Abstract
In large vessel occlusion (LVO) stroke, it is unclear whether severity of ischemia is involved in early post-thrombolysis recanalization over and above thrombus site and length. Here we assessed the relationships between perfusion parameters and early recanalization following intravenous thrombolysis administration in LVO patients. From a multicenter registry, we identified 218 thrombolysed LVO patients referred for thrombectomy with both (i) pre-thrombolysis MRI, including diffusion-weighted imaging (DWI), T2*-imaging, MR-angiography and dynamic susceptibility-contrast perfusion-weighted imaging (PWI); and (ii) evaluation of recanalization on first angiographic run or non-invasive imaging ≤ 3 h from thrombolysis start. Infarct core volume on DWI, PWI-DWI mismatch volume and hypoperfusion intensity ratio (HIR; defined as Tmax ≥ 10 s volume/ Tmax ≥ 6 s volume, low HIR indicating milder hypoperfusion) were determined using a commercially available software. Early recanalization occurred in 34 (16%) patients, and multivariable analysis was associated with lower HIR (P = 0.006), shorter thrombus on T2*-imaging (P < 0.001) and more distal occlusion (P = 0.006). However, the relationship between HIR and early recanalization was robust only for thrombus length <14 mm. In summary, the present study disclosed an association between lower HIR and early post-thrombolysis recanalization. Early post-thrombolysis recanalization is therefore determined not only by thrombus site and length but also by severity of ischemia.
Keywords: Cerebral perfusion, ischemic stroke, thrombolysis, thrombectomy, magnetic resonance imaging
Introduction
Perfusion imaging is of considerable interest in the acute stroke setting as it allows one to measure the presence and volume of infarct core – the already irreversibly injured tissue – and penumbra – the severely hypoperfused but still salvageable tissue – and thereby brings on powerful information on patient's response to reperfusion therapies such as intravenous thrombolysis (IVT) and mechanical thrombectomy, that may in turn help decision-making.1–3 Regarding IVT, perfusion imaging may improve the identification of patients likely to benefit.4,5 Regarding thrombectomy, several reports have shown that both infarct core volume, assessed using either MR-6 or CT-based approaches,7 and penumbral volume8,9 strongly predict three-month functional outcome within early time windows, i.e. ≤ 6 h, and that the clinical benefit of adding thrombectomy to medical therapy (including IVT or not) is related to infarct core volume.6,10 Moreover, perfusion imaging also guides thrombectomy decisions beyond 6 h, and based on the results of the DAWN and DEFUSE-3 trials,11,12 infarct core measurement is now recommended for thrombectomy eligibility in LVO patients seen 6–24 h from last known normal.13 Consequently, perfusion imaging is used in some centers to guide thrombectomy decisions both within and beyond the 6-h time window.14
The main therapeutic target of reperfusion therapies in acute stroke with LVO is early recanalization (i.e. within the very first few hours), because recanalization is strongly associated with smaller infarct growth,12,15 and consequently with improved functional outcome.9,15,16 However, IVT has limited efficacy to induce early recanalization (10–30% early recanalization rate in LVO patients17–20), which has led to the testing and subsequent licensing of thrombectomy added on IVT (so-called ‘bridging therapy’). Yet, the mechanisms underlying early recanalization failure following IVT, i.e., clot resistance, remain inadequately understood. Thrombus site is one key factor explaining resistance to IVT,17,19 while thrombus length, as determined on admission T2*-weighted MR or CT angiography (CTA),17,19–23 as well as thrombus perviousness on CTA,20,21,24,25 may also help predict recanalization. Finally, thrombus composition may also be involved.26,27 Due to the relatively low early recanalization rate following IVT together with its potential harmful effects, some authors have even called into question the implementation of IVT before thrombectomy in LVO patients.28,29 This issue will only be resolved by randomized controlled trials, and several trials testing thrombectomy alone vs. bridging are currently underway (e.g. SWIFT-DIRECT [NCT03192332] and MR-CLEAN NO-IV [ISRCTN80619088]). Identifying strong predictors of post-IVT early recanalization may help to identify LVO patients most likely to benefit from thrombolysis before thrombectomy. It is therefore important to investigate the mechanisms and predictors of post-IVT early recanalization.
Perhaps surprisingly, the potential predictive value of perfusion parameters for post-IVT early recanalization in LVO patients has been little studied so far. One study found that core volume was not associated with post-IVT early recanalization.26 Two studies have investigated the relationship between severity of hypoperfusion and post-IVT recanalization assessed at 24 h post-stroke30,31 which is not relevant to the current thrombectomy paradigm and in addition includes futile reperfusion, i.e. recanalization occurring too late to save sizeable penumbral volumes.
In the present primarily mechanistic study, we assessed the relationships between core volume and markers of hypoperfusion severity on one hand, and occurrence of early post-IVT recanalization on the other hand, in LVO patients undergoing multimodal admission MRI. In order to assess post-IVT early recanalization, we exploited a large multi-centric sample of LVO patients intended for thrombectomy since bridging therapy became standard-of-care, i.e. in whom early recanalization is routinely assessed on first-run conventional angiography.
Methods
Patients
From a large French multi-center registry (PREDICT-RECANAL) of consecutive LVO stroke patients referred for thrombectomy after IVT,19 we analyzed for the present study the data of the six centers that carry out perfusion-weighted imaging (PWI) as part of routine admission imaging (Sainte-Anne [Paris], Hospices civils [Lyon], Orléans, Tours, Montpellier and Nancy university hospitals). Among these six centers, the data were collected prospectively or retrospectively for three centers each. All centers had on-site endovascular capabilities except one, whose eligible patients were transferred to the nearest thrombectomy capable center (i.e. drip-and-ship, as opposed to mothership, paradigm). In line with French recommendations,32 MRI was implemented as first-line imaging in candidates for reperfusion therapy in all centers of the present study. CT and CTA were performed in case of contraindication to MRI. The stroke MRI protocol in the participating centers included diffusion-weighted imaging (DWI), T2*, intracranial MR angiography (MRA) and dynamic susceptibility-contrast PWI, whenever feasible with no delay. The PWI acquisition parameters used in each participating center are presented in Supplemental Table 1. The PWI data were not a basis for decision-making in routine except in borderline cases.
Inclusion criteria for the present study were therefore (1) patient admitted for acute stroke with LVO of the anterior circulation between May 2015 (when thrombectomy became routine care in these centers) and March 2017; (2) pre-IVT imaging with MRI, including DWI, T2*, MRA and PWI; (3) IVT with alteplase 0.9 mg/kg; and (4) evaluation of early recanalization before thrombectomy (see below).
In accordance with French legislation, approval by an Ethics Committee was not required as this study only implied retrospective analysis of anonymized data collected as part of routine care. However, each patient was informed by post of his/her participation in this study, and was offered the possibility to withdraw.
Clinical data
The following variables were extracted from the registries: age, sex, vascular risk factors and past medical history, pre-stroke medication, National Institutes of Health Stroke Scale (NIHSS) score on admission, time between symptom onset and start of IVT (onset-to-IVT time), and time elapsed between start of IVT and evaluation of early recanalization (see below).
Imaging data
A stroke neurologist with experience in neuroimaging (PS) reviewed the pre-IVT anonymized imaging of all included patients, blinded to recanalization status. The following variables were collected: (1) occlusion site, according to four categories: intracranial internal carotid artery T or L (ICA-T/L), M1 proximal, M1 distal and M2, where the M1 segment was defined as the first portion of the MCA up to the main bifurcation, and dichotomized as proximal or distal based on the MCA origin-to-thrombus distance (<10 mm and ≥10 mm, respectively)21,33; (2) length of the susceptibility vessel sign, a marker of thrombus on T2*, based on previously published methodology34; (3) DWI lesion volume, semi-automatically segmented by means of Olea Sphere software (Olea Medical SAS, La Ciotat, France) after applying a threshold of 620 × 10−6 mm2/s on apparent diffusion coefficient maps,35 with manual correction when necessary; (4) PWI-DWI mismatch volume, calculated as the volumetric difference between the time-to-maximum (Tmax) ≥ 6 s volume and the DWI lesion volume, the Tmax≥6 s volume being automatically segmented from PWI using Olea Sphere36 with manual correction whenever necessary; and (5) severity of hypoperfusion, assessed using the hypoperfusion intensity ratio (HIR),37 defined here as the proportion of the Tmax≥6 s volume with Tmax≥10 s (i.e. HIR = [Tmax≥10 s volume / Tmax ≥ 6 s volume] × 100),38–41 low HIR indicating milder hypoperfusion. Note that the HIR is derived only from perfusion maps, i.e. the Tmax volumes do not take into consideration the DWI lesion.
The inter-observer agreement for occlusion site and thrombus length for a larger cohort from which the present sample was extracted was high for both.19 Regarding perfusion data, an independent rater measured the Tmax ≥ 6 s and ≥ 10 s volumes in a random sub-sample of 25% (n = 53) of the total patient sample. Inter-rater reproducibility was determined by means of the intraclass correlation coefficient.
Early recanalization evaluation
Early recanalization was defined as recanalization occurring within 3 h after initiation of IVT, a delay that includes typical ‘drip-and-ship’ situations.42 In all participating centers, eligible patients were referred for thrombectomy as soon as possible after start of IVT. Consequently, early recanalization was evaluated on the first angiographic run carried out as part of intended thrombectomy. However, in some patients with significant improvement in neurological status before reaching the angiosuite, recanalization was evaluated using non-invasive vascular imaging (MRA or CTA). Two readers independently evaluated recanalization, blinded to clinical and imaging data. Discrepancies were resolved by consensus. On conventional angiography, recanalization was defined as 2b-3 on the modified Thrombolysis in Cerebral infarction scale for ICA-T/L or M1 occlusions, and 3 on the Arterial Occlusive Lesion scale for M2 occlusions.33 Otherwise, recanalization was defined as 3 on the Arterial Occlusive Lesion scale on CTA or MRA.
Statistical analysis
Continuous variables were described as mean ± standard deviation or median (interquartile range), as appropriate, and categorical variables as number (percentage). Univariable comparison of patients with and without early recanalization was performed using Student t or Mann–Whitney U tests for continuous variables, and Chi-square or Fisher exact test for categorical variables, as appropriate. Baseline variables associated with early recanalization in univariable analysis were candidates for inclusion into a multivariable binary logistic regression model, with early recanalization as dependent variable. We used a hybrid stepwise selection method, in which variables are entered into the model at P < 0.20, and retained only if they remained associated at P < 0.05 with the dependent variable, in such a way that each forward selection step can be followed by one or more backward elimination steps. The order in which the variables enter the model was entirely automated, based on the results of the Chi-square statistic. We alternatively used backward variable selection to ensure that the same candidate variables were included in the multivariable model. Covariates were assessed for potential collinearity and interaction effects. The overall performance of the model was estimated using c-statistic (i.e. the area under the receiver-operating characteristic curve), and internal cross-validation of the model was based on 5000 bootstrap replicates. Probability curves and contour plots were created based on the predictions of the final multivariable logistic model. Statistical analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC) and SPSS 16.0 (SPSS Inc.). Two-tailed P < 0.05 was considered statistically significant.
Results
Patients' characteristics
Across the six participating centers, 386 patients with ICA-T/L, M1 or M2 occlusion and eligible for thrombectomy received IVT during the study period. Of these, 168 were excluded (see Figure 1 for the reasons for exclusion), leaving 218 patients for the final analysis. Excluded patients with baseline MRI but without PWI or with poor quality PWI (n = 109) had similar age (P = 0.66), sex (P = 0.27), NIHSS score (P = 0.08), occlusion site (P = 0.57) and early recanalization rate (P = 0.60) than included patients. The intra-class correlation coefficient between the two observers was excellent for HIR, Tmax ≥ 6s and Tmax ≥ 10 s volumes (0.92 [95%CI 0.87–0.96], 0.99 [95%CI 0.98–0.99] and 0.98 [95%CI 0.96–0.99], respectively).
Figure 1.
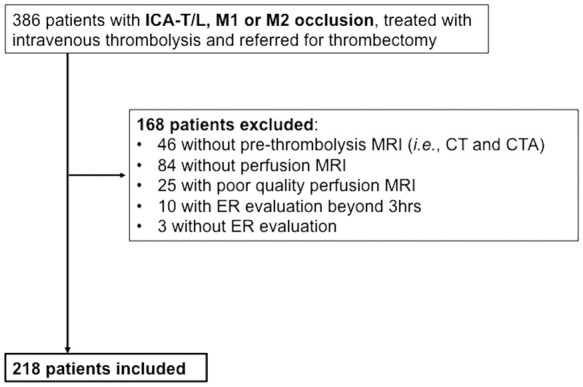
Study flow chart.
CT: computerized tomography; ER: early recanalization; ICA-T/L: intracranial internal carotid artery occlusion; M1: first segment of the middle cerebral artery; M2: second segment of the middle cerebral artery; MRI: magnetic resonance imaging.
The baseline characteristics of included patients are presented in Table 1. Patients were managed according to the mothership or drip-and-ship paradigms in 88% (191/218) and 12% (27/218), respectively. Early recanalization was evaluated on first angiographic run in 207/218 (95%) patients, and on non-invasive imaging in the remaining. Early recanalization occurred in 34/218 (16%) patients, with rates of 3% (1/40), 7% (6/91), 28% (11/39) and 33% (16/48) in ICA-T/L, proximal M1, distal M1 and M2 occlusions, respectively. Considering the similar incidence of early recanalization in ICA-T/L and proximal M1, and in distal M1 and M2 occlusions, respectively, these four subsets were collapsed into two categories for further analyses (distal M1 or M2 vs. ICA-T/L or proximal M1 occlusions).
Table 1.
Baseline characteristics of the population and univariable relationships with early recanalization.a
| Whole cohort n = 218 | Early recanalization n = 34 | No early recanalization n = 184 | P | |
|---|---|---|---|---|
| Patient history | ||||
| Age (years) | 72 (61–80) | 72 (59–83) | 72 (61–80) | 0.78 |
| Men | 120 (55) | 18 (53) | 102 (55) | 0.79 |
| Hypertension | 122 (56) | 19 (56) | 103 (56) | 0.99 |
| Diabetes mellitus | 35 (16) | 9 (27) | 26 (14) | 0.07 |
| Current smoking | 27 (12) | 5 (15) | 22 (12) | 0.66 |
| Antiplatelet use | 73 (34) | 13 (38) | 60 (33) | 0.52 |
| Statin use | 68 (31) | 14 (41) | 54 (29) | 0.17 |
| Pre-IVT characteristics | ||||
| NIHSS score | 16 (10–20) | 12 (6–17) | 16 (10–20) | <0.01 |
| Onset-to-IVT time (min) | 160 (130–192) | 163 (144–194) | 158 (129–192) | 0.48 |
| Pre-IVT MRI | ||||
| Occlusion site | <0.01 | |||
| ICA-T/L | 40 (18) | 1 (3) | 39 (21) | |
| Proximal M1 | 91 (42) | 6 (18) | 85 (46) | |
| Distal M1 | 39 (18) | 11 (32) | 28 (15) | |
| M2 | 48 (22) | 16 (47) | 32 (17) | |
| Thrombus lengthb (mm) | 12.6 (9.2–17.6) | 7.2 (5.8–8.9) | 14.0 (10.2–19.7) | <0.01 |
| DWI volume (ml) | 12 (5–23) | 9 (2–18) | 12 (5–29) | 0.04 |
| PWI-DWI mismatch volume (ml) | 62 (34–100) | 39 (17–63) | 71 (40–104) | <0.01 |
| Tmax ≥ 6s volume (ml) | 83 (45–127) | 44 (27–88) | 88 (57–130) | <0.01 |
| Tmax ≥ 10s volume (ml) | 31 (13–63) | 13 (4–37) | 37 (15–66) | <0.01 |
| HIR (%) | 43 (30–53) | 31 (19–49) | 43 (32–54) | <0.01 |
| Recanalization evaluation | ||||
| IVT to recanalization assessment time (min) | 62 (37–97) | 61 (44–118) | 62 (35–97) | 0.68 |
Categorical variables are expressed as numbers (%) and continuous variables as median (IQR).
Missing values: 20 patients without visible susceptibility vessel sign (4 with early recanalization and 16 without).
DWI: diffusion-weighted imaging; ICA-T/L: intracranial internal carotid artery occlusion; M1: first segment of the middle cerebral artery; M2: second segment of the middle cerebral artery.
Variables associated with early recanalization in univariable analysis
The univariable associations between early recanalization and baseline variables are presented in Table 1. The following variables were significantly associated with early recanalization occurrence: lower baseline NIHSS, more distal occlusions, shorter thrombus, smaller DWI lesion, Tmax ≥ 6s, Tmax ≥ 10s and PWI-DWI mismatch volumes, and lower HIR.
Multivariable analysis with early recanalization as the dependent variable
The multivariable model (n = 198 patients; 20 patients without susceptibility vessel sign were excluded for this analysis) is presented in Table 2. Lower HIR (P = 0.006), smaller thrombus length (P < 0.001) and more distal occlusion site (P=0.006) were associated with early recanalization occurrence. Other candidate variables for the multivariable model (i.e. with P < 0.20 in the univariable analysis), namely NIHSS score, DWI, Tmax ≥ 10s, Tmax ≥ 6s and PWI-DWI mismatch volumes, statin use and history of diabetes mellitus, were not retained in the multivariable model (see details in Supplemental Results). Given the presence of a significant (P = 0.02) interaction between thrombus length and HIR, the HIR×thrombus length interaction term was also included in the multivariable model. There was no notable multicollinearity within the multivariable model presented in Table 2 (largest variance inflation factor: 1.17, condition index: 7.42). The c-statistic of the final multivariable model was 0.913 (95%CI: 0.860–0.967). The internal cross-validation of the model based on 5000 bootstrap replicates showed a similar c-statistic (0.920; 95%CI: 0.862–0.965). Based on these results, and as an ancillary analysis, we compared the performance of the model for early recanalization prediction with and without adding HIR; the results are presented in Supplemental Results.
Table 2.
Variables associated with early recanalization in multivariable logistic regression.a
| β coefficient | Standard error | P | |
|---|---|---|---|
| HIR, per 10% increase | −1.19 | 0.43 | 0.006 |
| Thrombus length, per 1 mm increase | −0.78 | 0.22 | <0.001 |
| Occlusion site | 0.006 | ||
| ICA-T/L or M1 proximal | 0 (Reference) | – | |
| M1 distal or M2 | 1.54 | 0.56 | |
| Interaction term (HIR*thrombus length) | 0.09 | 0.04 | 0.02 |
Note: Due to the presence of an interaction term in the logistic model, results are presented as beta coefficients and standard error rather than odds ratios and 95% confidence intervals.
Twenty patients without visible susceptibility vessel sign were excluded from the model, which therefore included 198 patients (30 with early recanalization and 168 without).
HIR: hypoperfusion intensity ratio; ICA-T/L: intracranial internal carotid artery occlusion; M1: first segment of the middle cerebral artery; M2: second segment of the cerebral artery.
The predicted probability of post-IVT early recanalization as a function of HIR and occlusion site, illustrated for different thrombus lengths, is presented in Figure 2. Figure 3 displays the predicted probability of post-IVT early recanalization according to thrombus length, HIR and occlusion site, taking into account the interaction between HIR and thrombus length. To allow individual assessment, each patient's data according to early recanalization status are also plotted in the figure. Typical patients with and without early recanalization are shown in Figures 4 and 5, respectively.
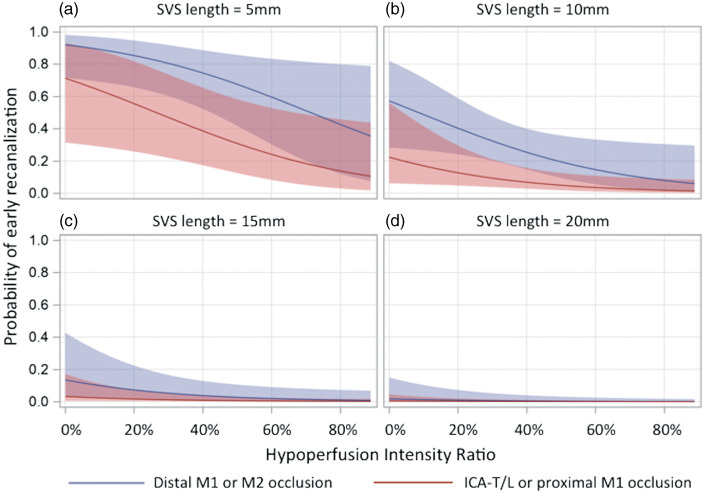
Figure 2.
Probability of post-thrombolysis early recanalization according to hypoperfusion intensity ratio, occlusion site and thrombus length. The regression curves are estimates of the probability of post-thrombolysis early recanalization according to the hypoperfusion intensity ratio for average patients with thrombus lengths of (a) 5 mm, (b) 10 mm, (c) 15 mm and (d) 20 mm. The red curve corresponds to ICA-T/L/proximal M1 occlusions, and the blue curve to distal M1/M2 occlusions. The shaded area corresponds to the 95% confidence interval (logistic regression model). Regression curves for patients with thrombus length >20 mm are not shown as no patient recanalized in this subgroup. ICA-T/L: intracranial internal carotid artery occlusion; M1: first segment of the middle cerebral artery; M2: second segment of the middle cerebral artery.
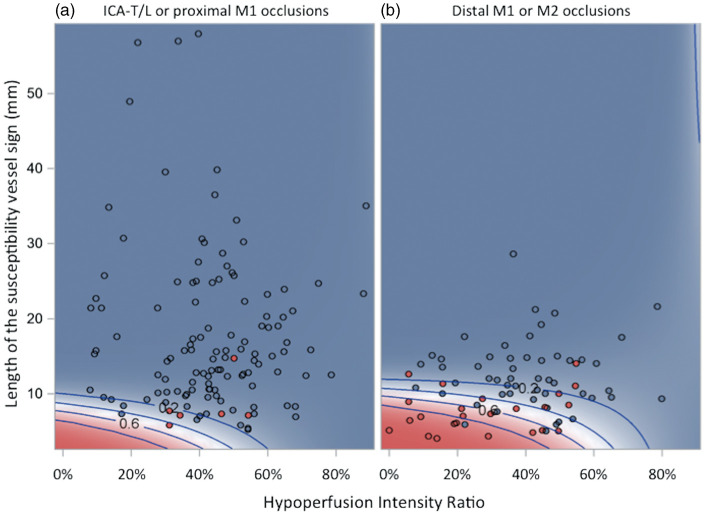
Figure 3.
Contour plots showing post-thrombolysis early recanalization probability according to hypoperfusion intensity ratio and thrombus length in patients with (a) ICA-T/L or proximal M1, and (b) distal M1 or M2 occlusions. Red and blue dots correspond to individual patients with and without early recanalization, respectively. The shading and the concentric contour curves depict the probability of post-thrombolysis early recanalization predicted by the multivariable logistic regression model (Table 2), which includes HIR, thrombus length, occlusion site in two categories and the HIR×thrombus length interaction term. Red and blue shading corresponds to predicted probabilities of early post-thrombolysis recanalization over or beyond 50%, respectively. The contour curves represent predicted probabilities of early recanalization equal to 80%, 60%, 40% and 20%. The interaction term in the model causes the curvature of the contour lines and shows how the effect of HIR on the predicted probability of early recanalization differs with thrombus length and vice versa.
HIR: hypoperfusion intensity ration; ICA-T/L: intracranial internal carotid artery occlusion; M1: first segment of the middle cerebral artery; M2: second segment of the middle cerebral artery.
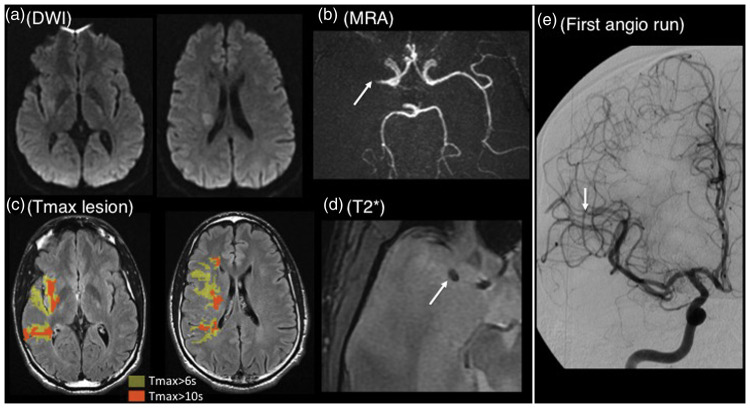
Figure 4.
Typical patient with early recanalization. Thirty-five years old patient with left hemiparesis; MRI obtained 180 min after stroke onset (baseline NIHSS=8). (a) Diffusion-weighted imaging showing a right-sided deep lesion in the middle cerebral artery territory (volume=4 ml); (b) MRA showing a right proximal M1 occlusion (arrow); (c) Tmax ≥ 6s lesion (yellow area, volume=61 ml) and Tmax ≥ 10s lesion (orange area, volume=19 ml) projected onto the fluid-attenuated inversion recovery sequence; corresponding HIR=31%; (d): T2*-imaging showing a small susceptibility vessel sign (arrow, 5 mm). Intravenous thrombolysis was started 210 min after stroke onset and the patient was immediately transported to the angiosuite, where the first angiographic run (e, performed 45 min after thrombolysis start) showed early recanalization (mTICI score = 2b).
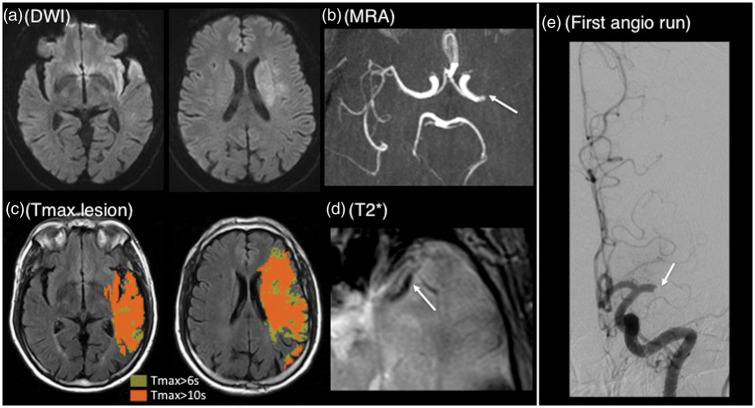
Figure 5.
Typical patient without early recanalization. Sixty-six years old patient with right hemiparesis and dysphasia; MRI obtained 90 min after stroke onset (baseline NIHSS=22). (a) Diffusion-weighted imaging showing a left-sided deep and superficial lesion in the MCA territory (volume=28 ml); (b) MRA showing a left proximal M1 occlusion (arrow); (c) Tmax ≥ 6 s lesion (yellow area, volume=176 ml) and Tmax ≥ 10 s lesion (orange area, volume=109 ml) projected onto the FLAIR sequence; corresponding HIR=62%; (d) T2*-imaging showing a long susceptibility vessel sign (arrow, 14 mm). IVT was started 115 min after stroke onset and the patient was immediately transported to the angiosuite, where the first angiographic run (e, performed 70 min after IVT start) showed persistent occlusion (mTICI score = 0).
Further investigation of the above HIR×thrombus interaction showed that it can be summarized as follows: lower odds of early recanalization with increasing HIR in patients with thrombus length up to approximately 13 mm, and an inverse tendency in patients with thrombus length ≥14 mm (data not shown). Close inspection of our datasets revealed that this effect is in fact entirely driven by 2 out of the 86 patients with thrombus length ≥14 mm, who both experienced early recanalization despite high HIR (see Figure 3). Even though apparently driven by these two patients, this interaction affects the interpretation of the above model, and we accordingly conducted a post hoc analysis including only the patients with thrombus length <14 mm (n = 112). As expected, there was no remaining HIR×thrombus interaction (P = 0.50), and the model yielded thrombus length, occlusion site and HIR as independent ER predictors (see Supplemental Table 2).
Discussion
In this large multicenter cohort of large vessel occlusion patients who underwent pre-thrombolysis PWI and were referred for thrombectomy following thrombolysis, multivariable analysis revealed that milder hypoperfusion severity – evaluated using the HIR – was associated with post-IVT early recanalization, together with smaller thrombus and more distal occlusion sites. As a note of caution, our data do not formally allow the evaluation of the effect of HIR in patients with thrombus length ≥14 mm, in whom the probability of early recanalization is, however, very close to zero.
Our study is the first to report an association between post-IVT early recanalization and HIR. This finding is, however, consistent with two previous studies that reported an association between other indexes of hypoperfusion severity and post-IVT recanalization,30,31 with the caveat that recanalization was evaluated at 24 h, which included futile recanalization and is not relevant anymore in the thrombectomy era. In addition, neither study adjusted the observed association for occlusion site or thrombus length.
Several mechanisms might explain the observed association between lower HIR and post-IVT early recanalization. First, good collaterals, which are strongly associated with lower HIR,37,38,43 may increase the odds of early recanalization via enhanced delivery of the thrombolytic agent to both ends of the thrombus.44–46 Second, non-fully occlusive thrombi, which likely are also associated with milder hypoperfusion and lower HIR, may enhance the odds of the thrombolytic agent permeating the thrombus.20,21,24,25 Last, severe hypoperfusion might favor more organized thrombi, which might in turn be more resistant to alteplase.31 The choice of Tmax cutoffs for the HIR in the present study was based on Olivot et al.,38 who found the so defined HIR to be strongly associated with infarct growth and functional outcome. As sensitivity analysis, we also assessed the HIR using Tmax ≥4 s instead of 6 s as it includes mild hypoperfusion and may better reflect good collaterals,47 which was similarly independently associated (data not shown).
Perfusion imaging has been shown to bring powerful prognostic information for three-month functional outcome after both IVT and thrombectomy, which may in turn help decision-making in early time windows,1,4,9 while infarct core measurement is now recommended beyond 6 h to guide indications for thrombectomy.13 Our results underline another potential utility of perfusion imaging, namely the use of HIR, which can be quickly determined using automated software.38 Relevant to this point, CTP- and MR-derived Tmax volumes have been found to be very similar when assessed in the same patients.48
Of note, the stepwise selection process did not retain other imaging markers of brain ischemia in the multivariable model, including the Tmax ≥ 10s volume, although replacing the HIR by the latter within the model resulted in similar discrimination (data not shown). Because the Tmax ≥ 10s volume is intrinsic to the HIR, the relationship between these two variables is by definition complex. Further studies should clarify the added value of the HIR over simple hypoperfusion volumes. Contrary to two recent studies,19,20 we found that the time elapsed between IVT start and recanalization assessment was not associated with early recanalization, which can be explained by the fact that the present study covered shorter time-intervals, namely the vast majority of patients were managed according to the mothership paradigm (see Results).
Our study has several strengths. First, it is based on a large multicentric sample of LVO patients referred for thrombectomy since bridging therapy became standard of care, thereby limiting potential selection biases typical of the pre-thrombectomy era. Also, patients with early neurological improvement, in whom early recanalization was evaluated using non-invasive vascular imaging, were also included, again limiting potential bias. Second, the method used for HIR determination was mostly automatized and therefore objective, using a licensed and widely available software, and accordingly the inter-rater reproducibility was excellent. Last, early recanalization was assessed independently by two experienced raters, reducing the risk of classification errors.
This study also has limitations. First, the decision to refer patients for thrombectomy was under the treating physician, which might have induced bias. For instance, patients with large core volumes may less likely be referred for thrombectomy. That said, the median DWI volume in our population (Table 1) was similar to both DWI6 and CT-perfusion7 median core volumes reported in recent thrombectomy trials. Second, one-third of patients from our MR-assessed population were excluded because PWI was not performed or was of poor quality, which may have impacted our results. Note, however, that the included and excluded MRI populations had similar baseline characteristics.
Conclusion
This mechanistic study revealed an association between milder hypoperfusion severity and early post-IVT recanalization. However, this association was statistically robust only for thrombus length <14 mm. Early post-thrombolysis recanalization is therefore determined not only by thrombus site and length but also by the severity of ischemia.
Supplemental Material
Supplemental Material for Relationships between brain perfusion and early recanalization after intravenous thrombolysis for acute stroke with large vessel occlusion by Pierre Seners, Guillaume Turc, Stéphanie Lion, Jean-Philippe Cottier, Tae-Hee Cho, Caroline Arquizan, Serge Bracard, Canan Ozsancak, Laurence Legrand, Olivier Naggara, Séverine Debiais, Yves Berthezene, Vincent Costalat, Sébastien Richard, Christophe Magni, Norbert Nighoghossian, Ana-Paula Narata, Cyril Dargazanli, Benjamin Gory, Jean-Louis Mas, Catherine Oppenheim and Jean-Claude Baron in Journal of Cerebral Blood Flow & Metabolism
Authors' contributions
Study conception and design: PS, CO and JCB. Data acquisition: PS, GT, SL, JPC, THC, CA, SB, CO, LL, ON, SD, YB, VC, SR, CM, NN, APN, CD, BG, JLM, CO, JCB. Data analysis and interpretation: PS, CO, JCB and GT. Statistical analysis: PS and GT. Article draft: PS and JCB. Critical revision of the manuscript for important intellectual content: PS, GT, SL, JPC, THC, CA, SB, CO, LL, ON, SD, YB, VC, SR, CM, NN, APN, CD, BG, JLM, CO, JCB. Approved the submitted version: PS, GT, SL, JPC, THC, CA, SB, CO, LL, ON, SD, YB, VC, SR, CM, NN, APN, CD, BG, JLM, CO, JCB.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: PS is funded by the Fondation pour la Recherche Médicale (FDM 41382).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Campbell BC, Parsons MW. Imaging selection for acute stroke intervention. Int J Stroke 2018; 13: 554–567. [DOI] [PubMed] [Google Scholar]
- 2.Olivot JM. Which imaging before reperfusion strategy? Rev Neurol 2017; 173: 584–589. [DOI] [PubMed] [Google Scholar]
- 3.Bang OY, Chung J-W, Son J, et al. Multimodal MRI-based triage for acute stroke therapy: challenges and progress. Front Neurol 2018; 9: 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bivard A, Levi C, Krishnamurthy V, et al. Perfusion computed tomography to assist decision making for stroke thrombolysis. Brain 2015; 138: 1919–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bivard A, Lou M, Levi CR, et al. Too good to treat? ischemic stroke patients with small computed tomography perfusion lesions may not benefit from thrombolysis. Ann Neurol 2016; 80: 286–293. [DOI] [PubMed] [Google Scholar]
- 6.Xie Y, Oppenheim C, Guillemin F, et al. Pretreatment lesional volume impacts clinical outcome and thrombectomy efficacy. Ann Neurol 2018; 83: 178–185. [DOI] [PubMed] [Google Scholar]
- 7.Campbell BC, Majoie CB, Albers GW, et al. Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol 2019; 18: 46–55. [DOI] [PubMed] [Google Scholar]
- 8.Wannamaker R, Guinand T, Menon BK, et al. Computed tomographic perfusion predicts poor outcomes in a randomized trial of endovascular therapy. Stroke 2018; 49: 1426–1433. [DOI] [PubMed] [Google Scholar]
- 9.Lansberg MG, Christensen S, Kemp S, et al. Computed tomographic perfusion to predict response to recanalization in ischemic stroke. Ann Neurol 2017; 81: 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albers GW. Late window paradox. Stroke 2018; 49: 768–771. [DOI] [PubMed] [Google Scholar]
- 11.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; 378: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 13.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 14.Fisher M, Goyal M. Variance of imaging protocols for patients with suspected acute ischemic stroke because of large-vessel occlusion. Stroke 2018; 49: 1805–1808. [DOI] [PubMed] [Google Scholar]
- 15.Lansberg MG, Straka M, Kemp S, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol 2012; 11: 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 2007; 38: 967–973. [DOI] [PubMed] [Google Scholar]
- 17.Seners P, Turc G, Maier B, et al. Incidence and predictors of early recanalization after intravenous thrombolysis: a systematic review and meta-analysis. Stroke 2016; 47: 2409–2412. [DOI] [PubMed] [Google Scholar]
- 18.Tsivgoulis G, Katsanos AH, Schellinger PD, et al. Successful reperfusion with intravenous thrombolysis preceding mechanical thrombectomy in large-vessel occlusions. Stroke 2018; 49: 232–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seners P, Turc G, Naggara O, et al. Post-thrombolysis recanalization in stroke referrals for thrombectomy: incidence, predictors, and prediction scores. Stroke 2018; 49: 2975–2982. [DOI] [PubMed] [Google Scholar]
- 20.Menon BK, Al-Ajlan FS, Najm M, et al. Association of clinical, imaging, and thrombus characteristics with recanalization of visible intracranial occlusion in patients with acute ischemic stroke. JAMA 2018; 320: 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra SM, Dykeman J, Sajobi TT, et al. Early reperfusion rates with IV tPA are determined by CTA clot characteristics. AJNR Am J Neuroradiol 2014; 35: 2265–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Behrens L, Mohlenbruch M, Stampfl S, et al. Effect of thrombus size on recanalization by bridging intravenous thrombolysis. Eur J Neurol 2014; 21: 1406–1410. [DOI] [PubMed] [Google Scholar]
- 23.Kaesmacher J, Giarrusso M, Zibold F, et al. Rates and quality of preinterventional reperfusion in patients with direct access to endovascular treatment. Stroke 2018; 49: 1924–1932. [DOI] [PubMed] [Google Scholar]
- 24.Ahn SH, d'Esterre CD, Qazi EM, et al. Occult anterograde flow is an under-recognized but crucial predictor of early recanalization with intravenous tissue-type plasminogen activator. Stroke 2015; 46: 968–975. [DOI] [PubMed] [Google Scholar]
- 25.Frolich AM, Psychogios MN, Klotz E, Schramm R, Knauth M, Schramm P. Antegrade flow across incomplete vessel occlusions can be distinguished from retrograde collateral flow using 4-dimensional computed tomographic angiography. Stroke 2012; 43: 2974–2979. [DOI] [PubMed] [Google Scholar]
- 26.Kimura K, Sakamoto Y, Aoki J, et al. Clinical and MRI predictors of no early recanalization within 1 hour after tissue-type plasminogen activator administration. Stroke 2011; 42: 3150–3155. [DOI] [PubMed] [Google Scholar]
- 27.De Meyer SF, Andersson T, Baxter B, et al. Analyses of thrombi in acute ischemic stroke: a consensus statement on current knowledge and future directions. Int J Stroke 2017; 12: 606–614. [DOI] [PubMed] [Google Scholar]
- 28.Fischer U, Kaesmacher J, Mendes Pereira V, et al. Direct mechanical thrombectomy versus combined intravenous and mechanical thrombectomy in large-artery anterior circulation stroke: a topical review. Stroke 2017; 48: 2912–2918. [DOI] [PubMed] [Google Scholar]
- 29.Bellwald S, Weber R, Dobrocky T, et al. Direct mechanical intervention versus bridging therapy in stroke patients eligible for intravenous thrombolysis: a pooled analysis of 2 registries. Stroke 2017; 48: 3282–3288. [DOI] [PubMed] [Google Scholar]
- 30.Nicoli F, Lafaye de Micheaux P, Girard N. Perfusion-weighted imaging-derived collateral flow index is a predictor of MCA M1 recanalization after i.v. thrombolysis. AJNR Am J Neuroradiol 2013; 34: 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nighoghossian N, Hermier M, Adeleine P, et al. Baseline magnetic resonance imaging parameters and stroke outcome in patients treated by intravenous tissue plasminogen activator. Stroke 2003; 34: 458–463. [DOI] [PubMed] [Google Scholar]
- 32.Haute Autorité de Santé. Stroke: early management (alert, prehospital phase, initial hospital phase, indications for thrombolysis). Clinical practice guidelines. May 2009. Available at: https://www.has-sante.fr/portail/upload/docs/application/pdf/2010-03/stroke_early_management_-_guidelines_-_english_version.pdf.
- 33.Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013; 44: 2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naggara O, Raymond J, Domingo Ayllon M, et al. T2* “susceptibility vessel sign” demonstrates clot location and length in acute ischemic stroke. PLoS One 2013; 8: e76727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purushotham A, Campbell BC, Straka M, et al. Apparent diffusion coefficient threshold for delineation of ischemic core. Int J Stroke 2015; 10: 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olea-Medical, www.olea-medical.com/en/ (2018, accessed 25 February 2019).
- 37.Bang OY, Saver JL, Alger JR, et al. Determinants of the distribution and severity of hypoperfusion in patients with ischemic stroke. Neurology 2008; 71: 1804–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olivot JM, Mlynash M, Inoue M, et al. Hypoperfusion intensity ratio predicts infarct progression and functional outcome in the DEFUSE 2 cohort. Stroke 2014; 45: 1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guenego A, Mlynash M, Christensen S, et al. Hypoperfusion ratio predicts infarct growth during transfer for thrombectomy. Ann Neurol 2018; 84: 616–620. [DOI] [PubMed] [Google Scholar]
- 40.Wouters A, Dupont P, Christensen S, et al. Association between time from stroke onset and fluid-attenuated inversion recovery lesion intensity is modified by status of collateral circulation. Stroke 2016; 47: 1018–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hernandez-Perez M, Puig J, Blasco G, et al. Dynamic magnetic resonance angiography provides collateral circulation and hemodynamic information in acute ischemic stroke. Stroke 2016; 47: 531–534. [DOI] [PubMed] [Google Scholar]
- 42.Mueller L, Pult F, Meisterernst J, et al. Impact of intravenous thrombolysis on recanalization rates in patients with stroke treated with bridging therapy. Eur J Neurol 2017; 24: 1016–1021. [DOI] [PubMed] [Google Scholar]
- 43.Bang OY, Saver JL, Buck BH, et al. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry 2008; 79: 625–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bang OY, Goyal M, Liebeskind DS. Collateral circulation in ischemic stroke: assessment tools and therapeutic strategies. Stroke 2015; 46: 3302–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang S, Zhang X, Yan S, et al. The velocity of collateral filling predicts recanalization in acute ischemic stroke after intravenous thrombolysis. Sci Rep 2016; 6: 27880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calleja AI, Cortijo E, Garcia-Bermejo P, et al. Collateral circulation on perfusion-computed tomography-source images predicts the response to stroke intravenous thrombolysis. Eur J Neurol 2013; 20: 795–802. [DOI] [PubMed] [Google Scholar]
- 47.Lee MJ, Son JP, Kim SJ, et al. Predicting collateral status with magnetic resonance perfusion parameters: probabilistic approach with a Tmax-derived prediction model. Stroke 2015; 46: 2800–2807. [DOI] [PubMed] [Google Scholar]
- 48.Lin L, Bivard A, Levi CR, et al. Comparison of computed tomographic and magnetic resonance perfusion measurements in acute ischemic stroke: back-to-back quantitative analysis. Stroke 2014; 45: 1727–1732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Relationships between brain perfusion and early recanalization after intravenous thrombolysis for acute stroke with large vessel occlusion by Pierre Seners, Guillaume Turc, Stéphanie Lion, Jean-Philippe Cottier, Tae-Hee Cho, Caroline Arquizan, Serge Bracard, Canan Ozsancak, Laurence Legrand, Olivier Naggara, Séverine Debiais, Yves Berthezene, Vincent Costalat, Sébastien Richard, Christophe Magni, Norbert Nighoghossian, Ana-Paula Narata, Cyril Dargazanli, Benjamin Gory, Jean-Louis Mas, Catherine Oppenheim and Jean-Claude Baron in Journal of Cerebral Blood Flow & Metabolism