Abstract
The clinical significance of ipsilateral thalamic diaschisis (ITD) occurring after stroke is unknown. To characterize ITD, we investigate its hemodynamic, structural, and clinical implications. A single-institution prospective cross-sectional study was conducted using 28 symptomatic cerebrovascular steno-occlusive patients undergoing both BOLD-CVR and Diamox-challenged 15(O)-H2O-PET. Follow-up was at least three months. In addition, 15 age-matched healthy subjects were included. ITD was diagnosed based on a BOLD-CVR thalamic asymmetry index (TAI) > +2 standard deviations from healthy subjects. Cerebral blood flow differences were assessed using a PET-based TAI before and after Diamox challenge. Thalamic volume masks were determined using Freesurfer. Neurological status at symptom onset and after three months was determined with NIHSS and mRS scores. ITD was diagnosed in 15 of 28 (57%) patients. PET-TAI before and after Diamox challenge were increased in patients with ITD, indicating an ipsilateral thalamic blood flow decrease. Patients with ITD exhibited a marked ipsilateral thalamic volume decrease as compared to patients without ITD and healthy subjects. Furthermore, patients with ITD had worse NIHSS and mRS at symptom onset and after three months follow-up, even after adjustment for stroke volume. The presence of ITD is characterized by thalamic volume reduction, reduced thalamic blood flow, and worse neurological performance unrelated to stroke volume.
Keywords: Ipsilateral thalamic diaschisis, BOLD MRI, 15(O)-H2O PET, cerebrovascular reactivity, thalamus
Introduction
Diaschisis, first introduced by the Russian-Swiss neuropathologist Constantin von Monakow in 1914, refers to a brain injury-induced drop in neuronal activity in distant but functionally connected brain regions.1 About 70 years later, the first diaschisis imaging study revealed a reduction in both blood flow and metabolism in the cerebellar hemisphere contralateral to a supratentorial stroke lesion (termed crossed cerebellar diaschisis).2 Since that time, others have shown the presence of another type of diaschisis, named ipsilateral thalamic diaschisis.3,4 Although multiple studies have shown that crossed cerebellar diaschisis present in patients after a supratentorial stroke is associated with worse neurological performance, the clinical correlate of ipsilateral thalamic diaschisis, however, remains a matter of debate.5,6
Ipsilateral thalamic diaschisis is thought to be caused by antero- or retrograde thalamo-cortical neuronal degeneration and may result in long-term structural changes, such as ipsilateral thalamic atrophy.7–9
Since ipsilateral thalamic diaschisis does not have an immediate clinical or anatomical correlate, it remains primarily a functional imaging diagnosis usually obtained with Positron Emission Tomography (PET). Further advancements in multimodal diaschisis imaging include MRI-based methods such as arterial spin labeling and blood oxygenation level–dependent MRI cerebrovascular reactivity (BOLD-CVR).10–13
To characterize ipsilateral thalamic diaschisis, we investigate the hemodynamic, structural, and clinical significance. To achieve this, we used BOLD-CVR to diagnose ipsilateral thalamic diaschisis and investigate thalamic blood flow and volumetric changes using Diamox challenged 15(O)-H2O PET imaging and high-resolution T1-weighted imaging. The clinical correlate was studied using two stroke severity scores. We show that presence of ipsilateral thalamic diaschisis in symptomatic patients with steno-occlusive disease is characterized by thalamic volume reduction, reduced thalamic blood flow and worse neurological performance not related to stroke volume.
Materials and methods
This study was conducted at the Clinical Neuroscience Center of a single university hospital. Ethical approval prior to the study was given by the local ethics board (Kantonale Ethikskommision Zürich, Kanton Zürich: KEK-ZH-Nr. 2012-0427). The procedures and analyses used in this study were carried out in accordance with the ethical standards of the Declaration of Helsinki (Ethical Principles for Medical Research Involving Human Subjects) from 1964 and subsequent amendments.14 We screened all datasets from patients with unilateral symptomatic steno-occlusive disease of the anterior cerebral circulation from an ongoing prospective BOLD-CVR database (January 2015–September 2018). All patients as well as healthy controls provided written informed consent prior to their participation. For this study, patients were selected on the basis of having symptomatic steno-occlusive disease and having undergone both Diamox challenged 15(O)-H2O PET and BOLD-CVR imaging within a time period of six weeks of each other. Moreover, if the subjects had ischemic lesions, they had to be confined to the middle or anterior cerebral artery territory. Patients with an interval more than six weeks between PET and BOLD-CVR imaging were excluded to avoid potential disease alteration. Other exclusion criteria included: subjects with a hemodynamic relevant vascular pathology of the posterior circulation, structural lesions in the posterior circulation or in the thalamus and the emergence of new neurological symptoms or a neurosurgical or endovascular intervention occurring prior to or within the time period between the BOLD-CVR and PET imaging.
To determine the normal ranges of thalamic BOLD-CVR, 15 age-matched healthy right-handed subjects (six females; age 60 ± 12) were extracted from the same BOLD-CVR database. The criteria for being included within this database were healthy adult subjects with no known neurological symptoms or intracranial pathologies. All subjects were asked to refrain from smoking and caffeine on the day of scanning.
Image acquisition and processing
BOLD-CVR and (15O)-H2O PET study
MRI data were acquired using a 3-tesla Skyra VD13 (Siemens Healthcare, Erlangen, Germany) with a 32-channel head coil. PET data were acquired using a full ring PET/CT-scanner in 3D mode (PET/CT Discovery STE, GE Healthcare, Chicago, IL, USA). The exact acquisition parameters and methods of processing of the BOLD and H2O PET images can be found in the supplementary folder. A more extensive review of the methodology is given in previous work.15,16 Requests to access the analysis methods and detailed results of this study may be made to the corresponding author (Dr Fierstra). To assess the supratentorial hemodynamic status, whole brain BOLD-CVR, BOLD-CVR of the supratentorial affected (i.e. ipsilateral to the symptomatic side) hemisphere and BOLD-CVR of the supratentorial unaffected hemisphere were determined.
ROI determination and volumetric calculations
Anatomical Regions of Interest (ROIs) were derived from the subjects' specific tissue parcellation using Freesurfer software17 (http://surfer.nmr.mgh.harvard.edu). The high-resolution T1-weighted images were uploaded onto Freesurfer and automatically segmented into different anatomical sub-regions according to the Destrieux atlas (file name: aparc+aseg2009s.mgz).18 From these parcellation maps, 3D thalamic masks were derived for BOLD-CVR and both PET calculations. We also obtained thalamic volumetric measurements (in mm3) of both the ipsilateral thalamus and the contralateral thalamus. A thalamic volume index was calculated as follows:
(ipsilateral thalamic volume − contralateral thalamus volume)/ipsilateral thalamus volume.
Failure to create a parcellation map due to excessive motion was an exclusion criterion for this study.
In our healthy cohort, which contained only right-handed subjects, the right thalamus was used for the ipsilateral thalamic volume and the left thalamic volume as the contralateral thalamic volume.
Ipsilateral thalamic diaschisis
A thalamic asymmetry index was calculated from the BOLD-CVR scan of each subject using the following equation
(CVR of the contralateral (to supratentorial stroke) thalamus − CVR of the ipsilateral thalamus) / CVR of the contralateral thalamus.
We calculated the average and standard deviation of the thalamic asymmetry index of the healthy control group. BOLD derived ipsilateral thalamic diaschisis was deemed present if the thalamic asymmetry index was larger than +2 standard deviations from our healthy cohort (i.e. positive asymmetry index). As a comparison, we also evaluated the thalamic asymmetry index in the PET baseline and the PET Diamox.
Stroke volume and location calculation
Each stroke volume was manually outlined using Brainlab software (Brainlab AG, Munich, Germany) using diffusion weighted imaging, T2-fluid-attentuated-inversion recovery imaging and T1-weighted imaging. When multiple stroke lesions were present, the stroke volume would represent the sum of all the lesions.
All stroke volume masks were binarized and transformed into Montreal Neurological Institute space using SPM. A subtraction map from ipsilateral thalamic diaschisis positive and ipsilateral thalamic diaschisis negative cumulative stroke location maps was created to visually inspect anatomical locations potentially associated with ipsilateral thalamic diaschisis.
Assessment of neurological and functional status
The National Institute of Health Stroke Scala (NIHSS) and modified Rankin scale (mRS) were used to evaluate the neurological status at the time of symptom onset and at 3 months of follow-up.
Statistical analysis
We performed statistical analysis using in-house scripts written in Matlab R2016b (the MathWorks, Inc., Natick, Massachusetts, United States; http://www.mathworks.com/). First, evaluation of normal distribution per variable was carried out via visual inspections of normality plots. Means of normally distributed continuous variables from the ipsilateral thalamic diaschisis positive and ipsilateral thalamic diaschisis negative groups were compared by an independent Student's two tailed t-test, where p < 0.05 was considered significant. Non-normal distributed, categorical ordinal and dichotomous variables were analyzed using the Mann-Whitney-U test. All normally distributed continuous variables are reported as mean ± standard deviation. Non-normally distributed variables as well as categorical ordinal variables are presented as median (interquartile range), whereas dichotomous variables are shown as frequency (% percentage). To evaluate thalamic cerebral blood flow reactivity in PET imaging, we compared the thalamic asymmetry index obtained with both PET measurements using a paired sample T-test.
A Pearson correlation was carried out in order to evaluate the correlation between the BOLD-CVR asymmetry index and both PET thalamic asymmetry indices. Second, a Spearman rank-order correlation analysis with and without partial correction for stroke volume was used to identify the relationship between the thalamic asymmetry index, detected by BOLD-CVR, and neurological outcome after three months, measured using NIHSS and mRS. Partial correction for stroke volume was conducted in order to cancel the effect of infarct volume on neurological status.19
Results
Study population
A flowchart describing the patient inclusion criteria is given in Figure 1. The baseline characteristics of all patients can be found in Tables 1 and 2. The mean age of all 28 included patients was 59 ± 14. We included 15 age-matched healthy subjects with a mean age of 58 ± 11 of which six healthy controls were females. Mean CVR for the whole brain was 0.20 ± 0.05 as compared to mean CVR of 0.14 ± 0.07 for the patient group (p = 0.005).
Figure 1.
Flow-chart of patient inclusion form the prospective database. BOLD: blood oxygen-level dependent, CVR: cerebrovascular reactivity, PET: positron emission tomography.
Table 1.
Patients' characteristics.
| Age | Sex | Lateralization | Time Interval between scans (Days) | Time interval after stroke (weeks/daysa) | Anatomical location of ischemia or parenchymal defect | Angiographical findings | ITD (+/−) | |
|---|---|---|---|---|---|---|---|---|
| 1 | 39 | F | Right | 32 | 91 | Right caudate nucleus | High-grade stenosis of both MCA | + |
| 2 | 62 | M | Right | 20 | 56 | Right basal ganglia | Right MCA occlusion | + |
| 3 | 45 | M | Left | 4 | 4 | Left postcentral gyrus and superior parietal lobule | Left MCA Stenosis, fusiform aneurysms of the left carotid T | + |
| 4 | 52 | M | Left | 18 | 5 | Left caudate nucleus putamen, posterior Insula, postcentral and centrum Semiovale | Stenosis MCA-1 bds., Occlusion left MCA-M2 | + |
| 5 | 77 | F | Left | 38 | 20 | Left caudate nucleus, inferior parietal lobule and centrum semiovale | Left MCA Stenosis | + |
| 6 | 77 | F | Left | 9 | 5a | Left caudate nucleus and centrum semiovale | Left ICA occlusion | + |
| 7 | 64 | M | Right | 6 | 6 | None | Right ICA occlusion, left ICA stenosis. | + |
| 8 | 50 | F | Left | 26 | 17 | Left caudate nucleus, claustrum, Globus Pallidum, Putamen, inferior temporal and medial temporal gyrus | Bilateral MCA stenosis | + |
| 9 | 65 | M | Right | 13 | 9 | Right lentiforme nucleus, corona radiata, intern capsule, precentral gyrus superior parietal lobule and inferior patieral lobule | Right ICA occlusion | + |
| 10 | 47 | M | Right | 10 | 1 | Right caudate nucleus, putamen, corona radiation, superior temporal gyrus, inferior parietal lobule and centrum Semiovale | Right M1 occlusion, no contralateral ICA stenosis | + |
| 11 | 72 | M | Right | 11 | 32 | None | Right ICA occlusion | + |
| 12 | 64 | M | Right | 5 | 1 | Right medial frontal gyrus precentral, postcentral and medial temporal gyrus | Right ICA occlusion, right MCA stenosis | + |
| 13 | 77 | M | Left | 2 | 1 | Left caudate nucleus, Insula, inferior frontal gyrus and subcentral gyrus | Left ICA and MCA occlusion | + |
| 14 | 50 | M | Right | 31 | 5 | Right centrum Semiovale and Globus pallidum | Right ICA occlusion | + |
| 15 | 50 | M | Left | 10 | 2 | Left ICA occlusion | + | |
| 16 | 80 | M | Right | 40 | 2 | Right precentral gyrus | Right ICA occlusion, no contralateral ICA stenosis | – |
| 17 | 58 | M | Left | 24 | 26 | None | Left MCA-M1 Stenosis | – |
| 18 | 45 | M | Right | 6 | 2 | Left superior frontal gyrus and centrum Semiovale | Right MCA Stenosis | – |
| 19 | 51 | M | Left | 26 | 42 | Left centrum Semiovale | Left ICA occlusion | – |
| 20 | 33 | F | Left | 16 | 4a | None | Left ICA dissection | – |
| 21 | 62 | M | Left | 3 | 74 | None | Left MCA-M1 occlusion | – |
| 22 | 44 | F | Right | 38 | 245 | None | Bilateral MCA/ACA stenosis | – |
| 23 | 69 | M | Left | 2 | 5a | Left medial frontal gyrus, inferior parietal lobule inferior and centrum semiovale | Left ICA occlusion | – |
| 24 | 52 | M | Left | 5 | 2 | Diffuse infarctions in left MCA territory | Left ICA occlusion | – |
| 25 | 46 | M | Right | 18 | 94 | Right medial frontal gyrus | Right ICA stenosis 90%, left ICA stenosis 50% | – |
| 26 | 74 | M | Right | 9 | 4a | Right caudate nucleus, putamen and insula | Right ICA occlusions, left ICA stenosis | – |
| 27 | 66 | F | Left | 18 | 2 | Left precentral gyrus. | Left ICA occlusion, no contralateral ICA stenosis | – |
| 28 | 76 | M | Right | 24 | 5 | Right semioval center | Bilateral ICA occlusion | – |
Note: aindicates days. All other numbers (i.e. without a) indicate weeks. ACA: Anterior cerebral artery; ICA: internal carotid artery; MCA: medial cerebral artery.
Table 2.
Demographics for ITD positive & negative group.
| Ipsilateral thalamic diaschisis positive group (n = 15) | Ipsilateral thalamic diaschisis negative group (n = 13) | p-Value | |
|---|---|---|---|
| Age (mean±SD) | 59 ± 13 | 59 ± 14 | 0.81 |
| Sex, n male (%) | 11 (73) | 11 (84) | 0.89 |
| Smoking, n (%) | 9 (69) | 8 (62) | 0.79 |
| Hypertension, n (%) | 10 (66) | 9 (69) | 0.93 |
| Hypercholesterolemia, n (%) | 4 (27) | 5 (38) | 0.62 |
| Obesity, n (%) | 1 (7) | 3 (23) | 0.47 |
| Diabetes, n (%) | 2 (13) | 1 (8) | 0.80 |
| Lateralization, n right (%) | 7(47) | 7(53) | 0.22 |
Note: n: Number; SD = standard deviation.
Thalamic asymmetry index
The mean BOLD-CVR of the healthy cohort reached an average of 0.21 ± 0.05 for the left thalamus and 0.22 ± 0.07 for the right thalamus compared to an average CVR of 0.14 ± 0.06 for the ipsilateral thalamus and 0.18 ± 0.06 for the contralateral thalamus in all patients. Only the ipsilateral thalamic BOLD-CVR of the patients was different when compared to the healthy cohort (p = 0.001). The thalamic asymmetry index for healthy subjects was 4.0% ± 8.4. We considered ipsilateral thalamic diaschisis present if the thalamic asymmetry index was larger than 21% (i.e. exceeding + 2 standard deviations of the average thalamic asymmetry index of the healthy control group, see the Materials and methods section).
Ipsilateral thalamic diaschisis determination
Exemplary imaging of one subject with ipsilateral thalamic diaschisis and one subject without ipsilateral thalamic diaschisis is illustrated in Figure 2. Stroke locations from the subtraction map are presented in the Supplementary Figure 1.
Figure 2.
Patient without ipsilateral thalamic diaschisis versus patient with ipsilateral thalamic diaschisis. Ipsilateral thalamic diaschisis negative: A 51-year-old male subject with recurrent transient ischemic attacks due to an ICA occlusion (white cross) on the left side. He had no lasting symptoms. On PET and BOLD-CVR imaging, signs of a left-sided supratentorial hemodynamic impairment can be seen in MCA territory on the left side. Taking only the thalamus (Figure A-G, A-H and A-I), no ipsilateral thalamic diaschisis can be appreciated for all imaging modalities. Ipsilateral thalamic diaschisis positive: A 65-year-old male subject with ischemia in the multiple right-sided infarctions due to a right ICA occlusion (white cross). He presented left-sided hemiparesis (NIHSS 6/42, mRS 3), most pronounced in this arm. Three months later, he experienced neurological improvement (NIHSS 2/42, mRS 1). For all imaging modalities, ipsilateral thalamic diaschisis can be appreciated with a decrease in both BOLD-CVR and PET-CBF on the left side. BOLD: blood oxygen-level dependent, CVR: cerebrovascular reactivity, DWI: diffusion weighted imaging, ICA: internal carotid artery, ITD: ipsilateral thalamic diaschisis, MCA: middle cerebral artery, MR angiography: magnetic resonance angiography, RS: modified Rankin Scale, NIHSS: National Institute of Health Stroke Scale, PET: Positron Emission Tomography.
Based on the BOLD-CVR cut-off point of 21%, 15 patients (57%) were classified as ipsilateral thalamic diaschisis positive. The vascular risk factors and hemispheric lateralization of all patients are shown in Table 2. No difference was found between both groups and any of the vascular risk factors.
BOLD-CVR and PET findings
CVR findings for patients with and without ipsilateral thalamic diaschisis can be found in Table 3. The thalamic asymmetry indices for BOLD-CVR and PET are also shown in Table 3. BOLD-CVR thalamic asymmetry index in ipsilateral thalamic diaschisis positive subjects was different to that seen in ipsilateral thalamic diaschisis negative subjects (34.65 ± 14.48 vs. 4.50 ± 8.60, p < 0.001). The PET baseline thalamic asymmetry index and PET Diamox thalamic asymmetry index followed the CVR thalamic asymmetry subjects by showing a clear difference between both groups. (PET baseline: 15.94 ± 9.03 vs. 1.64 ± 3.77, p < 0.001; PET Diamox: 16.58 ± 8.67 vs. 3.06 ± 3.74, p < 0.001).
Table 3.
Functional and outcome measurements.
| Ipsilateral thalamic diaschisis positive group (n = 15) | Ipsilateral thalamic diaschisis negative group (n = 13) | p-Value | |
|---|---|---|---|
| Mean CVR whole brain | 0.13 ± 0.07 | 0.15 ± 0.07 | 0.44 |
| Mean CVR affected supratentorial hemisphere | 0.10 ± 0.08 | 0.14 ± 0.07 | 0.19 |
| Mean CVR unaffected supratentorial hemisphere | 0.15 ± 0.07 | 0.16 ± 0.08 | 0.62 |
| CVR TAI (%) | 34.65 ± 14.48 | 4.50 ± 8.60 | <0.001a |
| PET TAI (%) baseline | 15.94 ± 9.03 | 1.64 ± 3.77 | <0.001a |
| PET TAI (%) Diamox | 16.58 ± 8.67 | 3.06 ± 3.74 | <0.001a |
| Ipsilateral thalamic volume (mm3) | 5644 ± 898 | 7049 ± 982 | 0.001a |
| Contralateral thalamic volume (mm3) | 6880 ± 813 | 7080 ± 1079 | 0.59 |
| Thalamic volume index (%) | −0.24 ± 0.21 | −0.01 ± 0.12 | 0.003a |
| Stroke volume (mm3) | 9231 ± 12905 | 1813 ± 2885 | 0.05 |
| NIHSS at symptom onset | 5 (7) | 0 (2) | 0.001a |
| mRS at symptom onset | 3 (2) | 0 (2) | 0.001a |
| NIHSS at three months' follow-up | 2 (3) | 0 (1) | 0.007a |
| mRS at three months' follow-up | 1 (1) | 0 (1) | 0.002a |
Note: Functional measures are shown as mean ± standard deviation, outcome as median (interquartile range). TAI: thalamic asymmetry index; CVR: cerebrovascular reactivity, defined as percentage BOLD signal change per mmHg CO2, N: number; mRS: modified Rankin scale; NIHSS: National Institute of Health Stroke Scale; PET: Positron Emission Tomography.
Significant p-value.
Between the PET baseline and PET Diamox, the thalamic asymmetry index did not differ and was similar before and after the Diamox challenge. This can be interpreted as an intact thalamic cerebral blood flow reactivity response. Both the baseline and Diamox asymmetry indices demonstrated a good correlation with the BOLD-CVR thalamic asymmetry index (PET baseline: R: 0.53, p = 0.004; PET Diamox: R: 0.52, p = 0.004).
Volumetric analysis of the thalamus
Volumetric measurements in healthy subjects showed an average left thalamic volume of 7494 ± 898 mm3 and a right thalamic volume of 6821 ± 598 mm3, with a thalamic volume index of −0.09 ± 0.08%. Findings for both patient groups are also presented in Table 1.
Patients with ipsilateral thalamic diaschisis had a marked reduction in ipsilateral thalamic volume as opposed to the thalamic diaschisis negative group (p = 0.001), as well as in comparison to the healthy cohort (p < 0.001). No differences were found for the contralateral thalamic volume as compared to both groups. The volumetric measurements of both thalamic volumes between the ipsilateral thalamic diaschisis negative group and the healthy cohort did not show any differences (p = 0.92).
Figure 3 shows the correlation between time of symptom onset and thalamic volume index for patients with and without ipsilateral thalamic diaschisis. A clear increase in thalamic volume index (i.e. larger difference between ipsi- and contralateral thalamic volumes) over time can be appreciated for the ipsilateral thalamic diaschisis patient group (R2 = −0.60, p = 0.001). No such increase can be seen in the patient group without ipsilateral thalamic diaschisis. (R2 = 0.26, p = 007). The graph clearly shows that the presence of ipsilateral thalamic diaschisis and a larger interval after stroke result in a larger ipsilateral thalamic volume reduction.
Figure 3.
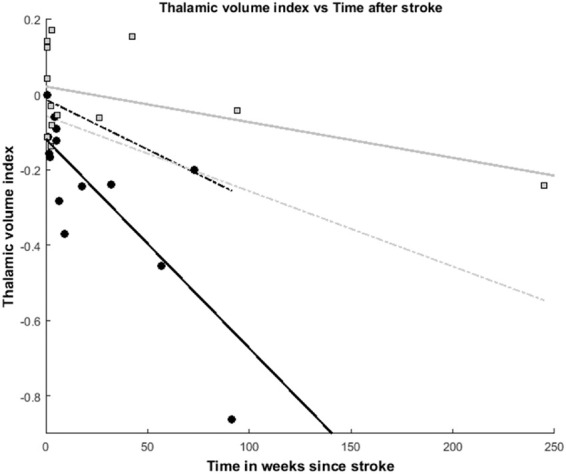
Thalamic volume vs. time after stroke. Thalamic volume index vs. time after stroke. Data points from each subject with ipsilateral thalamic diaschisis are presented with black dots, whereas the group without ipsilateral thalamic diaschisis is shown as gray squares. The gray dotted line represents the lower 95% confidence interval of the ipsilateral thalamic diaschisis negative group. The black dotted line represents the upper 95% confidence interval of the ipsilateral thalamic diaschisis positive group. A strong correlation between a decrease in thalamic volume index (i.e. increased difference between ipsilateral and contralateral volumes) is shown for the subjects with ipsilateral thalamic diaschisis (black line; r2 = 0.60, p = 0.001). Such a correlation was not found for the subjects without ipsilateral thalamic diaschisis (gray line; r2=0.26, p = 0.07).
Clinical status and outcome assessments
NHISS and mRS scores at symptom onset and after three months are presented in Table 3. Subjects with ipsilateral thalamic diaschisis presented with worse initial neurological status (NHISS: 5(7) versus 0(2), p = 0.001; mRS: 2(4) versus 0(1), p = 0.002). Moreover, the neurological performance at three months was also worse as compared to the ipsilateral thalamic negative subjects (NHISS: 2(3) versus 0(2), p = 0.007; mRS: 3(2) versus 0(0), p = 0.002).
Interestingly, the degree to which the BOLD-CVR thalamic asymmetry index correlates with initial neurological status as well as outcome after three months, indicates that a larger BOLD-CVR thalamic asymmetry index correlates with worse clinical performance.
Even after partial correction for stroke volume, the BOLD-CVR thalamic asymmetry index remained significantly correlated to MRS at symptom onset (rho=0.39, p = 0.04), NIHSS at three months (rho=0.41, p = 0.04) and MRS at three months (rho=0.52, p = 0.005). The correlation with NIHSS during symptom onset and BOLD-CVR thalamic asymmetry index showed a trend towards significance (rho = 0.34, p = 0.08).
Discussion
The presence of ipsilateral thalamic diaschisis strongly correlates with an increased thalamic volume asymmetry (i.e. larger volume difference between the affected and unaffected thalamus) in the absence of an apparent structural thalamic lesion on anatomical MRI, reduced thalamic blood flow, as well as a worse initial neurological status and a worse neurological performance after three months. The combination of hemodynamic alterations (i.e. BOLD cerebrovascular reactivity impairment, hypoperfusion of the thalamus with preserved CBF reactivity on PET imaging), and the presence of diaschisis and thalamic atrophy has not been reported within the same study cohort to the best of our knowledge. Furthermore, the ability to study cerebrovascular reactivity as well as CBF for the entire thalamus with (f)MRI is also a novel and further development of currently available imaging and analysis techniques.
Significance of ipsilateral thalamic diaschisis detection
The reduction of thalamic metabolism and cerebral blood flow following a supratentorial stroke in humans was first described in the early 1980.20 It was first named ipsilateral thalamic diaschisis by de Reuck et al. in 1995 with an incidence (∼50%) matching our population.3,21,22
More recently, the presence of ipsilateral thalamic diaschisis was determined in patients with acute middle cerebral artery stroke based on visual assessment of signs of hypoperfusion on ≥2 CT-perfusion maps.6 The occurrence of ipsilateral thalamic diaschisis (∼20%) in their patient cohort is significantly lower than our incidence. This difference could be explained by the difference in imaging modality with less imaging contrast as well as the fact that their diagnosis of ipsilateral thalamic diaschisis was carried out by visual inspection and inter-reader agreement. However, as such methodological differences imply inclusion of a completely different cohort of patients, differences in outcome can be expected. We found a strong relationship between ipsilateral thalamic diaschisis and neurological performance at symptom onset and three months follow-up. Correction for stroke volume resulted in a weaker association of ipsilateral thalamic diaschisis with neurological performance at symptom onset, but did not affect the association with neurological performance after three months. In work performed by others, infarct volume was correlated with neurological motor function outcome.23–25 Stroke volume as an independent variable appears to have more merit in the acute/subacute stroke phase, but not in the chronic phase.24–26
Structural consequences of ipsilateral thalamic diaschisis
Diaschisis was initially considered a reversible phenomenon; however, recent literature shows that diaschisis can be found years after the initial event and can cause histopathological and structural changes.9,27–29 With regard to the thalamus, studies have reported long-term structural thalamic effects following a supratentorial stroke.7,9,27,30 These findings, though were not correlated with functional imaging studies and associations were not directly made with diaschisis. Only a recent review hinted towards diaschisis as an underlying cause of thalamic atrophy.9 Our findings of a reduced ipsilateral thalamic volume over time contribute mounting evidence of structural consequences related to thalamic diaschisis. As expected, the thalamic volume reduction was therefore more clearly seen in patients with ipsilateral diaschisis in the chronic stages of stroke and less in the subacute stroke patients with ipsilateral diaschisis.
Pathophysiology of ipsilateral thalamic BOLD-CVR reduction
The exact pathophysiology causing ipsilateral thalamic diaschisis is still unknown. In most cases it is likely caused by a disruption of the thalamo-cortical or cortico-thalamic pathways.8 The functionality of these pathways, however, is not yet fully understood. Histologically, the thalamus can be divided into first and higher-order thalamic nuclei.31 First-order nuclei predominately receive input from subcortical structures, whereas higher-order structures receive information from cortical structures. Moreover, it is believed that higher order thalamic nuclei also act as a mediator between cortical structures.32 Therefore, the location of strokes localization causing thalamic diaschisis can be very diverse, which can be seen in supplementary Figure 1, and might appear alongside various neurological symptoms.7
In patients with ipsilateral thalamic diaschisis, we have shown that both the thalamic blood flow before and after Diamox challenge on PET imaging in the ipsilateral thalamus is reduced. Without apparent structural lesions on anatomical MRI, this may primarily be a consequence of neuronal deactivation. Such a neuronal deactivation would first lead to a physiological reduction of thalamic metabolism. In response, the decrease in metabolism would also reduce the need of thalamic blood flow. This phenomenon indicates that the vascular bed does not lose its remaining blood flow reactivity (i.e. cerebral blood flow increase in response to a vasoactive stimulus). In this study, the average thalamic asymmetry index is equal between the PET-baseline and PET Diamox study. This indicates a comparable ipsi- and contralateral thalamic blood flow increase (i.e. response) after the Diamox challenge. From this, we can infer the presence of a preserved thalamic cerebral blood flow reactivity in our patient cohort. This has also been confirmed by others in a similar patient cohort.4,13 Perhaps surprisingly, this finding is crucial for the capability of BOLD-CVR to detect ipsilateral thalamic diaschisis. Generally, BOLD-CVR and PET-derived cerebral blood flow reactivity has shown a good hemispheric correlation.15 However, whereas PET is primarily based on cerebral blood flow, the BOLD signal is primarily based on the amount of deoxyhemoglobin. Therefore, the BOLD signal is strongly influenced, not only by changes in cerebral blood flow (i.e. cerebral blood flow reactivity), but also by the local cerebral metabolic rate of oxygen and the oxygen extraction fraction.33 The local reduction in metabolism seen in the ipsilateral thalamus will lead to less deoxyhemoglobin. In this instance, even though a seemingly adequate vasoactive response can be induced, the outwash of deoxyhemoglobin will be reduced as compared to the contralateral thalamus. This reduction in outwash of deoxyhemoglobin will cause a reduced BOLD signal response. Consequently, while PET cerebral blood flow reactivity is seemingly intact, BOLD-CVR in the ipsilateral thalamus will be reduced as compared to the contralateral thalamus. This allows BOLD-CVR imaging to adequately detect diaschisis.13
Limitations
In this study, we only included patients with cerebrovascular steno-occlusive disease of the anterior cerebral circulation and excluded those patients with ischemic events located in the posterior circulation. Such pathologies may influence the thalamic cerebral blood flow reactivity and would have resulted in an inaccuracy of detection of ipsilateral thalamic diaschisis using BOLD-CVR. Importantly, the current study population does not represent the entire spectrum of anterior circulation stroke, i.e. only symptomatic patients with steno-occlusive disease (transient ischemic attack or stroke) were selected from our prospective BOLD-CVR database.
We included 28 datasets of 28 subjects with steno-occlusive disease with an ischemic event in the subacute and chronic phase, which resulted in a more heterogeneous cohort.5 This heterogeneity mostly presented itself in the volumetric analysis. However, as this was a study with the aim of characterizing ipsilateral thalamic diaschisis, we deemed it appropriate to include a more heterogeneous cohort. So far, though, our study provides further evidence of the previously described long-term effects of ipsilateral thalamic diaschisis.
Due to the relatively small number of subjects in both groups, a bilateral correction for asymmetry in the subtraction map was not performed. It is preferable that larger study cohorts investigating stroke locations associated with ipsilateral thalamic diaschisis include a bilateral asymmetry correction.
This study was carried out at one time point and varying time intervals after stroke. While diaschisis can be reversible, the correlation between clinical scores and the presence of ipsilateral thalamic diaschisis should be taken with extreme caution. Ideally, sequential longitudinal imaging studies should be conducted to study further the functional, structural and clinical consequences of ipsilateral thalamic diaschisis and its potential disappearance over time.
Conclusion
The presence of ipsilateral thalamic diaschisis in symptomatic patients with steno-occlusive disease, detected using BOLD-CVR, is characterized by thalamic volume reduction, reduced thalamic blood flow, and worse stroke severity scores at symptom onset as well as three months follow-up. This finding suggests that ipsilateral thalamic diaschisis may be an important clinical imaging marker in symptomatic patients with steno-occlusive disease.
Supplemental Material
Supplemental material, Supplemental Material1 for Characterizing ipsilateral thalamic diaschisis in symptomatic cerebrovascular steno-occlusive patients by Christiaan Hendrik Bas van Niftrik, Martina Sebök, Giovanni Muscas, Marco Piccirelli, Carlo Serra, Niklaus Krayenbühl, Athina Pangalu, Oliver Bozinov, Andreas Luft, Christoph Stippich, Luca Regli and Jorn Fierstra in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, Supplemental Material2 for Characterizing ipsilateral thalamic diaschisis in symptomatic cerebrovascular steno-occlusive patients by Christiaan Hendrik Bas van Niftrik, Martina Sebök, Giovanni Muscas, Marco Piccirelli, Carlo Serra, Niklaus Krayenbühl, Athina Pangalu, Oliver Bozinov, Andreas Luft, Christoph Stippich, Luca Regli and Jorn Fierstra in Journal of Cerebral Blood Flow & Metabolism
Authors' contributions
All authors conceived and designed the study, and analyzed and interpreted the data. BVN, MS, MP, GM, JF acquired specific part of the data. BVN constructed the figures. BVN and JF wrote the manuscript. All other authors (MS, MP, GM, CSe, NK, AP, OB, ARL, CSt and LR) critically revised the manuscript. All authors approved the final version of the manuscript and agree to be accountable for the accuracy of the work. JF supervised the study and is the guarantor.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: JF and CHBVN are supported by the Swiss Cancer League (KFS-3975-08-2016-R) and by Forschungskredit; Postdoc. Zurich University FK-16-040.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Finger S, Koehler PJ, Jagella C. The Monakow concept of diaschisis. Arch Neurol 2004; 61(2): 283–288. [DOI] [PubMed] [Google Scholar]
- 2.Baron JC, Bousser MG, Comar D, et al. “Crossed cerebellar diaschisis” in human supratentorial brain infarction. Trans Am Neurol Assoc 1981; 105: 459–461. [PubMed] [Google Scholar]
- 3.De Reuck J, Decoo D, Lemahieu I, Strijckmans K, Goethals P, Van Maele G. Ipsilateral thalamic diaschisis after middle cerebral artery infarction. J Neurol Sci 1995; 134: 130–135. [DOI] [PubMed] [Google Scholar]
- 4.Sakashita Y, Matsuda H, Kakuda K, Takamori M. Hypoperfusion and vasoreactivity in the thalamus and cerebellum after stroke. Stroke 1993; 24: 84–87. [DOI] [PubMed] [Google Scholar]
- 5.Sobesky J, Thiel A, Ghaemi M, et al. Crossed cerebellar diaschisis in acute human stroke: a PET study of serial changes and response to supratentorial reperfusion. J Cereb Blood Flow Metab 2005; 25: 1685–1691. [DOI] [PubMed] [Google Scholar]
- 6.Reidler P, Thierfelder KM, Fabritius MP, et al. Thalamic diaschisis in acute ischemic stroke: occurrence, perfusion characteristics, and impact on outcome. Stroke 2018; 49: 931–937. [DOI] [PubMed] [Google Scholar]
- 7.Kuchcinski G, Munsch F, Lopes R, et al. Thalamic alterations remote to infarct appear as focal iron accumulation and impact clinical outcome. Brain 2017; 140: 1932–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duering M, Schmidt R. Remote changes after ischaemic infarcts: a distant target for therapy?. Brain 2017; 140: 1818–1820. [DOI] [PubMed] [Google Scholar]
- 9.Baron JC, Yamauchi H, Fujioka M, Endres M. Selective neuronal loss in ischemic stroke and cerebrovascular disease. J Cereb Blood Flow Metab 2014; 34: 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strother MK, Anderson MD, Singer RJ, et al. Cerebrovascular collaterals correlate with disease severity in adult North American patients with Moyamoya disease. AJNR Am J Neuroradiol 2014; 35: 1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalela JA, Alsop DC, Gonzalez-Atavales JB, et al. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke 2000; 31: 680–687. [DOI] [PubMed] [Google Scholar]
- 12.Kang KM, Sohn CH, Choi SH, et al. Detection of crossed cerebellar diaschisis in hyperacute ischemic stroke using arterial spin-labeled MR imaging. PLoS One 2017; 12: e0173971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sebök M, Van Niftrik CHB, Piccirelli M, et al. BOLD cerebrovascular reactivity as a novel marker for crossed cerebellar diaschisis. Neurology 2018, pp. 1–10. [DOI] [PubMed] [Google Scholar]
- 14.WMA General Assembly. World Medical Asssociation declaration of Helsinki, Ferney-Voltaire, France: World Medical Association, 1964. [Google Scholar]
- 15.Fierstra J, van Niftrik C, Warnock G, et al. Staging hemodynamic failure with blood oxygen-level–dependent functional magnetic resonance imaging cerebrovascular reactivity. Stroke 2018; 49(3): 621–629. [DOI] [PubMed]
- 16.van Niftrik CHB, Piccirelli M, Bozinov O, et al. Iterative analysis of cerebrovascular reactivity dynamic response by temporal decomposition. Brain Behav 2017; 7(9): e00705, . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischl B, Salat DH, van der Kouwe AJ, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage 2004; 23: S69–S84. [DOI] [PubMed] [Google Scholar]
- 18.Destrieux C, Fischl B, Dale A, et al. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage 2010; 53: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serrati C, Marchal G, Rioux P, et al. Contralateral cerebellar hypometabolism: a predictor for stroke outcome?. J Neurol Neurosurg Psychiatry 1994; 57: 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feeney DM, Baron JC. Diaschisis. Stroke 1986; 17: 817–830. [DOI] [PubMed] [Google Scholar]
- 21.Kuhl DE, Phelps ME, Kowell AP, et al. Effects of stroke on local cerebral metabolism and perfusion: mapping by emission computed tomography of 18FDG and 13NH3. Ann Neurol 1980; 8: 47–60. [DOI] [PubMed] [Google Scholar]
- 22.Wise RJ, Bernardi S, Frackowiak RS, et al. Serial observations on the pathophysiology of acute stroke. The transition from ischaemia to infarction as reflected in regional oxygen extraction. Brain 1983; 106: 197–222. [DOI] [PubMed] [Google Scholar]
- 23.Saver JL, Johnston KC, Homer D, et al. Infarct volume as a surrogate or auxiliary outcome measure in ischemic stroke clinical trials. The RANTTAS Investigators. Stroke 1999; 30: 293–298. [DOI] [PubMed] [Google Scholar]
- 24.Stinear CM, Barber PA, Smale PR, et al. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain 2007; 130: 170–180. [DOI] [PubMed] [Google Scholar]
- 25.Cassidy JM, Tran G, Quinlan EB, et al. Neuroimaging identifies patients most likely to respond to a restorative stroke therapy. Stroke 2018; 49: 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schiemanck SK, Post MW, Witkamp TD, et al. Relationship between ischemic lesion volume and functional status in the 2nd week after middle cerebral artery stroke. Neurorehabil Neural Repair 2005; 19: 133–138. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Zhang Y, Xing S, et al. Secondary neurodegeneration in remote regions after focal cerebral infarction: a new target for stroke management?. Stroke 2012; 43: 1700–1705. [DOI] [PubMed] [Google Scholar]
- 28.Tien RD, Ashdown BC. Crossed cerebellar diaschisis and crossed cerebellar atrophy: correlation of MR findings, clinical symptoms, and supratentorial diseases in 26 patients. AJR Am J Roentgenol 1992; 158: 1155–1159. [DOI] [PubMed] [Google Scholar]
- 29.Carrera E, Tononi G. Diaschisis: past, present, future. Brain 2014; 137: 2408–2422. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa T, Yoshida Y, Okudera T, et al. Secondary thalamic degeneration after cerebral infarction in the middle cerebral artery distribution: evaluation with MR imaging. Radiology 1997; 204: 255–262. [DOI] [PubMed] [Google Scholar]
- 31.Sherman SM. The thalamus is more than just a relay. Curr Opin Neurobiol 2007; 17: 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherman SM. Thalamus plays a central role in ongoing cortical functioning. Nat Neurosci 2016; 19: 533–541. [DOI] [PubMed] [Google Scholar]
- 33.Davis TL, Kwong KK, Weisskoff RM, et al. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci U S A 1998; 95: 1834–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental Material1 for Characterizing ipsilateral thalamic diaschisis in symptomatic cerebrovascular steno-occlusive patients by Christiaan Hendrik Bas van Niftrik, Martina Sebök, Giovanni Muscas, Marco Piccirelli, Carlo Serra, Niklaus Krayenbühl, Athina Pangalu, Oliver Bozinov, Andreas Luft, Christoph Stippich, Luca Regli and Jorn Fierstra in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, Supplemental Material2 for Characterizing ipsilateral thalamic diaschisis in symptomatic cerebrovascular steno-occlusive patients by Christiaan Hendrik Bas van Niftrik, Martina Sebök, Giovanni Muscas, Marco Piccirelli, Carlo Serra, Niklaus Krayenbühl, Athina Pangalu, Oliver Bozinov, Andreas Luft, Christoph Stippich, Luca Regli and Jorn Fierstra in Journal of Cerebral Blood Flow & Metabolism




