Abstract
Parkinson’s disease (PD) and dementia with Lewy bodies (DLB) are progressive neurodegenerative diseases for which there is no disease-modifying treatment. PD and DLB are characterized by aggregation of the synaptic protein α-synuclein, and there is compelling evidence to suggest that progression of these diseases is associated with the trans-cellular spread of pathogenic α-synuclein through the brains of afflicted individuals. Therapies targeting extracellular, pathogenic α-synuclein may therefore hold promise for slowing or halting disease progression. In this regard, it has been suggested that highly-selective antibodies can be administered as therapeutic agents targeting pathogenic proteins. In the current study, we screened a series of antibodies using multiple selection criterion to identify those that selectively bind pathogenic α-synuclein and show potent inhibition of pathology seeding in a neuronal model of α-synucleinopathy. A lead antibody was tested in a mouse model of PD, and it was able to reduce the spread of α-synuclein pathology in the brain and attenuate dopamine reductions in the striatum. This study highlights the therapeutic potential of α-synuclein immunotherapy for the treatment of PD and DLB, and provides a framework for screening of α-synuclein antibodies to identify those with preferred properties.
Keywords: misfolded, SNCA, antibody, immunotherapy, animal model, transmission, protein spread, intervention
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurodegenerative disease that affects 1% of the worldwide population (Tanner and Goldman, 1996). Motor symptoms that are characteristic of the disease are often preceded by non-motor symptoms, including constipation, sleep disturbances and olfactory dysfunction (Poewe et al., 2017), and are often followed by cognitive decline, which can lead to a diagnosis of PD dementia (PDD) (Irwin et al., 2017). Dementia with Lewy bodies (DLB) bears symptom and pathology overlap with PD and PDD (Irwin et al., 2017; McKeith et al., 2017; Tsuboi et al., 2007), suggesting that these three disorders lie along a spectrum of neurodegenerative diseases collectively known as α-synucleinopathies (Jellinger, 2012; Postuma et al., 2016) due to the abnormal accumulation of normally synaptic α-synuclein protein into neuronal Lewy bodies (LBs) and axonal Lewy neurites (LNs) (Baba et al., 1998; Spillantini et al., 1998; Spillantini et al., 1997). α-Synuclein is not merely a bystander in these diseases since rare mutations (Appel-Cresswell et al., 2013; Kiely et al., 2013; Kruger et al., 1998; Pasanen et al., 2014; Polymeropoulos et al., 1997; Proukakis et al., 2013; Zarranz et al., 2004), duplications (Chartier-Harlin et al., 2004; Ibanez et al., 2004) and triplications (Singleton et al., 2003) of α-synuclein lead to familial PD.
Overexpression of α-synuclein in mice with (Giasson et al., 2002) or without (Masliah et al., 2000) familial mutations is sufficient to drive the formation of LB-like inclusions and neurodegeneration. Further, reduction in α-synuclein levels has been demonstrated to have a beneficial effect in neurotoxin-induced models of PD (Dauer et al., 2002; Zharikov et al., 2015). Together, these studies suggest that a reduction of α-synuclein, especially misfolded forms, may be a therapeutic strategy for treatment of PD and related α-synucleinopathies. Because α-synuclein is primarily localized in the neuronal cytoplasm (Maroteaux et al., 1988), it has been assumed that therapeutic molecules would need to cross not only the blood-brain-barrier (BBB), but also the neuronal plasma membrane, to interact with α-synuclein. However, a number of recent studies in humans (Beach et al., 2009; Braak et al., 2002; Braak et al., 2003; El-Agnaf et al., 2003; Kordower et al., 2008; Kurowska et al., 2011; Li et al., 2008; Li et al., 2010), animal models (Henderson et al., 2019a; Koller et al., 2017; Luk et al., 2012; Masuda-Suzukake et al., 2013; Paumier et al., 2015; Rey et al., 2017; Steiner et al., 2018) and cell culture (Luk et al., 2009; Nonaka et al., 2010; Tran et al., 2014; Volpicelli-Daley et al., 2011) suggest that misfolded α-synuclein species are released by neurons and can be taken up by nearby neurons, inducing the transcellular transmission of pathogenic α-synuclein (Henderson et al., 2019c). Thus, minimizing the impact of this pool of extracellular, pathogenic α-synuclein provides a unique and more easily accessed therapeutic opportunity for the treatment of PD, especially if pathological α-synuclein is also present in the peripheral areas such as the enteric nervous system, as has been suggested (Henderson et al., 2019c).
There are a number of potential approaches to inhibit the transmission of α-synuclein pathology. For example, extracellular α-synuclein could be targeted for vascular or glymphatic clearance, its uptake could be blocked, or glial cells could be modified to promote clearance of extracellular α-synuclein. At least some of these possible mechanisms might be facilitated through antibody-mediated immunotherapy (Henderson et al., 2019c). For example, antibodies might block neuronal α-synuclein uptake while also promoting glymphatic clearance to the periphery or glial clearance through binding to surface Fc receptors. Passive immunotherapy (treatment directly with antibodies, instead of injection of an immunogen) is a particularly attractive option because therapeutic antibodies have been demonstrated to be relatively safe (Schenk et al., 2017) and immunotherapy has been shown to promote clearance of extracellular targets (Schenk et al., 1999). Although antibodies have poor BBB penetration, there is evidence that sufficient brain levels can be achieved to affect disease biology, and technology is being developed to improve antibody penetrance into the central nervous system (Niewoehner et al., 2014; Vaikath et al., 2019). Indeed, immunotherapy has been shown to be effective at reducing brain pathology and improving symptoms in animal models (Bae et al., 2012; El-Agnaf et al., 2017; Games et al., 2014; Mandler et al., 2014; Masliah et al., 2005; Masliah et al., 2011; Sanchez-Guajardo et al., 2013; Shahaduzzaman et al., 2015; Spencer et al., 2014; Spencer et al., 2017; Spencer et al., 2016; Tran et al., 2014). Several immunotherapies targeting α-synuclein are currently in clinical trials for the treatment of PD (Schenk et al., 2017; Weihofen et al., 2019).
It is presently unclear what the preferred properties are for a therapeutic α-synuclein antibody. For example, targeting all α-synuclein species with a pan-α-synuclein antibody may have deleterious effects in the central nervous system (Collier et al., 2016; Gorbatyuk et al., 2010; Kanaan and Manfredsson, 2012) or in the blood, where α-synuclein is abundant in red blood cells (Scherzer et al., 2008). Moreover, the binding of a pan-α-synuclein antibody to non-pathogenic forms of the protein would reduce the antibody available for binding to misfolded species. For these reasons, we sought to create an antibody with high selectivity for pathogenic, misfolded α-synuclein. After first immunizing mice with misfolded α-synuclein and creating a library of B-cell hybridomas, antibodies produced by clonal hybridoma cultures were passed through a series of screening assays to select for antibodies that show high selectivity for misfolded α-synuclein and are able to inhibit the formation of LB-like structures in a primary neuron model of α-synucleinopathy. A top candidate antibody, Syn9048, was tested for efficacy in a wildtype mouse model of pathological α-synuclein transmission. Chronic 6-month administration of Syn9048 was well-tolerated by mice, and Syn9048 was able to preserve striatal dopamine levels and reduce α-synuclein pathology, especially in areas where α-synuclein inclusions likely resulted from transcellular spread of pathogenic α-synuclein. In all measures, Syn9048 showed improved efficacy over a previously validated α-synuclein antibody (Tran et al., 2014), suggesting that Syn9048 has desirable properties that may merit further advancement towards human studies.
RESULTS
Although α-synuclein passive immunotherapy is presently being pursued as a therapeutic approach for PD, there is some concern that pan-α-synuclein antibodies may have liabilities. For example, there are conflicting data on whether reducing overall α-synuclein levels in the brain would be deleterious to neuronal function (Collier et al., 2016; Dauer et al., 2002; Gorbatyuk et al., 2010; Kanaan and Manfredsson, 2012; Zharikov et al., 2015). The abundance of α-synuclein in red blood cells (Scherzer et al., 2008) also raises the possibility that a pan-α-synuclein antibody could cause on-target side effects in the blood, as antibody concentrations in the blood are approximately 1000-fold higher than in the brain (Banks et al., 2002). In addition, serum α-synuclein could act as a sink for a pan-α-synuclein antibodies, thereby reducing engagement with the intended target in the brain. Therefore, we sought to develop antibodies that are highly selective for pathogenic, misfolded α-synuclein. In order to develop these antibodies, mice were first immunized with misfolded α-synuclein pre-formed fibrils (PFFs) formed from recombinant human α-synuclein (Fig. 1A). Mice developed an immune response to the α-synuclein PFF immunogen, and antibody-producing B cells were harvested and fused to myeloma cells to generate antibody-producing hybridoma cells (Fig. 1B). Monoclonal hybridomas were isolated by limiting dilution, and antibody-containing supernatant from these clones was tested in several assays to determine which hybridoma clones were producing antibodies with preferred properties (Fig. 1C). Candidate antibodies were further subcloned to ensure monoclonality (Fig. 1D), with antibody properties confirmed through a screen to ensure retention of properties (Fig. 1E). Upon inspection of the results from the different screening assays, a preferred candidate antibody was selected for efficacy testing in an in vivo model of PD (Fig. 1F), where it was compared to a previously characterized immunotherapy antibody.
Fig. 1. Passive Immunotherapy Screen Schematic.
(A) Mice were immunized with α-synuclein pre-formed fibrils (PFFs) to induce an immune response and production of antibodies against pathogenic α-synuclein. (B) Antibody producing B cells were subsequently harvested from the spleen of immunized mice and fused with myeloma cells to produce hybridoma clonal cell lines expressing antibodies against α-synuclein. (C) Hybridomas were separated into individual clonal populations and the antibody-containing supernatant from each clone was passed through a primary screen to identify candidates with preferred properties. To confirm that antibodies would recognize pathogenic human α-synuclein, antibodies were used in IHC of human tissue. Preferred candidates should also have a preference for fibrillar α-synuclein, so they were screened in an indirect ELISA format for monomeric and fibrillar α-synuclein. Each antibody was epitope mapped and tested for immunogenicity against mouse and human α-synuclein so it could be utilized in a mouse disease model. Finally, antibodies were screened in a primary neuron immunotherapy assay for their ability to reduce α-synuclein pathology in cultured neurons. (D) Prioritized antibodies underwent a further two rounds of subcloning to ensure monoclonality. (E) Final clones underwent confirmation screening to ensure they maintained optimal properties and to identify a final candidate for the in vivo experiment. (F) A final candidate antibody was tested in a mouse model of PD for its ability to reduce α-synuclein pathology and toxicity. The final candidate was tested alongside and IgG control and an α-synuclein antibody with proven therapeutic efficacy.
Immunohistochemistry Reveals Antibodies That Preferentially Bind LBs
Preferred antibodies should have a high binding preference for misfolded LB α-synuclein over monomeric α-synuclein. In order to rapidly screen for antibody selectivity to pathological α-synuclein, amygdala sections from PD patients with abundant LB pathology were immunostained. Hybridoma supernatants were used undiluted or at a 1:3 dilution on screening slides processed in parallel to allow direct comparison of immunostaining. Most antibodies tested showed a preference for binding to LB α-synuclein over normal synaptic α-synuclein in the neuropil (Fig. 2A). A LB discrimination index (optical density of LB/optical density of all tissue) was developed to better compare the relative binding of antibodies to LB over the neuropil pool (Fig. 2B). A value of 1 indicates no discrimination of LBs over normal synaptic neuropil staining. While most of the antibodies had a preference for LBs, a cutoff of 1.5 was established to remove antibodies that showed relatively non-selective staining of LBs from further consideration.
Fig. 2. Immunohistochemistry Reveals Antibodies That Preferentially Bind LBs.
(A) Sections of amygdala tissue containing abundant Lewy body pathology were stained using undiluted or 1:3 diluted hybridoma supernatant. Representative staining of Lewy bodies is shown for each of the screened antibody supernatants. Scale bar=50 μm. (B) The optical density within Lewy bodies divided by the optical density of a 1 mm2 tissue section containing those Lewy bodies was defined as the Lewy Body Discrimination Index. Antibodies which preferentially recognized Lewy bodies score high on this measure. Most antibodies showed some enhanced recognition of Lewy bodies, with the exception of those antibodies scoring below 1.5, which show little apparent preference for Lewy bodies above neuropil α-synuclein staining.
Epitope Mapping Ensures that Preferred Antibodies Recognize Human and Mouse α-Synuclein
While preferred immunotherapy antibodies could eventually be used in humans, we sought to identify antibodies that could recognize both mouse and human α-synuclein to allow further characterization in mouse primary neuron and wildtype mouse models of PD. Antibodies were assayed by Western blot to determine whether they bound to human and mouse α-synuclein (Fig. 3). Multiple truncated forms of human α-synuclein (Fig. 3A) were also analyzed in parallel to determine the epitope within α-synuclein that was recognized by each antibody. All antibodies recognized some portion of the immunogenic C-terminus of α-synuclein, with a large proportion recognizing far C-terminal amino acids (120–140; Fig. 3B), consistent with previous reports of high antigenicity of C-terminal α-synuclein (Giasson et al., 2000; Vaikath et al., 2015). Another large group of antibodies recognized a slightly more internal epitope (110–120), and only a few antibodies recognized a region closer to the aggregation-prone NAC (non-Aβ component of Alzheimer’s disease amyloid (Ueda et al., 1993)) domain (residue 61–95) of α-synuclein (Fig. 3C). The majority of antibodies recognized mouse as well as human α-synuclein, although there were examples of antibodies in each epitope class that showed preferential binding to human α-synuclein. Only two antibodies showed evidence of cross-reactivity with β-synuclein (Fig. S1).
Fig. 3. Epitope Mapping Ensures that Preferred Antibodies Recognize Human and Mouse α-Synuclein.
(A) Schematic of recombinant α-synuclein fragments used to determine the epitope of the 9000 series antibodies. Full-length (FL) α-synuclein is 140 amino acids with an internal non-amyloid component (NAC) domain. Constructs used for testing epitopes had truncated N- or C-terminal human α-synuclein residues. (B) Most antibodies recognized both human and mouse α-synuclein to some extent. Further, all antibodies recognized α-synuclein with truncated N-termini, suggesting that they all recognize the C-terminus of α-synuclein. The most extreme of these did not recognize α-synuclein if even the last 10 amino acids were not included, suggesting that the epitope is between aa130–140. Many other antibodies recognized 1–130, but not 1–120, suggesting they have an epitope between 120–130. (C) Another large batch of antibodies did not recognize 1–110, suggesting they recognize 110–120. Finally, there were several antibodies that recognized all constructs except 1–89, suggesting the epitope lies between amino acids 90–102. *Peptide 1–110 was loaded in the last lane of these gels, thereby shifting the lanes leftward.
Sandwich ELISA Identifies Antibodies with a Preference for Misfolded α-Synuclein
Antibodies that showed selectivity for LBs in human tissue by immunohistochemistry and recognized both human and mouse α-synuclein underwent a further testing of selectivity by assessing their ability to bind to monomeric human α-synuclein and α-synuclein PFFs in a sandwich ELISA format. This assay retains the conformation of α-synuclein by allowing binding of α-synuclein that is in solution to immobilized antibody, and allows a broad range of affinity detection. Antibodies of interest were coated on an ELISA plate and either α-synuclein monomer or PFF were incubated with the antibodies at increasing α-synuclein concentrations to determine relative affinity of the antibodies for each form of α-synuclein. The previously characterized Syn211 antibody (Giasson et al., 2000) was also coated on each plate as a non-selective antibody control. Bound α-synuclein was detected with a monoclonal antibody (MJF-R1, Abcam, Cat#138501) and a goat-anti-rabbit IgG-HRP conjugate. Capture antibodies could be categorized almost evenly into three categories: 17 non-binding (Fig. 4A, Fig. S2), 18 non-selective (Fig. 4B, Fig. S3), 19 PFF-selective (Fig. 4C, Fig. S4). Since nearly all of the non-binding antibodies bound to LB pathology and detected α-synuclein by immunoblotting, the lack of activity in the sandwich ELISA would suggest that immobilization on ELISA plate wells affected their ability to capture α-synuclein. By fitting the absorbance values with a sigmoid dose curve, we calculated EC50 values for each antibody. We then calculated PFF preference values for each antibody using the following equation, with Syn211 as a non-selective control:
Fig. 4. Sandwich ELISA Identifies Antibodies with a Preference for Misfolded α-Synuclein.
Antibodies were assessed for a preference for misfolded α-synuclein by performing parallel sandwich ELISAs with either human α-synuclein monomer or α-synuclein PFFs as the antigen. Syn211 was also used on each plate as a non-selective control antibody. Using this method, three classes of antibodies could be distinguished—(A) antibodies which did not bind in this assay and therefore did not have a signal for either monomer or PFF; (B) antibodies which are non-selective and have a similar binding to α-synuclein monomer and PFF; and (C) antibodies which are PFF-selective, showing a markedly higher preference for α-synuclein PFF than monomer. The histograms shown are representative of the three classes, but all antibodies tested can be classified in one of these categories. Plots represent the means from 3 technical replicates, and error bars represent standard error.
Values are summarized in Supplementary Table 1. To confirm that the apparent conformation selectivity was not due to shared epitopes between the capture and the detection antibody that would reduce detection of α-synuclein monomers, four of the most selective antibodies were evaluated by sandwich ELISA using an alternate, polyclonal detection antibody, SNL4 (Fig. S5). This assay confirmed the conformation selectivity of the α-synuclein antibodies.
Primary Neuron Immunotherapy Assay Reveals Differential Potency of α-Synuclein Antibodies to Prevent LB-Like Pathology
While the intrinsic properties of antibodies are important, we sought to determine the ability of antibodies to prevent the induction of α-synuclein pathology, a property which is critical for therapeutic success. To accomplish this, a neuron immunotherapy assay was developed to allow co-treatment of antibodies with α-synuclein PFFs in a high-content format. After 10 days in culture, neurons were treated with purified antibodies of interest. Thirty minutes later, neurons were treated with human α-synuclein PFFs, which will seed the recruitment of endogenous mouse α-synuclein into LB- and LN-like inclusions (Henderson et al., 2017; Volpicelli-Daley et al., 2011). Neurons were fixed 7 days later and assayed for pathological pS129 α-synuclein and neuron number (NeuN; Fig. 5A). Antibodies showed a wide range of efficacy in this assay with 22 of the 41 antibodies causing over 75 percent reduction in pathology (Fig. 5B). The highest performing antibody, Syn9048, reduced pathology by a remarkable 97 percent.
Fig. 5. Primary Neuron Immunotherapy Assay Reveals Differential Potency of α-Synuclein Antibodies to Prevent LB-Like Pathology.
(A) Examples of pS129 α-synuclein pathology (green) and NeuN (magenta) immunostaining following treatment of primary neurons with α-synuclein PFFs and the noted representative antibodies. Scale bar=50 μm. (B) Quantification of pS129 α-synuclein area/neuron number normalized to IgG-treated neurons. Antibodies had varying effects on α-synuclein pathology and are ranked from least to most effective. Twenty-one of the antibodies showed statistically-significant reductions in pathology (Kruskal-Wallis test with Dunn’s multiple comparisons test: *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001). N=6–20/condition. Error bars represent standard error.
The thorough characterization of α-synuclein antibodies in multiple assays allowed us to compare antibodies quantitatively and select favored antibodies for subcloning and further screening (Fig. 6). We posited that preferred antibodies should recognize both mouse and human α-synuclein, bind LBs preferentially in human tissue, show greater affinity for misfolded α-synuclein than monomeric α-synuclein, and reduce PFF-seeded α-synuclein pathology in primary neurons. Based on these criteria, two antibodies (Syn9063 and Syn9048) were selected for subcloning, large-scale production and in vivo testing.
Fig. 6. Summary of Antibody Characteristics.
A summary of each of the antibody characteristics from different assays is shown as a heat map with the scale bars shown at the top of each column. Antibodies are mostly from IgG subclasses. Most antibodies recognize mouse α-synuclein, and all antibodies recognize human α-synuclein. The LB discrimination index, derived from immunohistochemistry reflects the ability of antibodies to bind selectively to LB α-synuclein. The α-synuclein PFF preference is derived from sandwich ELISA results and spanned over 100-fold differences to allow for selection of antibodies that have a preference for misfolded α-synuclein (NB = non-binding). Finally, the % inhibition measure, derived from the primary neuron assay, indicates antibody ability to reduce pathology in a cellular system. An ideal antibody would recognize both mouse and human α-synuclein, discriminate LBs from surrounding neuropil, have higher binding affinity for α-synuclein PFFs than monomer, and inhibit nearly all in vitro neuronal α-synuclein pathology. Based on these measures, Syn9048 and Syn9063 were chosen for further subcloning.
Subclones Retain Properties of Parent Clones
One of the key features of the selected antibodies was their preference for misfolded α-synuclein. Therefore, supernatants from clones derived during additional subcloning of Syn9063 and Syn9048 hybridomas were analyzed in the sandwich ELISA platform to identify clones producing the desired antibodies. All but one of the Syn9063 subclones showed a similar preference for α-synuclein PFFs as the parental clone (Fig. 7A). However, upon clonal expansion, Syn9063 subclones had very low antibody yields, precluding their use in vivo, where greater antibody amounts are needed. All Syn9048 clones showed high selectivity for α-synuclein PFFs (Fig. 7B) and clone #3 was used for further studies due to high yields of antibody obtained from roller cultures.
Fig. 7. Subclones Retain Properties of Parent Clones.
(A) Syn9063 subclones were assayed for PFF preference as previously described to ensure that all subclones showed the same selectivity as the parent clone. All but one of the subclones showed a high preference for α-synuclein PFFs. (B) All Syn9048 subclones showed similar selectivity for α-synuclein PFFs. Plots in panels (A) and (B) represent the means from 3 technical replicates, and error bars represent standard error. (C) Examples of pS129 α-synuclein pathology (green) and NeuN (magenta) immunostaining following treatment of primary neurons with α-synuclein PFFs and the noted molar ratios of purified Syn9048 clone #3 to α-synuclein monomer. Scale bar=50 μm. (D) Quantification of pS129 α-synuclein area/neuron number normalized to control IgG-treated neurons. Increasing molar ratios of Syn9048 reduced pS129 α-synuclein pathology in a dose-dependent manner and is fit by a sigmoidal dose curve with an IC50 of 0.006 (R2=0.9539). N=3/condition. Error bars represent standard error and the shaded area represents the 95% confidence interval of the sigmoidal fit.
To gain a further understanding of the concentration of antibody necessary to effectively inhibit α-synuclein pathology induction, purified Syn9048 was diluted over several log concentrations and assessed in the human α-synuclein PFF-seeded primary hippocampal neuron assay as described above. Syn9048 reduced neuronal α-synuclein pathology in a dose-dependent manner, with a molar antibody:α-synuclein ratio IC50 of 0.006 (1:166) (Fig. 7C, 7D). Syn9048 showed almost complete inhibition of α-synuclein pathology at a molar ratio of 0.03 or higher, suggesting that only one antibody per 33 α-synuclein monomer units is sufficient to fully inhibit α-synuclein seeding in neurons. Syn9048 was also capable of reducing α-synuclein pathology induced by mouse α-synuclein PFFs with a similar IC50 (Fig. S6), suggesting that it would be suitable for testing in a non-transgenic mouse model of PD.
In Vivo Immunotherapy Is Well-Tolerated and Improves Dopaminergic Tone
Several α-synuclein antibodies have been tested in animal models for their abilities to prevent PD-like pathology (Bae et al., 2012; El-Agnaf et al., 2017; Games et al., 2014; Masliah et al., 2011; Spencer et al., 2014). However, in the absence of prodromal disease biomarkers, patients with neurodegenerative disorders are unlikely to be given immunotherapy treatments prior to the symptomatic stages of disease when brain pathology is already established. Therefore, we sought to test the efficacy of Syn9048 for its ability to reduce pathology and rescue neuronal function after the initiation of pathology. A previously established model of α-synuclein pathology induction and transmission (Luk et al., 2012) was employed in which non-transgenic mice were injected with 5 μg α-synuclein PFFs in the dorsal striatum at 2–3 months of age. Mice were allowed 1 week for the BBB to recover and for pathogenic α-synuclein to be taken up by neurons and induce pathology prior to treatment with antibody.
The mice then received Syn9048 or isotype control antibody treatment (30 mg/kg intraperitoneally) weekly thereafter for 6 months (Fig. 8A, 16 mice/group). An additional group of mice was treated with a comparator antibody, Syn303, which has previously been validated for in vivo immunotherapy models (Tran et al., 2014). The one-week delay in antibody dosing after intrastriatal PFF injection was intended to minimize inhibition of initial PFF-seeding in neurons. Rather, we predicted that sites of secondary α-synuclein pathology formation that resulted from pathology transmission would be most affected by antibody treatment. Mice in all three groups gained weight steadily over the course of the study, suggesting that the passive immunotherapy was well-tolerated (Fig. 8B). Prior to sacrifice, mice were tested for motor performance on both an accelerating rotarod (Fig. S7A) and a grip strength meter (Fig. S7B). α-Synuclein PFF injection has been shown to induce deficits in both these assays previously (Henderson et al., 2019a; Luk et al., 2012). However, neither the Syn303 or Syn9048 groups showed significant improvements in behavioral measures in the current study.
Fig. 8. In Vivo Immunotherapy Is Well-Tolerated and Improves Dopaminergic Tone.
(A) Wildtype mice were injected with α-synuclein PFFs in the dorsal striatum at 2–3 month of age. One week after α-synuclein PFF injection, mice were injected with 30 mg/kg control IgG1, Syn303 or Syn9048. Mice were injected once weekly thereafter for 6 months, after which mice were sacrificed and assayed for pathology and motor behavior. (B) Average weight for mice in each antibody treatment group with standard error represented in shaded bands. Weights increased over time, but were not different by group (Two-way ANOVA: Time p=0.0001; Treatment p=0.7209). N=16/group. Error bars represent standard error. (C) Representative images of the SN stained with TH from mice treated with control IgG1, Syn303 or Syn9048. Scale bars = 500 μm. (D) Estimated TH+ neurons in the SN following manual quantification of neurons through the SN (C=contralateral; I=ipsilateral). Substantial neuron loss was observed ipsilateral to the injection site for all groups (Two-way ANOVA with Sidak’s multiple comparisons test, contra vs. ipsi: IgG1 p<0.0001; Syn303 p<0.0001; Syn9048 p<0.0001), but was not different between groups (Two-way ANOVA with Sidak’s multiple comparisons test Ipsi: IgG1 vs. Syn303 p=0.6266, IgG1 vs. Syn9048 p=0.3561). N=10–16/group. (E) The ratio of neurons remaining on the ipsilateral SN to the contralateral SN similarly showed no difference between groups (One-way ANOVA with Dunnett’s multiple comparison test: IgG1 vs. Syn303 p=0.6323, IgG1 vs. Syn9048 p=0.9084). (F) Dopamine levels measured from the dorsal striatum of injected mice were reduced ipsilateral to the injection site in IgG1-treated animals, but were preserved in Syn9048-treated mice (One-way ANOVA with Dunnett’s multiple comparison test: IgG1 vs. Naïve p=0.0283; IgG1 vs. Syn303 p=0.9791; IgG1 vs. Syn9048 p=0.0047). N=7–12/group. (G) DOPAC levels were similarly preserved by Syn9048 treatment (One-way ANOVA with Dunnett’s multiple comparison test: IgG1 vs. Naïve p=0.0469; IgG1 vs. Syn303 p=0.9969; IgG1 vs. Syn9048 p=0.0049). N=7–12/group. Error bars represent standard error.
Dopaminergic neuron loss in the substantia nigra (SN) is a primary feature of PD and is recapitulated in the PFF injection mouse model (Henderson et al., 2019a; Henderson et al., 2019b; Luk et al., 2012) (Fig. 8C). IgG1-treated mice have dramatic tyrosine hydroxylase (TH)-positive neuron loss ipsilateral to the injection site, and this neuron loss was not abrogated by either Syn303 or Syn9048 treatment (Fig. 8D, 8E). The dopaminergic neuron loss is consistent with these cells having direct connections to the injection site, such that antibody treatment one week after PFF injections would not be expected to block the initiation of pathology in these neurons. Dopaminergic tone is also reduced in the dorsal striatum in response to PFF injection (Fig. 8F). Somewhat surprisingly Syn9048, but not Syn303, was able to rescue the loss of striatal dopamine and DOPAC (Fig. 8F, 8G). Thus, while neurons are still lost, the function of remaining neurons may be improved by Syn9048 treatment, possibly due to reductions in α-synuclein pathology.
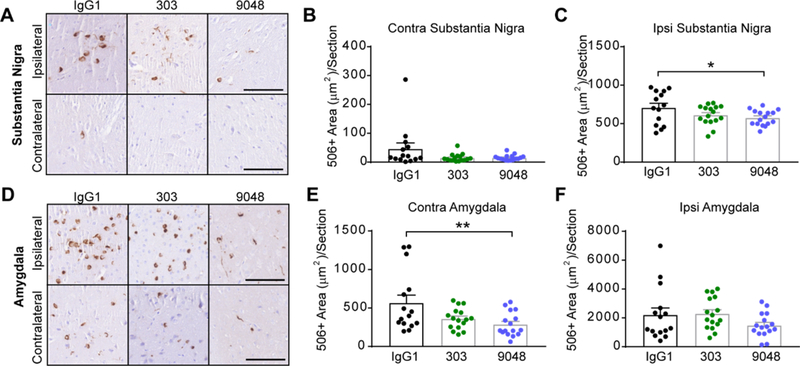
Syn9048 Reduces α-Synuclein Pathology in the SN and Amygdala
To better understand how α-synuclein pathology is changed in mice following passive immunotherapy, we performed quantitative pathology for misfolded α-synuclein (Syn506) in the SN (Fig. 9A, 9B, 9C) and amygdala (Fig. 9D, 9E, 9F). Syn303 and Syn9048 reduced mean pathology in the SN contralateral (Fig. 9B) and ipsilateral (Fig. 9C) to the injection site, although only the reduction by Syn9048 in the ipsilateral SN was statistically significant. A reduction in pathology was also more apparent in the contralateral amygdala (Fig. 9E) than in the ipsilateral amygdala (Fig. 9F), and was more substantial in Syn9048-treated animals than those treated with Syn303. Only the reduction by Syn9048 in the contralateral amygdala was statistically significant. The reduction in ipsilateral SN pathology by Syn9048, though modest may explain the improvement in striatal dopamine and DOPAC levels. Moreover, the greater effect of antibody treatment on contralateral α-synuclein pathology suggests that efficacy is greater at sites where extracellular α-synuclein spread is responsible for pathology induction.
Fig. 9. Syn9048 Reduces α-Synuclein Pathology in the SN and Amygdala.
(A) Mice injected with α-synuclein PFFs accumulate substantial α-synuclein pathology in the ipsilateral SN, as recognized by the conformation-selective antibody Syn506. Rare pathology is also observed in the contralateral SN at this time point. Scale bars=100 μm. (B) Syn506+ area/section was quantified throughout the contralateral SN, and was substantially, albeit not significantly, reduced (One-way ANOVA with Dunnett’s multiple comparison test: IgG1 vs. Syn303 p=0.0827; IgG1 vs. Syn9048 p=0.0847). N=14–16/group. (C) α-Synuclein pathology was also reduced with immunotherapy treatment in the ipsilateral SN (One-way ANOVA with Dunnett’s multiple comparison test: IgG1 vs. Syn303 p=0.0937; IgG1 vs. Syn9048 p=0.0444). N=14–16/group. (D) Mice injected with α-synuclein PFFs accumulate substantial α-synuclein pathology in the ipsilateral and contralateral amygdala, as recognized by the conformation-selective antibody Syn506. Scale bars=100 μm. (E) Syn506+ area/section was quantified throughout the contralateral amygdala, and was substantially reduced (One-way ANOVA with Dunnett’s multiple comparison test: IgG1 vs. Syn303 p=0.0521; IgG1 vs. Syn9048 p=0.0069). N=14–16/group. (F) α-Synuclein pathology was not reduced to the same extent in the ipsilateral amygdala (One-way ANOVA with Dunnett’s multiple comparison test: IgG1 vs. Syn303 p=0.8694; IgG1 vs. Syn9048 p=0.2464). N=14–16/group. All points represent individual mice and error bars represent standard error.
DISCUSSION
PD is an intractable disease with several symptomatic therapies available but no disease-modifying treatments. Even the most efficacious dopamine replacement therapy does not prevent disease progression, including the development of dementia which occurs in up to 80 percent of cases (Aarsland et al., 2003). The progression of symptoms from manageable to debilitating corresponds with the burden of α-synuclein pathology in higher cortical regions of the brain (Irwin et al., 2017). Therefore, if the spread of pathology through the brain could be slowed or prevented, the later disease stages may be curtailed, prolonging the period during which PD patients can successfully manage symptoms. The compelling data which suggest the presence of extracellular pathogenic α-synuclein species that can be transmitted from one brain region to another provide a novel opportunity for therapeutic intervention.
Previous research has identified several antibodies that are effective in animal models of PD at reducing α-synuclein aggregates (Bae et al., 2012; El-Agnaf et al., 2017; Games et al., 2014; Masliah et al., 2011; Shahaduzzaman et al., 2015; Spencer et al., 2014; Spencer et al., 2017; Spencer et al., 2016; Tran et al., 2014; Weihofen et al., 2019). These antibodies had differing selectivity profiles (α-synuclein oligomers, C-terminal truncated α-synuclein or fibrillar α-synuclein) and it is currently unclear whether antibodies directed against certain epitopes will be more efficacious than others. As it is generally acknowledged that only ~0.1% of administered antibody passes the BBB (Banks et al., 2002), there are likely to be benefits to utilizing α-synuclein antibodies that preferentially bind to pathogenic species and not the α-synuclein monomer that is present in the brain. This would increase the effective concentration of antibody that could target the α-synuclein that promotes disease progression, without loss of antibody in non-productive binding to monomers.
In the current study, we have characterized a group of antibodies that selectively bind misfolded forms of α-synuclein using a multi-technique screening workflow. Antibodies were generated using pathogenic α-synuclein PFFs as the immunogen. A similar strategy for generation of pathogenic α-synuclein-selective antibodies has been previously reported (Vaikath et al., 2015). Intriguingly, the antibodies generated in the current study and those reported by Vaikath et al. (Vaikath et al., 2015) show multiple similarities. As expected, both groups of antibodies have a high selectivity for misfolded α-synuclein. Despite the fact that recombinant α-synuclein PFFs were used as an antigen in both cases, both groups of antibodies recognize LBs in human tissue, suggesting that epitopes in recombinant α-synuclein fibrils are present and conserved in human disease. Finally, both groups of conformation-selective antibodies bind the C-terminus of α-synuclein. This is somewhat surprising, given that C-terminal residues do not appear to adopt a conserved conformation within fibrillar α-synuclein (Guerrero-Ferreira et al., 2018; Li et al., 2018a; Li et al., 2018b; Tuttle et al., 2016). However, it is possible that the accessibility of the C-terminus of fibrillar α-synuclein accounts for its high immunogenicity.
The antibodies generated by Vaikath et al. were later assessed for immunotherapy potential in transgenic mice overexpressing human α-synuclein driven by the mThy1 promoter (El-Agnaf et al., 2017). These antibodies were able to reduce α-synuclein levels to a varying degree in different regions of the brain, but spread of pathology could not be assessed in those experiments due to the broad expression of the transgene (El-Agnaf et al., 2017).
Antibodies in the current study were selected based on the criterion that they recognize both human and mouse α-synuclein, allowing them to be screened in a primary neuron model of α-synuclein aggregation and a non-transgenic mouse model of PD to assess effects on regions that incur α-synuclein inclusions early or late in pathology spread. While many of these antibodies may be useful tools for research and disease diagnosis, the primary goal of this study was to generate antibodies that would show therapeutic efficacy. We therefore assessed the top antibody candidate in a previously-described wildtype mouse model of PFF-seeded α-synuclein pathology, in which antibody dosing did not begin until neuronal α-synuclein pathology was already initiated. Syn9048 showed the ability to reduce α-synuclein pathology in multiple regions in the mouse brain, particularly in areas (contralateral SN and amygdala) where inclusions may form as the result of cell-to-cell spread. Despite not rescuing the dopaminergic neuron loss in this model, Syn9048 increased the dopamine levels in the striatum, suggesting that the reduction of pathology observed in the SN may improve the function of remaining neurons. These findings suggest that antibodies raised against pathogenic species of α-synuclein hold potential as immunotherapies, and the lead antibody, Syn9048, showed evidence of superiority relative to an earlier candidate, Syn303.
As PD patients do not usually come to clinic prior to the presence of substantial pathology and neuron loss, our study suggests that immunotherapy will not likely reverse existing pathology, but may halt the spread of pathology through the brain, preventing further motor and cognitive decline. Future studies assessing brain-wide spread patterns could help predict the maximal possible benefit of immunotherapy and could be used to determine when during disease progression immunotherapy would need to be administered to be most efficacious.
MATERIALS AND METHODS
Animals
All housing, breeding, and procedures were performed according to the NIH Guide for the Care and Use of Experimental Animals and approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Mice used for antibody generation were Balb/c; mice used for primary neuron culture were CD-1 (Charles River, Cat# CRL:22, RRID:IMSR_CRL:22), and mice used for in vivo studies were B6C3F1 (Charles River, Cat# CRL:31, RRID:IMSR_CRL:31).
Primary Hippocampal Neuron Cultures
Primary hippocampal neuron cultures were prepared as previously described (Henderson et al., 2017; Henderson et al., 2018) from embryonic day (E) 16–18 CD1 embryos. Dissociated hippocampal neurons were plated at 17,500 cells/well (96-well plate) in neuron media (Neurobasal medium (ThermoFisher 21103049) supplemented with B27 (ThermoFisher 17504044), 2 mM GlutaMax (ThermoFisher 35050061), and 100 U/mL penicillin/streptomycin (ThermoFisher 15140122).
α-synuclein PFFs
Purification of recombinant α-synuclein and generation of α-synuclein PFFs was conducted as described elsewhere (Luk et al., 2009; Volpicelli-Daley et al., 2014; Volpicelli-Daley et al., 2011). The pRK172 plasmid containing the gene of interest was transformed into BL21 (DE3) RIL-competent E. coli (Agilent Technologies Cat#230245). A single colony from this transformation was expanded in Terrific Broth (12 g/L of Bacto-tryptone, 24 g/L of yeast extract 4% (vol/vol) glycerol, 17 mM KH2PO4 and 72 mM K2HPO4) with ampicillin. Bacterial pellets from the growth were sonicated and sample was boiled to precipitate undesired proteins. The supernatant was dialyzed with 10 mM Tris, pH 7.6, 50 mM NaCl, 1 mM EDTA overnight. Protein was filtered with a 0.22 μm filter and concentrated using Amicon Ultra-15 centrifugal filter units (Millipore Sigma Cat# UFC901008). Protein was then loaded onto a Superdex 200 column and 1 mL fractions were collected. Fractions were run on SDS-PAGE and stained with Coomassie blue to select fractions that were highly enriched in α-synuclein. These fractions were combined and dialyzed in 10 mM Tris, pH 7.6, 50 mM NaCl, 1 mM EDTA overnight. Dialyzed fractions were applied to the MonoQ column (GE Health, HiTrap Q HP 645932) and run using a linear gradient from 25 mM NaCl to 1 M NaCl. Collected fractions were run on SDS-PAGE and stained with Coomassie blue. Fractions that were highly enriched in α-synuclein were collected and dialyzed into DPBS. Protein was filtered through a 0.22 μm filter and concentrated to 5 mg/mL with Amicon Ultra-15 centrifugal filter units (Millipore Sigma Cat# UFC901008). Monomer was aliquoted and frozen at −80°C. For preparation of α-synuclein PFFs, α-synuclein monomer was shaken at 1,000 rpm for 7 days. Conversion to PFFs was validated by sedimentation at 100,000 × g for 60 minutes and by Thioflavin T fluorescence.
α-synuclein PFF Treatments
Primary Neurons
For treatment of neurons, mouse α-synuclein PFFs, which were generated at a concentration of 5 mg/mL were vortexed and diluted with Dulbecco’s phosphate-buffered saline (DPBS, Corning Cat#21–031-CV) to 100 μg/mL. They were then sonicated on high for 10 cycles of 30 seconds on, 30 seconds off (Diagenode Biorupter UCD-300 bath sonicator). α-Synuclein PFFs were then diluted in neuron media to 5 μg/mL and added to neuron cultures at the noted concentrations.
Mice
All surgery experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania. Mouse α-synuclein PFFs, which were generated at a concentration of 5 mg/mL were vortexed and diluted with DPBS to 2 mg/mL. They were then sonicated on high for 10 cycles of 30 seconds on, 30 seconds off (Diagenode Biorupter UCD-300 bath sonicator). Mice were injected when 3 months old. Mice were injected unilaterally by insertion of a single needle into the right forebrain (coordinates: +0.2 mm relative to Bregma, +2.0 mm from midline) targeting the dorsal striatum (2.6 mm beneath the dura) with 5 μg α-synuclein PFFs (2.5 μL). Injections were performed using a 10 μL syringe (Hamilton, NV) at a rate of 0.4 μL/minute. After 6 months, mice were perfused transcardially with PBS, brains were removed and underwent overnight fixation in 70% ethanol in 150 mM NaCl, pH 7.4.
Antibody Production
Animal care and all procedures performed were conducted in accordance with the NIH Guide for the Care and Use of Experimental Animals and approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Murine monoclonal antibodies were raised as described previously (Gibbons et al., 2018) against sonicated, human α‐synuclein PFFs emulsified with complete Freund’s adjuvant (0.05 mg α‐synuclein /mouse) followed by 2 subsequent boosts of 0.025 mg α‐synuclein emulsified with incomplete Freund’s adjuvant 3 and 6 weeks following the initial injections. Nine weeks after initial antigen injection, mice received an intravenous boost of 0.025 mg/mouse. Four days after intravenous injection, spleens were dissociated into single cell suspensions and fused with SP2 cells by 1-minute treatment with a mixture of 50% polyethylene glycol and 5% DMSO (UPenn Cell Center Services Facility Cat# 1165). Hybridoma cells were selected for 7 days in medium containing 5.7 μM azaserine-10; 100 μM hypoxanthine (Sigma Cat# A9666) and cultured in Kennett’s HY (90% DMEM, 10% NCTC135, 4.15 g/L glucose, 3.55 g/L NaHCO3,), supplemented with 20% fetal bovine serum (FBS; Atlanta Biological Cat# E0118), 100 U/mL penicillin/100 μg/mL streptomycin (Gibco Cat# 15140–122); 2 mM L-glutamine (Corning Cat# 25–005-Cl) and OPI media supplement (1 mM oxoloacetate, 0.45 mM pyruvate, 0.2 U/mL insulin; Sigma Cat# 05003). Monoclonal populations were isolated by limiting dilution to 0.3 cells/well in 96-well plates. Antibodies were further characterized as described below. Hybridomas with selectivity for pathological misfolded α‐syn were expanded and subcloned at least twice.
Epitope Mapping
Recombinant α-synuclein constructs were produced in E. coli as previously established (Volpicelli-Daley et al., 2014). Total protein concentration in each sample was determined by a bicinchoninic acid colorimetric assay (Fisher Cat#23223 and 23224), using bovine serum albumin as a standard (Thermo Fisher Cat#23210). Protein was resolved on 5–20% gradient polyacrylamide gels using equal protein loading (250 ng α-synuclein/well). Proteins were transferred to 0.2 μm nitrocellulose membranes and detected with primary antibodies (1:1000). Primary antibodies were detected using IRDye 800 (LI-COR 925–32210) or IRDye 680 (LI-COR 925–68071) secondary antibodies, scanned on a LI-COR Odyssey Imaging System and analyzed using Image Studio software.
Primary Neuron Immunotherapy Assay
Neurons were treated using a modified version of the procedure previously described (Tran et al., 2014). Neurons were fed every three days after plating until 10 DIV. At that point, 125 μL of media was removed from each well, and sterile α-synuclein antibodies were added in 20 μL of fresh neuron media at incubated at 37°C for 30 minutes. 125 μg of freshly sonicated PFFs were added in an additional 20 μL of neuron media. Neurons were fed at 1 and 4 DPT and fixed and stained at 7-days post transduction as described previously (Tran et al., 2014). Data is reported as the normalized pS129 α-synuclein area divided by NeuN count. For initial clone selection, antibodies were included at a 1:1 molar ratio to α-synuclein PFFs. For more detailed characterization of high priority clones, antibodies were included at molar ratios ranging from 2:1 to 0.0003:1 (antibody:PFFs).
Immunocytochemistry
Primary neuron or cell line cultures were fixed with 4% paraformaldehyde, 4% sucrose in phosphate-buffered saline and washed five times in PBS. Immunostaining of neuronal cultures was carried out as described previously (Henderson et al., 2017; Henderson et al., 2018). Cells were permeabilized in 3% BSA + 0.3% TX-100 in PBS for 15 minutes at room temperature. After a PBS wash, cells were blocked for 50 minutes with 3% BSA in PBS prior to incubation with primary antibodies for 2 hours at room temperature. Primary antibodies used were targeting pS129 α-synuclein (81A, CNDR, 1:5,000) and NeuN (Millipore Cat#MAB377, 1:1,500). Cells were washed 5x with PBS and incubated with secondary antibodies for 1 hour at room temperature. After 5x wash with PBS, cells were incubated in DAPI (ThermoFisher Cat#D21490, 1:10,000) in PBS. 96-well plates were imaged on In Cell Analyzer 2200 (GE Healthcare) and analyzed in the accompanying software. A standard intensity-based threshold was applied to the pS129 α-synuclein channel equally across plates and positive area was quantified. For NeuN quantification, an object-based analysis was applied to identify objects of specified size and intensity. All quantification was optimized and applied equally across all conditions.
Sandwich ELISA
A 384-well Maxisorp clear plate (Thermo Fisher Scientific, Cat# 12565347) was coated with 30 μL per well (50 ng) of antibody in Takeda coating buffer, then plate was spun at 1000 × g for 1 minute and incubated overnight at 4°C. The plate was washed 4 times with PBST (PBS with 0.05% Tween) and blocked using Block Ace blocking solution (95 μL per well) (AbD Serotec) overnight at 4°C. Serial double dilutions of human wild-type α-synuclein fibrils and monomer were made in Buffer C (0.02 M sodium phosphate buffer, 2 mM EDTA, 0.4 M NaCl, 1% BSA, 0.005% thimerisol) starting at 256 μg/mL of monomer and 25.6 μg/mL of fibrils. Fibrils were then sonicated on high for 10 cycles of 30 seconds on, 30 seconds off (Diagenode Biorupter UCD-300 bath sonicator) prior to dilutions. Dilutions were added to each well (30 μL per well) and incubated overnight at 4°C. The plate was washed 4 times with PBST. 30 μL of MJF-R1 (1:3,000, Abcam Cat# ab138501) in Buffer C was added to each well and the plate was incubated for 4 hours at 37°C. After washing with PBST 4 times, 1:10k diluted goat-anti-rabbit IgG-HRP conjugate (Cell Signaling Technology) was added to the plate and the plate was incubated for 1 hour at 37°C. Following washing with PBST 4 times the plate was developed for 10–15 minutes using 30 μL per well of room temperature 1-Step Ultra TMB-ELISA Substrate Solution (Thermo Fisher Scientific, Cat# 34029) and the reaction was quenched using 10% phosphoric acid (30 μL per well). Plates were read on 384–450 nm on the SpectraMax M5 plate reader (Molecular Devices).
Antibody Purification (Small Scale Preparation)
Small amounts of antibodies for in vitro experiments were purified using the DynaBead magnetic system (Invitrogen, Cat#10003D) using 1 mL of Protein A and 1 mL of Protein G beads per 50 mL of supernatant. The beads were first washed 3 times with PBS, with 10 minutes of wash time on a rotator. The supernatant was added and incubated with the beads for 4 hours at room temperature or overnight at 4oC. The supernatant was removed and the beads were washed 3 times with PBS. Elution buffer (100 mM glycine, pH 2.8) was added to beads for 30 seconds and immediately neutralized with 1.5 M Tris-base. Buffer solution was removed and put into 50 kD filter Eppendorf tubes and a buffer exchange was completed 3 times spinning according to the manufacturer’s protocol. Antibodies were stored at 1 mg/mL.
Antibody Purification (Large Scale Preparation)
For large preparations of hybridoma supernatant, antibodies were purified using a HiTrap MabSelect SuRe column (GE Healthcare Life Sciences, Cat# 11003494) on an AKTA Pure FPLC system (GE Healthcare Life Sciences). Supernatant was sterile filtered with a 0.2 μm filter and loaded on the column. After washing, antibodies were eluted with 100 mM glycine, 150 mM NaCl, pH 3.0. Eluate was immediately neutralized with 1 M Tris-base, pH 9.0. The UV trace was used to select and pool fractions containing antibody. Antibody was concentrated using Amicon Ultra-15 50 K centrifugal filter units (Millipore Sigma, Cat# UFC905024) and dialyzed into phosphate-buffered saline, pH 7.2. Antibodies were then sterile filtered with a 0.2 μm filter, protein concentration in each sample was determined by a bicinchoninic acid colorimetric assay (Fisher Cat# 23223 and 23224), using bovine serum albumin as a standard (Thermo Fisher Cat# 23210). Samples were run on a 15% SDS-PAGE gel and coomassie stained to ensure presence of heavy and light changes and protein purity. After purification from hybridoma supernatant, antibodies were frozen in 1 mL aliquots and stored at −20°C.
In Vivo Antibody Administration
Immediately prior to use, antibodies were thawed and kept on ice until administration. Mice were weighed and antibodies were administered intraperitoneally to a final concentration of 30 mg/kg body weight. Injection side was alternated between injections, and mice were monitored for adverse events related to injection.
Behavior
Mouse all-limb grip strength was measured using the animal grip strength test (IITC 2200). For this test a grid is attached to a digital force transducer. Mice are moved to a quiet behavioral testing suite and allowed to acclimate for 1 hour. Each mouse is held by the base of the tail and allowed to grasp the grid with all limbs. Once the mouse clasps the grid, the mouse is slowly moved backwards, in line with the force transducer until the mouse releases the grid. The maximum grip force is recorded. The mouse is allowed to rest for several seconds, and then placed on the grid again. The maximum grip strength of 5 tests was recorded. No fatigue was observed during the test period, so the average of all 5 measures is reported.
An accelerating rotarod (MED-Associates) was used to assess motor coordination. Mice received two training sessions and two tests sessions. During the training sessions, mice were placed on a still rod. The rod then began to accelerate from 4 rotations per minute (rpm) to 40 rpm over 5 minutes. Mice were allowed to rest at least one hour between training and testing sessions. During the testing sessions, mice were treated as before, and the latency to fall was recorded. The trial was also concluded if a mouse gripped the rod and rotated with it instead of walking. Mice were allowed a maximum of 10 minutes on the rod.
Striatal Dopamine/DOPAC Detection
Following transcardial perfusion, a 1 mm coronal section was removed from the rostral brain between approximately Bregma and Bregma + 1 mm. The dorsal striatum was manually dissected from both the right (ipsilateral) and left (contralateral) side of the brain and flash frozen on dry ice for liquid chromatography-mass spectrometry (LC-MS) analysis of dopamine (DA) and dihydroxyphenylacetic acid (DOPAC). Frozen tissue was suspended in 10 μL Milli-Q water/mg tissue and sonicated at power level 1.5 using 15–20 short pulses (QSonica Microson™ XL-2000) until solution was homogenous. Lysate was briefly spun down and 30 μL was transferred to a new tube containing 30 μL 0.4 M perchloric acid. Remaining lysate was suspended in 2x RIPA buffer with protease inhibitors for assay of protein levels. Perchlorate sample were spun at 3000 × g and 4°C for 15 minutes. Two volumes of 0.4 M sodium acetate was added to supernatant and spin filtered through a 0.65 μm filter. DA and DOPAC were subsequently quantitated using a Waters Acquity UPLC-TQD LC-MS system. Ten microliters of each sample were injected onto a Waters Acquity HSS T3, C18, 1.8 μm, 2.1 × 100 mm column at 35°C and 0.4 mL/min. Mobile phase B was held at 0% B for 1 minute post injection and gradient separated from 0 to 25% B between 2 and 3 minutes followed by wash and equilibration steps (A: 0.1% (v/v) formic acid in water; B: acetonitrile with 0.1% (v/v) formic acid). Compounds were detected using multiple reaction monitoring of their specific collision-induced ion transitions (Dopamine, ES+ 154>137; 3,4-Dihydrophenylacetic acid, ES- 167>123). Peak areas were quantitated using Waters QuaLynx software against standard curves analyzed concurrently (Dopamine Hydrochloride (DA), Sigma Cat# H8502–5G; 3,4-Dihydrophenylacetic acid (DOPAC), Sigma Cat# 850217–1G) and normalized to total protein level.
Human Tissue
Human brain tissue was identified among deceased individuals with abundant LB pathology Center for Neurodegenerative Disease Research (CNDR) at the University of Pennsylvania.
Immunohistochemistry
After perfusion and fixation, brains were embedded in paraffin blocks, cut into 6 μm sections and mounted on glass slides. Slides were then stained using standard immunohistochemistry as described below. Slides were de-paraffinized with 2 sequential 5-minute washes in xylenes, followed by 1-minute washes in a descending series of ethanols: 100%, 100%, 95%, 80%, 70%. Slides were then incubated in deionized water for one minute prior to antigen retrieval as noted. After antigen retrieval, slides were incubated in 5% hydrogen peroxide in methanol to quench endogenous peroxidase activity. Slides were washed for 10 minutes in running tap water, 5 minutes in 0.1 M Tris, then blocked in 0.1 M Tris/2% fetal bovine serum (FBS). Slides were incubated in primary antibodies overnight. The following primary antibodies were used. For misfolded α-synuclein, Syn50648 was used at 0.4 ug/mL final concentration with microwave antigen retrieval (95°C for 15 minutes with citric acid based antigen unmasking solution (Vector H-3300). To stain midbrain dopaminergic neurons, Tyrosine hydroxylase (TH-16; Sigma-Aldrich T2928, RRID:AB_477569) was used at 1:5,000 with formic acid antigen retrieval.
Primary antibody was rinsed off with 0.1 M Tris for 5 minutes, then incubated with goat anti-rabbit (Vector BA1000, RRID:AB_2313606) or horse anti-mouse (Vector BA2000, RRID:AB_2313581) biotinylated IgG in 0.1 M Tris/2% FBS 1:1000 for 1 hour. Biotinylated antibody was rinsed off with 0.1 M Tris for 5 minutes, then incubated with avidin-biotin solution (Vector PK-6100, RRID:AB_2336819) for 1 hour. Slides were then rinsed for 5 minutes with 0.1 M Tris, then developed with ImmPACT DAB peroxidase substrate (Vector SK-4105, RRID:AB_2336520) and counterstained briefly with Harris Hematoxylin (Fisher 67–650-01). Slides were washed in running tap water for 5 minutes, dehydrated in ascending ethanol for 1 minute each: 70%, 80%, 95%, 100%, 100%, then washed twice in xylenes for 5 minutes and coversliped in Cytoseal Mounting Media (Fisher 23–244-256). Digitized slides were then used for quantitative pathology.
Quantitative Histology
To assay for antibody binding to Lewy body α-synuclein, undiluted hybridoma supernatant or supernatant diluted 1:3 in PBS was directly added to sections of human amygdala tissue with abundant Lewy bodies. Tissue was processed and developed in DAB reagent in parallel. A similar 1 mm2 section from each piece of tissue was then used to assay the ability of the antibody to preferentially bind Lewy bodies. Staining intensity was manually thresholded to only highlight Lewy bodies blind to antibody treatment and analyzed for mean optical density. Mean optical density for the whole section was then assayed using a standardized cutoff. The mean optical density of Lewy body staining divided by the mean optical density of the piece of tissue was calculated and is reported at the Lewy body discrimination index.
For mice, all section selection, annotation and quantification was done blinded to treatment group. All quantitation was performed in HALO quantitative pathology software (Indica Labs). Every 10th slide through the midbrain was stained with tyrosine hydroxylase (TH). TH-stained sections were used to annotate the SN, and cell counting was performed manually in a blinded manner for all sections. The sum of all sections was multiplied by 10 to estimate the total count that would be obtained by counting every section. The SN annotations drawn onto the TH-stained sections were then transferred to sequential sections that had been stained for misfolded α-synuclein (Syn506). Amygdala regions were also annotated on every 10th section through the length of the amygdala. A single analysis algorithm was then applied equally to all stained sections to quantify the percentage of area occupied by Syn506 staining. Specifically, the analysis included all DAB signal that was above a 0.157 optical density threshold, which was empirically determined to not include any background signal. This signal was then normalized to the total tissue area. A minimal tissue optical density of 0.02 was used to exclude any areas where tissue was split.
Statistical Analysis
All statistical analyses were done in GraphPad Prism 7. The analysis used for each data set is described in the figure legends. The number of samples (n) is noted in each figure legend. For primary neuron experiments, the n represents the number of independent wells assayed. For in vivo experiments, n represents the number of mice.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank members of our laboratories for critical discussions related to this paper. This study was supported by National Institute of Health grants: T32-AG000255, P30-AG10124, P50-NS053488, R01-NS088322. We also thank the patients and families without whom this study would not be possible.
CONFLICT OF INTEREST
This research was partially funded by GlaxoSmithKline.
REFERENCES
- Aarsland D, Andersen K, Larsen JP, Lolk A, and Kragh-Sorensen P (2003). Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Archives of neurology 60, 387–392. [DOI] [PubMed] [Google Scholar]
- Appel-Cresswell S, Vilarino-Guell C, Encarnacion M, Sherman H, Yu I, Shah B, Weir D, Thompson C, Szu-Tu C, Trinh J, et al. (2013). Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society 28, 811–813. [DOI] [PubMed] [Google Scholar]
- Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, and Iwatsubo T (1998). Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. The American journal of pathology 152, 879–884. [PMC free article] [PubMed] [Google Scholar]
- Bae EJ, Lee HJ, Rockenstein E, Ho DH, Park EB, Yang NY, Desplats P, Masliah E, and Lee SJ (2012). Antibody-aided clearance of extracellular alpha-synuclein prevents cell-to-cell aggregate transmission. J Neurosci 32, 13454–13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Terrell B, Farr SA, Robinson SM, Nonaka N, and Morley JE (2002). Passage of amyloid beta protein antibody across the blood-brain barrier in a mouse model of Alzheimer’s disease. Peptides 23, 2223–2226. [DOI] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, et al. (2009). Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta neuropathologica 117, 613–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, and Rub U (2002). Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages). Journal of neurology 249 Suppl 3, III/1–5. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, and Braak E (2003). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24, 197–211. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, et al. (2004). Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364, 1167–1169. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Redmond DE Jr., Steece-Collier K, Lipton JW, and Manfredsson FP (2016). Is Alpha-Synuclein Loss-of-Function a Contributor to Parkinsonian Pathology? Evidence from Non-human Primates. Frontiers in neuroscience 10, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, Staal R, Tieu K, Schmitz Y, Yuan CA, et al. (2002). Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A 99, 14524–14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agnaf O, Overk C, Rockenstein E, Mante M, Florio J, Adame A, Vaikath N, Majbour N, Lee SJ, Kim C, et al. (2017). Differential effects of immunotherapy with antibodies targeting alpha-synuclein oligomers and fibrils in a transgenic model of synucleinopathy. Neurobiology of disease 104, 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agnaf OM, Salem SA, Paleologou KE, Cooper LJ, Fullwood NJ, Gibson MJ, Curran MD, Court JA, Mann DM, Ikeda S, et al. (2003). Alpha-synuclein implicated in Parkinson’s disease is present in extracellular biological fluids, including human plasma. FASEB J 17, 1945–1947. [DOI] [PubMed] [Google Scholar]
- Games D, Valera E, Spencer B, Rockenstein E, Mante M, Adame A, Patrick C, Ubhi K, Nuber S, Sacayon P, et al. (2014). Reducing C-terminal-truncated alpha-synuclein by immunotherapy attenuates neurodegeneration and propagation in Parkinson’s disease-like models. J Neurosci 34, 9441–9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, and Lee VM (2002). Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron 34, 521–533. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Jakes R, Goedert M, Duda JE, Leight S, Trojanowski JQ, and Lee VM (2000). A panel of epitope-specific antibodies detects protein domains distributed throughout human alpha-synuclein in Lewy bodies of Parkinson’s disease. Journal of neuroscience research 59, 528–533. [DOI] [PubMed] [Google Scholar]
- Gibbons GS, Banks RA, Kim B, Changolkar L, Riddle DM, Leight SN, Irwin DJ, Trojanowski JQ, and Lee VMY (2018). Detection of Alzheimer Disease (AD)-Specific Tau Pathology in AD and NonAD Tauopathies by Immunohistochemistry With Novel Conformation-Selective Tau Antibodies. J Neuropathol Exp Neurol 77, 216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk OS, Li S, Nash K, Gorbatyuk M, Lewin AS, Sullivan LF, Mandel RJ, Chen W, Meyers C, Manfredsson FP, et al. (2010). In vivo RNAi-mediated alpha-synuclein silencing induces nigrostriatal degeneration. Molecular therapy : the journal of the American Society of Gene Therapy 18, 1450–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Ferreira R, Taylor NM, Mona D, Ringler P, Lauer ME, Riek R, Britschgi M, and Stahlberg H (2018). Cryo-EM structure of alpha-synuclein fibrils. eLife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson MX, Chung CH, Riddle DM, Zhang B, Gathagan RJ, Seeholzer SH, Trojanowski JQ, and Lee VMY (2017). Unbiased proteomics of early Lewy body formation model implicates active microtubule affinity-regulating kinases (MARKs) in synucleinopathies. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson MX, Cornblath EJ, Darwich A, Zhang B, Brown H, Gathagan RJ, Sandler RM, Bassett DS, Trojanowski JQ, and Lee VMY (2019a). Spread of α-synuclein pathology through the brain connectome is modulated by selective vulnerability and predicted by network analysis. Nature Neuroscience 22, 1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson MX, Peng C, Trojanowski JQ, and Lee VMY (2018). LRRK2 activity does not dramatically alter alpha-synuclein pathology in primary neurons. Acta neuropathologica communications 6, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson MX, Sengupta M, McGeary I, Zhang B, Olufemi MF, Brown H, Trojanowski JQ, and Lee VMY (2019b). LRRK2 inhibition does not impart protection from alpha-synuclein pathology and neuron death in non-transgenic mice. Acta neuropathologica communications 7, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson MX, Trojanowski JQ, and Lee VM (2019c). alpha-Synuclein Pathology in Parkinson’s Disease and Related alpha-Synucleinopathies. Neuroscience letters, 134316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P, Agid Y, Durr A, and Brice A (2004). Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet 364, 1169–1171. [DOI] [PubMed] [Google Scholar]
- Irwin DJ, Grossman M, Weintraub D, Hurtig HI, Duda JE, Xie SX, Lee EB, Van Deerlin VM, Lopez OL, Kofler JK, et al. (2017). Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. The Lancet Neurology 16, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA (2012). Neurobiology of cognitive impairment in Parkinson’s disease. Expert review of neurotherapeutics 12, 1451–1466. [DOI] [PubMed] [Google Scholar]
- Kanaan NM, and Manfredsson FP (2012). Loss of functional alpha-synuclein: a toxic event in Parkinson’s disease? Journal of Parkinson’s disease 2, 249–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely AP, Asi YT, Kara E, Limousin P, Ling H, Lewis P, Proukakis C, Quinn N, Lees AJ, Hardy J, et al. (2013). alpha-Synucleinopathy associated with G51D SNCA mutation: a link between Parkinson’s disease and multiple system atrophy? Acta neuropathologica 125, 753–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller EJ, Brooks MM, Golde TE, Giasson BI, and Chakrabarty P (2017). Inflammatory pre-conditioning restricts the seeded induction of alpha-synuclein pathology in wild type mice. Molecular neurodegeneration 12, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, and Olanow CW (2008). Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 14, 504–506. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, and Riess O (1998). Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nature genetics 18, 106–108. [DOI] [PubMed] [Google Scholar]
- Kurowska Z, Englund E, Widner H, Lindvall O, Li JY, and Brundin P (2011). Signs of degeneration in 12–22-year old grafts of mesencephalic dopamine neurons in patients with Parkinson’s disease. Journal of Parkinson’s disease 1, 83–92. [DOI] [PubMed] [Google Scholar]
- Li B, Ge P, Murray KA, Sheth P, Zhang M, Nair G, Sawaya MR, Shin WS, Boyer DR, Ye S, et al. (2018a). Cryo-EM of full-length alpha-synuclein reveals fibril polymorphs with a common structural kernel. Nature communications 9, 3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, et al. (2008). Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med 14, 501–503. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Widner H, Rehncrona S, Bjorklund A, Lindvall O, and Brundin P (2010). Characterization of Lewy body pathology in 12- and 16-year-old intrastriatal mesencephalic grafts surviving in a patient with Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society 25, 1091–1096. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhao C, Luo F, Liu Z, Gui X, Luo Z, Zhang X, Li D, Liu C, and Li X (2018b). Amyloid fibril structure of alpha-synuclein determined by cryo-electron microscopy. Cell research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, and Lee VM (2012). Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Song C, O’Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, and Lee VM (2009). Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A 106, 20051–20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler M, Valera E, Rockenstein E, Weninger H, Patrick C, Adame A, Santic R, Meindl S, Vigl B, Smrzka O, et al. (2014). Next-generation active immunization approach for synucleinopathies: implications for Parkinson’s disease clinical trials. Acta neuropathologica 127, 861–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux L, Campanelli JT, and Scheller RH (1988). Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci 8, 2804–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, Seubert P, Lee M, Goldstein J, Chilcote T, et al. (2005). Effects of alpha-synuclein immunization in a mouse model of Parkinson’s disease. Neuron 46, 857–868. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Mante M, Crews L, Spencer B, Adame A, Patrick C, Trejo M, Ubhi K, Rohn TT, et al. (2011). Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PloS one 6, e19338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, and Mucke L (2000). Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science 287, 1265–1269. [DOI] [PubMed] [Google Scholar]
- Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DM, and Hasegawa M (2013). Prion-like spreading of pathological alpha-synuclein in brain. Brain : a journal of neurology 136, 1128–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, et al. (2017). Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewoehner J, Bohrmann B, Collin L, Urich E, Sade H, Maier P, Rueger P, Stracke JO, Lau W, Tissot AC, et al. (2014). Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron 81, 49–60. [DOI] [PubMed] [Google Scholar]
- Nonaka T, Watanabe ST, Iwatsubo T, and Hasegawa M (2010). Seeded aggregation and toxicity of {alpha}-synuclein and tau: cellular models of neurodegenerative diseases. J Biol Chem 285, 34885–34898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasanen P, Myllykangas L, Siitonen M, Raunio A, Kaakkola S, Lyytinen J, Tienari PJ, Poyhonen M, and Paetau A (2014). Novel alpha-synuclein mutation A53E associated with atypical multiple system atrophy and Parkinson’s disease-type pathology. Neurobiol Aging 35, 2180 e2181–2185. [DOI] [PubMed] [Google Scholar]
- Paumier KL, Luk KC, Manfredsson FP, Kanaan NM, Lipton JW, Collier TJ, Steece-Collier K, Kemp CJ, Celano S, Schulz E, et al. (2015). Intrastriatal injection of pre-formed mouse alpha-synuclein fibrils into rats triggers alpha-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiology of disease 82, 185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, and Lang AE (2017). Parkinson disease. Nature reviews Disease primers 3, 17013. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. (1997). Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276, 2045–2047. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Marek K, Litvan I, Lang AE, Halliday G, et al. (2016). Abolishing the 1-year rule: How much evidence will be enough? Movement disorders : official journal of the Movement Disorder Society 31, 1623–1627. [DOI] [PubMed] [Google Scholar]
- Proukakis C, Dudzik CG, Brier T, MacKay DS, Cooper JM, Millhauser GL, Houlden H, and Schapira AH (2013). A novel alpha-synuclein missense mutation in Parkinson disease. Neurology 80, 1062–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey NL, George S, Steiner JA, Madaj Z, Luk KC, Trojanowski JQ, Lee VM, and Brundin P (2017). Spread of aggregates after olfactory bulb injection of alpha-synuclein fibrils is associated with early neuronal loss and is reduced long term. Acta neuropathologica. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Guajardo V, Annibali A, Jensen PH, and Romero-Ramos M (2013). alpha-Synuclein vaccination prevents the accumulation of parkinson disease-like pathologic inclusions in striatum in association with regulatory T cell recruitment in a rat model. J Neuropathol Exp Neurol 72, 624–645. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, et al. (1999). Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400, 173–177. [DOI] [PubMed] [Google Scholar]
- Schenk DB, Koller M, Ness DK, Griffith SG, Grundman M, Zago W, Soto J, Atiee G, Ostrowitzki S, and Kinney GG (2017). First-in-human assessment of PRX002, an anti-alpha-synuclein monoclonal antibody, in healthy volunteers. Movement disorders : official journal of the Movement Disorder Society 32, 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer CR, Grass JA, Liao Z, Pepivani I, Zheng B, Eklund AC, Ney PA, Ng J, McGoldrick M, Mollenhauer B, et al. (2008). GATA transcription factors directly regulate the Parkinson’s disease-linked gene alpha-synuclein. Proc Natl Acad Sci U S A 105, 10907–10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahaduzzaman M, Nash K, Hudson C, Sharif M, Grimmig B, Lin X, Bai G, Liu H, Ugen KE, Cao C, et al. (2015). Anti-human alpha-synuclein N-terminal peptide antibody protects against dopaminergic cell death and ameliorates behavioral deficits in an AAV-alpha-synuclein rat model of Parkinson’s disease. PloS one 10, e0116841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, et al. (2003). alpha-Synuclein locus triplication causes Parkinson’s disease. Science 302, 841. [DOI] [PubMed] [Google Scholar]
- Spencer B, Emadi S, Desplats P, Eleuteri S, Michael S, Kosberg K, Shen J, Rockenstein E, Patrick C, Adame A, et al. (2014). ESCRT-mediated uptake and degradation of brain-targeted alpha-synuclein single chain antibody attenuates neuronal degeneration in vivo. Molecular therapy : the journal of the American Society of Gene Therapy 22, 1753–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, Valera E, Rockenstein E, Overk C, Mante M, Adame A, Zago W, Seubert P, Barbour R, Schenk D, et al. (2017). Anti-alpha-synuclein immunotherapy reduces alpha-synuclein propagation in the axon and degeneration in a combined viral vector and transgenic model of synucleinopathy. Acta neuropathologica communications 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, Williams S, Rockenstein E, Valera E, Xin W, Mante M, Florio J, Adame A, Masliah E, and Sierks MR (2016). alpha-synuclein conformational antibodies fused to penetratin are effective in models of Lewy body disease. Annals of clinical and translational neurology 3, 588–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, and Goedert M (1998). Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neuroscience letters 251, 205–208. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, and Goedert M (1997). Alpha-synuclein in Lewy bodies. Nature 388, 839–840. [DOI] [PubMed] [Google Scholar]
- Steiner JA, Quansah E, and Brundin P (2018). The concept of alpha-synuclein as a prion-like protein: ten years after. Cell and tissue research 373, 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner CM, and Goldman SM (1996). Epidemiology of Parkinson’s disease. Neurologic clinics 14, 317–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran HT, Chung CH, Iba M, Zhang B, Trojanowski JQ, Luk KC, and Lee VM (2014). Alpha-synuclein immunotherapy blocks uptake and templated propagation of misfolded alpha-synuclein and neurodegeneration. Cell reports 7, 2054–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi Y, Uchikado H, and Dickson DW (2007). Neuropathology of Parkinson’s disease dementia and dementia with Lewy bodies with reference to striatal pathology. Parkinsonism & related disorders 13 Suppl 3, S221–224. [DOI] [PubMed] [Google Scholar]
- Tuttle MD, Comellas G, Nieuwkoop AJ, Covell DJ, Berthold DA, Kloepper KD, Courtney JM, Kim JK, Barclay AM, Kendall A, et al. (2016). Solid-state NMR structure of a pathogenic fibril of full-length human alpha-synuclein. Nature structural & molecular biology 23, 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, and Saitoh T (1993). Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci U S A 90, 11282–11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaikath NN, Hmila I, Gupta V, Erskine D, Ingelsson M, and El-Agnaf OMA (2019). Antibodies against alpha-synuclein: tools and therapies. Journal of neurochemistry. [DOI] [PubMed] [Google Scholar]
- Vaikath NN, Majbour NK, Paleologou KE, Ardah MT, van Dam E, van de Berg WD, Forrest SL, Parkkinen L, Gai WP, Hattori N, et al. (2015). Generation and characterization of novel conformation-specific monoclonal antibodies for alpha-synuclein pathology. Neurobiology of disease 79, 81–99. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, and Lee VM (2014). Addition of exogenous alpha-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous alpha-synuclein to Lewy body and Lewy neurite-like aggregates. Nature protocols 9, 2135–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, and Lee VM (2011). Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72, 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihofen A, Liu Y, Arndt JW, Huy C, Quan C, Smith BA, Baeriswyl JL, Cavegn N, Senn L, Su L, et al. (2019). Development of an aggregate-selective, human-derived alpha-synuclein antibody BIIB054 that ameliorates disease phenotypes in Parkinson’s disease models. Neurobiology of disease 124, 276–288. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, et al. (2004). The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55, 164–173. [DOI] [PubMed] [Google Scholar]
- Zharikov AD, Cannon JR, Tapias V, Bai Q, Horowitz MP, Shah V, El Ayadi A, Hastings TG, Greenamyre JT, and Burton EA (2015). shRNA targeting alpha-synuclein prevents neurodegeneration in a Parkinson’s disease model. The Journal of clinical investigation 125, 2721–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.