Abstract
Spatial attention is comprised of neural mechanisms that boost sensory processing at a behaviorally relevant location while filtering out competing information. The present review examines functional specialization in the network of brain regions that directs such preferential processing. This attention network includes both cortical (e.g., frontal and parietal cortices) and subcortical (e.g., the superior colliculus and the pulvinar nucleus of the thalamus) structures. Here, we piece together existing evidence that these various nodes of the attention network have dissociable functional roles by synthesizing results from electrophysiology and neuroimaging studies. We describe functional specialization across several dimensions (e.g., at different processing stages and within different behavioral contexts), while focusing on spatial attention as a dynamic process that unfolds over time. Functional contributions from each node of the attention network can change on a moment-to-moment timescale, providing the necessary cognitive flexibility for sampling from highly dynamic environments.
Keywords: attention, vision, electrophysiology, neuroimaging, monkey, human
INTRODUCTION
William James [1950 (1890), p. 404] described selective attention as a “withdrawal from some things in order to deal effectively with others.” Such preferential processing is needed because the brain has limited processing resources; that is, we cannot fully process all of the sensory information present in our complex environments. The brain therefore needs to filter sensory inputs using a variety of mechanisms, collectively referred to as selective attention (Buschman & Kastner 2015, Nobre & Kastner 2014). These mechanisms direct both the preferential processing of behaviorally relevant information and the filtering of distracting information. This review specifically focuses on visual spatial attention, the means through which a behaviorally relevant location receives preferential processing relative to other locations (Moran & Desimone 1985, Posner 1980). For example, when faced with a busy cityscape, you might use spatial attention to boost processing at a street corner where you agreed to meet a friend, while simultaneously suppressing processing of potentially distracting information such as passing cars.
Spatial attention is often compared to a spotlight that scans the visual environment, regularly pausing to illuminate potentially relevant locations (Posner 1980). In a natural setting, spatial attention is typically coupled to eye position; that is, we most often attend where we are looking. However, the metaphorical spotlight of spatial attention can also move independent of the eyes (i.e., covertly), with the eyes remaining fixed on a single location (Helmholtz 1867). In a laboratory setting, spatial attention is typically studied using such covert deployments, which helps to isolate attention-related modulation of sensory processing from motor-related processing (e.g., saccadic eye movements).
Task Designs for Investigating Spatial Attention
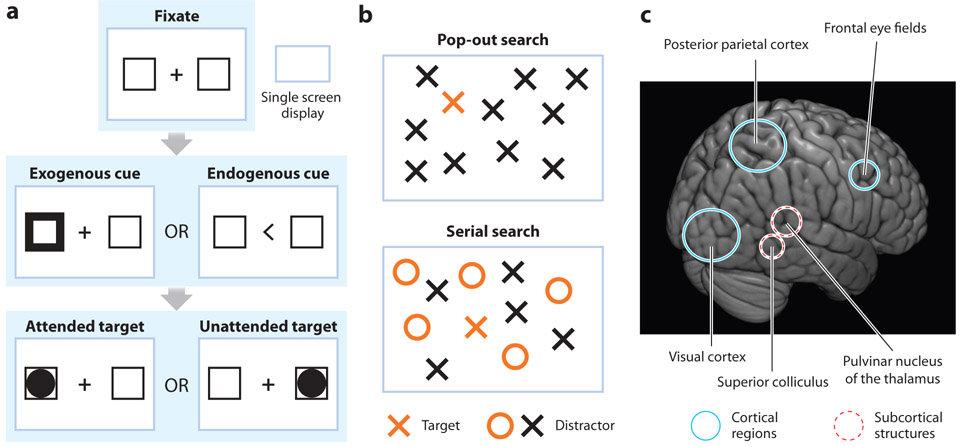
Experiments often manipulate the allocation of spatial attention by using a cue to indicate where a subsequent visual target is most likely to occur (Figure 1a). Behavioral performance and neural activity associated with the cued location (i.e., under conditions of directed spatial attention) are then compared with behavioral performance and neural activity associated with a noncued location (i.e., in the absence of spatial attention). Michael Posner was the first to use this task design, now often referred to as a Posner task or a Posner-like task. His seminal study demonstrated that spatial attention improves the accuracy and speed of behavioral performance at a cued location (Posner 1980). Subsequent studies have shown that spatial attention also influences perception, improving discriminability (Lu & Dosher 1998) and contrast sensitivity (Carrasco et al. 2004).
Figure 1.
Classic paradigms to study spatial attention and the attention network. (a) A Posner-like task asks participants to fixate a location while an exogenous (stimulus-driven attention) or endogenous (goal-directed attention) cue directs attentional allocation in anticipation of an upcoming target. Researchers then compare behavioral performance and neural activity when the target occurs at the attended location (i.e., validly cued) relative to when the target occurs at the unattended location (i.e., invalidly cued). (b) During a search task, participants identify a target (e.g., an orange cross) among an array of distractors (e.g., either a black cross or an orange circle). Pop-out search occurs when the target is unique (i.e., does not share features with the distractors), and a serial search occurs when the target shares features (e.g., color) with the distractors. (c) For both tasks, a large-scale network of cortical (solid circles) and subcortical (dashed circles) structures directs spatial attention.
Both stimulus properties and behavioral goals drive the dynamic allocation of spatial attention across different locations in the visual scene, for example, when searching for a behaviorally relevant target. Anne Treisman’s influential work described the behavior associated with stimulus-driven and goal-directed visual searches (Treisman & Gelade 1980). In visual-search tasks (Figure 1b), subjects are asked to report if a certain target stimulus (e.g., an orange X) is present in an array of stimuli (e.g., colored letters). If the target has a unique feature, such as being of a different color than the distractors, the search is completed quickly, independent of the number of items in the array. Such pop-out search is dominated by stimulus-driven or bottom-up factors (e.g., stimulus salience). In contrast, if a target shares features with distractors, search time increases as a function of the number of items in the array. This increase in search times reflects a serial search for the target that is dominated by goal-directed or top-down factors.
Functional Specialization in the Attention Network: An Overview
Both Posner-like tasks and visual-search tasks engage selection mechanisms that are linked to changes in neural activity across a large-scale network of brain regions (Corbetta & Shulman 2002, Petersen & Posner 2012). This attention network includes the visual cortex (Reynolds & Chelazzi 2004), parietal cortex (Bisley & Goldberg 2010), and frontal cortex (Squire et al. 2013), as well as subcortical structures such as the superior colliculus (Krauzlis et al. 2013) and the pulvinar nucleus of the thalamus (Saalmann & Kastner 2011) (Figure 1c). Researchers have made considerable progress in characterizing the function and local computations for individual nodes of the attention network, as well as considerable progress in characterizing how these nodes work together—despite their anatomical distance—to preferentially process sensory information. The allocation of spatial attention can be broken down into multiple operations (such as disengagement from the presently attended location, shift to a new location, and engagement at that new location), each of which may have a different neural substrate (Posner & Petersen 1990).
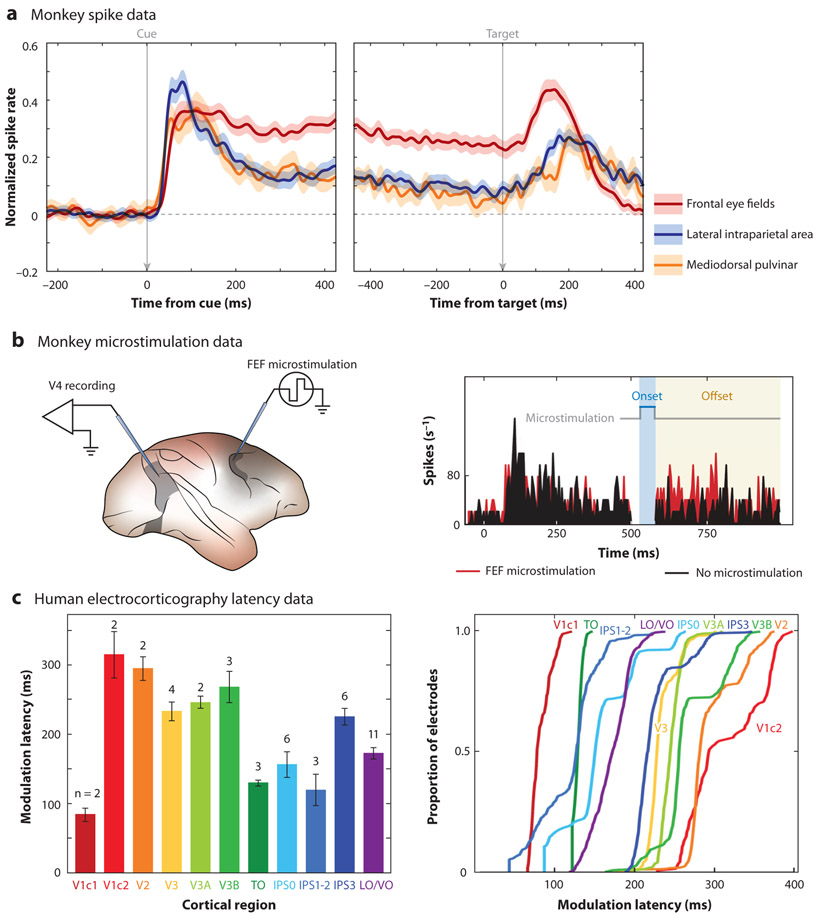
Neuroimaging and lesion studies in humans were among the first analyses to establish functional specialization among the brain regions that contribute to spatial attention (Corbetta & Shulman 2002, Corbetta et al. 2008, Posner & Petersen 1990). In comparison, electrophysiological studies in nonhuman primates—and to a lesser extent in humans—initially focused on the microstructure and mechanistic substrates within individual nodes of the attention network (e.g., functional contributions from specific cell types). These electrophysiological studies established how the allocation of spatial attention alters neural signals by, for example, changing spike rates and receptive field properties (Reynolds & Heeger 2009). Figure 2a illustrates increased spiking activity during the deployment of spatial attention (i.e., during a cue-target delay) across multiple nodes of the attention network. More recently, electrophysiological studies have begun to characterize the neural signatures of between-region interactions. These studies have investigated how nodes of the attention network dynamically interact to filter sensory information within different functional and behavioral contexts (Buschman & Kastner 2015).
Figure 2.
Attentional response modulation. Higher-order cortical and subcortical nodes of the attention network have been linked to attentional control. (a) Population peristimulus time histograms (PSTHs) from the monkey frontal eye fields (FEF), lateral intraparietal area (LIP), and mediodorsal pulvinar (mdPul) demonstrate increased spiking activity during the cue-target delay (i.e., in anticipation of an upcoming target), when receptive fields overlap the cued location. Such findings suggest that these nodes participate in the maintenance of spatial attention at a cued location. Panel adapted with permission from Fiebelkorn et al. (2019). (b) Stimulating FEF in monkeys leads to attention-like increases in spiking activity in the visual cortex (V4). The histogram on the right shows spiking activity in V4 both with (red) and without (black) microstimulation in FEF. The gray line above the spiking data shows the onset and offset of microstimulation. Such findings suggest that FEF generates attention-related signals that are fed back to the visual cortex. Panel adapted with permission from Moore & Armstrong (2003). (c) Results from electrocorticography (ECoG) recordings in humans with intractable epilepsy further suggest top-down attentional control from higher-order cortical nodes of the attention network. The latency of attention-related modulations in neural activity generally decreases at higher levels of the functional hierarchy, indicating that modulatory signals originate in higher-order cortices and are fed back to the visual cortex. The panels on the left and right use the same colors to represent each cortical region. Panel adapted with permission from Martin et al. (2019). Abbreviations: IPS0, intraparietal sulcus, area 0; IPS1-2, intraparietal sulcus, areas 1 and 2; IPS3, intraparietal sulcus, area 3; LO/VO, lateral occipital and ventral occipital; TO, cytoarchitectonic area in anterior temporal cortex; V1c1, visual area 1, early component; V1c2, visual area 1, late component; V2, visual area 2; V3, visual area 3; V3A, visual area 3A; V3B, visual area 3B.
Here, we synthesize the existing evidence for functional specialization from neuroimaging, lesion, and electrophysiological studies in both humans and nonhuman primates along several dimensions (e.g., goal-directed versus sensory-driven and sensory versus motor processing). We discuss how functional contributions from various nodes of the attention network are highly dynamic, even under unchanging task demands. Recent evidence has shown that spatial attention is characterized by moment-to-moment changes in functional contributions, with these changes providing critical flexibility during attention-related sampling.
FUNCTIONAL SPECIALIZATION BASED ON DIFFERENT PROCESSING STAGES
Results from neuroimaging and lesion studies in humans have largely identified the anatomically distributed network that mediates spatial attention (Corbetta et al. 1998, Kastner & Ungerleider 2000, Kastner et al. 1999, Nobre et al. 2000, Posner & Petersen 1990). The idea that there is functional specialization among the various nodes of this large-scale network was initially based on observations in patients with circumscribed brain lesions. For example, cortical lesions involving the posterior parietal cortex can lead to profound attentional deficits, such as visuo-spatial neglect, a syndrome associated with failure to direct attention to contralesional space (Posner et al. 1984, Steinmetz & Constantinidis 1995, Vallar & Perani 1986). In this section, we examine functional specialization at different processing stages, identifying the network nodes that direct spatial attention.
A Top-Down Model of Attentional Control
Neuroimaging studies have demonstrated that frontal and parietal cortices are a source for generating attention-related modulatory signals, which are then fed back to the visual cortex (Corbetta et al. 1998, Kastner & Ungerleider 2000, Kastner et al. 1999, Nobre et al. 2000). Attention-related activations of frontal and parietal cortices occur during the delay between a behaviorally relevant cue and a subsequent target stimulus (Kastner et al. 1999). These activations during the cue-target delay, which are observed in the absence of visual stimulation, suggest a role for frontal and parietal cortices in the maintenance and control of spatial attention. In contrast to activations in these higher-order cortical regions, activation of the visual cortex is primarily driven by sensory stimulation; that is, there is an enhanced response in the visual cortex when a sensory stimulus occurs at the attended (i.e., cued) location, but relatively weak activation during the cue-target delay (Kastner et al. 1999). Neuroimaging results thus indicate that higher-order nodes of the attention network, such as frontal and parietal cortices, direct modulations of sensory processing in the visual cortex.
This top-down model of attentional control has been further supported by causal manipulations in nonhuman primates (Armstrong et al. 2006, Gregoriou et al. 2014, Moore & Armstrong 2003, Moore & Fallah 2001, Rossi et al. 2007). For example, electrically stimulating neurons in a frontal region [i.e., the frontal eye fields (FEF)] led to both attention-like changes to neural activity in the visual cortex and improvements in behavioral performance (Armstrong et al. 2006, Moore & Armstrong 2003, Moore & Fallah 2001). This indicates that microstimulation of a higher-order cortical region mimics the effects of spatial attention on visual processing (Figure 2b). While these studies in monkeys have arguably provided the strongest empirical evidence for top-down attentional control, event-related potential (ERP) recordings in humans with damage to their dorsolateral prefrontal cortex have provided complementary results (Barcelo et al. 2000). Relative to controls, lesion patients demonstrated reduced visual responses that were coupled with behavioral deficits in the contralesional hemifield (i.e., a reduced ability to detect visual targets). These neural and behavioral deficits in humans were consistent with a disruption of attention-related, modulatory inputs from the dorsolateral prefrontal cortex.
Simultaneous recordings from higher-order cortical regions and the visual cortex have similarly provided evidence of top-down control from frontal (Gregoriou et al. 2009) and parietal cortices (Saalmann et al. 2007). Multisite recordings typically infer directionality by examining onset latencies (i.e., the order in which attention-related changes in neural activity occur across regions) or Granger causal influence (i.e., how well the neural signal in one region predicts the neural signal in another region). With regard to onset latencies, electrophysiological recordings in monkeys (Buffalo et al. 2010, Mehta et al. 2000), and more recently in humans (Martin et al. 2019), have shown that attention-related effects both occur earlier (Figure 2c) and are of greater magnitude in higher-order cortices (e.g., frontal and parietal cortices) than in the visual cortex. These results thus indicate that attention-related changes in neural processing propagate from higher-order cortices to the sensory cortex.
Electrophysiological studies in monkeys have further demonstrated that between-region synchronization during spatial attention is led or initiated by higher-order cortices (Gregoriou et al. 2009, Saalmann et al. 2007). For example, synchronization between frontal (i.e., FEF) and visual cortices (i.e., V4) during the deployment of spatial attention seems to be initiated by the frontal cortex (Gregoriou et al. 2009). Collectively, neuroimaging and electrophysiological studies investigating attention control have shown that the attention network can be divided into at least two parts: higher-order nodes that control spatial attention (e.g., frontal and parietal cortices) and lower-order nodes where attention-related changes in sensory processing are seemingly enacted (e.g., sensory cortex).
Subcortical Nodes Also Contribute to Attentional Control
Early accounts of functional specialization in the attention network also considered attentional control originating from its subcortical nodes. Based largely on a study of human patients with lesions, Posner & Petersen (1990) proposed that the allocation of covert spatial attention is comprised of three stages (i.e., disengagement from the presently attended location, shifting to a new location, and engagement at that new location), each seemingly associated with a different brain region. In addition to the parietal cortex, which was linked to disengagement from the present attentional focus (Fiebelkorn et al. 2019, Posner et al. 1984, Steinmetz & Constantinidis 1995), Posner & Petersen (1990) proposed that the superior colliculus and the pulvinar nucleus of the thalamus each have a role in controlling the allocation of spatial attention. Specifically, the superior colliculus appeared to be instrumental in shifting spatial attention to a new location (Krauzlis et al. 2013), and the pulvinar appeared to be important for engaging at a new attentional focus (Fiebelkorn et al. 2019, Petersen et al. 1987).
Patient data have thus provided evidence for functional specialization and attentional control in both cortical and subcortical nodes of the attention network (Posner et al. 1988). Most research on attention-related operations, however, has focused on cortical contributions. The notion of attentional control from subcortical nodes of the attention network has only recently been revisited, primarily by studies in nonhuman primates. We will discuss those studies in greater detail in the section titled Revisions of Cortico-Centric Biases in Functional Specialization.
The concept of functional specialization presented in this section ascribes functional contributions to single nodes of the attention network. We expand on this simplified account of functional specialization in the following sections. Regardless of the specific operation (or processing stage), attention-related sampling results from the integrated efforts of multiple brain regions. Both neuroimaging and electrophysiology studies have demonstrated, for example, that increased neural activity simultaneously occurs among multiple network nodes during the maintenance of spatial attention at a cued location (Figure 2a,b) (Bisley & Goldberg 2003; Fiebelkorn et al. 2018, 2019; Ignashchenkova et al. 2004; Kastner et al. 1999; Saalmann et al. 2012). Multiple nodes of the attention network therefore seem to be contributing during a single processing stage (i.e., during engagement at the cued location).
In the following section, we begin to describe evidence that spatial attention is characterized by a dynamic reweighting of relative contributions from multiple nodes of the attention network, with control shifting across nodes during different attention-related operations (Buschman & Miller 2007; Fiebelkorn et al. 2018, 2019). We specifically focus on studies investigating whether functional contributions from different nodes shift based on the behavioral context (i.e., based on task demands).
FUNCTIONAL SPECIALIZATION BASED ON BEHAVIORAL CONTEXT
Mechanisms of spatial attention mediate the filtering of our complex environment based on behavioral relevance, which depends both on the physical properties of the stimuli (e.g., salience) and on how behavioral goals relate to those stimuli. The allocation of spatial attention can therefore be either stimulus driven or goal directed (Koch & Ullman 1985, Treisman & Gelade 1980). For example, while you are looking for a friend at the street corner where you agreed to meet (i.e., goal-directed attention), the salient flash of a moving vehicle in your peripheral vision might capture your attention (stimulus-driven attention).
Neuroimaging Evidence for Context-Specific Specialization
Neuroimaging studies investigating whether the brain regions that direct spatial attention differ across goal-directed and stimulus-driven operational modes have yielded somewhat mixed results. For example, Rosen et al. (1999) reported that both goal-directed (or endogenous) and stimulus-driven (or exogenous) attention involve increased activation of bilateral parietal and dorsal premotor cortices, including FEF. Although there was relatively greater activation during goal-directed attention, the specific brain regions involved were largely the same across the two behavioral contexts. There was only one brain region, within the dorsolateral prefrontal cortex, that was exclusively activated during goal-directed attention.
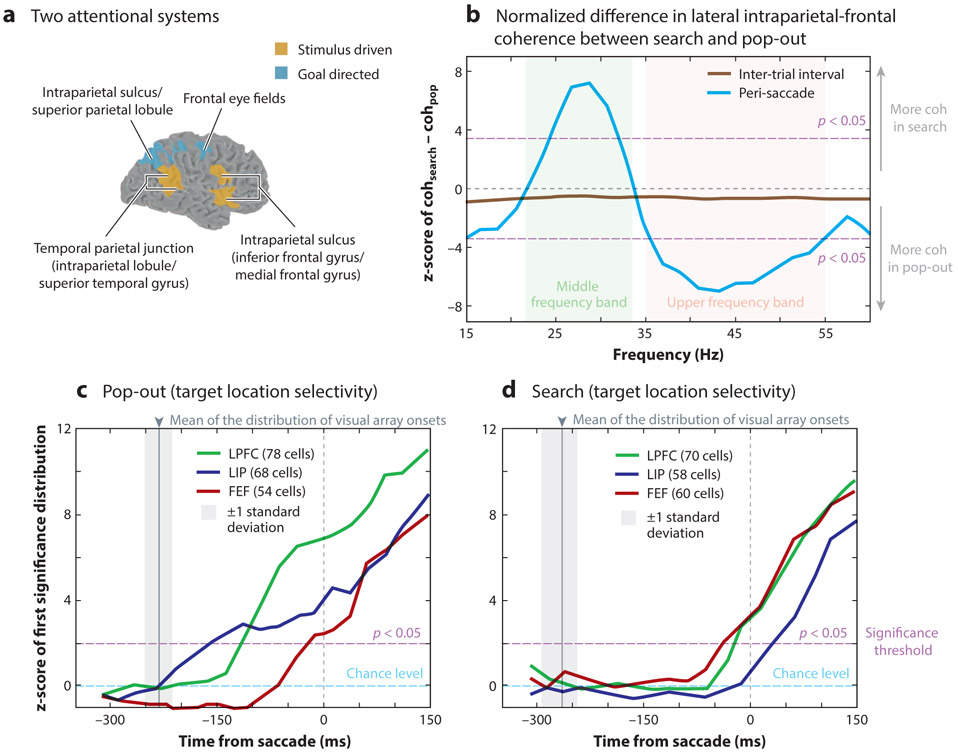
In contrast, Maurizio Corbetta and colleagues have provided evidence for two partially segregated cortical networks that direct spatial attention: a dorsal frontoparietal network for goal-directed attention that includes (a) the intraparietal sulcus, superior parietal lobule, and dorsal frontal cortex in both hemispheres and (b) a ventral frontoparietal network for stimulus-driven attention that is largely lateralized and includes the temporal parietal junction (TPJ) and ventral frontal cortex in the right hemisphere (Corbetta & Shulman 2002, Corbetta et al. 2000) (Figure 3a). Their findings demonstrated that the dorsal network was activated when spatial attention was allocated and maintained at a cued location (i.e., during goal-directed attention), while the ventral network was activated when a target unexpectedly appeared at a noncued location (i.e., during stimulus-driven attention).
Figure 3.
Attentional response modulation based on goal-directed and stimulus-driven context. (a) Some neuroimaging studies in humans have provided evidence of overlapping networks for directing either stimulus-driven (orange) or goal-directed (blue) attention. Panel adapted with permission from Corbetta & Shulman (2002). (b,c,d) Electrophysiological recordings in monkeys have provided evidence that the temporal dynamics of interactions between higher-order cortical hubs of the attention network shift between stimulus-driven (i.e., pop-out search) and goal-directed (i.e., serial search) attention. (b) Prior to a saccadic eye movement (i.e., peri-saccade), coherence (coh), a measure of functional connectivity, is stronger at higher frequencies during stimulus-driven attention and stronger at lower frequencies during goal-directed attention. In addition, attentional control shifts from the LIP during stimulus-driven attention to the FEF during goal-directed attention. Panel adapted with permission from Buschman & Miller (2007). The target location is first signaled (c) by LIP during a pop-out search and (d) by FEF during a serial search. Abbreviations: FEF, frontal eye fields; LIP, lateral intraparietal area; LPFC, lateral prefrontal cortex.
Additional evidence has shown that responses in the ventral network are neither specific to a sensory domain nor specific to a behavioral task. During an oddball task, for example, the ventral network is activated not only in response to infrequently occurring visual stimuli, but also in response to infrequently occurring auditory or tactile stimuli (Downar et al. 2000). The ventral frontoparietal network therefore appears to be more generally engaged in the detection of salient (or infrequently occurring) events, regardless of the specific task demands (Corbetta et al. 2008).
Electrophysiological Evidence for Context-Specific Specialization
The neuroimaging studies described above focused on detecting whether different brain regions comprise the attention network during different behavioral contexts. In comparison, electrophysiological studies—with better temporal and spatial resolution—have largely focused on detecting context-specific changes in neural activity within nodes of the network that are engaged during multiple behavioral contexts. Many of the same brain regions are involved during goal-directed and stimulus-driven attention, but the specific contribution of each brain region changes depending on the behavioral context (Buschman & Miller 2007, Fiebelkorn et al. 2019).
Such context-specific changes in functional contributions are observable, for example, through changes in the relative onset latency of attention-related effects following a spatial cue. Buschman & Miller (2007) simultaneously recorded from the frontal cortex (i.e., dorsolateral prefrontal cortex and FEF) and the lateral intraparietal area (LIP) in the posterior parietal cortex while monkeys performed two attention-related tasks. The first task promoted stimulus-driven attention (i.e., a pop-out task), and the second task promoted goal-directed attention (i.e., a serial search task). Although both regions signaled the target location, parietal neurons signaled the target location earlier during the stimulus-driven task, while frontal neurons signaled the target location earlier during the goal-directed task (Figure 3c) (see also Ibos et al. 2013). These results thus provided evidence that the parietal cortex leads the frontal cortex during stimulus-driven attention, while the frontal cortex leads the parietal cortex during goal-directed attention (but see Katsuki & Constantinidis 2012).
Buschman & Miller (2007) also demonstrated context-specific changes in the interactions (i.e., functional connectivity) between FEF and LIP. In electrophysiological studies, between-region interactions are typically measured as either synchronization between spiking activity in one brain region and local field potentials (LFPs) in another brain region (i.e., spike-LFP coherence), or as synchronization between LFPs across brain regions (i.e., LFP-LFP coherence). LFPs arise from electrical currents associated with synaptic activity (i.e., action potentials and postsynaptic potentials) emerging from the summed activity of neural populations. Prior to measuring between-region synchronization, LFPs are often filtered to isolate neural activity in a specific frequency range. The remaining signal can capture frequency-specific neural oscillations, which are sometimes conceptualized as rhythmic alternations between high- and low-excitability states (Helfrich et al. 2018). The specific frequencies associated with between-region synchronization provide clues, for example, regarding the directionality of functional connectivity in different behavioral contexts (Bastos et al. 2015, Buffalo et al. 2011, Riddle et al. 2019, van Kerkoerle et al. 2014).
Buschman & Miller (2007) specifically demonstrated that synchronization between FEF and LIP was weighted toward the gamma range (reaching statistical significance from 33 to 55 Hz) during stimulus-driven attention and toward the beta range (reaching statistical significance from 22 to 34 Hz) during goal-directed attention (Figure 3b). While the neural mechanisms that produce such synchronization within these different frequency bands have yet to be fully described (Mejias et al. 2016), gamma-band activity has been linked to feedforward connectivity (Bastos et al. 2015, Buffalo et al. 2011, van Kerkoerle et al. 2014), and beta-band activity has been linked to feedback connectivity (Bastos et al. 2015). The results obtained by Buschman & Miller (2007) therefore suggest that stimulus-driven attention is associated with increased feedforward connectivity, while goal-directed attention is associated with increased feedback connectivity.
Spatial Priority Maps Guide Attentional Allocation
According to computational theories, the allocation of spatial attention is based on priority maps, with peaks in those priority maps defined by both the stimulus-driven properties of the visual environment (e.g., salience) and behavioral goals (Fecteau & Munoz 2006, Itti & Koch 2001, Koch & Ullman 1985). Evidence for such priority maps has been found in multiple nodes of the attention network, including frontal cortices (Serences & Yantis 2007, Sprague & Serences 2013, Thompson & Bichot 2005) and parietal cortices (Bisley & Goldberg 2010, Gottlieb et al. 1998, Serences & Yantis 2007, Sprague & Serences 2013), but little is known about whether region-specific priority maps contribute differently to the allocation of spatial attention (i.e., whether these region-specific maps encode different information about attentional priority).
In order to address whether different brain regions have distinct roles in representing attentional priority, a recent investigation used simultaneous electrophysiological recordings in frontal (i.e., FEF) and parietal (i.e., LIP) cortices in monkeys trained to overtly scan (i.e., with eye movements) a cluttered visual scene, searching for a specific target stimulus (Sapountzis et al. 2018). Whereas neurons in both cortical regions encoded the similarity of stimuli to the searched-for target, the encoding of this information in the frontal neurons was integrated with oculomotor decisions; that is, attentional priority in the frontal neurons was strongly influenced by the decision to fixate a target-like stimulus (i.e., saccade selection). These findings therefore not only provide further evidence of functional specialization in the attention network based on behavioral context, but they also introduce another dimension of functional specialization. The attention network directs both sensory and motor functions of environmental sampling (Corbetta et al. 1998). In this context, the results described above (Sapountzis et al. 2018) show that attentional priority in the frontal neurons, relative to the parietal neurons, is strongly influenced by motor functions of the attention network (see the section titled Functional Specialization Based on Sensory and Motor Processing).
Both neuroimaging and electrophysiological studies have provided evidence of functional specialization within the attention network based on differences in task demands (i.e., whether the allocation of spatial attention is driven by stimulus properties or behavioral goals). Electrophysiological studies have further demonstrated context-specific patterns of activation (i.e., onset latencies) and between-region synchronization (i.e., functional connectivity) within network nodes that are active across multiple behavioral contexts (Buschman & Miller 2007, Ibos et al. 2013). In the next section, we discuss electrophysiological evidence that the attention network is also highly dynamic during unchanging task demands (e.g., under conditions that promote sustained attention at a single location). We specifically discuss evidence that spatial attention, rather than being a continuous process, samples the visual environment in rhythmic cycles, with these rhythmic cycles reflecting an alternation between sensory and motor functions of the attention network (Fiebelkorn & Kastner 2019b).
FUNCTIONAL SPECIALIZATION BASED ON SENSORY AND MOTOR PROCESSING
The network of brain regions that directs attention-related boosts in sensory processing also directs exploratory eye movements. Both neuroimaging studies (Corbetta et al. 1998, Nobre et al. 2000) and electrophysiological studies (Barash et al. 1991, Bruce & Goldberg 1985, Robinson et al. 1991) have provided evidence for this functional overlap, leading researchers to investigate how the same network of brain regions directs both the sensory and the motor aspects of environmental sampling.
A Premotor Theory of Attention
One potential explanation for a shared network is that the sensory and motor functions of environmental sampling reflect a single control mechanism. The influential premotor theory of attention proposed that covert boosts in sensory processing might simply reflect a weaker activation of the same neural circuits that guide saccadic eye movements (Rizzolatti et al. 1987). In other words, the premotor theory of attention suggests that there is no functional specialization associated with the sensory and motor functions directed by the attention network. Spatial attention and saccades might be inseparably linked (Rizzolatti et al. 1987), with covert boosts in sensory processing reflecting the intention to make a saccade (Andersen & Buneo 2002).
Work by Tirin Moore (and colleagues) was initially taken as strong evidence for this premotor characterization of covert spatial attention (Armstrong et al. 2006, Moore & Armstrong 2003, Moore & Fallah 2001). Stimulating a region of FEF leads to saccades with a consistent trajectory and end point (Figure 2b). Stimulating the same region of FEF below the threshold necessary to evoke a saccade leads to attention-like effects in regions of the visual cortex that represent the end point of the would-be saccade (i.e., the unexecuted saccade). This apparent link between the oculomotor system and covert attention has recently received further support. Lowet et al. (2018b) found that attention-related boosts in sensory processing only occur following small, fixational eye movements (i.e., microsaccades) toward the cued location. These findings thus suggest that covert attentional shifts might require engagement of the oculomotor system.
While the findings described above support the premotor theory of attention, other evidence argues against interpreting covert spatial attention as being inseparably coupled to saccadic preparation (Smith & Schenk 2012). First, there is functional specialization within the microstructure of FEF as well as other nodes of the attention network. For example, cell types demonstrate saccade-related responses, visual-sensory responses, or both saccade-related responses and visual-sensory responses. Only those neurons with a visual-sensory response (i.e., visual and visual-movement neurons) show attention-related changes in spiking activity (Fiebelkorn et al. 2018, 2019; Gregoriou et al. 2012; Thompson et al. 2005). Because all cell types are activated during stimulation, it is unclear how each cell type contributes to either attention-related boosts in sensory processing or saccadic eye movements. Second, studies in both humans and monkeys have shown that spatial attention and saccades can be uncoupled (Juan et al. 2004,2008). Such findings indicate that that these sensory and motor functions of the attention network, rather than having a shared control mechanism, have separate control mechanisms (Smith & Schenk 2012). This evidence therefore challenges the premotor theory of attention, but it remains undeniable that attention-related boosts in sensory processing and exploratory eye movements are tightly linked during natural viewing.
A Rhythmic Theory of Attention
We recently proposed a rhythmic theory of attention to describe the relationship between sensory and motor functions of the attention network (Fiebelkorn & Kastner 2019b). We specifically proposed that low-frequency oscillations in the attention network are temporally organizing neural activity into two rhythmically alternating states associated with either sensory or motor functions (Fiebelkorn et al. 2018, 2019), avoiding potential functional conflicts between the visual-sensory and the oculomotor systems (Fiebelkorn & Kastner 2019b).
Classic studies of spatial attention assumed that its effects on sensory processing and behavioral performance were continuous during attentional deployment (i.e., that attentional deployment was stable over time). Recent work, however, has shown that spatial attention is discontinuous, sampling the visual environment in rhythmic cycles (Busch & VanRullen 2010, Fiebelkorn et al. 2018, Helfrich et al. 2018,Jia et al. 2017, Landau et al. 2015). This rhythmic sampling occurs in the absence of environmental rhythms and despite unchanging task demands (Fiebelkorn & Kastner 2019b).
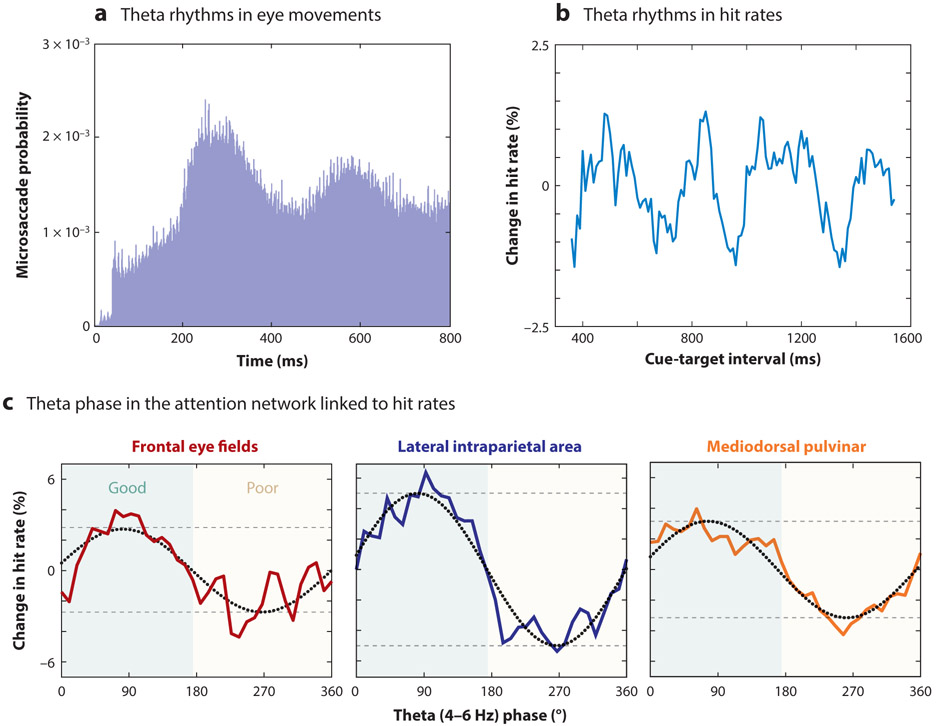
Evidence of rhythmic sampling during spatial attention can be observed directly in behavioral data (Figure 4a), with alternating periods of relatively enhanced or diminished perceptual sensitivity (Fiebelkorn et al. 2013, Landau & Fries 2012, Song et al. 2014, VanRullen et al. 2007). These behavioral oscillations are linked to intrinsic theta-band activity (3–8 Hz) in cortical and subcortical nodes of the attention network (Fiebelkorn et al. 2018, 2019; Helfrich et al. 2018): Theta rhythms in the attention network organize two alternating attentional states (Figure 4b), associated with either better or worse visual-target detection.
Figure 4.
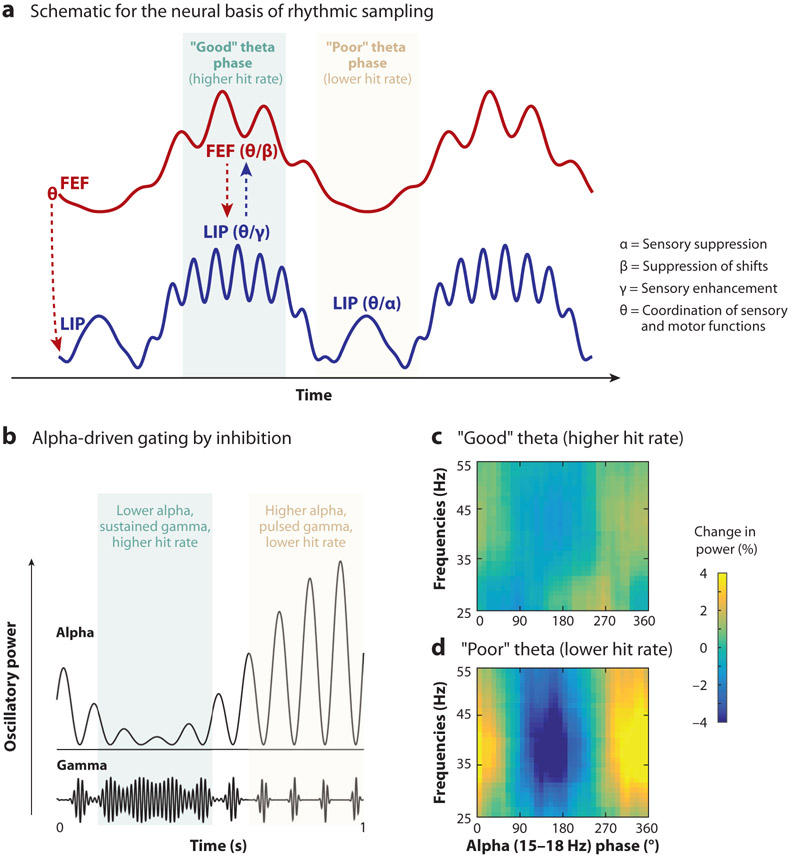
The role of theta rhythms in environmental sampling. Both the sensory and the motor aspects of environmental sampling are linked to theta rhythms. Evidence of theta-rhythmic sampling in monkeys, for example, has been observed (a) in the probability of microsaccades (panel adapted with permission from Bosman et al. 2009) and (b) in hit rates at different time points following a spatial cue (panel adapted with permission from Fiebelkorn et al. 2018). (c) Behavioral oscillations have been linked to the phase of theta-band activity (3–8 Hz) in both higher-order cortical and subcortical nodes of the attention network (panel adapted with permission from Fiebelkorn et al. 2018, 2019). Theta rhythms organize neural activity in the attention network into alternating periods of either enhanced (i.e., during the “good” theta phase; green shaded area) or relatively diminished (i.e., during the “poor” theta phase; yellow shaded area) perceptual sensitivity. These plots show behavioral performance as a function of oscillatory phase in the frontal eye fields (red solid line), the lateral intraparietal area (blue solid line), and the mediodorsal pulvinar (orange solid line). Phase-detection functions (colored solid lines) were fit with one-cycle sine waves (black dotted lines), and the amplitude of these sine waves (distance between dashed gray lines) provided an estimate of the strength of the relationship between theta phase and the likelihood of visual target detection. We have proposed that periods of enhanced perceptual sensitivity are associated with attention-related sampling at a behaviorally relevant location (e.g., a cued location), while periods of relatively diminished perceptual sensitivity are associated with an increased likelihood of attentional shifts and/or eye movements (Fiebelkorn & Kastner 2019b).
The first attentional state, associated with better visual-target detection (i.e., enhanced perceptual sensitivity), is characterized by increased gamma-band activity from LIP and increased beta-band activity from FEF (Figure 5a). In the previous section, we discussed both of these frequency bands in the context of feedforward (gamma) and feedback (beta) connectivity, but these frequency bands have also been consistently linked to specific sensory and motor functions. Increased gamma-band activity has been repeatedly associated with attention-related enhancements in sensory processing (Bichot et al. 2005, Fries 2009, Fries et al. 2001, Womelsdorf et al. 2006), while increased beta-band activity has been associated with the suppression of motor processing (Pogosyan et al. 2009, Zhang et al. 2008), including the suppression of attentional shifts and/or eye movements (Fiebelkorn et al. 2018, Gregoriou et al. 2012). The first attentional state therefore appears to reflect attention-related sampling at the cued location, that is, both enhanced sensory processing at the cued location (gamma) and a decreased likelihood of shifting away from the cued location (beta). Lending further support to this proposal, gamma-band activity during the first attentional state has been linked to neurons with visual-sensory responses, while beta-band activity has been linked to neurons with both visual-sensory and saccade-related responses (Fiebelkorn et al. 2018).
Figure 5.
Brain rhythms shape attention-related sampling. (a) Theta-band activity (3–8 Hz) in the attention network organizes neural activity into rhythmically alternating attentional states characterized by differences in temporal dynamics and behavioral performance. Periods of better behavioral performance (i.e., the “good” theta phase) are characterized by increases in both gamma- and beta-band activity (>35 Hz and 15–35 Hz), associated with sensory enhancement and the suppression of attentional shifts, respectively. Periods of worse behavioral performance (i.e., the “poor” theta phase) are characterized by increases in alpha-band activity (9–14 Hz), associated with sensory suppression. The arrows represent the directionality of frequency-specific activity (e.g., from FEF to LIP). Panel adapted with permission from Fiebelkorn et al. (2018). (b) Increases in alpha-band activity (e.g., observed during rhythmic periods of worse behavioral performance) are thought to gate visual processing by disrupting feedforward sensory signals. This disruption in visual processing can be observed as coupling between the phase of alpha-band activity and the power of gamma-band activity, with gamma-band activity being an established proxy for feedforward sensory processing. Panel adapted with permission from Osipova et al. (2008). (c,d) In the context of rhythmic sampling during spatial attention, there is evidence of alpha-driven gating in LIP during periods of worse behavioral performance. We have proposed that this rhythmically occurring, alpha-driven gating of visual sensory processing at an attended location is associated with an increased likelihood of attentional shifts. Panels adapted with permission from Fiebelkorn et al. (2019). Abbreviations: FEF, frontal eye fields; LIP, lateral intraparietal area.
The second attentional state, associated with worse visual-target detection (i.e., diminished perceptual sensitivity), is characterized by increased alpha-band activity (9–14 Hz) from LIP (Figure 5a). In opposition to increased gamma-band activity, increased alpha-band activity has been repeatedly associated with the suppression of sensory processing (Foxe & Snyder 2011, Haegens et al. 2011, Worden et al. 2000) (see the next section for further discussion of the links between gamma-band activity and enhanced sensory processing and alpha-band activity and suppressed sensory processing). We have proposed that the alpha-driven suppression of sensory processing during the second attentional state occurs in anticipation of a potential attentional shift (Fiebelkorn & Kastner 2019b), increasing the likelihood that a stimulus (or location) outside the presently attended location will receive attentional priority. Rhythmic sampling thus promotes a more active exploration of the environment (Schroeder et al. 2010) by providing windows of opportunity when it is easier to disengage from the presently attended location and shift to another location (Fiebelkorn & Kastner 2019b).
In line with the proposal that rhythmically occurring periods of diminished perceptual sensitivity are associated with an increased likelihood of attentional shifts (Fiebelkorn & Kastner 2019b), recent work has linked both covert (i.e., in the absence of eye movement) and overt (i.e., with eye movements) shifts to theta-band activity in various brain regions (Bosman et al. 2009, Dugue et al. 2016, Hogendoorn 2016, Lowet et al. 2018a, Otero-Millan et al. 2008) (Figure 4a). Hogendoorn (2016), for example, asked human participants to monitor two locations for the presentation of a near-threshold visual target that was flashed at different latencies relative to voluntary saccades between the two locations. The near-threshold visual target only occurred at the presently fixated location. The results revealed a theta rhythm in behavioral performance, with saccades seemingly occurring during periods of worse visual-target detection. These findings thus provide further evidence for the rhythmic theory of attention, which posits alternating periods that promote either sampling (i.e., sensory function) or shifting (i.e., motor function).
Whereas some nodes of the attention network seem to be more strongly weighted toward either sensory functions (e.g., visual cortices) or motor functions (e.g., the superior colliculus), frontal and parietal nodes represent a nexus between sensory and motor function. Periods of sampling might therefore be characterized by increased functional connectivity between frontal and parietal cortices and sensory nodes of the attention network (e.g., the visual cortex), while periods associated with an increased likelihood of shifting might be characterized by increased functional connectivity between frontal and parietal cortices and motor nodes of the attention network (e.g., the superior colliculus) (Fiebelkorn & Kastner 2019b). This means that state-specific patterns of functional connectivity during rhythmic sampling likely reflect functional specialization among nodes of the attention network. In the section titled The Neural Basis of Attentional Control Evolves over Time, we discuss supporting evidence that functional connectivity rhythmically shifts in the attention network despite unchanging task demands (Fiebelkorn et al. 2019).
Rhythmic sampling is a fundamental property of spatial attention, which has been demonstrated across multiple species and behavioral tasks (Fiebelkorn et al. 2018, Helfrich et al. 2018, Landau & Fries 2012). Recent research has provided some evidence that low-frequency oscillations might similarly contribute to functional flexibility outside the attention network, either when processing competing environmental stimuli (Caruso et al. 2018) or when holding multiple items in working memory (Lisman 2010). Low-frequency oscillations might generally serve to temporally organize neural activity associated with either stimuli or functions that are competing for representation (Kienitz et al. 2018, Rollenhagen & Olson 2005).
In this section, we have described evidence of functional specialization in the attention network associated with the sensory and motor aspects of environmental sampling. Such functional specialization has been observed both at the level of single neurons, with functionally defined cell types, and at the network level, with temporally organized shifts in spatiotemporal dynamics. Below we focus exclusively on the sensory functions of the attention network, examining functional specialization associated with either attention-related enhancement of sensory processing or attention-related suppression of sensory processing.
FUNCTIONAL SPECIALIZATION BASED ON ENHANCEMENT OR SUPPRESSION
Visual information from cluttered environments cannot be fully represented in the visual system due to limited processing capacity. As a consequence, simultaneously presented visual stimuli are not processed independently in the visual cortex but instead interact with each other. In the extrastriate cortex, for example, monkey electrophysiology and human neuroimaging studies have shown that multiple stimuli presented simultaneously evoke less activity than when the same stimuli are presented in isolation (Kastner et al. 1998, Moran & Desimone 1985, Reynolds et al. 1999). Responses to a pair of stimuli, for example, appeared to be a weighted average of the responses evoked by each of the stimuli when presented alone (Luck et al. 1997, Reynolds et al. 1999).
Such suppressive interactions among simultaneously presented stimuli have been found throughout the visual system, and they have been interpreted as a neural signature of the competition for neural representation in the context of limited processing resources (Desimone & Duncan 1995). This competition can be biased by either behavioral goals or physical stimulus properties (e.g., salience). For example, electrophysiological studies have shown that when a monkey directs attention to one of two competing stimuli, neural responses in the extrastriate cortex are biased toward the response evoked by the attended stimulus (i.e., the response obtained when the attended stimulus was presented alone) (Recanzone & Wurtz 2000, Reynolds et al. 1999). These findings suggest that goal-directed effects can overcome competitive interactions to strengthen stimulus representations that are important for behavioral goals (see Beck & Kastner 2009 for corroborating evidence from human neuroimaging studies). Alternatively, neural responses in the extrastriate cortex to a pair of simultaneously presented stimuli can be dominated by the higher-contrast stimulus, suggesting that competition can also be biased in favor of stimulus-driven factors such as stimulus salience (Beck & Kastner 2009, Reynolds & Desimone 2003).
Both goal-directed and stimulus-driven factors thus influence the competition for limited processing resources. As a consequence of the interplay between these factors, behaviorally relevant information is enhanced, while competing information is suppressed. In this section, we present evidence that these complementary mechanisms of attention-related enhancement and suppression are associated with different neural signals and possibly with different nodes of the attention network.
The Temporal Dynamics of Sensory Enhancement and Suppression
As discussed in previous sections, the biophysical properties of neurons or interactions between neurons (and neural populations) can create rhythmic patterns of neural activity. These neural oscillations occur across multiple frequency bands, with activity within specific frequency bands being consistently linked to specific functions (Bastos et al. 2015, Buschman & Miller 2007). For example, attention-related enhancement of sensory processing has been repeatedly linked to increases in gamma-band oscillations (>35 Hz) (Fries 2009), while attention-related suppression of sensory processing has been repeatedly linked to increases in alpha-band oscillations (9–14 Hz) (Foxe & Snyder 2011).
Gamma-band activity is typically higher in cortical regions processing the attended stimulus (Bichot et al. 2005, Fries et al. 2001). This increased local synchronization might serve to amplify the influence that neurons in one brain region have on neurons in another brain region by temporally aligning spiking activity associated with the attended stimulus (or location): Simultaneous spikes have a greater chance of eliciting further spiking activity from a shared synaptic target.
In support of this notion, the deployment of spatial attention is associated not only with increased local gamma-band synchronization but also with increased between-region gamma-band synchronization (Bastos et al. 2015, Gregoriou et al. 2009), potentially improving between-region communication (Bosman et al. 2012). Gamma-band synchronization may facilitate the transfer of information between regions by optimally aligning periods of increased excitability (Fries 2015), that is, periods when neurons are relatively depolarized and therefore closer to their firing thresholds. Evidence from electrophysiological recordings indicates that multiple higher-order nodes of the attention network control facilitatory increases in gamma-band activity (Gregoriou et al. 2009, Saalmann et al. 2007, Zhou et al. 2016). Attention-related enhancements in sensory processing have been associated with both higher-order cortical regions (Gregoriou et al. 2009, Kastner et al. 1999, Moore & Armstrong 2003, Saalmann et al. 2007) and subcortical structures (Fiebelkorn et al. 2019, Saalmann et al. 2012, Zenon & Krauzlis 2012).
In contrast to gamma-band activity, alpha-band activity is typically higher in cortical regions processing task-irrelevant (i.e., competing) stimuli (Haegens et al. 2011, Worden et al. 2000), reflecting an active suppression of sensory processing (Kelly et al. 2006). Electrophysiological recordings in monkeys have demonstrated that increased alpha-band activity in the sensory cortex is associated with lower spike rates (Haegens et al. 2011, Johnston et al. 2019), further supporting a link between alpha-band activity and sensory suppression.
Rather than with a continuous disruption of sensory processing, however, increased alpha-band activity seems to be associated with a phasic disruption of sensory processing. Jensen & Mazaheri (2010) proposed that increased alpha-band activity rhythmically disrupts the processing of feedforward sensory information (Figure 5b). Evidence of such gating by inhibition has been shown through alpha-related disruptions both in behavioral performance (Mathewson et al. 2009) and in visual-sensory processing (Fiebelkorn et al. 2019, Spaak et al. 2012). In both cases, the evidence suggests that attention-related alpha-band activity operates more as a picket fence (or gate) than a wall (Mathewson et al. 2009).
Suppression at the Focus of Spatial Attention
Most research has described the enhancement of sensory processing inside the metaphorical spotlight of spatial attention (i.e., at behaviorally relevant locations) and the suppression of sensory processing outside the spotlight of spatial attention (i.e., at to-be-ignored locations). Recent evidence, however, indicates that even within the spotlight of spatial attention—when task conditions promote sustained attention at a single location—periods of enhancement alternate with periods of relative suppression (Fiebelkorn & Kastner 2019b). These alternating periods are associated with changes in both gamma- and alpha-band activity (Figure 5a), with periods of sensory enhancement characterized by increased gamma-band activity in frontal and parietal cortices and periods of sensory suppression characterized by increased alpha-band activity in the parietal cortex. Fiebelkorn et al. (2018) proposed that the periodic, alpha-related suppression of visual processing at the presently attended (i.e., cued) location temporarily alters the spatial priority map, increasing the likelihood that attentional resources will shift to another location.
Rhythmically occurring periods of relative sensory suppression at the focus of spatial attention that are associated with rhythmic sampling (see the previous section) may explain a well-established attentional phenomenon. Inhibition of return (IOR) has been hypothesized to encourage the exploration of novel stimuli (Klein 2000). Following a spatial cue, there is an immediate facilitation of behavioral performance (e.g., reaction times) relative to noncued locations. After approximately 300 ms, however, behavioral performance at the cued location becomes worse than behavioral performance at noncued locations. This pattern of better-then-worse behavioral performance might reflect the alternating periods of enhanced and suppressed sensory processing associated with rhythmic sampling during spatial attention (Fiebelkorn & Kastner 2019b). In this view, IOR and rhythmically occurring sensory suppression might share the same neural basis, with some evidence suggesting shared sources in the parietal cortex (Bourgeois et al. 2012, Fiebelkorn et al. 2019, Vivas et al. 2006).
The suppression of sensory processing has been linked to multiple nodes of the attention network. While electrophysiological recordings in both humans and monkeys indicate that the parietal cortex might control the alpha-driven, pulsed inhibition of sensory processing (Capotosto et al. 2015, Fiebelkorn et al. 2019, Halgren et al. 2017), there is also some evidence linking the frontal cortex to attention-related suppression (Helfrich et al. 2017, Suzuki & Gottlieb 2013). For example, responses to distractors are more strongly suppressed in the frontal than in the parietal cortex, and inactivations of the frontal cortex are associated with a larger increase in distractibility than inactivations of the parietal cortex (Suzuki & Gottlieb 2013).
Future research will need to further investigate the microstructure and network-level interactions that differentially support enhancement and suppression. In this section, we largely focused on differences in the temporal dynamics associated with these complementary processes, but even these differences are not entirely consistent. For example, we described alpha-band activity in the context of sensory suppression (Foxe & Snyder 2011). It should be noted, however, that some increases in alpha-band activity are associated with sensory enhancement rather than sensory suppression (Bollimunta et al. 2008, Fiebelkorn et al. 2019, Saalmann et al. 2012). Control of this “good” alpha-band activity has been linked to the pulvinar nucleus of the thalamus (Fiebelkorn et al. 2019, Saalmann et al. 2012). In the next section we discuss the role of such subcortical regions in supporting attention-related function.
REVISIONS OF CORTICO-CENTRIC BIASES IN FUNCTIONAL SPECIALIZATION
Early models of attentional control, such as the one by Posner & Petersen (1990), included subcortical structures, such as the superior colliculus and the pulvinar nucleus of the thalamus. Empirical research in nonhuman primates and humans during the last 40 years, however, has largely focused on the role of the cortex in attentional control. This cortico-centric bias stems, in part, from issues with either accessing or imaging deep brain structures for electrophysiological and neuroimaging approaches (Parvizi 2009). As a result of this cortico-centric bias, attention-related function has been mainly attributed to the cortex, but this viewpoint has recently shifted toward a more global account. Recent evidence has shown that subcortical structures also play an important role in attentional control (Fiebelkorn et al. 2019, Halassa & Kastner 2017, Lovejoy & Krauzlis 2010, Saalmann et al. 2012, Zenon & Krauzlis 2012). The role of the superior colliculus in attentional control has been reviewed in detail elsewhere (Krauzlis et al. 2013); here, we specifically focus on different regions of the thalamus.
Attention-Related Modulation in the Thalamus
The lateral geniculate nucleus (LGN) is the thalamic relay nucleus of the visual system that conveys visual information from the sensory periphery (i.e., the retina) to the cortex (Sherman et al. 2006). According to classic views, thalamic relays operate passively, with no influence from cognitive processes such as spatial attention (Lehky & Maunsell 1996). Human neuroimaging studies, however, have contradicted this assumption, providing compelling evidence of attention-related modulation in the LGN (O’Connor et al. 2002, Schneider & Kastner 2009). These studies have demonstrated enhanced responses to attended stimuli, attenuated responses to ignored stimuli, and enhanced baseline activity in anticipation of a target (O’Connor et al. 2002).
Single-cell recordings in the monkey’s LGN and thalamic reticular nucleus (TRN) have corroborated such findings from neuroimaging studies (McAlonan et al. 2008). The TRN consists of a thin sheet of inhibitory neurons that is anatomically positioned between the cortex and the thalamus, providing inhibitory input to other thalamic nuclei (e.g., the pulvinar and LGN). McAlonan et al. (2008) demonstrated that attention-related modulation occurs earlier in the TRN than in the LGN, consistent with the TRN serving as a source of attention-related modulation in the LGN. This TRN-to-LGN modulation, which occurs less than 40 ms after stimulus onset, might be attributable to direct connections between higher-order (e.g., frontal) cortex and the TRN (Wimmer et al. 2015). Attention-related inhibitory input from TRN to the LGN may thus serve as an early thalamic gatekeeper (Crick 1984), representing the first stage at which the attention network can filter visual information.
Attentional Control from the Thalamus
Unlike the LGN, which receives substantial projections from the sensory periphery, the pulvinar is almost exclusively interconnected with the cortex, making it a higher-order thalamic nucleus. The pulvinar is the largest nucleus in the primate thalamus, having undergone dramatic expansion during evolution on a scale comparable with the expansion of the prefrontal cortex (Jones 2001). Directly connected cortical areas are generally indirectly connected via the pulvinar, through cortico-thalamo-cortical pathways (Sherman et al. 2006, Shipp 2003). This intricate pattern of connectivity perfectly positions the pulvinar to coordinate cortico-cortical interactions.
Early studies of patients with pulvinar lesions demonstrated a slowing of orienting responses to visual space on the contralesional side, suggesting a functional role for the pulvinar in spatial attention (Danziger et al. 2001, Rafal & Posner 1987, Ward et al. 2002). Indeed, the pulvinar seemed to be a critical subcortical node of the attention network. These patient studies were paralleled by electrophysiology studies in monkeys, which demonstrated attention-related changes in spiking activity among pulvinar neurons, similar to effects observed in the cortex (Petersen et al. 1985,1987).
Until recently, the specific computations or functions that the pulvinar might exert on cortico-cortical interactions remained elusive, becoming one of the enigmatic puzzles of the neuroscience field (Fiebelkorn & Kastner 2019a). Recent studies, however, using simultaneous recordings from the pulvinar and interconnected cortical regions, have led to a breakthrough in understanding the functional role or cortico-pulvino-cortical pathways (Fiebelkorn et al. 2019, Purushothaman et al. 2012, Saalmann et al. 2012, Zhou et al. 2016).
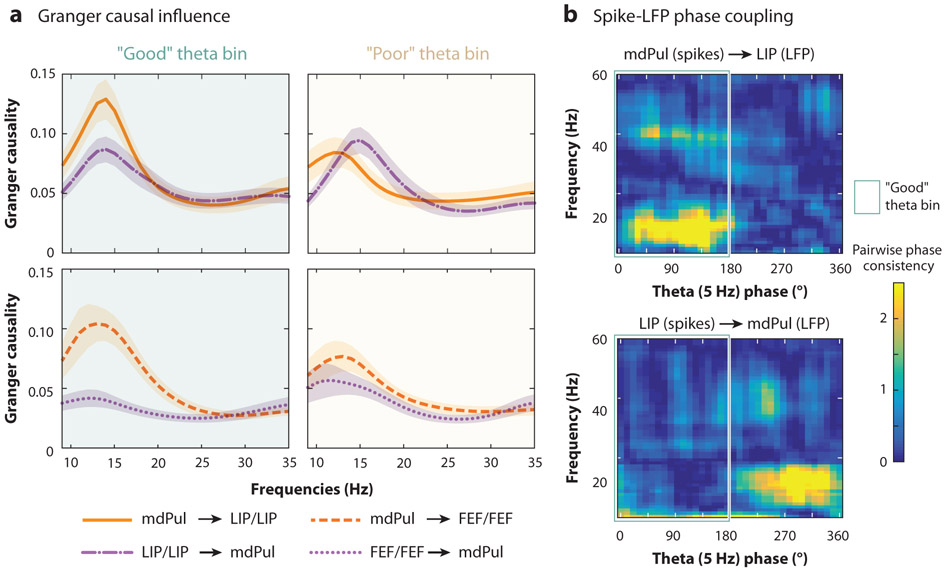
Saalmann et al. (2012), for example, trained monkeys to perform a variant of the Eriksen Flanker task, with the animals discriminating between shape stimuli presented at a cued location. The researchers then simultaneously recorded from single neurons and local populations of neurons (i.e., local field potentials) in directly connected cortical regions along the ventral visual pathway (areas V4 and TEO) and their shared projection zone in the ventrolateral pulvinar. Their findings revealed attention-related functional connectivity in the alpha/low beta range (8–20 Hz), with Granger causal influence indicating that the pulvinar synchronizes neural activity in V4 and TEO. This pulvinar-driven synchronization of visual cortices may serve to optimize between-region communication during the deployment of spatial attention. The function of the pulvinar in the attention network might therefore be that of a cortical timekeeper, suggesting that subcortical nodes can exert control over cortical nodes. Studies pairing lesions in the pulvinar with recordings in visual cortices have further shown that pulvino-cortical inputs are required for normal sensory function in the cortex (Purushothaman et al. 2012, Zhou et al. 2016).
Different regions of the pulvinar are interconnected with different regions of the cortex (Saalmann & Kastner 2011). Whereas the ventrolateral portion of the pulvinar is interconnected with visual cortices, the dorsomedial portion of the pulvinar is interconnected with higher-order cortices (i.e., frontal and parietal cortices). Fiebelkorn et al. (2019) recently demonstrated that the apparent functional role of the pulvinar as a cortical timekeeper extends to higher-order cortical nodes of the attention network. Simultaneous recordings from FEF, LIP, and the mediodorsal pulvinar showed patterns of pulvinar-driven synchronization similar to those previously observed among visual cortices and the ventrolateral pulvinar. Here, however, attentional control from the pulvinar (i.e., pulvino-cortical synchronization) was specifically observed during rhythmically occurring periods of relative engagement at a cued location (Figure 6) associated with enhanced perceptual sensitivity (Fiebelkorn et al. 2019). In the next section, we more specifically discuss a dynamic shifting in attentional control between the pulvinar and higher-order cortices, which reflects a shifting among the different attentional modes or operations that unfolds over time. We conclude by focusing on functional specialization both in space (i.e., at different nodes of the attention network) and in time.
Figure 6.
The role of the pulvinar in spatial attention. While there has historically been a cortico-centric bias in attention research, recent work has shown that subcortical structures can also direct attention-related function. The mediodorsal pulvinar (mdPul), for example, seems to specifically organize higher-order cortical nodes of the attention network during theta-rhythmic periods associated with better behavioral performance (i.e., during the “good” theta phase). This apparent directionality between mdPul and higher-order cortices is observable in both (a) Granger causal influence and (b) spike-LFP phase coupling. Attentional control seems to shift to LIP during theta-rhythmic periods associated with relatively worse behavioral performance (i.e., during the “poor” theta phase). The green outline indicates the “good” theta phase. These findings demonstrate moment-to-moment changes in functional connectivity, reflecting functional specialization within different nodes of the attention network. Figure adapted with permission from Fiebelkorn et al. (2019). Abbreviations: FEF, frontal eye fields; LFP, local field potential; LIP, lateral intraparietal area.
THE NEURAL BASIS OF ATTENTIONAL CONTROL EVOLVES OVER TIME
Spatial attention involves multiple processing stages. For example, Posner & Petersen (1990) proposed three stages of attentional allocation: (a) disengagement from the previously attended location, (b) shift to another location, and (c) engagement at that new location. But what happens after attention is engaged at a new location? Will attention-related sampling continue at the same location until there is a change in either visual stimulus properties (i.e., the visual scene) or behavioral goals? And if so, will attentional control be maintained by the same node(s) of the attention network? Here, we summarize evidence for functional specialization in the context of recent work that describes the temporal organization of attention-related sampling.
As discussed above, spatial attention can be allocated based on either stimulus properties or behavioral goals, with these two factors contributing to an attentional priority map (Fecteau & Munoz 2006). Experimenters can bias the allocation of spatial attention toward one of these two factors by using either exogenous (e.g., with a flashed stimulus) or endogenous (e.g., with an arrow) cueing (Figure 1a). Previous research indicates that the spatial and/or temporal dynamics of attentional allocation differ depending on the underlying motivation (Buschman & Miller 2007, Corbetta & Shulman 2002, Ibos et al. 2013), but these studies did not consider an important characteristic of spatial attention as it unfolds over time. Spatial attention is an intrinsically rhythmic process, with attentional control rhythmically shifting among nodes of the attention network, even under unchanging task conditions (Fiebelkorn & Kastner 2019b). While the initial allocation of attention to a specific location might be made based on behavioral goals, the neural substrates associated with that allocation of attention will change over time, even if attention-related sampling continues at the same location (Fiebelkorn et al. 2018, 2019; Helfrich et al. 2018; Wimmer et al. 2016).
For example, theta rhythms in the attention network organize neural activity during spatial attention into two rhythmically alternating attentional states (Fiebelkorn et al. 2018). State-specific changes in both perceptual sensitivity and neural signals (e.g., the specific frequency components that comprise the neural signals) are indicative of alternating periods of either enhanced or suppressed visual processing at a cued location. Whereas rhythmically occurring periods of enhancement occur at the peak in the attentional priority map, intervening periods of relative suppression at this same peak temporarily reweight the priority map. This relative suppression increases the likelihood that stimulus properties outside the present focus of spatial attention will generate an attentional shift; that is, the rhythmic suppression of visual processing at the present focus of attention (e.g., at a cued location) helps to prevent us from becoming overly focused on any single location (Fiebelkorn & Kastner 2019b). Periods of relatively suppressed visual processing thus provide critical cognitive flexibility during attention-related sampling.
Alternating attentional states during spatial attention are also associated with a rhythmic reweighting of functional connectivity in the attention network (Figure 6). The mediodorsal division of the pulvinar, for example, coordinates neural activity in higher-order cortex during periods associated with enhanced visual processing (i.e., sensory sampling), while the parietal cortex influences neural activity in the mediodorsal pulvinar during periods associated with suppressed visual processing (i.e., a higher likelihood of attentional shifting) (Fiebelkorn et al. 2019). These findings are in agreement with the functional roles first proposed by Posner & Petersen (1990) for the pulvinar and parietal cortex. These alternating attentional states could be characterized as periods of pulvinar-driven engagement at a behaviorally relevant location alternating with periods of parietal-driven disengagement.
The rhythmic reweighting of attentional control during spatial attention likely also involves shifts in functional connectivity among other nodes of the attention network. Frontal regions, such as FEF, might coordinate attention-related boosts in the sensory processing during periods of relative engagement (Squire et al. 2013), while regions typically associated with the motor output of attention (e.g., saccadic eye movement), such as the superior colliculus (Gandhi & Katnani 2011, Krauzlis et al. 2013), might be more strongly engaged during periods of relative disengagement (i.e., when there is a higher likelihood of an attentional shift).
CONCLUSION
This review has described evidence for functional specialization in the attention network across several dimensions: stimulus-driven versus goal-directed attention, sensory versus motor functions of the attention network, enhancement versus suppression of sensory processing, and cortical versus subcortical control of attention-related processes. It is important to recognize that although these dimensions have often been studied in isolation, they are interrelated and combine to influence the deployment of spatial attention on a moment-to-moment timescale: Navigating our complex environment requires a fluid transition between attentional modes and operations.
Imagine again a busy cityscape, where you agreed to meet your friend at a specific street corner. Approaching that street corner, you use goal-directed spatial attention to enhance sensory processing. Every approximately 250 ms, visual processing at this behaviorally relevant location is relatively suppressed as a result of rhythmic sampling during spatial attention. This relative suppression at the present focus of spatial attention provides a window of opportunity in which a salient stimulus at another location, such as someone waving their arms in your peripheral vision, has an increased likelihood of generating a stimulus-driven attentional shift. This attentional shift, either covert or overt, is preceded by the disengagement of attention-related resources from the previously attended location and followed by the engagement of attention-related resources at a new location (i.e., the location of your friend). The attention network supports all of these attention-related processes. However, each step in the above scenario involves a different combination or weighting of network nodes, neural populations, and neural mechanisms.
Here, we have synthesized results from multiple methodologies that have each made substantial contributions to our understanding of functional specialization in the attention network. Given the spatial and temporal complexity of attention-related sampling, a full understanding of how the various nodes of the attention network work together to direct the brain’s limited resources will likely require a considerable investment in multisite electrophysiological recordings. Such recordings from the primate brain can capture network interactions at a spatial and temporal resolution that matches the known spatiotemporal dynamics of attention-related processes. However, this systems-level approach will continue to be informed by findings from other methodologies, including neuroimaging, lesion, and behavioral studies. These other critical approaches provide foundational discoveries, for example, by identifying target structures (i.e., nodes of the attention network) and developing incisive task designs for further unraveling functional specialization in the attention network.
SUMMARY POINTS.
Preferential processing of locations in the visual scene leads to changes in neural activity (e.g., spike rates and receptive field properties) and subsequent improvements in behavioral outcomes (e.g., response times and hit rates).
A large-scale network of both cortical and subcortical structures directs preferential processing of locations (i.e., spatial attention), and different nodes of that network are associated with different attention-related functions.
While there is considerable evidence of top-down attentional control, with higher-order brain regions (e.g., frontal and parietal cortices) modulating processing in the sensory cortex, the specific brain regions directing attentional deployment vary depending on the processing stage (e.g., engagement versus disengagement of attention) and behavioral context (e.g., stimulus-driven versus goal-directed attention).
Low-frequency oscillations (i.e., rhythmic changes in neural activity) might temporally isolate sensory and motor functions of the attention network, with a rhythmic reweighting of functional connectivity among nodes specialized for either sampling (i.e., sensory function) or shifting (i.e., motor function).
Attentional selection and control is not only based on cortical computations but also arises from subcortical nodes of the attention network, such as the pulvinar nucleus of the thalamus and the superior colliculus.
Matching the dynamic nature of natural environments, attention-related processes unfold over time, with an ongoing reweighting of functional contributions from the various nodes of the attention network.
ACKNOWLEDGMENTS
This work was supported by a training fellowship to I.C.F. (F32EY023465) and by grants from the National Institute of Mental Health (R01MH064063), Silvio O. Conte Center (P50MH109429), National Eye Institute (R01EY017699, R21EY023565), and James S. McDonnell Foundation to S.K.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Andersen RA, Buneo CA. 2002. Intentional maps in posterior parietal cortex. Annu. Rev. Neurosci 25:189–220 [DOI] [PubMed] [Google Scholar]
- Armstrong KM, Fitzgerald JK, Moore T. 2006. Changes in visual receptive fields with microstimulation of frontal cortex. Neuron 50:791–98 [DOI] [PubMed] [Google Scholar]
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. 1991. Saccade-related activity in the lateral intraparietal area. II. Spatial properties. J. Neurophysiol 66:1109–24 [DOI] [PubMed] [Google Scholar]
- Barcelo F, Suwazono S, Knight RT. 2000. Prefrontal modulation of visual processing in humans. Nat. Neurosci 3:399–403 [DOI] [PubMed] [Google Scholar]
- Bastos AM, Vezoli J, Bosman CA, Schoffelen JM, Oostenveld R, et al. 2015. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron 85:390–401 [DOI] [PubMed] [Google Scholar]
- Beck DM, Kastner S. 2009. Top-down and bottom-up mechanisms in biasing competition in the human brain. Vis. Res 49:1154–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichot NP, Rossi AF, Desimone R. 2005. Parallel and serial neural mechanisms for visual search in macaque area V4. Science 308:529–34 [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. 2003Neuronal activity in the lateral intraparietal area and spatial attention. Science 299:81–86 [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. 2010. Attention, intention, and priority in the parietal lobe. Annu. Rev. Neurosci 33:1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimunta A, Chen Y, Schroeder CE, Ding M. 2008. Neuronal mechanisms of cortical alpha oscillations in awake-behaving macaques. J. Neurosci 28:9976–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman CA, Schoffelen JM, Brunet N, Oostenveld R, Bastos AM, et al. 2012. Attentional stimulus selection through selective synchronization between monkey visual areas. Neuron 75:875–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman CA, Womelsdorf T, Desimone R, Fries P. 2009. A microsaccadic rhythm modulates gamma-band synchronization and behavior. J. Neurosci 29:9471–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois A, Chica AB, Migliaccio R, Thiebaut de Schotten M, Bartolomeo P. 2012. Cortical control of inhibition of return: evidence from patients with inferior parietal damage and visual neglect. Neuropsychologia 50:800–9 [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. 1985. Primate frontal eye fields. I. Single neurons discharging before saccades. J. Neurophysiol 53:603–35 [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Fries P, Landman R, Buschman TJ, Desimone R.2011. Laminar differences in gamma and alpha coherence in the ventral stream. PNAS 108:11262–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalo EA, Fries P, Landman R, Liang H, Desimone R. 2010A backward progression of attentional effects in the ventral stream. PNAS 107:361–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch NA, VanRullen R. 2010. Spontaneous EEG oscillations reveal periodic sampling of visual attention. PNAS 107:16048–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Kastner S. 2015. From behavior to neural dynamics: an integrated theory of attention. Neuron 88:127–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. 2007. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 315:1860–62 [DOI] [PubMed] [Google Scholar]
- Capotosto P, Spadone S, Tosoni A, Sestieri C, Romani GL, et al. 2015. Dynamics of EEG rhythms support distinct visual selection mechanisms in parietal cortex: a simultaneous transcranial magnetic stimulation and EEG study. J. Neurosci 35:721–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Ling S, Read S. 2004. Attention alters appearance. Nat. Neurosci 7:308–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso VC, Mohl JT, Glynn C, Lee J, Willett SM, et al. 2018. Single neurons may encode simultaneous stimuli by switching between activity patterns. Nat. Commun 9:2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, et al. 1998. A common network of functional areas for attention and eye movements. Neuron 21:761–73 [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. 2000. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat. Neurosci 3:292–97 [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. 2008. The reorienting system of the human brain: from environment to theory of mind. Neuron 58:306–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. 2002. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci 3:201–15 [DOI] [PubMed] [Google Scholar]
- Crick F 1984. Function of the thalamic reticular complex: the searchlight hypothesis. PNAS 81:4586–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danziger S, Ward R, Owen V, Rafal R. 2001. The effects of unilateral pulvinar damage in humans on reflexive orienting and filtering of irrelevant information. Behav. Neurol 13:95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. 1995. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci 18:193–222 [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. 2000. A multimodal cortical network for the detection of changes in the sensory environment. Nat. Neurosci 3:277–83 [DOI] [PubMed] [Google Scholar]
- Dugue L, Roberts M, Carrasco M. 2016. Attention reorients periodically. Curr. Biol. 26:1595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau JH, Munoz DP. 2006. Salience, relevance, and firing: a priority map for target selection. Trends Cogn. Sci 10:382–90 [DOI] [PubMed] [Google Scholar]
- Fiebelkorn IC, Kastner S. 2019a. The puzzling pulvinar. Neuron 101:201–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebelkorn IC, Kastner S. 2019b. A rhythmic theory of attention. Trends Cogn. Sci 23:87–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebelkorn IC, Pinsk MA, Kastner S. 2018. A dynamic interplay within the frontoparietal network underlies rhythmic spatial attention. Neuron 99:842–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebelkorn IC, Pinsk MA, Kastner S. 2019. The mediodorsal pulvinar coordinates the macaque fronto-parietal network during rhythmic spatial attention. Nat. Commun 10:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebelkorn IC, Saalmann YB, Kastner S. 2013. Rhythmic sampling within and between objects despite sutained attention at a cued location. Curr. Biol 23:2553–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Snyder AC. 2011. The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front. Psychol 2:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P 2009. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu. Rev. Neurosci 32:209–24 [DOI] [PubMed] [Google Scholar]
- Fries P 2015. Rhythms for cognition: communication through coherence. Neuron 88:220–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. 2001. Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291:1560–63 [DOI] [PubMed] [Google Scholar]
- Gandhi NJ, Katnani HA. 2011. Motor functions of the superior colliculus. Annu. Rev. Neurosci. 34:205–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb JP, Kusunoki M, Goldberg ME. 1998. The representation of visual salience in monkey parietal cortex. Nature 391:481–84 [DOI] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Desimone R.2012. Cell-type-specific synchronization of neural activity in FEF with V4 during attention. Neuron 73:581–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. 2009. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science 324:1207–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Rossi AF, Ungerleider LG, Desimone R. 2014. Lesions of prefrontal cortex reduce attentional modulation of neuronal responses and synchrony in V4. Nat. Neurosci 17:1003–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Nacher V, Luna R, Romo R, Jensen O. 2011. Alpha-oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. PNAS 108:19377–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Kastner S. 2017. Thalamic functions in distributed cognitive control. Nat. Neurosci 20:1669–79 [DOI] [PubMed] [Google Scholar]
- Halgren M, Ulbert I, Bastuji H, Fabo D, Eross L, et al. 2017. The generation and propagation of the human alpha rhythm. bioRxiv 202564. 10.1101/202564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich RF, Fiebelkorn IC, Szczepanski SM, Lin JJ, Parvizi J, et al. 2018. Neural mechanisms of sustained attention are rhythmic. Neuron 99:829–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich RF, Huang M, Wilson G, Knight RT. 2017. Prefrontal cortex modulates posterior alpha oscillations during top-down guided visual perception. PNAS 114:9457–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogendoorn H 2016. Voluntary saccadic eye movements ride the attentional rhythm. J. Cogn. Neurosci 28:1625–35 [DOI] [PubMed] [Google Scholar]
- Ibos G, Duhamel JR, Ben Hamed S. 2013A functional hierarchy within the parietofrontal network in stimulus selection and attention control. J. Neurosci 33:8359–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignashchenkova A, Dicke PW, Haarmeier T, Thier P. 2004. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nat. Neurosci 7:56–64 [DOI] [PubMed] [Google Scholar]
- Itti L, Koch C. 2001. Computational modelling of visual attention. Nat. Rev. Neurosci 2:194–203 [DOI] [PubMed] [Google Scholar]
- James W 1950. (1890). The Principles of Psychology. New York: H. Holt & Co. [Google Scholar]
- Jensen O,Mazaheri A. 2010. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Hum. Neurosci 4:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Liu L, Fang F, Luo H. 2017. Sequential sampling of visual objects during sustained attention. PLOS Biol. 15:e2001903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K,Ma L, Schaeffer L, Everling S.2019. Alpha-oscillations modulate preparatory activity in marmoset area 8Ad. J. Neurosci 39:1855–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. 2001. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 24:595–601 [DOI] [PubMed] [Google Scholar]
- Juan CH, Muggleton NG, Tzeng OJ, Hung DL, Cowey A,Walsh V. 2008. Segregation of visual selection and saccades in human frontal eye fields. Cereb. Cortex 18:2410–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan CH, Shorter-Jacobi SM, Schall JD. 2004. Dissociation of spatial attention and saccade preparation. PNAS 101:15541–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, De Weerd P, Desimone R, Ungerleider LG. 1998. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science 282:108–11 [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. 1999. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22:751–61 [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. 2000. Mechanisms of visual attention in the human cortex. Annu. Rev. Neurosci 23:315–41 [DOI] [PubMed] [Google Scholar]
- Katsuki F, Constantinidis C.2012. Early involvement of prefrontal cortex in visual bottom-up attention. Nat. Neurosci 15:1160–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SP, Lalor EC, Reilly RB, Foxe JJ. 2006. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distractor suppression during sustained visuospatial attention. J. Neurophysiol 95:3844–51 [DOI] [PubMed] [Google Scholar]
- Kienitz R, Schmiedt JT, Shapcott KA, Kouroupaki K, Saunders RC, Schmid MC. 2018. Theta rhythmic neuronal activity and reaction times arising from cortical receptive field interactions during distributed attention. Curr. Biol 28:2377–87.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RM. 2000. Inhibition of return. Trends Cogn. Sci 4:138–47 [DOI] [PubMed] [Google Scholar]
- Koch C, Ullman S. 1985. Shifts in selective visual attention: towards the underlying neural circuitry. Hum. Neurobiol 4:219–27 [PubMed] [Google Scholar]
- Krauzlis RJ, Lovejoy LP, Zenon A. 2013. Superior colliculus and visual spatial attention. Annu. Rev. Neurosci 36:165–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau AN, Fries P 2012. Attention samples stimuli rhythmically. Curr. Biol 22:1000–4 [DOI] [PubMed] [Google Scholar]
- Landau AN, Schreyer HM, van Pelt S, Fries P. 2015. Distributed attention is implemented through theta-rhythmic gamma modulation. Curr. Biol 25:2332–37 [DOI] [PubMed] [Google Scholar]
- Lehky SR,Maunsell JH. 1996. No binocular rivalry in the LGN of alert macaque monkeys. Vis. Res 36:1225–34 [DOI] [PubMed] [Google Scholar]
- Lisman J 2010. Working memory: the importance of theta and gamma oscillations. Curr. Biol. 20:R490–92 [DOI] [PubMed] [Google Scholar]
- Lovejoy LP, Krauzlis RJ. 2010. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat. Neurosci 13:261–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowet E, Gips B, Roberts MJ, De Weerd P, Jensen O, van der Eerden J. 2018a. Microsaccade-rhythmic modulation of neural synchronization and coding within and across cortical areas V1 and V2. PLOS Biol. 16:e2004132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowet E, Gomes B, Srinivasan K, Zhou H, Schafer RJ, Desimone R. 2018b. Enhanced neural processing by covert attention only during microsaccades directed toward the attended stimulus. Neuron 99:207–14.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZL, Dosher BA. 1998. External noise distinguishes attention mechanisms. Vis. Res 38:1183–98 [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. 1997. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J. Neurophysiol 77:24–42 [DOI] [PubMed] [Google Scholar]
- Martin AB, Yang X, Saalmann YB, Wang L, Shestyuk A, et al. 2019. Temporal dynamics and response modulation across the human visual system in a spatial attention task: an ECoG study. J. Neurosci 39:333–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T. 2009. To see or not to see: Prestimulus alpha phase predicts visual awareness. J. Neurosci 29:2725–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH. 2008. Guarding the gateway to cortex with attention in visual thalamus. Nature 456:391–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AD, Ulbert I, Schroeder CE. 2000. Intermodal selective attention in monkeys. I. Distribution and timing of effects across visual areas. Cereb. Cortex 10:343–58 [DOI] [PubMed] [Google Scholar]
- Mejias JF,Murray JD, Kennedy H, Wang XJ. 2016. Feedforward and feedback frequency-dependent interactions in a large-scale laminar network of the primate cortex. Sci. Adv 2:e1601335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T,Armstrong KM. 2003. Selective gating of visual signals by microstimulation of frontal cortex. Nature 421:370–73 [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M. 2001. Control of eye movements and spatial attention. PNAS 98:1273–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran J, Desimone R. 1985. Selective attention gates visual processing in the extrastriate cortex. Science 229:782–84 [DOI] [PubMed] [Google Scholar]
- Nobre AC, Gitelman DR, Dias EC, Mesulam MM. 2000. Covert visual spatial orienting and saccades: overlapping neural systems. Neuroimage 11:210–16 [DOI] [PubMed] [Google Scholar]
- Nobre K, Kastner S. 2014. The Oxford Handbook of Attention. Oxford, UK: Oxford Univ. Press [Google Scholar]
- O’Connor DH, Fukui MM, Pinsk MA, Kastner S. 2002. Attention modulates responses in the human lateral geniculate nucleus. Nat. Neurosci 5:1203–9 [DOI] [PubMed] [Google Scholar]
- Osipova D, Hermes D,Jensen O. 2008. Gamma power is phase-locked to posterior alpha activity. PLOS ONE 3:e3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero-Millan J, Troncoso XG, Macknik SL, Serrano-Pedraza I, Martinez-Conde S. 2008. Saccades and microsaccades during visual fixation, exploration, and search: foundations for a common saccadic generator. J. Vis 8:21. [DOI] [PubMed] [Google Scholar]
- Parvizi J 2009. Corticocentric myopia: old bias in new cognitive sciences. Trends Cogn. Sci 13:354–59 [DOI] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. 2012. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci 35:73–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Robinson DL, Keys W. 1985. Pulvinar nuclei of the behaving rhesus monkey: visual responses and their modulation. J. Neurophysiol 54:867–86 [DOI] [PubMed] [Google Scholar]
- Petersen SE, Robinson DL, Morris JD. 1987. Contributions of the pulvinar to visual spatial attention. Neuropsychologia 25:97–105 [DOI] [PubMed] [Google Scholar]
- Pogosyan A, Gaynor LD, Eusebio A, Brown P. 2009. Boosting cortical activity at beta-band frequencies slows movement in humans. Curr. Biol 19:1637–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. 1980. Orienting of attention. Q.J. Exp. Psychol 32:3–25 [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. 1990. The attention system of the human brain. Annu. Rev. Neurosci 13:25–42 [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE, Fox PT, Raichle ME. 1988. Localization of cognitive operations in the human brain. Science 240:1627–31 [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. 1984. Effects of parietal injury on covert orienting of attention. J. Neurosci 4:1863–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman G, Marion R, Li K, Casagrande VA. 2012. Gating and control of primary visual cortex by pulvinar. Nat. Neurosci 15:905–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafal RD, Posner MI. 1987. Deficits in human visual spatial attention following thalamic lesions. PNAS 84:7349–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Wurtz RH. 2000. Effects of attention on MT and MST neuronal activity during pursuit initiation. J. Neurophysiol 83:777–90 [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L. 2004. Attentional modulation of visual processing. Annu. Rev. Neurosci 27:611–47 [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L, Desimone R. 1999. Competitive mechanisms subserve attention in macaque areas V2 and V4. J. Neurosci 19:1736–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Desimone R. 2003. Interacting roles of attention and visual salience in V4. Neuron 37:853–63 [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Heeger DJ. 2009. The normalization model of attention. Neuron 61:168–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle J, Hwang K, Cellier D, Dhanani S, D’Esposito M. 2019. Causal evidence for the role of neuronal oscillations in top-down and bottom-up attention. J. Cogn. Neurosci 31:768–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umilta C. 1987. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia 25:31–40 [DOI] [PubMed] [Google Scholar]
- Robinson DL, McClurkin JW, Kertzman C, Petersen SE. 1991. Visual responses of pulvinar and collicular neurons during eye movements of awake, trained macaques. J. Neurophysiol 66:485–96 [DOI] [PubMed] [Google Scholar]
- Rollenhagen JE, Olson CR. 2005. Low-frequency oscillations arising from competitive interactions between visual stimuli in macaque inferotemporal cortex. J. Neurophysiol 94:3368–87 [DOI] [PubMed] [Google Scholar]
- Rosen AC, Rao SM, Caffarra P, Scaglioni A, Bobholz JA, et al. 1999. Neural basis of endogenous and exogenous spatial orienting: a functional MRI study. J. Cogn. Neurosci 11:135–52 [DOI] [PubMed] [Google Scholar]
- Rossi AF, Bichot NP, Desimone R, Ungerleider LG. 2007. Top-down attentional deficits in macaques with lesions of lateral prefrontal cortex. J. Neurosci 27:11306–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Kastner S. 2011. Cognitive and perceptual functions of the visual thalamus. Neuron 71:209–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Pigarev IN, Vidyasagar TR. 2007. Neural mechanisms of visual attention: how top-down feedback highlights relevant locations. Science 316:1612–15 [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. 2012. The pulvinar regulates information transmission between cortical areas based on attention demands. Science 337:753–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapountzis P, Paneri S, Gregoriou GG. 2018. Distinct roles of prefrontal and parietal areas in the encoding of attentional priority. PNAS 115:E8755–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider KA, Kastner S. 2009. Effects of sustained spatial attention in the human lateral geniculate nucleus and superior colliculus. J. Neurosci 29:1784–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CE, Wilson DA, Radman T, Scharfman H, Lakatos P. 2010. Dynamics of active sensing and perceptual selection. Curr. Opin. Neurobiol 20:172–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Yantis S. 2007. Spatially selective representations of voluntary and stimulus-driven attentional priority in human occipital, parietal, and frontal cortex. Cereb. Cortex 17:284–93 [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW, Sherman SM. 2006. Exploring the Thalamus and Its Role in Cortical Function. Cambridge, MA: MIT Press [Google Scholar]
- Shipp S 2003. The functional logic of cortico-pulvinar connections. Philos. Trans. R. Soc. B 358:1605–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DT, Schenk T. 2012. The premotor theory of attention: time to move on? Neuropsychologia 50:1104–14 [DOI] [PubMed] [Google Scholar]
- Song K, Meng M, Chen L, Zhou K, Luo H. 2014. Behavioral oscillations in attention: rhythmic alpha pulses mediated through theta band. J. Neurosci. 34:4837–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaak E, Bonnefond M, Maier A, Leopold DA, Jensen O. 2012. Layer-specific entrainment of gamma-band neural activity by the alpha rhythm in monkey visual cortex. Curr. Biol. 22:2313–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague TC, Serences JT. 2013Attention modulates spatial priority maps in the human occipital, parietal and frontal cortices. Nat. Neurosci 16:1879–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire RF, Noudoost B, Schafer RJ, Moore T. 2013. Prefrontal contributions to visual selective attention. Annu. Rev. Neurosci 36:451–66 [DOI] [PubMed] [Google Scholar]
- Steinmetz MA, Constantinidis C. 1995. Neurophysiological evidence for a role of posterior parietal cortex in redirecting visual attention. Cereb. Cortex 5:448–56 [DOI] [PubMed] [Google Scholar]
- Suzuki M, Gottlieb J. 2013. Distinct neural mechanisms of distractor suppression in the frontal and parietal lobe. Nat. Neurosci 16:98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP. 2005. A visual salience map in the primate frontal eye field. Prog. Brain Res 147:251–62 [DOI] [PubMed] [Google Scholar]
- Thompson KG, Biscoe KL, Sato TR. 2005. Neuronal basis of covert spatial attention in the frontal eye field. J. Neurosci. 25:9479–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman AM, Gelade G. 1980. A feature-integration theory of attention. Cogn. Psychol 12:97–136 [DOI] [PubMed] [Google Scholar]
- Vallar G, Perani D. 1986. The anatomy of unilateral neglect after right-hemisphere stroke lesions: a clinical/CT-scan correlation study in man. Neuropsychologia 24:609–22 [DOI] [PubMed] [Google Scholar]
- van Kerkoerle T, Self MW, Dagnino B, Gariel-Mathis MA, Poort J, et al. 2014. Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. PNAS 111:14332–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRullen R, Carlson T, Cavanagh P. 2007. The blinking spotlight of attention. PNAS 104:19204–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivas AB, Humphreys GW, Fuentes LJ. 2006. Abnormal inhibition of return: a review and new data on patients with parietal lobe damage. Cogn. Neuropsychol. 23:1049–64 [DOI] [PubMed] [Google Scholar]
- von Helmholtz H 1867. Handbuch derphysiologischen Optik. Leipzig, Ger.: Voss [Google Scholar]
- Ward R, Danziger S, Owen V, Rafal R. 2002. Deficits in spatial coding and feature binding following damage to spatiotopic maps in the human pulvinar. Nat. Neurosci. 5:99–100 [DOI] [PubMed] [Google Scholar]
- Wimmer K, Ramon M, Pasternak T, Compte A. 2016. Transitions between multiband oscillatory patterns characterize memory-guided perceptual decisions in prefrontal circuits. J. Neurosci. 36:489–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer RD, Schmitt LI, Davidson TJ, Nakajima M, Deisseroth K, Halassa MM. 2015. Thalamic control of sensory selection in divided attention. Nature 526:705–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P, Mitra PP, Desimone R. 2006. Gamma-band synchronization in visual cortex predicts speed of change detection. Nature 439:733–36 [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. 2000. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J. Neurosci 20:RC63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenon A, Krauzlis RJ. 2012. Attention deficits without cortical neuronal deficits. Nature 489:434–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen Y, Bressler SL, Ding M. 2008. Response preparation and inhibition: the role of the cortical sensorimotor beta rhythm. Neuroscience 156:238–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Schafer RJ, Desimone R. 2016. Pulvinar-cortex interactions in vision and attention.Neuron 89:209–20 [DOI] [PMC free article] [PubMed] [Google Scholar]