Abstract
Age is the strongest risk factor for cerebrovascular disease; however, age-related changes in cerebrovascular function are still not well understood. The objective of this study was to measure cerebral vasomotor reactivity (CVMR) during hypo- and hypercapnia across the adult lifespan. One hundred fifty-three healthy participants (21–80 years) underwent measurements of cerebral blood flow velocity (CBFV) via transcranial Doppler, mean arterial pressure (MAP) via plethysmograph, and end-tidal CO2 (EtCO2) via capnography during hyperventilation (hypocapnia) and a modified rebreathing protocol (hypercapnia). Cerebrovascular conductance (CVCi) and resistance (CVRi) indices were calculated from the ratios of CBFV and MAP. CVMRs were assessed by the slopes of CBFV and CVCi in response to changes in EtCO2. The baseline CBFV and CVCi decreased and CVRi increased with age. Advanced age was associated with progressive declines in CVMR during hypocapnia indicating reduced cerebral vasoconstriction, but increases in CVMR during hypercapnia indicating increased vasodilation. A negative correlation between hypo- and hypercapnic CVMRs was observed across all subjects (CBFV%/ EtCO2: r = −0.419, CVCi%/ EtCO2: r = −0.442, P < 0.0001). Collectively, these findings suggest that aging is associated with decreases in CBFV, increases in cerebrovascular resistance, reduced vasoconstriction during hypocapnia, but increased vasodilatory responsiveness during hypercapnia.
Keywords: Aging, cerebral vasomotor reactivity, hypercapnia, hypocapnia, transcranial Doppler
Introduction
Cerebral blood flow (CBF) is highly sensitive to partial pressure of arterial carbon dioxide (PaCO2). Elevated PaCO2 (hypercapnia) increases, whereas reduced PaCO2 (hypocapnia) decreases CBF.1 Cerebral vasomotor reactivity (CVMR) reflects the ability of cerebral blood vessels to dilate or constrict to regulate CBF in response to changes in PaCO2.1
CVMR during hypercapnia can be assessed either by using stepwise increases in inspiratory air concentration of CO2 or a rebreathing method in which a progressive increase in PaCO2 is induced by having subjects rebreathe his/her own expired air.2–4 Similar results of CVMR between the two methods have been reported previously.5 Clinically, CVMR measured by the rebreathing method mimics the conditions during sleep apnea where PaCO2 progressively builds up with breathing cessations.6 On the other hand, CVMR during hypocapnia is commonly assessed by asking study participants to perform a short period of hyperventilation of room air to induce progressive decreases in PaCO2.5,7,8 Measurement of CVMR has been used widely in clinical and research assessment of cerebrovascular function.9
Age is the strongest risk factor for cerebrovascular disease10; however, age-related changes in CVMR are still not well understood.8,11–13 In our previous study, we have observed that older adults have reduced cerebral blood flow velocity (CBFV) at rest, increased hypercapnic CVMR, and decreased hypocapnic CVMR when compared with young individuals.13 Conversely, other studies have reported reduced or no change in hypercapnic CVMR with age.8,11,12 These discrepancies may reflect the limitations of relatively small sample size, differences in methods used to assess CVMR, or both in these studies.
Measurement of CVMR is also influenced by the marked changes in systemic arterial blood pressure (BP) during hypo- or hypercapnia which are likely to be mediated by the central and peripheral chemoreceptor responses to changes in PaCO2.14 Recent evidence suggests that aging15 and clinical conditions, such as cardiovascular disease16,17 and sleep apnea,18 may alter peripheral chemoreceptor sensitivity and BP responses to hypercapnia, thus underscoring the necessity to understand the systemic effects of BP responses on CVMR.
The purpose of this study was to extend our previous studies to determine the age-related differences in CVMR during hypo- and hypercapnia across the adult lifespan in a large sample of 153 healthy adults aged 21–80 years. On the basis of our previous study, we hypothesized that advanced age is associated with (1) reduced CBFV and increased cerebrovascular resistance at rest, and (2) reduced hypocapnic CVMR, but increased hypercapnic CVMR in healthy human subjects.
Materials and methods
Subjects
One hundred fifty-three healthy participants aged between 21 and 80 years were recruited through flyers and newspaper advertisements from the Dallas-Fort Worth metropolitan areas. Exclusion criteria were the presence of ischemic or structural heart disease screened by 12-lead ECG and echocardiography, office BP>140/90 mmHg confirmed by ambulatory BP monitoring, carotid artery atherosclerotic plaque or stenosis with > 50% occlusion imaged by ultrasound, diabetes mellitus screened by the presence of symptoms, use of antidiabetic drugs, or fasting blood glucose >126 mg/dL, body mass index (BMI) > 40 kg/m2, current smoking or a history of smoking within the last two years, active alcohol or drug abuse, history of brain trauma, and the presence or history of cerebrovascular (e.g. stroke), neurological, psychiatric, or inflammatory disease, pregnancy or breast-feeding women. To minimize the confounding effects of aerobic exercise training on CVMR,19,20 individuals who participate in structured aerobic exercise training program (i.e. moderate intensity, aerobic exercise training over the past two years) were also excluded.
This study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas, and was performed in accordance with the guidelines of the Declaration of Helsinki and Belmont Report. All subjects provided informed written consent prior to participation.
Instrumentation and data acquisition
CBFV was measured from the middle cerebral artery (MCA) using a 2-MHz transcranial Doppler (TCD) probe (Multi-Dop X2, Compumedics/DWL, Singen, Germany). The probe was securely attached by an individually created mold to fit the facial bone structure and keep the position and angle of the probe unchanged during the study.21 End-tidal CO2 (EtCO2), an estimate of PaCO2,22 and breathing frequency were monitored using a capnography (Carpnograd, Novamatrix, Wallingford, CT, USA). Arterial blood oxygen saturation (SaO2) was measured by a pulse oximeter (Biox 3700, Ohmeda Monitoring Systems, Boulder, CO, USA). Brachial BP was intermittently measured from the right upper arm using an electrosphygmomanometer (Suntech, Morrsville, NC, USA). Beat-to-beat mean arterial pressure (MAP) was continuously monitored from the middle finger of the left hand using a Finapres (Finapres Medical Systems, Amsterdam, The Netherlands). The finger pressure transducer was fixed at the heart level during the study. Heart rate (HR) was recorded via a three-lead ECG system (Hewlett-Packard, Palo Alto, CA, USA). All data were collected with a sampling frequency of 1000 Hz and stored in a computer for off-line analysis using a data acquisition and analysis software (Acknowledge, BIOPAC systems, Goleta, CA, USA).
Experimental procedures
All experiments were performed in an environmentally controlled laboratory with an ambient temperature of 22℃. Subjects refrained from high intensity exercise, caffeinated beverages, or alcohol >24 h before experiment. After subjects have rested in the supine position for >10 min, a nose clip was placed and subjects breathed through a mouthpiece with a Y valve, with one end connected to the mouthpiece, one end open to room air, and one end connected to a 5L rebreathing bag.2,5,13
First, baseline CBFV, HR, MAP, and EtCO2 were recorded simultaneously for 3 min. During these measurements, subjects were instructed to breathe normally and avoid body movement or Valsalva maneuvers. After baseline data collection, subjects were coached by an investigator to perform voluntary hyperventilation for 20 s (1 breath/second) which induced a brief period of hypocapnia. Following hyperventilation, a > 5-min recovery period was provided until all hemodynamic variables returned to the baseline level.5 Then, a modified rebreathing protocol was used to induce hypercapnia.2 Briefly, at the end of a deep inspiration, the Y valve of the mouthpiece was switched to the rebreathing bag to induce a progressive increase in EtCO2 for 3 min.2 During rebreathing, a small amount of oxygen was added to the rebreathing bag based on each subject's basal metabolic rate (estimated using the Harris-Benedict formula) to maintain constant arterial blood oxygen saturation.2 Intermittent brachial cuff BP was measured at baseline and during the rebreathing protocol to corroborate finger arterial pressure measurement. The rebreathing protocol was tolerated by all subjects.
Data analysis
Baseline data were obtained by averaging a 1-min steady-state data segment under resting condition before hyperventilation and rebreathing protocol. Cerebrovascular conductance index (CVCi) and resistance (CVRi) indices were calculated from the ratio of mean CBFV and MAP. CVCi was calculated to account for the effects of changes in MAP on CBFV during hypo- and hypercapnia.2 The magnitude of absolute changes in CBFV, CVCi, MAP, HR, and EtCO2 during hypo- and hypercapnia is presented as ΔCBFV, ΔCVCi, ΔMAP, ΔHR, and ΔEtCO2, respectively. The percentage changes in hypo- and hypercapnic ΔCBFV% and ΔCVCi% were calculated relative to their corresponding baseline values. The pulsatility index was calculated as systolic minus diastolic CBFV divided by mean CBFV.
Hypocapnic CVMR
During hyperventilation, maximal hemodynamic changes were calculated from the average of three breath cycles after the reduction of EtCO2 reached nadir. Then, CVMR was calculated as the ratio of ΔCFBV% to ΔEtCO2 and ΔCVCi% to ΔEtCO2.2 The breath-by-breath analysis was not performed during hypocapnia because rapid changes in EtCO2 during hyperventilation may not accurately reflect the concurrent changes in PaCO2. Thus, maximal reductions in CBFV% and CVCi% in response to the maximal reduction in EtCO2 were used to assess CVMR when these variables reached the steady-state. Cardiovascular reactivity to changes in EtCO2 was calculated as the ratio of ΔMAP to the corresponding changes in EtCO2.
Hypercapnic CVMR
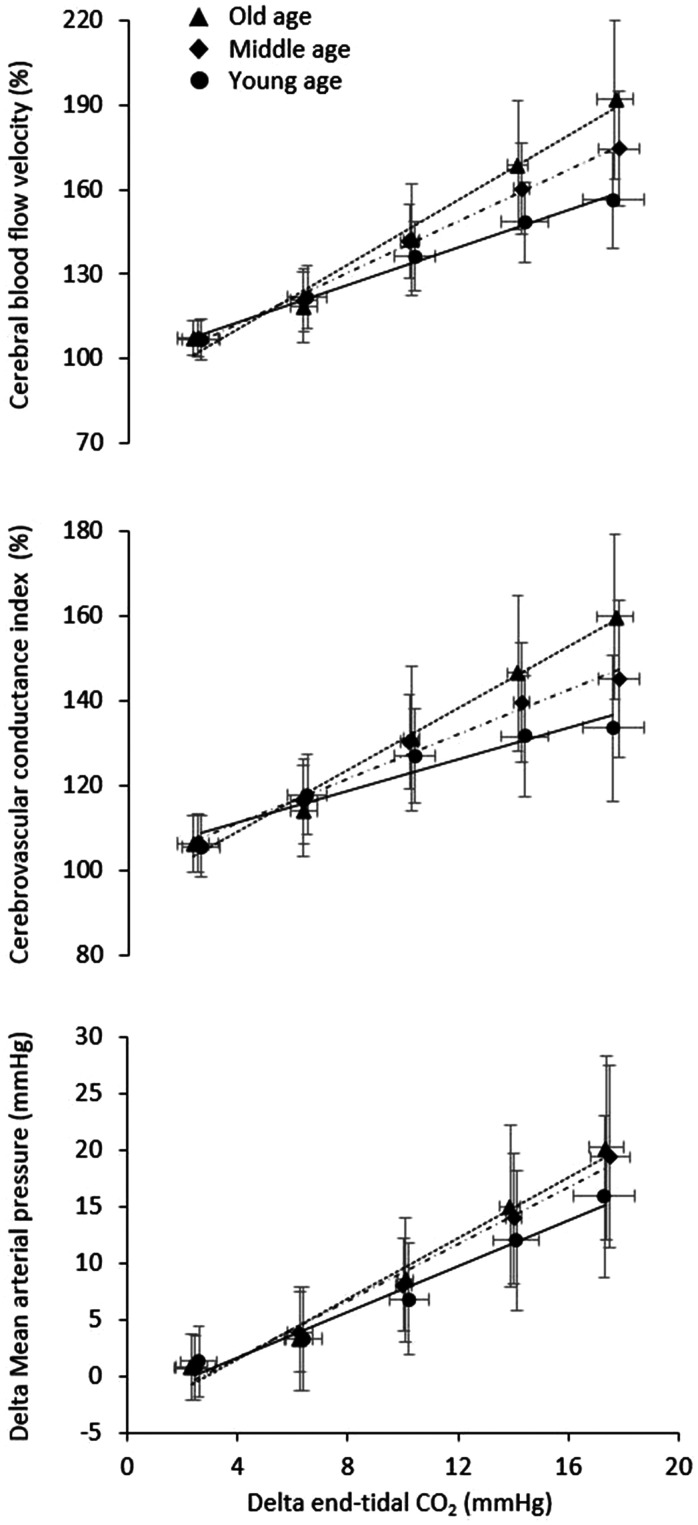
Baseline data for hypercapnia were obtained by averaging a 1-min steady-state data segment before rebreathing protocol. Breath-by-breath data were extracted for analysis during rebreathing.2,23 Due to a deep inspiration performed when switching a Y valve, a brief reduction in both EtCO2 and CBFV was observed at the beginning of rebreathing. Hence, to address the effect of hypercapnia, only those data of ΔCBFV% (>100%) and ΔEtCO2 ( > baseline level) were included in analysis. Linear regression analysis of ΔCBFV% vs. ΔEtCO2 and ΔCVCi% vs. ΔEtCO2 was performed within each subject and then group averaged for statistical analysis. The slopes of these regression lines were used as the estimates of CVMR during hypercapnia.2 Cardiovascular reactivity to ΔEtCO2 was assessed by the slope of linear regression between ΔMAP and ΔEtCO2. Of note, the rebreathing protocol used in the current study was shorter than the protocol used in our previous studies (5 minutes) and induced moderate increase in EtCO2 and CBFV, thus providing the rationale for using the linear regression analysis.2 The results of linear fitting were examined by the coefficient of determination (R2). For data visualization, group-averaged bin plots of CBFV%, CVCi%, and MAP were created based on every 4 mmHg increase in EtCO2 from the baseline (Figure 1).
Figure 1.
Group-averaged bin plots showing the cerebral blood flow velocity (CBFV, %), cerebrovascular conductance index (CVCi, %), mean arterial pressure (MAP, mmHg), and end-tidal CO2 (EtCO2, mmHg)) during rebreathing in young, middle-aged, and older adults. The error bars represent standard deviations. Each bin represents 4 mmHg change in EtCO2.
Statistical analysis
The χ-square test was used to examine the group differences in categorical variables. Two-way analysis of variance (ANOVA) was used to test the main effects of age and sex as well as the interaction effect of age×sex. After observing no notable effects of sex or age×sex on hypo- and hypercapnic CVMR measures, we used one-way ANOVA to focus upon the age-related group differences. Furthermore, linear mixed model (LMM) analysis was conducted to examine the contributions of hypercapnic changes in ΔEtCO2 and ΔMAP to ΔCBFV% and also account for potential correlations between repeated measures using an unstructured covariance structure.24 From the LMM analysis, the unstandardized regression coefficients for EtCO2 and MAP were compared among the age groups using one-way ANOVA. The Bonferroni method was used to correct for multiple pairwise comparisons. Pearson's product-moment correlation analysis was used to examine the relation between hypo- and hypercapnic CVMRs. Data are presented as mean ± standard deviation. An α-level of 0.05 was set as the criterion for statistical significance. All statistical analyses were performed using SPSS 19.0 (SPSS, Chicago, IL, USA).
Results
Baseline hemodynamics
Data from 36 subjects were excluded from analysis because of poor TCD, finger pressure, and/or EtCO2 signals during hyperventilation and/or rebreathing. This resulted in the final sample of 117 subjects and their characteristics and baseline hemodynamic data are shown in Table 1. The men and women distributions were similar across the age groups. The brachial systolic BP increased with age, whereas diastolic BP peaked in middle age, and then decreased in the old group. With increasing age, mean, systolic, and diastolic CBFV as well as CVCi were decreased progressively, while the PI was increased. Older adults showed lower EtCO2 than the younger subjects, while SaO2 was similar across the age groups. Women showed higher mean, systolic, and diastolic CBFVs and CVCi than men.
Table 1.
Subject characteristics and baseline cerebral and systemic hemodynamics.
| Young |
Middle age |
Old |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Men | Women | Men | Women | Men | Women | Age | Sex | Age×sex |
| n | 16 | 20 | 24 | 20 | 15 | 22 | 0.423 | ||
| Age (years) | 34 ± 7 | 33 ± 6 | 54 ± 6 | 59 ± 5* | 71 ± 3 | 70 ± 4 | <0.001 | 0.354 | 0.031 |
| Height (cm) | 177 ± 8 | 163 ± 6 | 177 ± 7 | 165 ± 9 | 176 ± 5 | 160 ± 7 | 0.136 | <0.001 | 0.475 |
| Body mass (kg) | 81 ± 10 | 62 ± 12 | 90 ± 13 | 71 ± 10 | 87 ± 10 | 70 ± 9 | 0.001 | <0.001 | 0.930 |
| BMI (kg/m2) | 26 ± 4 | 23 ± 3 | 28 ± 4 | 26 ± 4 | 28 ± 3 | 27 ± 4 | 0.001 | 0.009 | 0.467 |
| Systolic BP (mmHg) | 114 ± 11 | 108 ± 7 | 117 ± 12 | 116 ± 7 | 123 ± 13 | 122 ± 13 | 0.001 | 0.170 | 0.552 |
| Diastolic BP (mmHg) | 71 ± 10 | 69 ± 8 | 76 ± 7 | 74 ± 6 | 74 ± 8 | 70 ± 9 | 0.034 | 0.051 | 0.925 |
| Baseline hemodynamics | |||||||||
| EtCO2 (mmHg) | 40 ± 3 | 38 ± 3 | 38 ± 3 | 38 ± 3 | 37 ± 3 | 37 ± 2 | 0.006 | 0.101 | 0.487 |
| SaO2 (%) | 98 ± 1 | 99 ± 1 | 99 ± 1 | 99 ± 1 | 99 ± 1 | 99 ± 1 | 0.417 | 0.489 | 0.117 |
| Heart rate (bpm) | 68 ± 11 | 69 ± 9 | 69 ± 12 | 71 ± 8 | 65 ± 9 | 69 ± 7 | 0.375 | 0.183 | 0.683 |
| MAP (mmHg) | 89 ± 14 | 91 ± 14 | 93 ± 11 | 96 ± 11 | 98 ± 14 | 98 ± 11 | 0.014 | 0.446 | 0.812 |
| Mean CBFV (cm/s) | 56 ± 6 | 65 ± 8 | 49 ± 9 | 53 ± 10 | 43 ± 6 | 49 ± 9 | <0.001 | <0.001 | 0.449 |
| Systolic CBFV (cm/s) | 85 ± 12 | 95 ± 12 | 74 ± 13 | 79 ± 15 | 69 ± 9 | 77 ± 13 | <0.001 | 0.001 | 0.695 |
| Diatolic CBFV (cm/s) | 39 ± 5 | 42 ± 6 | 32 ± 6 | 33 ± 7 | 26 ± 5 | 28 ± 6 | <0.001 | 0.036 | 0.510 |
| Pulsatility index (a.u.) | 0.81 ± 0.17 | 0.82 ± 0.08 | 0.85 ± 0.10 | 0.88 ± 0.07 | 1.01 ± 0.17 | 0.99 ± 0.14 | <0.001 | 0.858 | 0.653 |
| CVCi (cm/s×mmHg) | 0.64 ± 0.12 | 0.73 ± 0.14 | 0.54 ± 0.12 | 0.56 ± 0.13 | 0.45 ± 0.08 | 0.51 ± 0.12 | <0.001 | 0.020 | 0.507 |
| CVRi (mmHg/cm/s) | 1.61 ± 0.31 | 4.42 ± 0.28 | 1.96 ± 0.49 | 1.89 ± 0.45 | 2.32 ± 0.46 | 2.08 ± 0.46 | <0.001 | 0.039 | 0.660 |
Note: Data are mean ± standard deviation. *P < 0.05: compared with middle-aged men. Bold values represent P < 0.05.
BPM: beats per minute; BMI: body mass index; BP: blood pressure; CBFV: cerebral blood flow velocity; CVCi: cerebrovascular conductance index; CVRi: cerebrovascular resistance index; EtCO2: end-tidal CO2; MAP: mean arterial pressure; SaO2: arterial blood oxygen saturation.
Hypocapnic CVMR
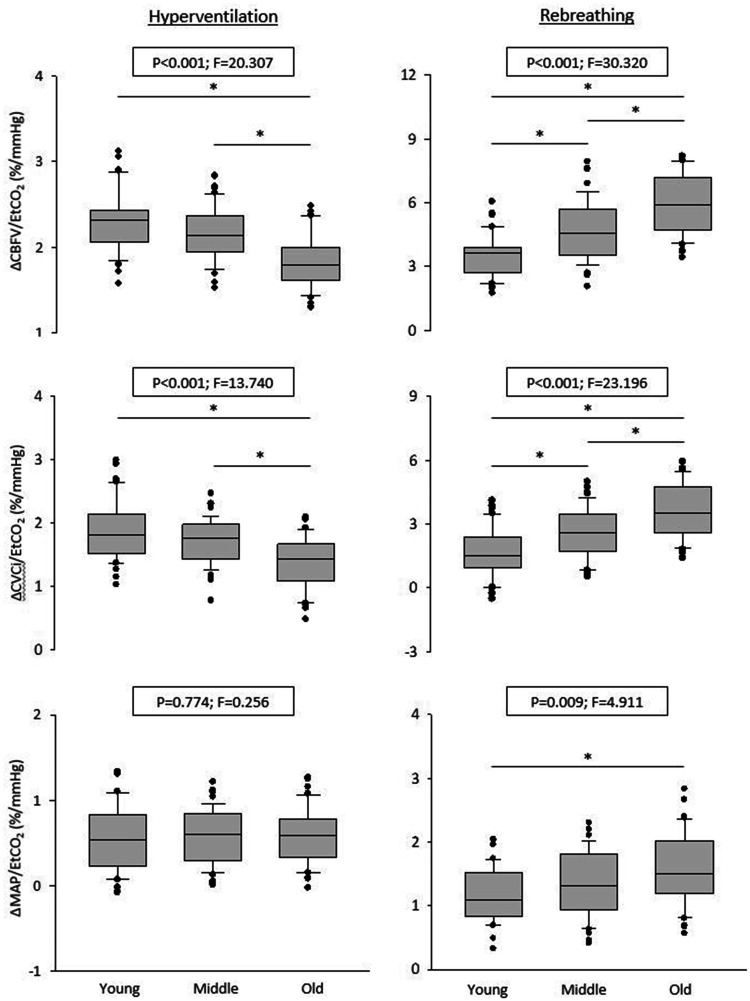
Hemodynamic data during hyperventilation are presented in Table 2. During hyperventilation, EtCO2, MAP, CBFV, and CVCi decreased, while HR increased from the baseline. Despite similar reductions in EtCO2 across all age groups, the magnitude of CBFV% and CVCi% reductions was attenuated in the old group. The HR response was attenuated in older groups, while the MAP response was similar across all groups. Figure 2 presents the age-related differences in hypocapnic CVMR and cardiovascular reactivity. The ΔCBFV%/ΔEtCO2 and ΔCVCi%/ΔEtCO2 were significantly attenuated in the old group compared with the young and middle-aged groups, while ΔMAP/ΔEtCO2 remained at a similar level.
Table 2.
Cerebral and systemic hemodynamics during hypocapnia.
| Young |
Middle age |
Old |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Men | Women | Men | Women | Men | Women | Age | Sex | Age× sex |
| ΔEtCO2 (mmHg) | −19 ± 4 | −18 ± 3 | −19 ± 3 | −18 ± 4 | −19 ± 4 | −18 ± 3 | 0.997 | 0.745 | 0.983 |
| ΔHeart rate (bmp) | 19 ± 12 | 19 ± 8 | 14 ± 7 | 11 ± 6 | 6 ± 5 | 8 ± 8 | <0.001 | 0.955 | 0.434 |
| ΔMAP (mmHg) | −11 ± 7 | −9 ± 7 | −11 ± 7 | −11 ± 6 | −11 ± 7 | −10 ± 6 | 0.773 | 0.628 | 0.808 |
| ΔCBFV (cm/s) | −24 ± 7 | −27 ± 6 | −20 ± 5 | −21 ± 7 | −15 ± 5 | −17 ± 5 | <0.001 | 0.109 | 0.912 |
| ΔCVCi (cm/s×mmHg) | −0.23 ± 0.09 | −0.26 ± 0.10 | −0.18 ± 0.07 | −0.18 ± 0.07 | −0.12 ± 0.06 | −0.13 ± 0.07 | <0.001 | 0.358 | 0.747 |
| ΔCBFV (%) | −36 ± 13 | −35 ± 10 | −32 ± 7 | −31 ± 7 | −26 ± 10 | −25 ± 9 | <0.001 | 0.380 | 0.949 |
| ΔCVCi (%) | −44 ± 12 | −42 ± 8 | −40 ± 6 | −39 ± 7 | −34 ± 9 | −34 ± 7 | <0.001 | 0.580 | 0.993 |
| ΔCBFV/ΔEtCO2 | |||||||||
| (cm/s/mmHg) | 1.31 ± 0.27 | 1.46 ± 0.32 | 1.06 ± 0.21 | 1.15 ± 0.30 | 0.80 ± 0.21 | 0.90 ± 0.25 | 0.001 | 0.028 | 0.866 |
| (%/mmHg) | 2.34 ± 0.36 | 2.26 ± 0.35 | 2.16 ± 0.33 | 2.14 ± 0.28 | 1.85 ± 0.32 | 1.82 ± 0.30 | <0.001 | 0.481 | 0.902 |
| ΔCVCi/ΔEtCO2 | |||||||||
| (cm/s×mmHg/mmHg) | 0.012 ± 0.004 | 0.014 ± 0.005 | 0.009 ± 0.003 | 0.010 ± 0.003 | 0.006 ± 0.002 | 0.007 ± 0.003 | <0.001 | 0.227 | 0.646 |
| (%/mmHg) | 1.89 ± 0.52 | 1.88 ± 0.47 | 1.74 ± 0.39 | 1.71 ± 0.35 | 1.40 ± 0.41 | 1.37 ± 0.41 | <0.001 | 0.770 | 0.995 |
| ΔMAP/ΔEtCO2 | |||||||||
| (mmHg/mmHg) | 0.57 ± 0.37 | 0.50 ± 0.41 | 0.57 ± 0.34 | 0.60 ± 0.30 | 0.60 ± 0.34 | 0.56 ± 0.34 | 0.796 | 0.665 | 0.783 |
Note: Data are mean ± standard deviation. Bold values represent P < 0.05.
BPM: beats per minute; CBFV: mean cerebral blood flow velocity; CVCi: cerebrovascular conductance index; EtCO2: end-tidal CO2; MAP: mean arterial pressure.
Figure 2.
Box-and-whisker plots showing the slopes of mean cerebral blood flow velocity (CBFV), cerebrovascular conductance index (CVCi), and mean arterial pressure (MAP) in response to changes in end-tidal CO2 (EtCO2) during hypo- and hypercapnia in young, middle-aged, and older adults. *P < 0.05.
Hypercapnic CVMR
Hemodynamic data during rebreathing are summarized in Table 3. Despite similar increases in EtCO2 across all age groups, elevations of ΔCBFV% and ΔCVCi% were greater in the older groups. Consistently, hypercapnic CVMRs measured from the slopes of ΔCBFV% vs. EtCO2 and ΔCVCi% vs. EtCO2 were steeper in the older compared with younger groups (Figures 1 and 2). The goodness of line fit for ΔEtCO2 with ΔCBFV% and ΔCVCi% yielded excellent coefficients of determination. The slope of ΔMAP vs. ΔEtCO2 was greater in the older group than in the young group, while the HR response was similar across the groups.
Table 3.
Cerebral and systemic hemodynamics during hypercapnia.
| Young |
Middle age |
Old |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Men | Women | Men | Women | Men | Women | Age | Sex | Age× sex |
| ΔEtCO2 (mmHg) | 19 ± 3 | 18 ± 2 | 19 ± 2 | 19 ± 3 | 19 ± 3 | 18 ± 3 | 0.681 | 0.403 | 0.500 |
| ΔSaO2 (%) | −0.6 ± 0.6 | −0.5 ± 0.7 | −0.6 ± 0.6 | −0.6 ± 0.6 | −0.7 ± 0.6 | −0.5 ± 0.5 | 0.906 | 0.448 | 0.700 |
| ΔHeart rate (bmp) | 8 ± 7 | 9 ± 6 | 7 ± 6 | 8 ± 7 | 4 ± 3 | 8 ± 5 | 0.153 | 0.084 | 0.497 |
| ΔMAP (mmHg) | 17 ± 7 | 17 ± 7 | 20 ± 7 | 21 ± 9 | 22 ± 8 | 25 ± 9 | 0.002 | 0.351 | 0.779 |
| ΔCBFV (cm/s) | 34 ± 9 | 38 ± 13 | 37 ± 8 | 40 ± 11 | 42 ± 11 | 46 ± 13 | 0.010 | 0.093 | 0.941 |
| ΔCVCi (cm/s×mmHg) | 0.22 ± 0.11 | 0.23 ± 0.08 | 0.23 ± 0.07 | 0.24 ± 0.06 | 0.27 ± 0.08 | 0.26 ± 0.09 | 0.056 | 0.753 | 0.882 |
| ΔCBFV (%) | 60 ± 17 | 59 ± 19 | 77 ± 17 | 78 ± 25 | 103 ± 34 | 95 ± 25 | <0.001 | 0.516 | 0.679 |
| ΔCVCi (%) | 35 ± 18 | 34 ± 17 | 46 ± 17 | 47 ± 20 | 64 ± 20 | 56 ± 19 | <0.001 | 0.359 | 0.566 |
| ΔCBFV/ΔEtCO2 | |||||||||
| Slope (cm/s/mmHg) | 1.98 ± 0.48 | 2.17 ± 0.85 | 2.24 ± 0.80 | 2.49 ± 0.67 | 2.65 ± 0.65 | 2.92 ± 0.95 | 0.001 | 0.105 | 0.975 |
| Slope (%/mmHg) | 3.53 ± 0.99 | 3.50 ± 1.08 | 4.71 ± 1.51 | 4.67 ± 1.40 | 6.07 ± 1.49 | 5.85 ± 1.46 | <0.001 | 0.699 | 0.945 |
| R2 | 0.88 ± 0.08 | 0.82 ± 0.23 | 0.92 ± 0.06 | 0.91 ± 0.05 | 0.95 ± 0.02 | 0.92 ± 0.05 | 0.004 | 0.104 | 0.456 |
| ΔCVCi/ΔEtCO2 | |||||||||
| Slope (cm/s×mmHg/mmHg) | 0.011 ± 0.007 | 0.011 ± 0.007 | 0.012 ± 0.006 | 0.015 ± 0.005 | 0.017 ± 0.005 | 0.016 ± 0.007 | 0.003 | 0.485 | 0.678 |
| Slope (%/mmHg) | 1.80 ± 1.14 | 1.58 ± 1.24 | 2.46 ± 1.28 | 2.76 ± 1.15 | 3.94 ± 1.12 | 3.43 ± 1.37 | <0.001 | 0.532 | 0.337 |
| R2 | 0.60 ± 0.31 | 0.59 ± 0.31 | 0.73 ± 0.27 | 0.76 ± 0.21 | 0.89 ± 0.07 | 0.84 ± 0.13 | <0.001 | 0.869 | 0.729 |
| # of data points | 37 ± 10 | 36 ± 9 | 32 ± 11 | 34 ± 10 | 35 ± 9 | 37 ± 9 | 0.236 | 0.556 | 0.792 |
| ΔMAP/ΔEtCO2 | |||||||||
| Slope (mmHg/mmHg) | 1.18 ± 0.49 | 1.20 ± 0.40 | 1.42 ± 0.51 | 1.28 ± 0.54 | 1.47 ± 0.55 | 1.63 ± 0.60 | 0.015 | 0.876 | 0.443 |
| R2 | 0.80 ± 0.24 | 0.83 ± 0.08 | 0.86 ± 0.10 | 0.84 ± 0.12 | 0.84 ± 0.11 | 0.86 ± 0.08 | 0.421 | 0.556 | 0.615 |
Note: Data are mean ± standard deviation. Bold values represent P < 0.05.
BPM: beats per minute; CBFV: mean cerebral blood flow velocity; CVCi: cerebrovascular conductance index; EtCO2: end-tidal CO2; MAP: mean arterial pressure; R2: coefficient of determination; SaO2: arterial blood oxygen saturation.
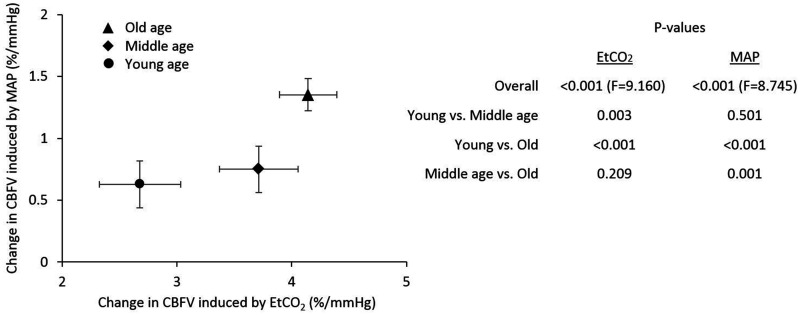
The LMM analysis demonstrated the greater contribution of ΔEtCO2 to ΔCBFV% in the middle-aged and older groups than in the young group. The contribution of ΔMAP was also greater in the old group compared with young and middle-aged groups (Figure 3).
Figure 3.
Linear mixed model analysis exhibiting the contributions of hypercapnic changes in end-tidal CO2 (EtCO2) and mean arterial pressure (MAP) to cerebral blood flow velocity (CBFV) in young, middle-aged, and older groups. The table shows P-values for the overall effect of group x EtCO2 and group x MAP as well as their posthoc tests. The error bars represent standard errors.
Relationship between hypo- and hypercapnic CVMRs
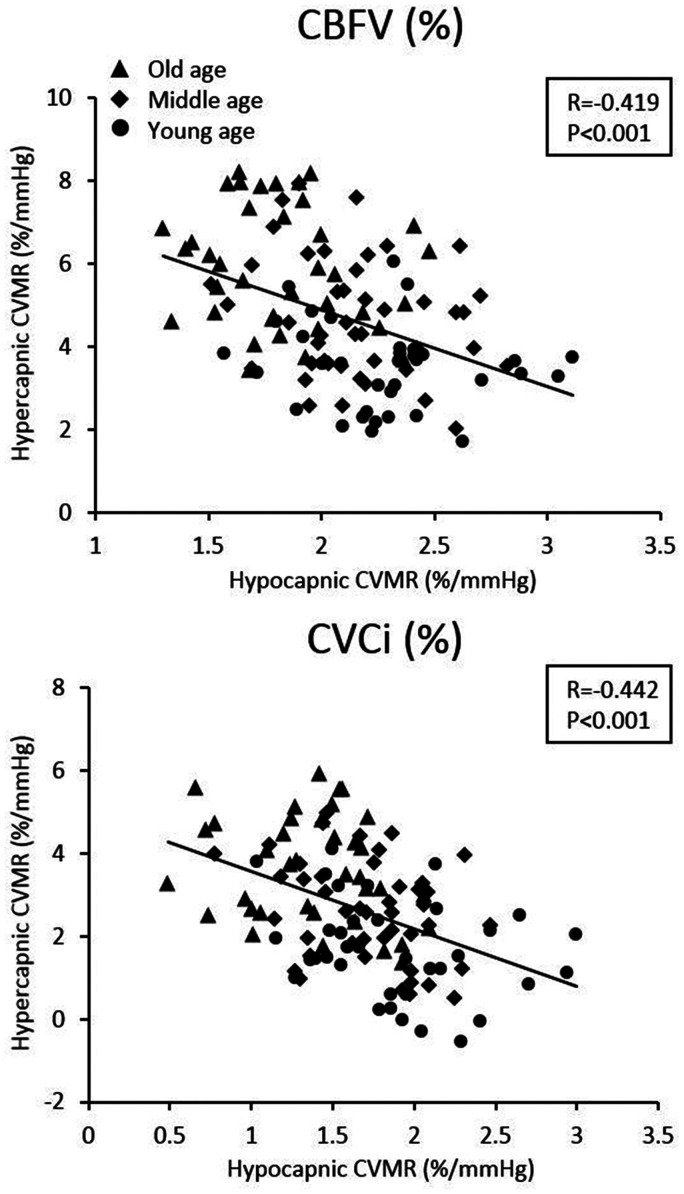
Negative correlations were observed between hypo- and hypercapnic CVMRs, as measured by both CBFV% and CVCi% across all subjects (Figure 4). These correlations indicate that the greater reductions in CBFV or CVCi during hypocapnia are associated with the smaller increases in CBFV or CVCi during hypercapnia.
Figure 4.
Simple correlation between hypo- and hypercapnic cerebral vasomotor reactivity (CVMR) across all subjects. CVMRs were calculated from the slope of cerebral blood flow velocity (CBFV, %) vs. end-tidal CO2 (mmHg) and cerebrovascular conductance index (CVCi, %) vs. end-tidal CO2 (mmHg).
Discussion
This study examined the effects of normal aging on cerebro- and cardiovascular reactivity to hypo- and hypercapnia across the adult lifespan. The main findings from the present study are as follows. First, aging is associated with reduced CBFV and CVCi and increases in CVRi under resting conditions. Second, aging is associated with a progressive decline in hypocapnic CVMR. Third, hypercapnic CVMR is augmented with increasing age. Fourth, hypo- and hypercapnic CVMRs are negatively correlated with each other across all age groups. Collectively, these findings suggest that normal aging is associated with increases in cerebrovascular resistance, leading to the reduced cerebral vasoconstrictive capacity while augmenting the dilatory responsiveness.
Age and baseline hemodynamics
CBF declines with age.25–27 The magnitude of age-related decline in CBF is approximately 0.3–0.6% per year across the adult lifespan as measured by positron emission tomography or phase-contrast magnetic resonance imaging.28 Using TCD, the percentage of CBFV declines we observed in this study was consistent with these previous studies and decreased by ∼23% in men and ∼25% in women between the age range of 21 to 80 years. These estimates equate to the rate of reductions by ∼0.6% in men and ∼0.7% in women per year.
The age-related reductions in CBF may be related to the decreases in brain metabolic rate or increases in cerebrovascular resistance and impedance. Age increases systolic BP and MAP while diastolic BP peaks around middle age and decreases in the older age.29 These systemic changes in BP may cause cerebrovascular remodeling and influence cerebral perfusion pressure.30 A recent study showed a negative correlation between age and intracranial pressure in patients with traumatic brain injury.30 If this finding can be extrapolated to normal adults, age may indeed increase cerebral perfusion pressure through the effects of elevated MAP and reduced intracranial pressure. Consequently, cerebrovascular bed in older adults may undergo compensatory remodeling which increases vascular resistance and impedance and protects the downstream capillary beds and vulnerable brain tissues from over perfusion.
The lower resting EtCO2 we observed from older adults is consistent with previous findings.31,32 In a systematic review of 26 cross-sectional studies, blood PaCO2 decreased by ∼3 mmHg in healthy subjects aged between 20 and 80 years.32 Later, Dhokalia et al. also demonstrated that EtCO2 decreases by 3 mmHg in old adults compared with younger individuals. Further, cross-sectional studies have reported that plasma concentration of bicarbonate is negatively correlated with age, whereas blood concentration of hydrogen is positively correlated with age.32 These observations then suggest that decreases in EtCO2 with normal aging are due to a compensatory response to progressive metabolic acidosis in the blood.31
Cardiovascular and CVMR with aging
In the present study, hypocapnic CVMR (vasoconstriction) was reduced in older adults compared with young individuals. This observation is consistent with previous findings under similar experimental conditions (hyperventilation).7,8 For example, Gotoh et al.7 continuously measured the jugular blood flow as an index of CBF during voluntary hyperventilation, and Yamaguchi et al.8 measured regional CBF by 133Xe inhalation methods during hyperventilation. Both studies demonstrated that the cerebral vasoconstrictor capacity is reduced in older adults compared with young individuals.
In addition, we observed that increases in HR during hypocapnia were attenuated in older adults, while reductions in MAP were similar across the age groups. It is possible that decrease in EtCO2 by hypocapnia stimulates parasympathetic neural system via chemoreceptor and causes vasodilation which can lead to decreased MAP.33 In response to reductions of BP, HR is increased by the activation of baroreceptor reflex (negative feedback system). Thus, blunted baroreflex sensitivity with aging may have attenuated HR response to hypocapnia in older adults.15
In contrast, we observed that hypercapnic CVMR (vasodilation) was enhanced in older adults compared with young individuals. These observations are consistent with our previous report from a small group of young and older participants.13 However, these findings are not consistent with some of the previous studies using the steady-state (i.e. stepwise increases in inspiratory air concentration of CO2) and breath-holding techniques.19,34,35 We suspect a few possible explanations for this discrepancy.
First, rebreathing method may evoke different autonomic and cardiovascular responses compared with the breath-holding and steady-state methods. In terms of the CO2 stimulus, the magnitude of PaCO2 elevation during rebreathing (∼16 mmHg) is greater than the breath-holding method (∼5 mmHg when holding breath for 15 seconds) and the steady-state method (∼8 mmHg when using the typical 5% CO2 gas).36,37 In addition, it has been shown that rebreathing method can elicit the greater responses of peripheral chemoreceptor reflex and sympatho-excitation than the steady-state method.38,39 On the other hand, breath-holding may evoke greater BP elevation due to the Valsalva effects.40 Despite these methodological differences, our previous studies have demonstrated that CVMR assessed by the rebreathing and steady-state methods are similar in healthy young individuals,5 although there is currently no investigation that compared rebreathing and breath-holding methods for CVMR assessment.
Second, our observations of an inverse relationship between hypo- and hypercapnic CVMRs may provide an explanation. In older adults, cerebrovascular disease and/or dysfunction, as manifested, for example, by endothelial dysfunction and blood vessel wall smooth muscle degeneration, increases the basal cerebrovascular tone and risks of cerebral ischemia.41 Increases in basal cerebrovascular tone may shift the operating (baseline) point of the PaCO2-CBF relationship downward closer to the ischemic threshold and decreases the hypocapnic cerebral vasoconstrictor reserve. On the other hand, the downward shift of the operating point may result in a greater reserve for cerebral vasodilation (Figure 4).
Third, we have observed that advancing age is associated with increases in BP responses to hypercapnia, thus increasing the driving force of CBF. Consistent with our observations, peripheral chemoreceptor sensitivity and sympathetic neural responses during hypercapnia were higher in patients with chronic heart failure than in healthy adults.16,17 Further investigations are needed to understand the physiological mechanisms of age-related differences in CVMR during hypo- and hypercapnia.
Methodological considerations
Prior studies demonstrated that hypo- and hypercapnic CVMRs are asymmetric and that hyperventilation performed immediately before rebreathing may attenuate the magnitude of hypercapnic cerebral vasodilation.5,42–44 Because of these reasons, hyperventilation and rebreathing protocols were conducted separately in this study. In addition, we used a relatively short period of rebreathing protocol (i.e. 3 min) to induce moderate but sufficiently large increases in EtCO2 to reduce the possibility of discomfort associated with prolonged CO2 rebreathing. Both CBFV and CVCi responses to moderate changes in EtCO2 can be approximated by a linear relationship to simplify the data analysis (Figure 1).2,5 Further, linear mixed model was used to examine the independent contributions of hypercapnic changes in ΔEtCO2 and ΔMAP to ΔCBFV% and also account for potential correlations between repeated measures.13
It is possible that age alters pulmonary gas exchange efficiency and arterial-pulmonary gradient of CO2 level, especially during rebreathing where EtCO2 increases rapidly.45 Although all age groups had similar magnitudes of EtCO2 changes during both hyperventilation and rebreathing, PaCO2 may be different if age influences pulmonary gas exchange.45 However, no differences in EtCO2 and PaCO2 at rest have been reported from young and older adults,46 and potential effects of age on the differences between EtCO2 and PaCO2 during rebreathing are likely to be small given a continued rebreathing period of 3 min for pulmonary gas exchange to reach an equilibrium.
Study limitations
There are a few important study limitations which need to be discussed. First, changes in CBFV reflect changes in CBF only if the insolated MCA diameter stays relatively constant. The direct measurement of the MCA diameter during moderate changes in arterial pressure and CO2 during craniotomy did not show significant changes. In contrast, recent studies using high resolution magnetic resonance angiographic studies showed MCA dilation during moderate hypercapnia (∼15 mmHg) which suggests the potential underestimation of CVMR assessed by TCD.47 In this study, the magnitude of changes in EtCO2 during both hyperventilation and rebreathing was similar across all the age groups; thus, we assume that hypo- and hypercapnic effects on the MCA diameter are similar across all age groups. In this regard, a recent magnetic resonance angiographic study reported a slight increase in the MCA dimeter with healthy aging.48 If this is the case, the age-related differences in the MCA diameter may have led to an underestimate of CVMR during rebreathing hypercapnia in older adults. Second, we much acknowledge that similar to previous cross-sectional aging studies, the age-related differences in CVMR observed in this study only suggest, but cannot imply a causal relation between age and CVMR. Third, although our study sample was vigorously screened for cardiovascular disease and risk factors, other factors such as lifestyle may influence our findings. In this regard, we have excluded individuals who participate in structured aerobic exercise program; however, dietary factor was not considered.49
Conclusions
This study demonstrated the presence of substantial age-related differences in cerebral and cardiovascular hemodynamics under resting and hypo- and hypercapnic conditions. We found that CBFV and CVCi are decreased and CVRi is increased at rest in older adults compared with younger adults. Furthermore, advanced age progressively decreased hypocapnic CVMR while increasing hypercapnic CVMR. Notably, we also observed negative correlations between hypo- and hypercapnic CVMRs. Collectively, these findings suggest that aging increases cerebrovascular resistance at rest, thus contributing to the reduction of basal CBF. The increased cerebrovascular resistance at rest diminishes the vasoconstrictor reserve during hypocapnia but augments the dilatory capacity in older adults. From the clinical perspective, these findings suggest that older adults with decreased cerebral vasoconstrictor reserve may have an elevated risk of hypoperfusion or ischemia, as their basal cerebrovascular tone is likely closer to an ischemic threshold. In the future research, investigating the association between hypocapnic CVMR and ischemic brain markers (e.g. white matter hyperintensity) may advance our understanding of the age-related increase in cerebrovascular disease.
Acknowledgements
The authors thank all our study participants for their willingness, time, and effort devoted to this study.
Authors' contributions
T Tomoto, RZ, and T Tarumi conceived and designed the paper; RZ and T Tarumi contributed materials and ideas; T Tomoto, JR, MT, RZ, and T Tarumi analyzed and refined both text and intellectual content. All of the authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by R01AG033106, R01HL102457, and K99HL133449. This study was supported in part by the National Institute of Health (R01AG033106, R01HL102457, and K99HL133449).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest 1948; 27: 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claassen JA, Zhang R, Fu Q, et al. Transcranial Doppler estimation of cerebral blood flow and cerebrovascular conductance during modified rebreathing. J Appl Physiol 2007; 102: 870–877. [DOI] [PubMed] [Google Scholar]
- 3.Duffin J, McAvoy GV. The peripheral-chemoreceptor threshold to carbon dioxide in man. J Physiol 1988; 406: 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Read DJ, Leigh J. Blood-brain tissue Pco2 relationships and ventilation during rebreathing. J Appl Physiol 1967; 23: 53–70. [DOI] [PubMed] [Google Scholar]
- 5.Brothers RM, Lucas RA, Zhu YS, et al. Cerebral vasomotor reactivity: steady-state versus transient changes in carbon dioxide tension. Exp Physiol 2014; 99: 1499–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberger AL, Amaral LA, Glass L, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation 2000; 101: E215–220. [DOI] [PubMed] [Google Scholar]
- 7.Gotoh F, Meyer JS, Takagi Y. Cerebral effects of hyperventilation in man. Arch Neurol 1965; 12: 410–423. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi F, Meyer JS, Sakai F, et al. Normal human aging and cerebral vasoconstrictive responses to hypocapnia. J Neurol Sci 1979; 44: 87–94. [DOI] [PubMed] [Google Scholar]
- 9.Willie CK, Colino FL, Bailey DM, et al. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 2011; 196: 221–237. [DOI] [PubMed] [Google Scholar]
- 10.Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol 2012; 22: R741–752. [DOI] [PubMed] [Google Scholar]
- 11.Galvin SD, Celi LA, Thomas KN, et al. Effects of age and coronary artery disease on cerebrovascular reactivity to carbon dioxide in humans. Anaesth Intensive Care 2010; 38: 710–717. [DOI] [PubMed] [Google Scholar]
- 12.Ito H, Kanno I, Ibaraki M, et al. Effect of aging on cerebral vascular response to Paco2 changes in humans as measured by positron emission tomography. J Cereb Blood Flow Metab 2002; 22: 997–1003. [DOI] [PubMed] [Google Scholar]
- 13.Zhu YS, Tarumi T, Tseng BY, et al. Cerebral vasomotor reactivity during hypo- and hypercapnia in sedentary elderly and Masters athletes. J Cereb Blood Flow Metab 2013; 33: 1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cullen DJ, Eger EI, 2nd. Cardiovascular effects of carbon dioxide in man. Anesthesiology 1974; 41: 345–349. [DOI] [PubMed] [Google Scholar]
- 15.Paleczny B, Niewinski P, Rydlewska A, et al. Age-related reflex responses from peripheral and central chemoreceptors in healthy men. Clin Auton Res 2014; 24: 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niewinski P, Engelman ZJ, Fudim M, et al. Clinical predictors and hemodynamic consequences of elevated peripheral chemosensitivity in optimally treated men with chronic systolic heart failure. J Card Fail 2013; 19: 408–415. [DOI] [PubMed] [Google Scholar]
- 17.Ponikowski P, Chua TP, Anker SD, et al. Peripheral chemoreceptor hypersensitivity: an ominous sign in patients with chronic heart failure. Circulation 2001; 104: 544–549. [DOI] [PubMed] [Google Scholar]
- 18.Narkiewicz K, van de Borne PJ, Pesek CA, et al. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation 1999; 99: 1183–1189. [DOI] [PubMed] [Google Scholar]
- 19.Bailey DM, Marley CJ, Brugniaux JV, et al. Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke 2013; 44: 3235–3238. [DOI] [PubMed] [Google Scholar]
- 20.Tarumi T, Gonzales MM, Fallow B, et al. Cerebral/peripheral vascular reactivity and neurocognition in middle-age athletes. Med Sci Sports Exerc 2015; 47: 2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giller CA, Giller AM. A new method for fixation of probes for transcranial Doppler ultrasound. J Neuroimaging 1997; 7: 103–105. [DOI] [PubMed] [Google Scholar]
- 22.Razi E, Moosavi GA, Omidi K, et al. Correlation of end-tidal carbon dioxide with arterial carbon dioxide in mechanically ventilated patients. Arch Trauma Res 2012; 1: 58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hancock SM, Mahajan RP, Athanassiou L. Noninvasive estimation of cerebral perfusion pressure and zero flow pressure in healthy volunteers: the effects of changes in end-tidal carbon dioxide. Anesth Analg 2003; 96: 847–851. [DOI] [PubMed] [Google Scholar]
- 24.Dumville J, Panerai RB, Lennard NS, et al. Can cerebrovascular reactivity be assessed without measuring blood pressure in patients with carotid artery disease? Stroke 1998; 29: 968–974. [DOI] [PubMed] [Google Scholar]
- 25.Kety SS. Human cerebral blood flow and oxygen consumption as related to aging. J Chronic Dis 1956; 3: 478–486. [DOI] [PubMed] [Google Scholar]
- 26.Leenders KL, Perani D, Lammertsma AA, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 1990; 113(Pt 1): 27–47. [DOI] [PubMed] [Google Scholar]
- 27.Xing CY, Tarumi T, Meijers RL, et al. Arterial pressure, heart rate, and cerebral hemodynamics across the adult life span. Hypertension 2017; 69: 712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buijs PC, Krabbe-Hartkamp MJ, Bakker CJ, et al. Effect of age on cerebral blood flow: measurement with ungated two-dimensional phase-contrast MR angiography in 250 adults. Radiology 1998; 209: 667–674. [DOI] [PubMed] [Google Scholar]
- 29.Hart EC, Joyner MJ, Wallin BG, et al. Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol 2012; 590: 2069–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czosnyka M, Balestreri M, Steiner L, et al. Age, intracranial pressure, autoregulation, and outcome after brain trauma. J Neurosurg 2005; 102: 450–454. [DOI] [PubMed] [Google Scholar]
- 31.Dhokalia A, Parsons DJ, Anderson DE. Resting end-tidal CO2 association with age, gender, and personality. Psychosom Med 1998; 60: 33–37. [DOI] [PubMed] [Google Scholar]
- 32.Frassetto L, Sebastian A. Age and systemic acid-base equilibrium: analysis of published data. J Gerontol A Biol Sci Med Sci 1996; 51: B91–99. [DOI] [PubMed] [Google Scholar]
- 33.Burnum JF, Hickam JB, Mc IH. The effect of hypocapnia on arterial blood pressure. Circulation 1954; 9: 89–95. [DOI] [PubMed] [Google Scholar]
- 34.Matteis M, Troisi E, Monaldo BC, et al. Age and sex differences in cerebral hemodynamics: a transcranial Doppler study. Stroke 1998; 29: 963–967. [DOI] [PubMed] [Google Scholar]
- 35.Murrell CJ, Cotter JD, Thomas KN, et al. Cerebral blood flow and cerebrovascular reactivity at rest and during sub-maximal exercise: effect of age and 12-week exercise training. Age 2013; 35: 905–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bright MG, Donahue MJ, Duyn JH, et al. The effect of basal vasodilation on hypercapnic and hypocapnic reactivity measured using magnetic resonance imaging. J Cereb Blood Flow Metab 2011; 31: 426–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tancredi FB, Hoge RD. Comparison of cerebral vascular reactivity measures obtained using breath-holding and CO2 inhalation. J Cereb Blood Flow Metab 2013; 33: 1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohan RM, Amara CE, Cunningham DA, et al. Measuring central-chemoreflex sensitivity in man: rebreathing and steady-state methods compared. Respir Physiol 1999; 115: 23–33. [DOI] [PubMed] [Google Scholar]
- 39.Shoemaker JK, Vovk A, Cunningham DA. Peripheral chemoreceptor contributions to sympathetic and cardiovascular responses during hypercapnia. Can J Physiol Pharmacol 2002; 80: 1136–1144. [DOI] [PubMed] [Google Scholar]
- 40.Novak P. Assessment of sympathetic index from the Valsalva maneuver. Neurology 2011; 76: 2010–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci 2004; 5: 347–360. [DOI] [PubMed] [Google Scholar]
- 42.Ide K, Eliasziw M, Poulin MJ. Relationship between middle cerebral artery blood velocity and end-tidal PCO2 in the hypocapnic-hypercapnic range in humans. J Appl Physiol 2003; 95: 129–137. [DOI] [PubMed] [Google Scholar]
- 43.Low DA, Wingo JE, Keller DM, et al. Cerebrovascular responsiveness to steady-state changes in end-tidal CO2 during passive heat stress. J Appl Physiol 2008; 104: 976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandit JJ, Mohan RM, Paterson ND, et al. Cerebral blood flow sensitivities to CO2 measured with steady-state and modified rebreathing methods. Respir Physiol Neurobiol 2007; 159: 34–44. [DOI] [PubMed] [Google Scholar]
- 45.Guenard H, Marthan R. Pulmonary gas exchange in elderly subjects. Eur Respir J 1996; 9: 2573–2577. [DOI] [PubMed] [Google Scholar]
- 46.Williams JS, Babb TG. Differences between estimates and measured PaCO2 during rest and exercise in older subjects. J Appl Physiol 1997; 83: 312–316. [DOI] [PubMed] [Google Scholar]
- 47.Verbree J, Bronzwaer AS, Ghariq E, et al. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol 2014; 117: 1084–1089. [DOI] [PubMed] [Google Scholar]
- 48.Bullitt E, Zeng D, Mortamet B, et al. The effects of healthy aging on intracerebral blood vessels visualized by magnetic resonance angiography. Neurobiol Aging 2010; 31: 290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edwards DG, Farquhar WB. Vascular effects of dietary salt. Curr Opin Nephrol Hypertens 2015; 24: 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]