Abstract
Severe allergic eosinophilic asthma can be characterized by inadequate control, despite the regular use of high dosages of inhaled corticosteroids/long-acting β2-adrenergic agonists combinations, and the very frequent utilization of oral corticosteroids. Therefore, under these circumstances, an add-on biological treatment with monoclonal antibodies directed against suitable molecular targets, involved in the pathobiology of type-2 airway inflammation, is very useful. Within such a context, our case report refers to a 46-year-old woman with severe allergic eosinophilic asthma and relapsing nasal polyps, not eligible to add-on biological therapy with omalizumab because of her very high serum levels of immunoglobulins E (IgE). She is currently under treatment with the humanized monoclonal antibody benralizumab (30 mg subcutaneous injection, administered every 4 weeks for the first three doses, and every 8 weeks thereafter), an eosinophil-depleting anti-interleukin-5-receptor biologic. Our patient experienced relevant clinical and functional improvements already after the first dose, and subsequently striking changes were recorded after the second and third doses, including remarkable increases in asthma control test scores and forced expiratory volume in 1 s values, associated with a complete depletion of blood eosinophils and the interruption of oral corticosteroid intake, as well as with the concomitant disappearance of nasal polyps after the second dose. In conclusion, this case study suggests that benralizumab can exert a very rapid and effective therapeutic action in patients with severe eosinophilic asthma and nasal polyposis.
Keywords: Severe eosinophilic asthma, interleukin-5 receptor, nasal polyps, benralizumab
Introduction
According to a recent document jointly elaborated by the European Respiratory Society (ERS) and the American Thoracic Society (ATS), asthma is considered to be severe when its control requires high dosages of ICS/LABA (inhaled corticosteroids/long-acting β2-adrenergic agonists) combinations, eventually integrated by the addition of other drugs (i.e. leukotriene modifiers, tiotropium, theophylline, oral corticosteroids (OCS)), or when the disease remains uncontrolled despite such intense treatments.1 In the latter case, international guidelines recommend an add-on biological therapy consisting of one monoclonal antibody, to be chosen among currently available anti-IgE, anti-interleukin (IL)-5, anti-IL-5 receptor, or anti-IL-4 receptor agents.2 In particular, benralizumab is an eosinophil-depleting humanized IgG1k antibody, whose Fab fragments bind to the α subunit of the IL-5 receptor (IL-5Rα) expressed by eosinophils, basophils, and group 2 innate lymphoid cells (ILC2), thus impeding its interaction with IL-5.3–6 Moreover, through its Fc constant region, benralizumab binds to the FcγRIIIa receptor expressed by natural killer (NK) cells, thereby inducing them to release high amounts of pro-apoptotic proteins such as granzyme B and perforin, which implement eosinophil apoptosis triggered by the so-called antibody-dependent cell-mediated cytotoxicity (ADCC).3–5 The pre-marketing randomized clinical trials SIROCCO and CALIMA have previously shown that benralizumab effectively prevents exacerbations of refractory eosinophilic asthma and also improves symptom control and lung function.7,8 Furthermore, the ZONDA study demonstrated that benralizumab can significantly decrease OCS consumption.9 It is also noteworthy that the therapeutic effects of benralizumab appear to be independent of both atopic status and serum IgE levels;10 therefore, benralizumab can be equally effective in the treatment of allergic as well as non-allergic eosinophilic asthma. Less known are the effects of benralizumab on nasal polyps.
On the basis of the above-mentioned considerations, we decided to treat with benralizumab a woman with severe allergic eosinophilic asthma and nasal polyps, not eligible to add-on therapy with omalizumab because of her too high serum levels of IgE. In this patient, we evaluated the impact of the first three doses of benralizumab on symptom control, lung function, blood eosinophils, OCS intake, and nasal polyps.
Approval by Ethical Committee is not required for this case report. The patient provided written informed consent for the publication of information referring to her disease, reported in this article.
Case report
During last November, a non-smoker 46-year-old woman referred as outpatient to our Respiratory Unit at ‘Magna Graecia’ University Hospital located in Catanzaro, Italy. She complained of persistent dyspnoea, wheezing, and cough. These symptoms dated from adolescence and often exacerbated as recurrent asthma attacks. Asthma control test (ACT) score was 11. The patient also suffered from nasal obstruction and hyposmia; indeed, she had undergone two surgical procedures of nasal polypectomy in February 2011 and May 2018, respectively. On chest examination, widespread expiratory wheezes were clearly heard.
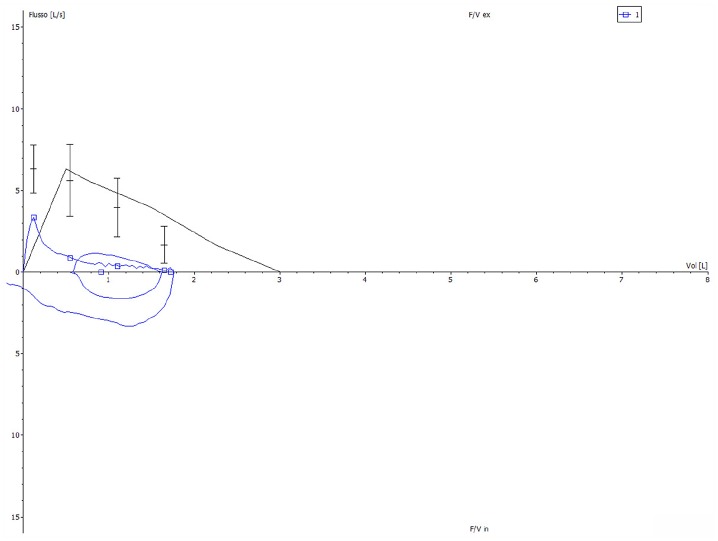
On 19 November 2018, lung function tests documented a partially reversible severe obstructive ventilatory defect, involving both large and small airways, which was characterized by a deep concavity of the flow–volume curve (Figure 1). Forced expiratory volume in 1 s (FEV1) was 0.91 L (35% of predicted value), FEV1/FVC (forced vital capacity) ratio was 52.64%, peak expiratory flow (PEF) was 3.33 L/s (52.7% pred.), and the average expiratory flow over the middle half of the FVC manoeuvre (MMEF 75/25) was 0.27 L/s (8.1% pred.).
Figure 1.
Baseline flow–volume curve, characterized by a marked expiratory airflow limitation.
Skin prick tests were positive for house dust mite. Serum IgE levels were 2760 IU/mL. Rhinoscopy showed the presence of nasal polyps prolapsing into nasal cavities (Figure 2, left panel).
Figure 2.
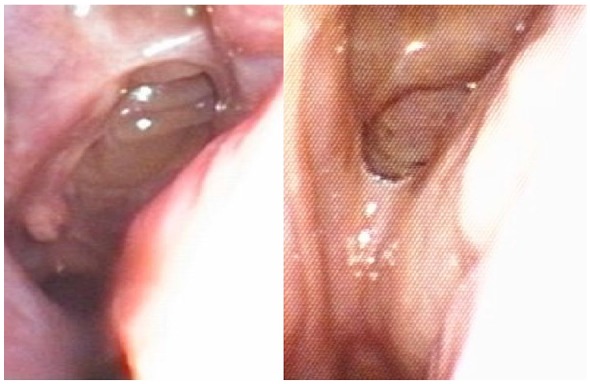
Rhinoscopic evidence of nasal polyps (left panel), which disappeared after 8 weeks of add-on treatment with benralizumab (right panel).
The treatment regimen of our patient included regular therapy with a pressurized metered dose inhaler containing 200/6 µg beclomethasone dipropionate/formoterol fumarate dehydrate (two puffs twice daily), 2.5 µg tiotropium respimat inhaler (two puffs daily), montelukast 10 mg tablets (one tablet daily), prednisone 25 mg tablets (half tablet daily), and mometasone furoate 50 µg/dose nasal spray (two doses for each nostril, once daily).
Because of the largely unsatisfactory asthma control, despite the above intense pharmacological treatment, we considered the option of an add-on biological therapy. Due to her too high serum IgE levels (2760 IU/mL), omalizumab could not be prescribed. Therefore, an anti-IL-5 receptor therapy with benralizumab was started, consisting of a 30-mg subcutaneous injection administered every 4 weeks for the first three times, and every 8 weeks thereafter.
Results
We further evaluated our patient 4 weeks after receiving the first dose of benralizumab, just before administering the second dose (Table 1). Her ACT score slightly enhanced to 14, and a 240-mL increase in FEV1 was measured. Because of a subjective improvement in global health status, 2 weeks after benralizumab first injection she spontaneously tapered down the daily dosage of prednisone to 6.25 mg. Blood eosinophil count dropped to 200 cells/µL. Moreover, the patient continued the regular ICS/LABA treatment twice daily, but did not need to inhale additional puffs. On the basis of such positive results, we suggested to interrupt OCS therapy. Much more impressive were the data recorded 4 weeks after the second dose (8th week with respect to baseline), and 8 weeks after the third one (16th week with respect to baseline) (Table 1). Indeed, ACT score progressively increased, and 16 weeks after the first dose of benralizumab overcame the critical threshold of 20, indicative of a good overall asthma control. Furthermore, between the 4th and 16th week from the first benralizumab administration FEV1 values boosted, thus slightly exceeding a very remarkable 1-L increase. In parallel with FEV1, also PEF values markedly and progressively increased after the second and the third injections of benralizumab (between the 4th and 16th week with respect to baseline). These noticeable clinical and functional progresses were associated with a complete depletion of blood eosinophils. Moreover, our patient referred a relevant improvement of her hyposmia, and rhinoscopy evidenced the disappearance of the relapsing nasal polyps (Figure 2, right panel). No adverse event or side effect was observed during the first 16 weeks of treatment with benralizumab.
Table 1.
Effects of add-on treatment with benralizumab (first injection performed after detection of baseline parameters).
| Baseline | 4 weeks | 8 weeks | 16 weeks | |
|---|---|---|---|---|
| ACT | 11 | 14 | 18 | 24 |
| FEV1 (L, % pred.) | 0.91 (35%) | 1.15 (45%) | 1.78 (69%) | 1.95 (76%) |
| PEF (L/s, % pred.) | 3.33 (53%) | 3.23 (51%) | 4.83 (77%) | 5.64 (89%) |
| Blood eosinophils (cells/μL) | 400 | 200 | 0 | 0 |
| Serum IgE (IU/mL) | 2760 | 2710 | 2630 | 2600 |
| Prednisone intake (mg/day) | 12.5 | 6.25 | 0 | 0 |
ACT: asthma control test; FEV1: forced expiratory volume in 1 s; PEF: peak expiratory flow.
Discussion
Taken together, the real-life clinical, functional, hematologic, and rhinoscopic observations referring to this case report clearly indicate that benralizumab induced in our patient a rapid and striking improvement of both severe asthma and nasal polyposis. Such findings corroborate, emphasize, and further extend the results of several randomized, placebo-controlled trials.11 Of course, the excellent therapeutic actions of benralizumab reported in the present case report are mainly due to the rapid and effective eosinophil-depleting properties of this biologic, which depend on its peculiar dual mechanism of action (i.e. IL-5-receptor blockade and antibody-dependent eosinophil apoptosis).4,5 The very quick and effective drop of blood eosinophils elicited by benralizumab was paralleled, in our patient, by a concomitant improvement of asthma symptom control, demonstrated by the marked increase in ACT score. In particular, in real-life contexts, ACT appears to be more practical and useful than the Asthma Control Questionnaire (ACQ) utilized in randomized controlled trials;12,13 indeed, when compared to ACQ, the ACT questionnaire seems to be simpler and more easily usable by patients, thus being likely quicker in providing detailed and reliable information on both entity and recurrence of asthma symptoms. Moreover, the very rapid decrease in the use of day-time and night-time rescue medication, experienced by our patient, is consistent with a recent analysis of pooled results from the SIROCCO and CALIMA phase 3 studies.14 Such an excellent improvement in symptom control made it possible for our patient a quite fast OCS withdrawal, namely a better OCS sparing effect than that one reported by phase 3 ZONDA trial.9
The present case report also shows that the very positive impact of benralizumab on asthma control was associated with an extraordinary functional result, expressed by a FEV1 increase which, after 16 weeks with respect to baseline, exceeded 1 L. This finding is largely greater than the average FEV1 improvements recorded during pre-marketing randomized controlled trials.15 Therefore, our observation further confirms that the therapeutic responses to anti-asthma biological drugs are strictly individual, being absolutely dependent on the specific endotype of each patient. The most relevant FEV1 enhancements were paralleled by similar PEF increases, consistently with the results of a recent comprehensive evaluation of the data obtained by SIROCCO, CALIMA, and ZONDA studies.16
Moreover, benralizumab exerted a very effective therapeutic action on nasal polyps, which had relapsed in our patient after two previous surgical treatments. This effect of benralizumab is very important, because nasal polyposis is one of the most relevant comorbidities of severe asthma. Nasal polyps are characterized by eosinophilic infiltration of polyp tissue.17 It can thus be argued that also the valuable benefits experienced by our patient with regard to her nasal polyposis were dependent on benralizumab-induced resolution of type-2 airway eosinophilic inflammation. On the other hand, the presence of nasal polyposis is one of the most reliable predictive factors of a positive therapeutic response to treatment with benralizumab.18
In conclusion, the present case report clearly shows that in a real-life setting benralizumab was very effective as add-on biological therapy of severe asthma and nasal polyposis. In particular, it appears that benralizumab exerted its therapeutic effects to a much greater extent with respect to randomized controlled trials. Hence, this observation strongly suggests the opportunity of carrying out real-world studies, focusing on the efficacy and safety of benralizumab investigated in daily clinical practice.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient for her anonymized information to be published in this article.
ORCID iD: Girolamo Pelaia  https://orcid.org/0000-0001-9288-8913
https://orcid.org/0000-0001-9288-8913
References
- 1. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. [DOI] [PubMed] [Google Scholar]
- 2. Global Initiative for Asthma, 2019. Update, www.ginasthma.org
- 3. Matera MG, Calzetta L, Rinaldi B, et al. Pharmacokinetic/pharmacodynamic drug evaluation of benralizumab for the treatment of asthma. Expert Opin Drug Metab Toxicol 2017; 13(9): 1007–1013. [DOI] [PubMed] [Google Scholar]
- 4. Pelaia C, Vatrella A, Bruni A, et al. Benralizumab in the treatment of severe asthma: design, development and potential place in therapy. Drug Des Devel Ther 2018; 12: 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pelaia C, Calabrese C, Vatrella A, et al. Benralizumab: from the basic mechanism of action to the potential use in the biological therapy of severe eosinophilic asthma. Biomed Res Int 2018; 2018: 4839230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sehmi R, Lim HF, Mukherjee M, et al. Benralizumab attenuates airway eosinophilia in prednisone-dependent asthma. J Allergy Clin Immunol 2018; 141(4): 1529–1532. [DOI] [PubMed] [Google Scholar]
- 7. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016; 388: 2115–2127. [DOI] [PubMed] [Google Scholar]
- 8. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016; 388: 2128–2141. [DOI] [PubMed] [Google Scholar]
- 9. Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med 2017; 376(25): 2448–2458. [DOI] [PubMed] [Google Scholar]
- 10. Chipps BE, Newbold P, Hirsch I, et al. Benralizumab efficacy by atopy status and serum immunoglobulin E for patients with severe, uncontrolled asthma. Ann Allergy Asthma Immunol 2018; 120(5): 504–511. [DOI] [PubMed] [Google Scholar]
- 11. Chia YL, Yan L, Yu B, et al. Relationship between benralizumab exposure and efficacy for patients with severe eosinophilic asthma. Clin Pharmacol Ther 2019; 106(2): 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jia CE, Zhang HP, Lv Y, et al. The asthma control test and the asthma control questionnaire for assessing asthma control: systematic review and meta-analysis. J Allergy Clin Immunol 2013; 131: 695–703. [DOI] [PubMed] [Google Scholar]
- 13. Pelaia C, Busceti MT, Solinas S, et al. Real-life evaluation of the clinical, functional, and hematological effects of mepolizumab in patients with severe eosinophilic asthma: results of a single-centre observational study. Pulm Pharmacol Ther 2018; 53: 1–5. [DOI] [PubMed] [Google Scholar]
- 14. O’Quinn S, Xu X, Hirsch I. Daily patient-reported health status assessment improvements with benralizumab for patients with severe, uncontrolled eosinophilic asthma. J Asthma Allergy 2019; 12: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kupczyk M, Kuna P. Benralizumab: an anti-IL-5 receptor α monoclonal antibody in the treatment of asthma. Immunotherapy 2018; 10: 349–359. [DOI] [PubMed] [Google Scholar]
- 16. Chupp G, Lugogo NL, Kline JN, et al. Rapid onset of effect of benralizumab on morning peak expiratory flow in severe, uncontrolled asthma. Ann Allergy Asthma Immunol 2019; 122(5): 478–485. [DOI] [PubMed] [Google Scholar]
- 17. Newton JR, Ah-See KW. A review of nasal polyposis. Ther Clin Risk Manag 2008; 4: 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bleecker ER, Wechsler ME, FitzGerald JM, et al. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur Respir J 2018; 52(4): 1800936. [DOI] [PMC free article] [PubMed] [Google Scholar]