Abstract
Background
Levels of antibodies induced by the measles virus–containing vaccine have been shown to decline over time, but there is no formal recommendation about testing immunized subjects (in particular, healthcare workers [HCWs]) to investigate the persistence of measles immunoglobulin G (IgG).
Methods
This study aims to evaluate the long-term immunogenicity of measles vaccine in a sample of medical students and residents of the University of Bari who attended the Hygiene Department for a biological risk assessment (April 2014–June 2018).
Results
Two thousand immunized (2 doses of measles-mumps-rubella [MMR] vaccine) students and residents were tested; 305 of these (15%) did not show protective anti-measles IgG. This proportion was higher among subjects who received vaccination at ≤15 months (20%) than in those who received vaccination at 16–23 months (17%) and at ≥24 months (10%) (P < .0001). After an MMR vaccine booster dose, we noted a seroconversion of 74% of seronegative HCWs. The overall seroconversion rate after a second dose (booster) was 93%. No serious adverse events were noted after the booster doses.
Conclusions
An important proportion of subjects immunized for measles do not show a protective IgG titer in the 10 years after vaccination. Our management strategy seems consistent with the purpose of evidencing immunological memory.
Keywords: healthcare workers, booster dose, duration of immunization
Measles is a viral vaccine-preventable disease that may adversely affect infants and adults. The World Health Organization (WHO) reported 173 330 cases of measles in 2017 worldwide (almost 90 000 deaths in 2016) [1]. According to postlicensure data, 1 dose of measles-mumps-rubella (MMR) vaccine is 93% effective and receipt of 2 doses is 97% effective against measles [2]. Since the introduction of global mass vaccination, the vaccine showed high safety [3], cost savings [4], and efficacy; cases of measles decreased 99.9%, with >20 000 000 saved lives [5].
In Italy, single-antigen measles vaccine was introduced in the 1970s and 1980s [6]. Since 2003, the national vaccination schedule has recommended a universal mass vaccination using 2 doses of MMR vaccine, following the Centers for Disease Control and Prevention (CDC) recommendations (the first dose at 12–15 months and the second at the age of 5–6 years) [2]. Although this very important vaccination campaign was carried out [7], measles elimination has never been reached due to suboptimal vaccine coverage [8]. Coverage achieved in newborns cohorts ranged between 85.4% and 91.6% for 1 dose during the period 2007–2017 [8, 9], far from the minimum coverage (95%) planned by public health institutions for the goal of measles circulation interruption [10]. In this context, in 2017 a measles epidemic has broken out, with >7000 cases, 12 confirmed deaths, and >400 cases among healthcare workers (HCWs) [11, 12]. The median age of cases is 26 years, and >70% of cases occur in people aged >14 years [11, 12]; 1.5%–6% of cases were reported in fully vaccinated subjects, although we do not know if they had circulating antibodies at the time of contact with the virus.
The question of the long-term persistence of anti-measles immunoglobulin G (IgG) among vaccinated subjects has been poorly investigated; the few recommendations available regarded HCWs. Nonseroprotected HCWs are an important issue in public health, representing a risk both for themselves and for patients, and many studies in the literature assert this concept [13–15].
Prelicensure studies have indicated that protective levels of antibodies induced by the MMR vaccine persist lifelong; according to results of more recent studies, the levels of antibodies have been shown to decline over time and may persist for 15–20 years [16]. Furthermore, immunization strategies could influence the effectiveness and the long-term immunogenicity of the MMR vaccine, as recent studies have shown for pertussis [17]. In particular, the reduction of natural booster could be related with a decline of the IgG level in fully vaccinated persons.
Our study was carried out in Apulia (southern Italy, around 4 000 000 inhabitants), where the anti-MMR vaccine coverage is around 91% (in the year 2017, birth cohort 2015) [8] and where in 2002–2003 a large outbreak (around 20 000 cases) was documented [18], followed by many outbreaks in subsequent years [19, 20] with documented cases of nosocomial transmission [13, 20]; many studies have evaluated the prevalence of anti-measles IgG on Apulian blood donors, showing a value around 5%–6% of nonimmunization, with the highest prevalence of no seroprotection in young adults (18–26 years old) [21, 22]. These data show how Apulia (but the context is not so different from the rest of Italy) is a region with a real risk of measles circulation, and therefore it is necessary that HCWs are evaluated for immunization status and the long-term persistence of antibodies against measles.
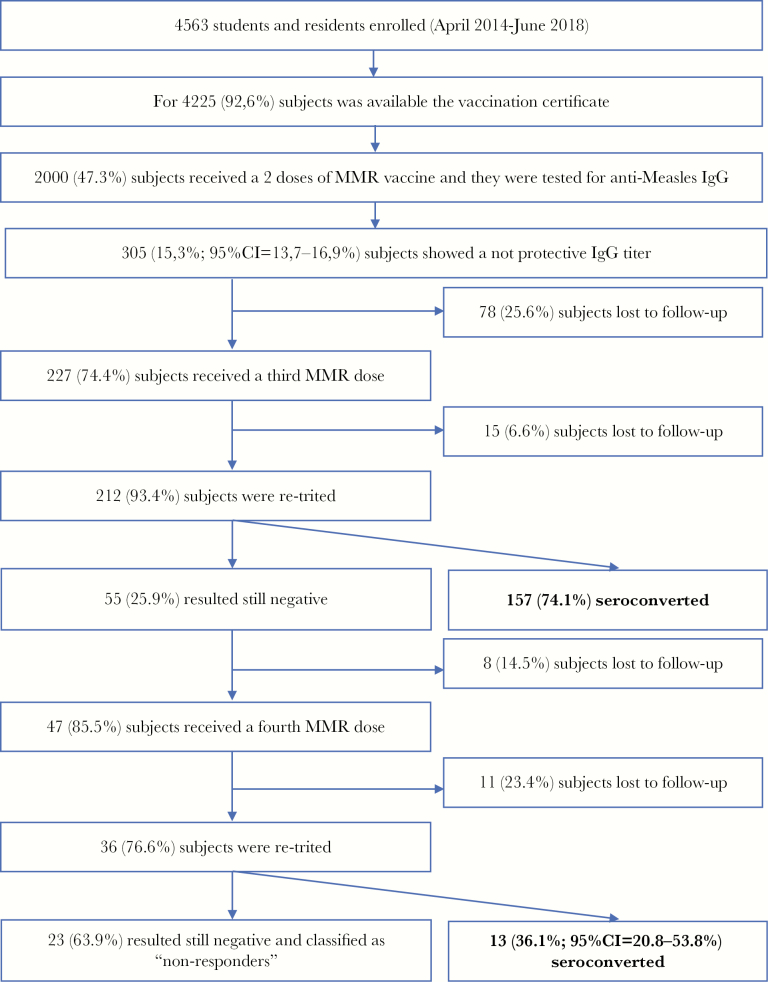
Flowchart 1.
Study population.
METHODS
This was a retrospective cohort study. This study aimed to evaluate the long-term immunogenicity of the measles component of MMR vaccination and the effectiveness of the strategy for the management of immunized subjects who did not show IgG against measles (reported as “nonresponders”). On the model of the hepatitis B vaccine [23], we define nonresponder as a person without a history of measles infection who has a documented history of an age-appropriate primary course of MMR vaccine, but with a current anti-measles nonseroprotective titer (≤16.5 AU/mL).
According to the Italian Ministry of Health’s recommendations [24], in April 2014 the Hygiene Department of the Bari Policlinico University Hospital planned a biological risk prevention program for students and residents (physicians in postgraduate training) of the Medical School of the University of Bari. The study sample is composed of students and residents who attended the Hygiene Department from April 2014 to June 2018. Subjects without an available vaccination history, never vaccinated, or vaccinated with a single dose of MMR vaccine at baseline and with a history of measles infection were excluded from the study. Informed consent was routinely collected during clinical procedures. People enrolled in the study gave informed consent on the use of data collected for clinical procedures for publication. This research was carried out according to the principles of the Helsinki Declaration. We considered in this survey only students and residents who had received, at the time of the enrollment, 2 doses of MMR vaccine (vaccine basal routine). The vaccination status of enrolled subjects was assessed by the Regional Immunization Database (GIAVA) [21]. GIAVA is a computerized vaccination registry that allows, for every Apulian inhabitant, to ascertain the vaccination history and eventually generate the immunization schedule.
For each enrolled participant, a 5-mL serum sample was collected to assess the immunity/susceptibility status for measles and tested by chemiluminescence immunoassay, using LIAISON measles IgG, a semiquantitative method, performed with a standardized commercial method (Diasorin). The LIAISON measles IgG cutoff value (>16.5 AU/mL) equates to 175 mIU/mL (WHO Third International Standard for Anti-measles, National Institute for Biological Standards and Control code 97/648) [21, 25].
Tested subjects who showed a nonprotective IgG titer received a booster dose of MMR vaccine (M-M-RVAXPRO, administered subcutaneously in the deltoid). Subjects with equivocal tests were retested and, if still equivocal, they were classified as negative. Twenty to 25 days after vaccination, a new blood test was performed to retest IgG titers; if the value found in reevaluation exceeded the cutoff, the subject was classified as seroconverted; if the titer was still negative, another vaccine dose (28 days after the first booster) was administered and after 20–25 days an additional measurement of IgG was performed. Subjects still seronegative were definitively classified as nonresponders; they received the recommendation to be evaluated for measles infection in all cases of exposure, and eventually to receive immunoglobulin. This management is consistent with specific protocols applied in some US medical schools [26]. Subjects who received the booster doses underwent 1 month of follow-up to assess the insurgence of any adverse effects.
For every enrolled subject a specific form was built, including information on patient ID, sex, age at enrollment, dates of the routine MMR vaccine, measles IgG titer, date of first booster dose, IgG titer after first booster, date of second booster dose, and IgG titer after second booster dose. Compiled forms were entered into a database created with an Excel spreadsheet, and data analysis was performed using Stata MP15 software.
Continuous variables were described as mean (standard deviation [SD]) and range, and categorical variables as proportions, with the 95% confidence interval (CI) when appropriate. The skewness and kurtosis test was used to evaluate the normality of continuous variables, but any of them was normally distributed or normalizable. The Kruskal–Wallis test was used to compare continuous variables between 3 distinct groups: (1) subjects who received the first dose of MMR routine vaccine at age ≤15 months; (2) subjects who received the first dose of MMR routine vaccine at age 16–23 months; and (3) subjects who received the first dose of MMR routine vaccine at age ≥24 months.
These groups were investigated for differences in the response to the vaccine between subjects who received the first MMR dose as provided by CDC recommendations (13–15 months), subjects who received the first dose at an older age (≥2 years), and subjects vaccinated between 16 and 23 months (ie, the modal case in the general population, considering some delay in the vaccination appointment); the Dunn multiple comparison test with Bonferroni correction was used to compare 2 groups at a time. The Wilcoxon rank-sum test was used to compare the continuous variables between the sexes. The χ 2 and exact Fisher tests were used to compare the proportions.
To assess the determinants of being seroprotected at the time of enrollment (seroconversion after the vaccine basal routine), univariate logistic regression was used, considering the seroprotection as outcome and sex (male vs female), age at enrollment (years), age at the time of the first vaccination at the basal routine, age at the time of second vaccination at basal routine (years), time from the first to the second dose of vaccine at the basal routine (months), time from the first dose of vaccination to the antibody titer evaluation (months), and time from the second dose of vaccination to the antibody titer evaluation (months) as determinants; odds ratios (ORs) were calculated with 95% CIs. For the previous outcome, a multivariate logistic regression model was built, using as determinant the group variable adjusted for the variables associated in the univariate logistic regression. Adjusted ORs (aORs) were calculated, with the 95% CI.
To assess the determinants of seroconversion after a booster dose, univariate logistic regression was used, considering the seroconversion after the third MMR dose in fully vaccinated subjects as outcome and as determinants the sex (male vs female), age at enrollment (years), age at the time of the first vaccination at the basal routine, age at the time of second vaccination at basal routine (years), time from the first to the second dose of vaccine at the basal routine (months), time from the first dose of vaccine at basal routine to the booster dose (months), and time from the second dose of vaccine at basal routine to the booster dose (months); the ORs (95% CIs) were calculated. For the previous outcome, a multivariate logistic regression model was built, using as determinant the group variable adjusted for the variables associated in the univariate logistic regression. The aOR values were calculated, with the 95% CI. The Box–Tidwell test was used to evaluate the linearity of independent variables and log odds; the Hosmer–Lemeshow test was used to evaluate the goodness-of-fit of the multivariate logistic regression models.
Protective antibody survival (PAS) was evaluated as the time elapsed from the first dose of routine MMR vaccination to the evaluation of antibody titer (years). The Kaplan–Meier curve was used to evaluate PAS and the log-rank test was used to evaluate the differences between groups. The median time of PAS and the incidence rate per 100 person-years of loss of seroprotection, both with 95% CIs, were estimated; the incidence rate ratios (IRRs) with 95% CIs were calculated considering as denominator the subjects vaccinated at ≤15 months and as numerator those vaccinated at 16–23 months or at ≥2 years.
To evaluate the determinants of PAS, the multivariate Cox semiparametric regression was used, considering as risk predictors the sex (male vs female), age at enrollment (years), age at the first dose of routine vaccine divided per group (≤15, 16–23, or ≥24 months), age at the second dose of routine vaccine (years), and time from the second dose of MMR vaccine to the antibody titer evaluation (months). The adjusted hazard ratio (aHR) values were calculated, with 95% CIs. The Schoenfeld and scaled Schoenfeld residuals tests were used to evaluate the proportionality assumption of the multivariate Cox semiparametric regression model.
For all tests, a 2-sided P value < .05 was considered statistically significant.
RESULTS
Between April 2014 and June 2018, 4563 students and residents were tested. The immunization status, downloaded by GIAVA, was available for 4225 of 4563 (92.6%) subjects, and 2000 of these (47.3%) received a complete MMR vaccination schedule. Of these 2000, 360 (18.0%) received the first dose of routine vaccine at age ≤15 months, 958 (47.9%) at age 16–23 months, and 682 (34.1%) at age ≥24 months.
Of the 2000 subjects, 1387 (69.4%) subjects were female and the proportion of females did not differ among groups in analysis (P > .05; Table 1). The mean age at enrollment was 21.1 (SD, 2.4) years (range, 18.0–38.0 years) with a difference between subjects vaccinated at ≤15 months and vaccinated at ≥24 months (P < .0001) and between subjects vaccinated at 16–23 months and vaccinated at ≥24 months (P < .0001) (Table 1). All of the subjects with a complete baseline vaccination routine were tested for anti-measles IgG. No one reported a history of measles.
Table 1.
Characteristics of the Sample, by Vaccination Group
| Variable | Total (N = 2000) | Immunized at ≤15 mo (n = 360) | Immunized at 16–23 mo (n = 958) | Immunized at ≥24 mo (n = 682) | P Value | |||
|---|---|---|---|---|---|---|---|---|
| Overall |
≤15 vs 16–23 mo | ≤15 vs ≥24 mo | 16–23 vs ≥24 mo | |||||
| Age at enrollment, y, mean ± SD (range) | 21.1 ± 2.4 (18.0–38.0) | 20.7 ± 2.0 (18.0–28.0) | 20.8 ± 2.2 (18.0–35.0) | 21.8 ± 2.8 (18.0–38.0) | < .0001 | .749 | < .0001 | < .0001 |
| Female sex, No. (%) | 1387 (69.4) | 239 (66.4) | 680 (71.0) | 468 (68.6) | .240 | .463 | .106 | .304 |
| Protective IgG titer at enrollment, No. (% [95% CI]) | 1695 (84.8 [83.1–86.3]) | 288 (80.0 [75.5–84.0]) | 794 (82.9 [80.3–85.2]) | 613 (89.9 [87.4–92.0]) | < .0001 | < .0001 | < .0001 | .224 |
| GMT IgG value at enrollment, AU/mL (95% CI) | 77.2 (73.0–81.6) | 58.5 (51.2–66.9) | 71.7 (65.6–77.9) | 99.1 (90.7–108.2) | < .0001 | .007 | < .0001 | < .0001 |
| Seroconverted after third MMR booster dose, No. (% [95% CI]) | 157/212 (74.1 [67.6–79.8]) | 40/52 (76.9 [62.8–80.2]) | 80/111 (72.1 [66.0–80.1]) | 37/49 (75.5 [61.1–86.7]) | .777 | .512 | .868 | .651 |
| IgG value after third MMR booster dose in seronegative subjects at enrollment, GMT, AU/mL (95% CI) | 46.1 (39.1–54.4) | 51.1 (36.5–71.5) | 43.7 (34.4–55.7) | 46.7 (34.1–64.0) | .762 | .693 | 1.000 | 1.000 |
Abbreviations: CI, confidence interval; GMT, geometric mean titer; IgG, immunoglobulin G; MMR, measles-mumps-rubella vaccine; SD, standard deviation.
Of the 2000 subjects, 1695 (84.8% [95% CI, 83.1%–86.3%]) showed a protective IgG titer; this percentage did not differ between males (512/613; 83.5% [95% CI, 80.3%–86.4%]) and females (1183/1387; 85.3% [95% CI, 83.3%–87.1%]). The proportion of subjects with detectable titer of anti-measles IgG was lower (P < .0001) among those vaccinated at ≤15 months (80.0% [95% CI, 75.5%–84.0%]) than in those vaccinated at 16–23 months (82.9% [95% CI, 80.3%–85.2%]) or ≥24 months (89.9% [95% CI, 87.4%–92.0%]) (Table 1). The overall IgG geometric mean titer was 77.2 (95% CI, 73.0–81.6), with differences among groups (P < .0001; Table 1). Two hundred twenty-seven of 305 (85.6%) seronegative subjects received a booster dose and of these, 212 (93.4%) were reevaluated. In 157 of 212 (74.1% [95% CI, 67.6%–79.8%]) a seroconversion was noted, without differences among the groups in analysis (P > .05) (Table 1). The IgG geometric mean titer value after a booster dose was 46.1 (95% CI, 39.1–54.4), without significant differences between groups (P > .05; Table 1). Forty-seven of 55 (85.5%) subjects who were still seronegative received another booster dose, and 36 of these (76.6%) were reevaluated: 13 (36.1% [95% CI, 20.8%–53.8%]) seroconverted (the study population is described in flowchart 1). Overall, the seroconversion rate after a second dose was of 93.4% (95% CI, 89.0%–96.5%).
The multivariate logistic regression showed that seropositivity at enrollment was associated with the time from the second dose of MMR vaccine to the antibody titer evaluation (aOR, 0.99 [95% CI, .98–.99]) and the time (months) from the first to the second dose of MMR vaccine (aOR, 0.99 [95% CI, .99–1.00]), whereas there were no associations with the other determinants (P > .05; Table 2).
Table 2.
Univariate and Multivariate Logistic Regression Analysis of Determinants of Seropositivity at Enrollment
| Determinants | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | aOR (95% CI) | P Value | |
| Age at enrollment, y | .97 (.92–1.02) | .194 | … | |
| Sex (male vs female) | .9 (.7–1.1) | .311 | … | |
| Age at the first dose of routine MMR vaccination | … | |||
| ≤15 mo (baseline) | 1.00 | 1.00 | ||
| 16–23 mo | 1.21 (.89–1.65) | .225 | 1.17 (.86–1.60) | .319 |
| ≥24 mo | 2.22 (1.55–3.18) | < .0001 | 1.49 (.95–2.32) | .080 |
| Age, y, at the second dose of routine MMR vaccine | 1.05 (1.02–1.09) | .005 | 1.01 (.94–1.09) | .753 |
| Time, mo, from the first to second dose of MMR vaccine | 1.00 (.99–1.00) | .019 | .99 (.99–1.00) | .014 |
| Time, mo, from the first dose of MMR vaccine to antibody titer evaluation | .99 (.98–.99) | < .0001 | a | |
| Time, mo, from the second dose of MMR vaccine to antibody titer evaluation | .99 (.98–.99) | < .0001 | .99 (.98–.99) | < .0001 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; MMR, measles-mumps-rubella; OR, odds ratio.
aOmitted because of collinearity; Hosmer–Lemeshow χ 2 = 5.1 (P = .747).
Univariate logistic regression showed that the outcome of seroconversion after a booster dose was associated with sex (male vs female; OR, 0.52 [95% CI, .28–.98]; z = 2.0; P = .044), whereas it was not associated with the other determinants (P > .05); the multivariate model confirmed the association with sex (male vs female; aOR, 0.52 [95% CI, .3–.9]; z = 2.0; P = .042), whereas the age at first dose of routine vaccination seemed not to be significant (P > .05; Hosmer–Lemeshow χ 2 = 3.4; P = .492; Table 3).
Table 3.
Univariate and multivariate logistic regression analysis of determinants of seroconversion after booster MMR dose
| Determinants | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95%CI) | p-value | aOR (95%CI) | p-value | |
| Age at enrollment (years) | 0.92 (0.80-1.06) | 0.231 | ||
| Gender (male vs. female) | 0.52 (0.28-0.98) | 0.044 | 0.52 (0.27-0.98) | 0.042 |
| Age at the first dose of MMR vaccine routine | ||||
| • ≤15 months (baseline) | 1.00 | - | 1.00 | - |
| • 16-23 months | 0.77 (0.36-1.66) | 0.512 | 0.79 (0.36-1.70) | 0.539 |
| • ≥24 months | 0.90 (0.36-2.25) | 0.826 | 0.98 (0.39-2.49) | 0.871 |
| Age at the second dose of MMR vaccine routine (years) | 0.99 (0.98-1.00) | 0.054 | ||
| Time from the first to the second dose of MMR vaccine (months) | 1.00 (0.99-1.01) | 0.772 | ||
| Time from the first dose of MMR vaccine to the antibody titer evaluation (months) | 1.00 (1.00-1.01) | 0.379 | ||
| Time from the second dose of MMR vaccine to the antibody titer evaluation (months) | 1.01 (1.00-1.02) | 0.291 |
Hosmer-Lemeshow chi-square=3.4; p=0.492
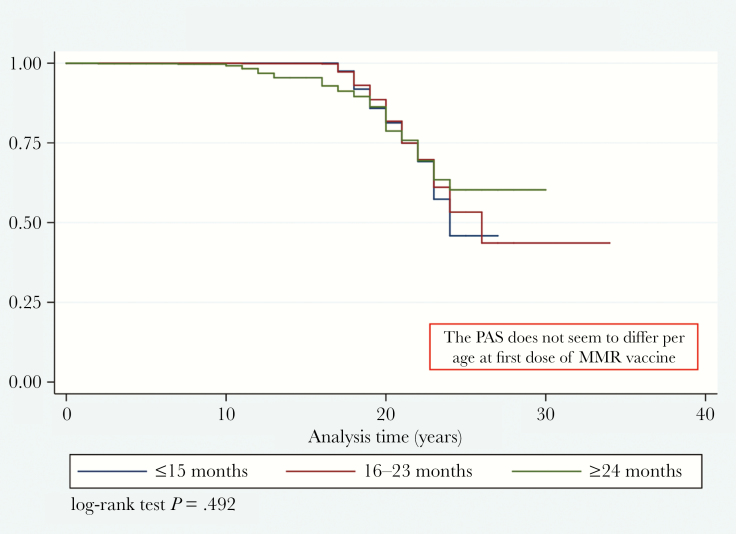
The mean time of PAS was 17.8 (SD, 4.0) years (range, 0.0–34.0 years); the IgG loss in 25% of fully vaccinated subjects was estimated to be 21 years (95% CI, 21–22 years), and the incidence rate per 100 person-years of seronegativity was 8.6 (95% CI, 7.7–9.6). The PAS did not seem to differ by age at first dose of MMR vaccine (z = 2.5; P = .285; Figure 1); the incidence rate per 100 person-years of losing IgG was 10.2 (95% CI, 8.1–12.9) among subjects vaccinated at ≤15 months, 8.9 (95% CI, 7.6–10.3) in those vaccinated at 16–23 months, and 6.9 (95% CI, 5.4–8.7) in those vaccinated at ≥24 months, with an IRR of 0.86 (95% CI, .65–1.16; P = .154) in the comparison between those vaccinated at 16–23 months and ≤15 months and an IRR of 0.67 (95% CI, .47–.94; P = .009) in the comparison between those vaccinated at ≥24 months and ≤15 months. The results of multivariate Cox semiparametric regression analysis are described in Table 4.
Figure 1.
Kaplan–Meier measles protective antibody survival estimates, by group. Abbreviations: MMR, measles-mumps-rubella vaccine; PAS, protective antibody survival.
Table 4.
Multivariate Cox Semiparametric Regression Analysis of Risk Predictors of Measles Immunoglobulin G Protective Antibody Survival
| Determinants | Multivariate Analysis | |
|---|---|---|
| aHR (95% CI) | P Value | |
| Sex (male vs female) | 1.12 (.88–1.42) | .370 |
| Age, y, at enrollment | .61 (.51–.87) | < .0001 |
| Age at the first dose of routine MMR vaccine | ||
| ≤15 mo (baseline) | 1.00 | |
| 16–23 mo | 1.24 (.93–1.64) | .141 |
| ≥24 mo | 4.08 (2.82–5.90) | < .0001 |
| Age, y, at the second dose of routine MMR vaccine | .67 (.51–.87) | .003 |
| Time, mo, from the second dose of MMR vaccine to the antibody titer evaluation | .99 (.95–.99) | .005 |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; MMR, measles-mumps-rubella.
For the purpose of adapting our study to the international guidelines, we have considered subjects with IgG values ≥120 mIU/mL as seroprotected [27] and recalculated the proportion of nonseroprotected subjects according to this cutoff. A total of 1781 of 2000 (89.1% [95% CI, 87.6%–90.4%]) subjects showed a protective IgG titer; this proportion was lower (P < .0001) among those vaccinated at age ≤15 months (86.1% [95% CI, 82.1%–89.5%]) than in those vaccinated at 16–23 months (87.5% [95% CI, 85.2%–89.5%]) and at ≥24 months (92.8% [95% CI, 90.6%–94.6%]).
DISCUSSION
In our study, carried out in a sample of 2000 subjects, >15% of students and residents enrolled did not show IgG for measles and 1 or more booster doses were needed to elicit circulating anti-measles IgG. This value differs from the evidence in the literature: a 2015 review indicates that 6% of HCWs in Europe are seronegative for measles [28], a 2013 French study estimates this value to be 8% [28], whereas a 2013 Spanish study reports a susceptibility rate of 2% [29]. These studies did not consider vaccination status in the assessment.
The lack of IgG among immunized subjects could be explained by 2 different mechanisms: (1) primary vaccination failure, due to failures in vaccine attenuation, vaccination regimens, or administration; or (2) loss of circulating antibodies, characterized by a loss of protection after initial effectiveness and accelerated by the poor circulation of pathogens [23].
The administration of a dose booster could be also a strategy to discriminate between these 2 scenarios. Indeed, theoretically, a seroconversion after booster dose could imply the persistence of immunological memory and therefore a loss of immunogenicity, while a nonresponse to the booster could imply a primary vaccination failure; this point is just a hypothesis and further studies are needed to clarify it. Regarding the determinants of seroconversion after basal vaccination routine, the time (months) from the second dose of MMR vaccine to the antibody titer evaluation appears to be a significant factor (OR, 0.99; P = .014); in particular, it seems that a shorter time is associated with a better persistence of circulating anti-measles antibodies. Of particular interest is the finding of an association between being female and a better response to the third MMR dose compared to males. Sex differences in the response to vaccines is a topic studied by many authors in the literature, and the scientific community agrees in recognizing that immunological, hormonal, genetic, microbiota, and environmental differences between males and females may also affect the outcome of vaccination, with males seeming to be less immunoresponsive compared to females [30–32]. The estimated value in which half of the vaccinated subjects lose seroprotection is >25 years, without important differences among the groups analyzed, and antibody levels tend to decline almost 15 years after the first dose of MMR vaccine. Considering that we have analyzed only 1 observation per case, the persistence of circulating antibodies could be shorter than observed in our study, so the duration of immunity may be lower than that reported in the literature (around 15 years) [27]. The administration of the first dose of MMR vaccine at ≥2 years of age seems to be associated with a lower risk of loss of immunity (IRR, 0.67 [95% CI, .47–.94]) compared to the other ages in the analysis. The Cox analysis suggests that a younger age at the time of the first and second doses of MMR vaccine, an older age at enrollment, and a longer time from the second dose of MMR vaccine to the antibody titer evaluation seem to be risk factors for the persistence of circulating antibodies.
In summary, 93.4% of subjects without circulating anti-measles IgG at enrollment gained a protective titer after 1 or 2 booster doses. Our strategy seemed consistent with the purpose of eliciting protection in a subgroup with high risk of exposure (HCWs) and additional risk of complications (young adults). The low percentage of nonresponders received a specific recommendation of being evaluated in case of exposure, in particular if male or at high risk of complications.
The time between vaccination doses and the antibody titer evaluation is a determinant for persisting circulating antibodies; in particular, the time from the second dose to the antibody titer evaluation seems to be the main determinant of the persistence of circulating antibodies, with a major role compared to age at the time of the first dose of MMR vaccine. Therefore, the antibody titer and immunity decrease over the years, as already described for the anti–hepatitis B virus, anti-pertussis, and anti-mumps vaccines [17, 24, 33]. To the best of our knowledge, the introduction of booster dose(s) in fully vaccinated but not measles-seroprotected subjects among HCWs is a topic not studied in the literature. Few official recommendations are available regarding the use of a third dose of MMR vaccine in other contexts; indeed, many studies showed that a booster dose of MMR vaccine in fully vaccinated subjects is safe and effective in preventing mumps infection in subjects exposed in close settings during outbreaks [33]. The strengths of our study include the relevant sample size, the topic being poorly studied in the literature, and the comparison based on the age of the first dose; furthermore, the issue of vaccinations in nonresponder HCWs is of great importance in future decisions on vaccination strategies. The major limitation is related to the impossibility to analyze the subjects’ immunostatus in relation to the commercial type of MMR vaccine, and it was not possible to evaluate if the subject had ever come into contact with wild virus. Moreover, a topic of discussion in the literature is the role of cell-mediated immunity in long-term response to the vaccine and protection against measles [34]; therefore, hypothetically, a vaccinated subject not providing circulating antibodies could be immune. Ruckdeschel et al [35] asserted that the lymphocyte responsiveness to measles complement fixation antigen seen in 2 pediatric residents who had negative anti-measles IgG titers and who had frequent exposure to patients affected by measles is the in vitro correlate of their clinical protection against infection. Nevertheless, a recent study asserts that the contribution of T cells to protection is generally considered minor in comparison to neutralizing antibodies [36], so more studies are needed to clarify this point.
The full MMR vaccination routine is not an absolute guarantee of immunity against the wild viruses; a 2014 study [37] describes a measles outbreak investigation in a hospital that included 8 infected HCWs, of whom 6 were subjects who received 2 doses of measles vaccine. Rota et al [38] described a measles outbreak in 2009 in Pennsylvania and Virginia that resulted in the exposure and apparent infection of 2 physicians, both fully vaccinated with 2 doses of MMR vaccine. We cannot know if these subjects were vaccine failures or they lost circulating antibodies over the years, but in both cases our management should have prevented the disease. From this point of view, it becomes fundamental to verify the serological state and implement the appropriate measures of prophylaxis in seronegative cases, even in the vaccinated HCWs. Given these assumptions, inclusion of the screening model described in the routine assessment of the biological risk of medical students (before medical internship) and HCWs (in the context of occupational medical examination) may be a winning strategy in preventing measles nosocomial infection.
Notes
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Mumps reported cases 2018. http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tsincidencemumps.html. Accessed 24 September 2018. [Google Scholar]
- 2. Centers for Disease Control and Prevention. Measles, mumps, and rubella (MMR) vaccination: what everyone should know.https://www.cdc.gov/vaccines/vpd/mmr/public/index.html. Accessed 22 September 2018.
- 3. Centers for Disease Control and Prevention. Measles, mumps, and rubella (MMR) vaccine safety.https://www.cdc.gov/vaccinesafety/vaccines/mmr-vaccine.html. Accessed 15 September 2018.
- 4. Zhou F, Reef S, Massoudi M, et al. . An economic analysis of the current universal 2-dose measles-mumps-rubella vaccination program in the United States. J Infect Dis 2004; 189(Suppl 1):S131–45. [DOI] [PubMed] [Google Scholar]
- 5. Dabbagh A, Patel MK, Dumolard L, et al. . Progress towards regional measles elimination—worldwide, 2000–2016. Wkly Epidemiol Rec 2017; 92:649–59.29076714 [Google Scholar]
- 6. Bechini A, Boccalini S, Tiscione E, et al. . Progress towards measles and rubella elimination in Tuscany, Italy: the role of population seroepidemiological profile. Eur J Public Health 2012; 22:133–9. [DOI] [PubMed] [Google Scholar]
- 7. European Centre for Disease Prevention and Control. Number of measles cases in EU and EEA countries, 2016 data https://ecdc.europa.eu/en/publications-data/number-Measles-cases-eu-and-eea-countries-2016-data.
- 8. D’Ancona F, D’Amario C, Maraglino F, Rezza G, Ricciardi W, Iannazzo S. Introduction of new and reinforcement of existing compulsory vaccinations in Italy: first evaluation of the impact on vaccination coverage in 2017. Euro Surveill 2018; 23. doi:10.2807/1560-7917.ES.2018.23.22.1800238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Porretta A, Quattrone F, Aquino F, et al. . A nosocomial measles outbreak in Italy, February–April 2017. Euro Surveill 2017; 22:30597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization European Region. Eliminating measles and rubella and preventing congenital rubella infection.http://www.euro.who.int/__data/assets/pdf_file/0008/79028/E87772.pdf. Accessed 24 September 2018.
- 11. European Centre for Disease Prevention and Control. Monthly measles and rubella monitoring report—February 2018. https://www.ecdc.europa.eu/sites/portal/files/documents/Monthly%20Measles%20and%20Rubella%20monitoring%20report%20%20February%202018.pdf. Accessed 23 September 2018.
- 12. Epicentro, the Italian portal of Epidemiology for Public Health. Measles and rubella news. Monthly update. Report number 44, October 2018. https://www.epicentro.iss.it/morbillo/bollettino/RM_News_2018_45%20.pdf. Accessed 21 September 2018.
- 13. Tafuri S, Germinario C, Rollo M, Prato R. Occupational risk from measles in healthcare personnel: a case report. J Occup Health 2009; 51:97–9. [DOI] [PubMed] [Google Scholar]
- 14. Botelho-Nevers E, Cassir N, Minodier P, et al. . Measles among healthcare workers: a potential for nosocomial outbreaks. Euro Surveill 2011; 16. pii:19764. [PubMed] [Google Scholar]
- 15. Sydnor E, Perl TM. Healthcare providers as sources of vaccine-preventable diseases. Vaccine 2014; 32:4814–22. [DOI] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention. Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60(RR-7):1–45. [PubMed] [Google Scholar]
- 17. Warfel JM, Edwards KM. Pertussis vaccines and the challenge of inducing durable immunity. Curr Opin Immunol 2015; 35:48–54. [DOI] [PubMed] [Google Scholar]
- 18. Lopalco PL, Prato R, Pastore R, Martinelli D, Caputi G, Germinario C. Epidemiological analysis of measles in the Apulian region based on the use of current data sources. J Prev Med Hyg 2005; 46:132. [Google Scholar]
- 19. Prato R, Chironna M, Caputi G, et al. . An outbreak of measles in Apulia, Italy, November 2006–January 2007. Euro Surveill 2007; 12:E070405.1. [DOI] [PubMed] [Google Scholar]
- 20. Caputi G, Tafuri S, Chironna M, et al. . An outbreak of measles including nosocomial transmission in Apulia, south-east Italy, January–March 2008—a preliminary report. Euro Surveill 2008; 13. pii:18839. [PubMed] [Google Scholar]
- 21. Tafuri S, Gallone MS, Gallone MF, Pappagallo MT, Larocca AMV, Germinario C. Monitoring the process of measles elimination by serosurveillance data: the Apulian 2012 study. Vaccine 2016; 34:2092–5. [DOI] [PubMed] [Google Scholar]
- 22. Gallone MS, Germinario C, Larocca AMV, Tafuri S. Long time immunogenicity of measles vaccine in the vaccination era: an open question. Hum Vaccin Immunother 2017; 13:117–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wiedermann U, Garner-Spitzer E, Wagner A. Primary vaccine failure to routine vaccines: why and what to do? Hum Vaccin Immunother 2016; 12:239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bianchi FP, Gallone MS, Gallone MF, et al. . HBV seroprevalence after 25 years of universal mass vaccination and management of non-responders to the anti-hepatitis B vaccine: an Italian study among medical students. J Viral Hepat 2019; 26:136–44. [DOI] [PubMed] [Google Scholar]
- 25. DiaSorin. The Diagnostic Specialist. LIAISON Measles IgG and IgM. The fully automated solution for antibody detection https://www.diasorin.com/sites/default/files/allegati_prodotti/ese_brochure_liaison_Measles_0413_low.pdf. Accessed 15 October 2018.
- 26. Cabrillo College. Clinical compliance basics. Health screening. measles, mumps, rubella (MMR).https://sites.google.com/a/cabrillo.edu/cabrillo-allied-health-clinical-compliance/home/page-1/Measles-mumps-rubella. Accessed 4 November 2018.
- 27. McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013; 62:1–34. [PubMed] [Google Scholar]
- 28. Haviari S, Bénet T, Saadatian-Elahi M, André P, Loulergue P, Vanhems P. Vaccination of healthcare workers: a review. Hum Vaccin Immunother 2015; 11:2522–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Freund R, Krivine A, Prévost V, et al. . Measles immunity and measles vaccine acceptance among healthcare workers in Paris, France. J Hosp Infect 2013; 84:38–43. [DOI] [PubMed] [Google Scholar]
- 30. Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol 2017; 33:577–99. [DOI] [PubMed] [Google Scholar]
- 31. Ruggieri A, Anticoli S, D’Ambrosio A, Giordani L, Viora M. The influence of sex and gender on immunity, infection and vaccination. Ann Ist Super Sanita 2016; 52:198–204. [DOI] [PubMed] [Google Scholar]
- 32. Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg 2015; 109:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marin M, Marlow M, Moore KL, Patel M. Recommendation of the Advisory Committee on Immunization Practices for use of a third dose of mumps virus-containing vaccine in persons at increased risk for mumps during an outbreak. MMWR Morb Mortal Wkly Rep 2018; 67:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dhiman N, Ovsyannikova IG, Jacobson RM, et al. . Correlates of lymphoproliferative responses to measles, mumps, and rubella (MMR) virus vaccines following MMR-II vaccination in healthy children. Clin Immunol 2005; 115:154–61. [DOI] [PubMed] [Google Scholar]
- 35. Ruckdeschel JC, Graziano KD, Mardiney MR Jr. Additional evidence that the cell-associated immune system is the primary host defense against measles (rubeola). Cell Immunol 1975; 17:11–8. [DOI] [PubMed] [Google Scholar]
- 36. Griffin DE. The immune response in measles: virus control, clearance and protective immunity. Viruses 2016; 8:E282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hahné SJ, Nic Lochlainn LM, van Burgel ND, et al. . Measles outbreak among previously immunized healthcare workers, the Netherlands, 2014. J Infect Dis 2016; 214:1980–6. [DOI] [PubMed] [Google Scholar]
- 38. Rota JS, Hickman CJ, Sowers SB, Rota PA, Mercader S, Bellini WJ. Two case studies of modified measles in vaccinated physicians exposed to primary measles cases: high risk of infection but low risk of transmission. J Infect Dis 2011; 204(Suppl 1):S559–63. [DOI] [PubMed] [Google Scholar]