P<0.001) and operative time (202±13 vs. 154±3 min, P=0.009) (16). Based on these results, we concluded that the ablation with microwave system provides a better local tumor control with faster ablation cycles.
Oncological outcomes
The first multicenter randomized prospective trial, evaluating the effect of RFA of non-resectable CRLM, was conducted in Europe between 2002 and 2007. While the initial report in 2012 revealed significant improvement in progression-free survival (PFS) without OS benefit, the final analysis with longer median follow-up (9.7 years) demonstrated significant improvement in both OS (8-year OS 35.9% vs. 8.9%; P=0.01, median OS 45.6 vs. 40.5 months) and PFS (3-year PFS 27.6% vs. 10.6%, P=0.025, median PFS 16.8 vs. 9.9 months) (13,45). It can be challenging to analyze the effect of ablation with OS given multiple available treatment options before or after ablative therapy, although OS is a commonly used end point in cancer studies. Albeit limitations with smaller sample size, this is the first randomized study demonstrating significant survival benefit with aggressive ablative therapy compared to palliative chemotherapy alone for patients with unresectable CRLM.
There have been multiple non-randomized retrospective and prospective studies for ablation in patients with CRLM. Table 1 showed recent studies with median OS and DFS, although these studies need to be considered carefully due to possible selection bias, different patient number, and different methods of statistical analysis (9,22,27,42,46-48). In our own experience, the patients with unresectable disease who had laparoscopic RFA exhibited median disease-free survival (DFS) of 6 months and median OS of 24 months (48). When we analyzed the subset of patients with small (<3 cm) solitary colorectal liver metastasis, we found that the DFS and OS were similar (47). The ASR in this cohort was 18% and with a close follow up strategy, those patients who developed ASR were identified early and salvage therapies with either resection or repeat ablation were performed.
Table 1. Summary of the literatures on outcomes in CRLM after RFA and MWA.
| Authors | Year | Patients | Modality | Follow-up (months)* | LR rate (%)§ | DFS (months)* | OS (months)* |
|---|---|---|---|---|---|---|---|
| Shady et al. (percutaneous) (46) | 2016 | 162 | RFA | 55 | 48 | 26 (LTPFS) | 36 |
| Leung et al. (42) | 2015 | 140 | MWA | 20.5 | 7.9 | NA | 57 |
| Groeschl et al. (22) | 2014 | 198 | MWA | 19 | 5.2 | 24.5 | 32.1 |
| Aliyev et al. (tumors ≤3 cm) (47) | 2013 | 44 | RFA | 32 | 18 | 25 | 47¶ |
| Kennedy et al. (27) | 2013 | 130 | RFA | 42.2 | 4.3 | 17.4 | 40.4 |
| Martin et al. (9) | 2010 | 50 | MWA | 36 | 6 | 12 | 36 |
| Siperstein et al. (48) | 2007 | 234 | RFA | 24 | 18 | 6 | 24 |
§, LR rate is calculated per lesions; *, follow-up, DFS and OS were reported as median unless otherwise specified; ¶, cancer-specific survival. CRLM, colorectal cancer liver metastasis; RFA, radiofrequency ablation, MWA, microwave ablation; LR, local recurrence; DFS, disease-free survival; OS, overall survival; NR, not reached; LTPFS, local tumor progression free survival; NA, not assessed.
Additionally, showing that the oncologic outcomes of patients with small solitary colorectal metastasis were similar between ablation and resection, we looked at opportunities for cost savings. Combined with significantly shorter operative time and shorter length of stay, the total cost for initial ablative therapy was significantly less than liver resection in this very selected patient population (49,50).
Discussion
The exact role of thermal ablation therapy continues to evolve, as the technology improves and this treatment modality remains as an important option in the armamentarium of the surgeon treating CRLM. For unresectable disease, as demonstrated in CLOCC trial, ablation contributes to the local control and OS, with minimal disruption to the systemic chemotherapy (13). Although a few studies have suggested comparable results compared to hepatectomy for resectable disease (15,47,51,52), further evidence is necessary to recommend ablation versus hepatectomy. The COLLISION trial is an ongoing phase III randomized trial comparing thermal ablation and liver resection on patients with small (<3 cm) CRLM (14). Another ongoing trial, the LAVA trial, has also been designed to compare liver resection and ablative therapy for the patients with CRLM (53). These new randomized trials might provide more evidence for this dilemma. Certain scenarios might be appropriate for the performance of ablation in some patients, such as with the intention for parenchymal preservation, down staging as a part of two-stage treatment, or the treatment of patients with poor risk for a major liver resection. In these scenarios, we suggest to consider MWA and monitor the patient closely to recognize local treatment failures early.
Nevertheless, ablative therapy and liver resection should not be mutually exclusive. For example, both modalities should be utilized in the management of bilobar diseases with improved perioperative outcomes (51,52,54). Additionally, concomitant ablative therapy with hepatectomy may spare the patients from having two-stage hepatectomy. Philips et al. reported that single-stage hepatectomy and MWA resulted in outcome similar to that with two-stage hepatectomy (OS: single-stage 38.4 months vs. two-stage 42.2 months; P=0.132) with less morbidity for bilobar CRLM (55).
In conclusion, with EORTC-CLOCC trial, ablative therapy has established its position in patients with unresectable but ablatable lesions (13). The role of the thermal ablation will continue to evolve in patients with resectable and ablatable lesions owing to newly emerging technology, in addition to new systemic treatment options, including immunotherapy for metastatic CRC.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004;239:818-25; discussion 825-7. 10.1097/01.sla.0000128305.90650.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoo PS, Lopez-Soler RI, Longo WE, et al. Liver resection for metastatic colorectal cancer in the age of neoadjuvant chemotherapy and bevacizumab. Clin Colorectal Cancer 2006;6:202-7. 10.3816/CCC.2006.n.036 [DOI] [PubMed] [Google Scholar]
- 3.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-Year Survival After Resection of Colorectal Liver Metastases Defines Cure. J Clin Oncol 2007;25:4575-80. 10.1200/JCO.2007.11.0833 [DOI] [PubMed] [Google Scholar]
- 4.Siperstein A, Garland A, Engle K, et al. Laparoscopic radiofrequency ablation of primary and metastatic liver tumors. Technical considerations. Surg Endosc 2000;14:400-5. 10.1007/s004640000067 [DOI] [PubMed] [Google Scholar]
- 5.Gillams A, Goldberg N, Ahmed M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontieres meeting 2013. Eur Radiol 2015;25:3438-54. 10.1007/s00330-015-3779-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finlay IG, Seifert JK, Stewart GJ, et al. Resection with cryotherapy of colorectal hepatic metastases has the same survival as hepatic resection alone. Eur J Surg Oncol 2000;26:199-202. 10.1053/ejso.1999.0776 [DOI] [PubMed] [Google Scholar]
- 7.Korpan NN. Hepatic cryosurgery for liver metastases. Long-term follow-up. Ann Surg 1997;225:193-201. 10.1097/00000658-199702000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin RC, Scoggins CR, McMasters KM. Microwave hepatic ablation: initial experience of safety and efficacy. J Surg Oncol 2007;96:481-6. 10.1002/jso.20750 [DOI] [PubMed] [Google Scholar]
- 9.Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol 2010;17:171-8. 10.1245/s10434-009-0686-z [DOI] [PubMed] [Google Scholar]
- 10.Alonzo M, Bos A, Bennett S, et al. The Emprint Ablation System with Thermosphere Technology: One of the Newer Next-Generation Microwave Ablation Technologies. Semin Intervent Radiol 2015;32:335-8. 10.1055/s-0035-1564811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berber E. Laparoscopic microwave thermosphere ablation of malignant liver tumors: an initial clinical evaluation. Surg Endosc 2016;30:692-8. 10.1007/s00464-015-4261-3 [DOI] [PubMed] [Google Scholar]
- 12.Zaidi N, Okoh A, Yigitbas H, et al. Laparoscopic microwave thermosphere ablation of malignant liver tumors: An analysis of 53 cases. J Surg Oncol 2016;113:130-4. 10.1002/jso.24127 [DOI] [PubMed] [Google Scholar]
- 13.Ruers T, Van Coevorden F, Punt CJ, et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J Natl Cancer Inst 2017. doi: . 10.1093/jnci/djx015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puijk RS, Ruarus AH, Vroomen L, et al. Colorectal liver metastases: surgery versus thermal ablation (COLLISION) - a phase III single-blind prospective randomized controlled trial. BMC Cancer 2018;18:821. 10.1186/s12885-018-4716-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hof J, Wertenbroek MW, Peeters PM, et al. Outcomes after resection and/or radiofrequency ablation for recurrence after treatment of colorectal liver metastases. Br J Surg 2016;103:1055-62. 10.1002/bjs.10162 [DOI] [PubMed] [Google Scholar]
- 16.Takahashi H, Kahramangil B, Kose E, et al. A comparison of microwave thermosphere versus radiofrequency thermal ablation in the treatment of colorectal liver metastases. HPB (Oxford) 2018;20:1157-62. 10.1016/j.hpb.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 17.Wong SL, Mangu PB, Choti MA, et al. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol 2010;28:493-508. 10.1200/JCO.2009.23.4450 [DOI] [PubMed] [Google Scholar]
- 18.Petre EN, Sofocleous C. Thermal Ablation in the Management of Colorectal Cancer Patients with Oligometastatic Liver Disease. Visc Med 2017;33:62-8. 10.1159/000454697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berber E, Siperstein A. Local recurrence after laparoscopic radiofrequency ablation of liver tumors: an analysis of 1032 tumors. Ann Surg Oncol 2008;15:2757-64. 10.1245/s10434-008-0043-7 [DOI] [PubMed] [Google Scholar]
- 20.Hof J, Joosten HJ, Havenga K, et al. Radiofrequency ablation is beneficial in simultaneous treatment of synchronous liver metastases and primary colorectal cancer. PLoS One 2018;13:e0193385. 10.1371/journal.pone.0193385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulier S, Ruers T, Jamart J, et al. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? An update. Dig Surg 2008;25:445-60. 10.1159/000184736 [DOI] [PubMed] [Google Scholar]
- 22.Groeschl RT, Pilgrim CH, Hanna EM, et al. Microwave ablation for hepatic malignancies: a multiinstitutional analysis. Ann Surg 2014;259:1195-200. 10.1097/SLA.0000000000000234 [DOI] [PubMed] [Google Scholar]
- 23.Mulier S, Ni Y, Jamart J, et al. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg 2005;242:158-71. 10.1097/01.sla.0000171032.99149.fe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Birsen O, Aliyev S, Aksoy E, et al. A critical analysis of postoperative morbidity and mortality after laparoscopic radiofrequency ablation of liver tumors. Ann Surg Oncol 2014;21:1834-40. 10.1245/s10434-014-3526-8 [DOI] [PubMed] [Google Scholar]
- 25.Foroutani A, Garland AM, Berber E, et al. Laparoscopic ultrasound vs triphasic computed tomography for detecting liver tumors. Arch Surg 2000;135:933-8. 10.1001/archsurg.135.8.933 [DOI] [PubMed] [Google Scholar]
- 26.Berber E, Pelley R, Siperstein AE. Predictors of survival after radiofrequency thermal ablation of colorectal cancer metastases to the liver: a prospective study. J Clin Oncol 2005;23:1358-64. 10.1200/JCO.2005.12.039 [DOI] [PubMed] [Google Scholar]
- 27.Kennedy TJ, Cassera MA, Khajanchee YS, et al. Laparoscopic radiofrequency ablation for the management of colorectal liver metastases: 10-year experience. J Surg Oncol 2013;107:324-8. 10.1002/jso.23268 [DOI] [PubMed] [Google Scholar]
- 28.Sindram D, Lau KN, Martinie JB, et al. Hepatic tumor ablation. Surg Clin North Am 2010;90:863-76. 10.1016/j.suc.2010.04.014 [DOI] [PubMed] [Google Scholar]
- 29.Lu DS, Raman SS, Limanond P, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol 2003;14:1267-74. 10.1097/01.RVI.0000092666.72261.6B [DOI] [PubMed] [Google Scholar]
- 30.Takahashi H, Akyuz M, Aksoy E, et al. Local recurrence after laparoscopic radiofrequency ablation of malignant liver tumors: Results of a contemporary series. J Surg Oncol 2017;115:830-4. 10.1002/jso.24599 [DOI] [PubMed] [Google Scholar]
- 31.van Duijnhoven FH, Jansen MC, Junggeburt JM, et al. Factors influencing the local failure rate of radiofrequency ablation of colorectal liver metastases. Ann Surg Oncol 2006;13:651-8. 10.1245/ASO.2006.08.014 [DOI] [PubMed] [Google Scholar]
- 32.Pathak S, Jones R, Tang JM, et al. Ablative therapies for colorectal liver metastases: a systematic review. Colorectal Dis 2011;13:e252-65. 10.1111/j.1463-1318.2011.02695.x [DOI] [PubMed] [Google Scholar]
- 33.Yu NC, Raman SS, Kim YJ, et al. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol 2008;19:1087-92. 10.1016/j.jvir.2008.03.023 [DOI] [PubMed] [Google Scholar]
- 34.Pillai K, Akhter J, Chua TC, et al. Heat sink effect on tumor ablation characteristics as observed in monopolar radiofrequency, bipolar radiofrequency, and microwave, using ex vivo calf liver model. Medicine (Baltimore) 2015;94:e580. 10.1097/MD.0000000000000580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heerink WJ, Solouki AM, Vliegenthart R, et al. The relationship between applied energy and ablation zone volume in patients with hepatocellular carcinoma and colorectal liver metastasis. Eur Radiol 2018;28:3228-36. 10.1007/s00330-017-5266-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi H, Kahramangil B, Berber E. Local recurrence after microwave thermosphere ablation of malignant liver tumors: results of a surgical series. Surgery 2018;163:709-13. 10.1016/j.surg.2017.10.026 [DOI] [PubMed] [Google Scholar]
- 37.Mulier S, Mulier P, Ni Y, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg 2002;89:1206-22. 10.1046/j.1365-2168.2002.02168.x [DOI] [PubMed] [Google Scholar]
- 38.Correa-Gallego C, Fong Y, Gonen M, et al. A retrospective comparison of microwave ablation vs. radiofrequency ablation for colorectal cancer hepatic metastases. Ann Surg Oncol 2014;21:4278-83. 10.1245/s10434-014-3817-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brudvik KW, Jones RP, Giuliante F, et al. RAS Mutation Clinical Risk Score to Predict Survival After Resection of Colorectal Liver Metastases. Ann Surg 2019;269:120-6. 10.1097/SLA.0000000000002319 [DOI] [PubMed] [Google Scholar]
- 40.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18; discussion 318-21. 10.1097/00000658-199909000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Margonis GA, Sasaki K, Gholami S, et al. Genetic And Morphological Evaluation (GAME) score for patients with colorectal liver metastases. Br J Surg 2018;105:1210-20. 10.1002/bjs.10838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leung U, Kuk D, D'Angelica MI, et al. Long-term outcomes following microwave ablation for liver malignancies. Br J Surg 2015;102:85-91. 10.1002/bjs.9649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machi J, Oishi AJ, Sumida K, et al. Long-term outcome of radiofrequency ablation for unresectable liver metastases from colorectal cancer: evaluation of prognostic factors and effectiveness in first- and second-line management. Cancer J 2006;12:318-26. 10.1097/00130404-200607000-00011 [DOI] [PubMed] [Google Scholar]
- 44.Hildebrand P, Leibecke T, Kleemann M, et al. Influence of operator experience in radiofrequency ablation of malignant liver tumours on treatment outcome. Eur J Surg Oncol 2006;32:430-4. 10.1016/j.ejso.2006.01.006 [DOI] [PubMed] [Google Scholar]
- 45.Ruers T, Punt C, Van Coevorden F, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol 2012;23:2619-26. 10.1093/annonc/mds053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shady W, Petre EN, Gonen M, et al. Percutaneous Radiofrequency Ablation of Colorectal Cancer Liver Metastases: Factors Affecting Outcomes--A 10-year Experience at a Single Center. Radiology 2016;278:601-11. 10.1148/radiol.2015142489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aliyev S, Agcaoglu O, Aksoy E, et al. Efficacy of laparoscopic radiofrequency ablation for the treatment of patients with small solitary colorectal liver metastasis. Surgery 2013;154:556-62. 10.1016/j.surg.2013.03.009 [DOI] [PubMed] [Google Scholar]
- 48.Siperstein AE, Berber E, Ballem N, et al. Survival after radiofrequency ablation of colorectal liver metastases: 10-year experience. Ann Surg 2007;246:559-65; discussion 565-7. 10.1097/SLA.0b013e318155a7b6 [DOI] [PubMed] [Google Scholar]
- 49.Cassera MA, Potter KW, Ujiki MB, et al. Computed tomography (CT)-guided versus laparoscopic radiofrequency ablation: a single-institution comparison of morbidity rates and hospital costs. Surg Endosc 2011;25:1088-95. 10.1007/s00464-010-1322-5 [DOI] [PubMed] [Google Scholar]
- 50.Takahashi H, Akyuz M, Kahramangil B, et al. A Comparison of the Initial Cost Associated With Resection Versus Laparoscopic Radiofrequency Ablation of Small Solitary Colorectal Liver Metastasis. Surg Laparosc Endosc Percutan Tech 2018;28:371-4. 10.1097/SLE.0000000000000577 [DOI] [PubMed] [Google Scholar]
- 51.Karanicolas PJ, Jarnagin WR, Gonen M, et al. Long-term outcomes following tumor ablation for treatment of bilateral colorectal liver metastases. JAMA Surg 2013;148:597-601. 10.1001/jamasurg.2013.1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meijerink MR, Puijk RS, van Tilborg A, et al. Radiofrequency and Microwave Ablation Compared to Systemic Chemotherapy and to Partial Hepatectomy in the Treatment of Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cardiovasc Intervent Radiol 2018;41:1189-204. 10.1007/s00270-018-1959-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gurusamy K, Corrigan N, Croft J, et al. Liver resection surgery versus thermal ablation for colorectal LiVer MetAstases (LAVA): study protocol for a randomised controlled trial. Trials 2018;19:105. 10.1186/s13063-018-2499-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agcaoglu O, Aliyev S, Karabulut K, et al. Complementary use of resection and radiofrequency ablation for the treatment of colorectal liver metastases: an analysis of 395 patients. World J Surg 2013;37:1333-9. 10.1007/s00268-013-1981-1 [DOI] [PubMed] [Google Scholar]
- 55.Philips P, Groeschl RT, Hanna EM, et al. Single-stage resection and microwave ablation for bilobar colorectal liver metastases. Br J Surg 2016;103:1048-54. 10.1002/bjs.10159 [DOI] [PubMed] [Google Scholar]