Abstract
Certain diabetic and hypertensive patients started on intravitreal vascular endothelial growth factor inhibition for diabetic retinopathy may experience worsening of hypertension and proteinuria. The etiology of this is the newly recognized absorption of intravitreally injected vascular endothelial growth factor inhibitors, and the susceptibility of patients with pre-existing renal disease to exacerbations depends on the degree of systemic absorption. There are eighteen reported cases of worsening hypertension, woresening proteinuria, worsening renal function, thrombotic microangiopathy, and glomerular disease noted after initiation of intravitreal vascular endothelial growth factor blockade. This nineteenth case demonstrates worsening hypertension and proteinuria with the start of bevacizumab. Both blood pressure and proteinuria parameters showed overall improvement with switching to the less absorbed and lower potency agent ranibizumab. There was a slight rise in serum creatinine after bevacizumab therapy, which stabilized at a new baseline, and the serum creatinine remained stable on ranibizumab. There were no other nephrotoxic exposures that explained the mild rise in serum creatinine. Because of improvement in renal function and proteinuria, a renal biopsy was deferred for the time. This case re-demonstrates the risk of worsening proteinuria with vascular endothelial growth factor inhibitors when given intravitreally in some patients. The demonstration of improvement in blood pressure and proteinuria with the use of lower potency agents like ranibizumab is novel and an important concept confirming observations from pharmacokinetic studies. The switch to ranibizumab offers a therapeutic option when proteinuria worsens with intravitreal vascular endothelial growth factor blockade, and the patient requires ongoing intravitreal therapy for treatment of diabetic retinopathy.
Keywords: Proteinuria, vascular endothelial growth factor inhibitors, bevacizumab, aflibercept, ranibizumab, diabetic nephropathy, diabetic retinopathy
Introduction
Systemic injections of vascular endothelial growth factor (VEGF) inhibitors, which are angiogenesis inhibitors, have long been a mainstay of adjuvant chemotherapy. The utilization of these agents came with several dose-limiting toxicities including hypertension, proteinuria, and in some cases glomerular diseases including diverse causes of nephrotic syndrome and thrombotic microangiopathy.1 The use of three of these agents intravitreally has been done both on and off Food and Drug Administration (FDA) label.
Bevacizumab is used off label intravitreally, while aflibercept and ranibizumab are FDA approved.2 Their indications are for age-related macular degeneration, central retinal vein occlusion, and diabetic macular edema/proliferative diabetic retinopathy.2 Recent drug level and systemic VEGF measurements after intravitreal use of these drugs have noted changes in VEGF levels and serum drug levels near the biological 50% inhibitory concentration.3 To date, 19 patients including this case have been reported with worsening hypertension, thrombotic microangiopathy, or glomerular disease after intravitreal injections of bevacizumab, aflibercept, and rarely of ranibizumab.2 According to pharmacodynamic testing, ranibizumab has a lower potency, a much shorter half-life, and a lesser degree of absorption than bevacizumab or aflibercept. However, VEGF blockade or blockade of associated pathways to any degree can result in proteinuria.1–4
We report a case of a 37-year-old female with early-onset type 2 diabetes and diabetic retinopathy and nephropathy who developed an increase in blood pressures and proteinuria on bevacizumab. These measurements subsequently improved with switch to the lower potency intravitreally administered anti-VEGF agent: ranibizumab.
Case report
The patient is a 37-year-old Native American female with obesity, a strong family history of type 2 diabetes and metabolic syndrome, was diagnosed with type 2 diabetes mellitus since the age of 19 years. She presented to University of California, Los Angeles (UCLA) nephrology clinic at 35 years of age for worsening serum creatinine and microalbuminuria. She has type 2 diabetes mellitus with a hemoglobin A1c of 6%–7%. Her blood pressure and proteinuria level had been stable, but started to rise at 32 years of age. At the time of presentation, urine albumin/creatinine ratios increased to 2.1 g/g from a baseline of 1.6–1.7 g/g just before referral to nephrology. A simultaneous total protein collected over 24 h was 3.43 g/day.
The patient reported that her home blood pressure used to be well controlled (averaged around 140 mm Hg systolic blood pressure, 80–85 mm Hg diastolic blood pressure), but has gotten worse over the last 5 years. Blood pressure monitoring was done at home daily once in the a.m. and once in the p.m. with a calibrated measuring device placed correctly—relative to the brachial artery. She noted that her blood pressure had increased remarkably to 160 mm Hg at home with occasions of 170–180 mm Hg and diastolic blood pressure of 90–100 mm Hg.
The patient had only microvascular findings, there was no neuropathy, there was no cardiovascular disease and peripheral arterial disease, and she was and remained sedentary due to her job and an inability to exercise despite counseling. Her height was 5 ft 8 in. (172 cm), her weight was 236 lbs (107 kg), and her body mass index (BMI) was 35.9 kg/m2. Physical exam was only notable for 1+ bilateral lower extremity edema which improved subsequently to trace bilateral lower extremity edema.
Initially, she was only on benazepril 40 mg daily, and there was no alteration of dosage or type of angiotensin receptor blockers throughout time frame of this report, 2017–2019. In addition, patient was on besifloxacin eye drops, one drop in injected eye three times daily for 7 days after anti-VEGF injection, dorzolamide 2% one drop in both eyes twice daily, insulin lispro 18 units subcutaneously three times a day, and insulin detemir was changed to 54 units subcutaneously once a day. Empagliflozin 25 mg daily, ketorolac 0.5% ophthalmic solution one drop in each eye twice daily, metformin 500 mg twice a day, and simvastatin 10 mg at bedtime. Subsequently, insulin detemir was changed to glargine insulin 40–50 units subcutaneously at bedtime.
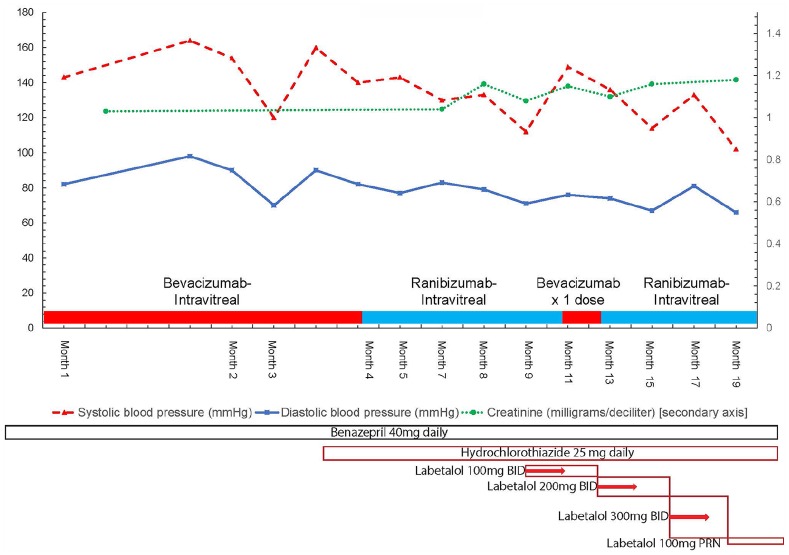
Anti-hypertensives were added at a later date as well (see Figures 1 and 2 for timing details). Between 2017 and 2019, blood pressure medications were changed, hydrochlorothiazide 25 mg was added in end of month 3 from presentation, and labetalol 100 mg twice a day was added in month 8–9, up titrated to 200 mg twice a day in month 13, and up to 300 mg twice a day in month 16. As patient was switched to ranibizumab, blood pressure dropped to 100–110 in month 18, and labetalol 300 mg twice a day was tapered down to 100 mg on an as needed basis in month 18.
Figure 1.
Trends of serum creatinine (mg/dL), systolic blood pressure (mm Hg), and diastolic blood pressure (mm Hg) versus date.
BID, twice daily; mg, milligrams; PRN, as needed.
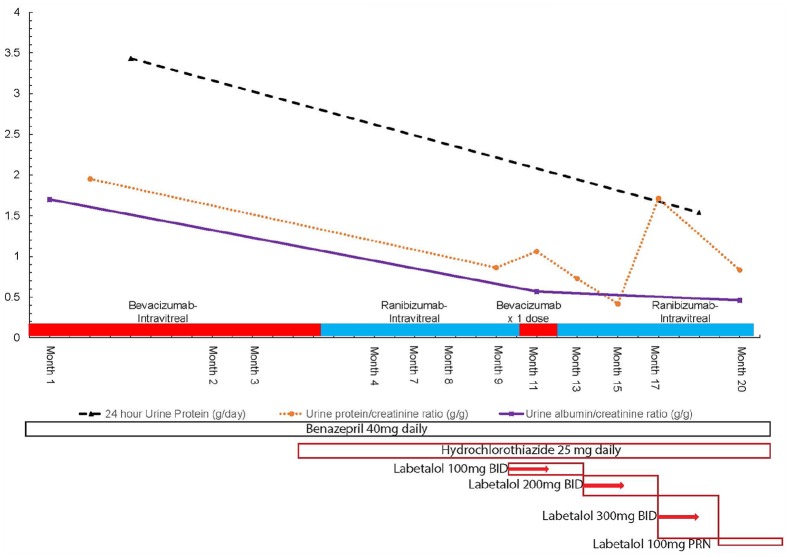
Figure 2.
Trends of daily urine protein excretion (grams protein/24 h), urine total protein to creatinine ratio (grams protein/gram creatinine), urinary albumin/creatinine ratio (mcg albumin/mg creatinine, equivalent to grams albumin/gram creatinine) versus date.
BID, twice daily; mg, milligrams; PRN, as needed.
Serum creatinine had also slightly increased from 1.04 to 1.1–1.2 range between 2017 and 2018. A full acute kidney injury workup was unremarkable, urinalysis showed only proteinuria without hematuria and pyuria, and no casts were observed on microscopic analysis. Renal structural evaluation was obtained with a renal ultrasound showing no nephrolithiasis, hydronephrosis, and bilateral kidneys measuring 11 cm × 6 cm without finding of simple or complex cysts or renal masses. A full serological workup including anti-nuclear antibody, double stranded antibody, anti-ribonucleoprotein/anti-smith antibody, anti-neutrophil cytoplasmic antibody (C and P ANCA), acute hepatitis panel, human immunodeficiency virus serology, syphilis serology, rheumatoid factor, anti-citric citrullinated peptide, complement (C3, C4, total complement-Ch50), cryoglobulin titer (cryocrit), and anti-scleroderma antibody (anti-SCL-70) were all within normal range. Myeloma labs (kappa-to-lambda ratio of free light chains) were also within normal range.
Further history revealed that she has not been using herbal medications, supplements, or non-steroidal anti-inflammatory agents. Upon review of her medications, she disclosed that she had been treated with bevacizumab injections (1 mg in alternating eyes) for diabetic proliferative retinopathy by a local ophthalmologist every 2 weeks. We decided to hold further bevacizumab use and switch to ranibizumab after further discussion with the ophthalmologist. Upon switching to ranibizumab, her blood pressure improved and later normalized on the same blood pressure medication regimen. Her ranibizumab injections were interrupted once with a dose of bevacizumab in month 11 from presentation, until her insurance approved a further supply. Her proteinuria parameters have improved in the longer term, now averaging 0.5–1 g protein/g creatinine. There was, however, an outlier value with a recent uptick to 1.7 g obtained temporally the same week as a ranibizumab injection. This value soon decreased to 0.83 g/g of total-protein-to-creatinine ratio and 0.46 g/g urinary-albumin-to-creatinine ratio. Her blood pressure has also decreased now to 102/66 mm Hg, while her serum creatinine remains stable at 1–1.1 mg/dL. Her 24-h protein levels have dropped from 3.4 to 1.5 g/day. Figures 1 and 2 detail the trends of patient’s blood pressure, serum creatinine, and urine protein excreted per day cross-referenced with the timing of intravitreal anti-VEGF agent injections.
Discussion
The systemic effects of intravenous anti-VEGF inhibition have been demonstrated. The literature contains an extensive database showing worsening of hypertension, proteinuria, and renal function with systemic use of anti-VEGF agents for oncologic purposes. In some cases, these drugs have triggered various glomerular diseases as well.1 Intravitreal use of these drugs has been increasingly noted to contribute to the risk of vascular events,5 and there is a growing body of evidence that these agents may contribute to proteinuria, and in some studies hypertension.6 The now established finding that these anti-VEGF agents are absorbed systemically in significant quantities when given intravitreally is equally concerning.3 New literature reports suggest that they may induce temporary or continual intravascular VEGF depletion.7
Molecularly VEGF depletion affects trophic signals to podocytes and endothelial cells through upregulation of (v-rel avian reticuloendotheliosis viral oncogene homolog A) Rel-A and causes inflammation via renal angiotensin aldosterone system (RAAS) upregulation and (nuclear factor kappa light chain enhancer of activated B cells) NF-κB signaling.2,8 Nephrotic syndrome can be caused via podocyte cytoskeleton effects can be observed as via disruption of C-MIP (though this is classically seen more with inhibition of the closely related tyrosine kinase pathway).1,2,8 That is not all, as VEGF blockade can also induce changes in pro-thrombotic pathways (Diacyl-glycerol (DAG)-Kinase epsilon), in addition it affects nitric oxide signaling.2,8 These are very well documented for systemic anti-VEGF toxicity and increasingly being shown in patients receiving intravitreal VEGF injections which are now known to be systemically absorbed according to Avery et al.3,9
An additional study by Tschulakow et al.10 also shows that not all VEGF blocking agents present the same risk. He shows that ranibizumab is present for shorter periods of time at simian glomeruli than aflibercept, effects systemic VEGF less, and that ranibizumab does not induce changes in endothelial fenestrations of glomerular capillaries seen with longer acting anti-VEGF agents like aflibercept. That does not mean that ranibizumab is risk free, but it presents a safer compromise in patients with diabetic nephropathy and diabetic retinopathy needing treatment.2,8
This case suggests that bevacizumab can worsen previously stable proteinuria in a diabetic patient. It also illustrates the improvement of observed proteinuria with a lower potency agent like ranibizumab which has a shorter half-life and is less absorbed.3 It was previously postulated that switching patients from bevacizumab and aflibercept to ranibizumab may decrease absorption and risk of systemic side effects after intravitreal injections.8 Supplemental Figure 3 shows structural and pharmacologic information regarding the different classes of VEGF blocking agents used intravitreally for diabetic macular edema and other indications.
The rationale we derive for attributing the improvement in blood pressure and proteinuria to changing VEGF blocking agents being given intravitreally is as follows. Only non-RAAS blocking agents were added to control blood pressure due to the rising serum creatinine. The patient’s dose of benazepril was never changed throughout the course of treatment with a near 50% magnitude drop in proteinuria and albuminuria measurements. There were also no other non-dihydropyridine calcium channel blockers, the only other group of agents proven to have a significant effect on proteinuria. These changes are not easily explainable by the hydrochlorothiazide and labetalol added as anti-hypertensive agents, particularly because the blood pressure was not in the hypertensive emergency range (>200 mm Hg) initially.
When bevacizumab was given one time by the ophthalmologist due to ranibizumab unavailability in month 11, systolic and diastolic blood pressure rose in tandem for about 1 month and then improved. The urine protein-to-creatinine ratio rose when checked 1–2 months after the lone bevacizumab injection re-challenge as well, but it is difficult to see the effect as cleanly as with blood pressure. The change to ranibizumab also allowed the labetalol to be titrated down to 100 mg taken on an as needed basis (relatively infrequently 2–3 times per week) in month 18. Even more compelling is that there was a persistence of all three urinary protein parameters improving even when the patient is on the PRN labetalol taken only a few times a week. The lower urine albumin/creatinine ratio, urine 24-h protein, and spot urine protein/creatinine ratio in month 20 (in Figure 2) seem consistently improved relative to earlier baseline on bevacizumab despite removing the labetalol to only occasional use in month 18. These factors plausibly suggest that some other factor was responsible for changes in blood pressure and proteinuria improvement. It is reasonable to conclude that the change from a potent to a less potent VEGF inhibitor being used intravitreally (and absorbed) can affect these parameters in the observed manner.
This case is the nineteenth case showing changes in proteinuria, hypertensive control, renal function, or development of glomerular disease after intravitreal VEGF blockade.8 A more recent prospective trial showed a rise in diastolic blood pressure after intravitreal bevacizumab injections, along with elevated platelets and hemoglobin. Although the change in urinary-albumin-to-creatinine ratio was not statistically significant, 45% of patients who received bevacizumab (18/40 patients) showed an increase in urinary-albumin-to-creatinine ratio. It is possible that only certain patients will have proteinuria aggravation due to different levels of absorption, genetic factors, or differences at which level of VEGF depletion side effects manifest.11
Differences in absorption levels from intravitreal injections and differences in response to VEGF blockade suggest that response to these agents is heterogeneous.2,3,6,8 This is analogous to experience in patients with pre-eclampsia where reactions to VEGF depletion by sFLt-1 produces a spectrum of worsening hypertension and proteinuria.12 This is likely dependent on hitherto unexplored genetic factors involving VEGF receptor signaling, downstream mediators, and unknown factors modulating vitreal absorption.2,8,13 More basic and clinical investigation to discover which patients are most susceptible to the effects of intravitreal VEGF blockade, and the genetics that may underlie this susceptibility, is required.2
Conclusion
This report serves to demonstrate that switching to ranibizumab maybe helpful in patients who demonstrate worsening hypertension and proteinuria after receiving intravitreal bevacizumab or aflibercept for diabetic proliferative retinopathy and diabetic macular edema. This is due to the shorter half-life and relatively lower level of systemic absorption of ranibizumab relative to the more potent and longer acting anti-VEGF agents. This corroborates the suggested guidelines for management of worsening proteinuria and hypertension that maybe seen in selected patients who experience VEGF depletion from intravitreal injections.
Supplemental Material
Supplemental material, Figure_3_Structures_of_anti_VEGF_agents_Supplemental_Figure for Intravitreal bevacizumab-induced exacerbation of proteinuria in diabetic nephropathy, and amelioration by switching to ranibizumab by Ramy M Hanna, Lama Abdelnour, Huma Hasnain, Umut Selamet and Ira Kurtz in SAGE Open Medical Case Reports
Footnotes
Consent to participate: This research work does not contain human subjects research.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: I.K. was supported, in part, by funds from the National Institutes of Health (NIH) (R01-DK077162), the Allan Smidt Charitable Fund, the Factor Family Foundation, and the Ralph Block Family Foundation.
Informed consent: We have retroactively obtained written informed consent that is required to publish patient information from patient, with no images to be published.
ORCID iD: Ramy M Hanna  https://orcid.org/0000-0003-1807-8909
https://orcid.org/0000-0003-1807-8909
Supplemental material: Supplemental material for this article is available online.
References
- 1. Hanna RM, Lopez E, Wilson J. Minimal change disease onset observed after bevacizumab administration. Clin Kidney J 2016; 9(2): 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanna RM, Lopez E, Hasnain H, et al. Three patients with injection of intravitreal vascular endothelial growth factor inhibitors and subsequent exacerbation of chronic proteinuria and hypertension. Clin Kidney J 2019; 12(1): 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Avery R, Castellarin A, Steinle N, et al. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina 2017; 37(10): 1847–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hanna RM, Selamet U, Hasnain H. Development of focal segmental glomerulosclerosis and thrombotic microangiopathy in a liver transplant patient on sorafenib for hepatocellular carcinoma: a case report. Transplant Proc 2018; 50(10): 4033–4037. [DOI] [PubMed] [Google Scholar]
- 5. Hanhart J, Comaneshter Freier Dror Y, Vinker S. Mortality in patients treated with intravitreal bevacizumab for age-related macular degeneration. BMC Ophthalmol 2017; 17(1): 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rasier R, Artunay O, Yuzbasioglu E, et al. The effect of intravitreal bevacizumab (avastin) administration on systemic hypertension. Eye 2009; 23(8): 1714–1718. [DOI] [PubMed] [Google Scholar]
- 7. Jampol LM, Glassman AR, Liu D. Plasma vascular endothelial growth factor concentrations after intravitreous anti-vascular endothelial growth factor therapy for diabetic macular edema. Ophthalmology 2018; 125(7): 1054–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hanna RM, Barsoum M, Arman F, et al. Nephrotoxicity induced by intravitreal vascular endothelial growth factor (VEGF) inhibitors: emerging evidence. Kidney Int 2019; 96(3): 572–580. [DOI] [PubMed] [Google Scholar]
- 9. Avery RL, Castellarin AA, Steinle NC, et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 2014; 98(12): 1636–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tschulakow A, Christner S, Julien S, et al. Effects of a single intravitreal injection of aflibercept and ranibizumab on glomeruli of monkeys. PLoS ONE 2014; 9(11): e113701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bagheri S, Dormanesh B, Farid M, et al. Proteinuria and renal dysfunction after intravitreal injection of bevacizumab in patients with diabetic nephropathy: a prospective observational study. Galen Med J 2018; 7: e1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thadhani R, Hagmann H, Schaarschmidt W, et al. Removal of soluble Fms-like tyrosine kinase-1 by dextran sulfate apheresis in preeclampsia. J Am Soc Nephrol 2016; 27(3): 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao L, Grob S, Avery R, et al. Common variant in VEGFA and response to anti-VEGF therapy for neovascular age-related macular degeneration. Curr Mol Med 2013; 13(6): 929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Figure_3_Structures_of_anti_VEGF_agents_Supplemental_Figure for Intravitreal bevacizumab-induced exacerbation of proteinuria in diabetic nephropathy, and amelioration by switching to ranibizumab by Ramy M Hanna, Lama Abdelnour, Huma Hasnain, Umut Selamet and Ira Kurtz in SAGE Open Medical Case Reports