Abstract
Background
Infection with multiple cytomegalovirus (CMV) strains (mixed infection) was reported in a variety of hosts. As the virus genetic diversity in primary CMV infection and the changes over time remain incompletely defined, we examined CMV diversity and changes in diversity over time in healthy adolescent females who participated in a phase 2 CMV gB/MF59 vaccine trial.
Methods
CMV genetic diversity was determined by genotyping of 5 genes—gB (UL55), gH (UL75), gN (UL73), US28, and UL144—in urine, saliva, and plasma samples from 15 study subjects.
Results
At the time of primary infection, 5 of 12 (42%) urine samples had multiple virus strains, and 50% of vaccine recipients were infected with gB1 genotype (vaccine strain). Mixed infection was documented in all 15 subjects within 3 months after primary infection, and the majority had different CMV genotypes in different compartments. Changes in genotypes over time were observed in all subjects.
Conclusions
Infection with multiple CMV genotypes was common during primary infection and further diversification occurred over time. Infection with gB1 genotype in vaccine recipients suggests a lack of strain-specific protection from the vaccine. As only 5 polymorphic genes were assessed, this study likely underestimated the true genetic diversity in primary CMV infection.
Keywords: cytomegalovirus, primary infection, strains, diversity
By utilizing direct examination of samples from different sites of shedding, we demonstrate that infection with multiple cytomegalovirus (CMV) strains is common after primary CMV infection. Understanding the importance of CMV diversity in primary infection is critical for future vaccine efforts.
Cytomegalovirus (CMV) is an important human pathogen that is the most frequent cause of congenital infection and significant disease in immunocompromised patients including solid organ transplant recipients [1]. In the United States, an estimated 20 000–30 000 infants are born with congenital CMV each year, with 10%–15% of these infants developing significant neurological sequelae, the most common being hearing loss [2]. The Institute of Medicine of the National Academy of Sciences listed the development of a CMV vaccine to prevent congenital infections as the highest priority based on cost savings and health benefits [3].
CMV has a large genome (~236 kb) with >200 protein-coding open reading frames. It has been shown to be highly genetically diverse with polymorphisms scattered across the virus genome [4]. Studies have documented the presence of multiple CMV virus strains in a variety of population groups, including immunocompetent adults, children attending day care, human immunodeficiency virus–infected individuals, allograft recipients, and infants with congenital CMV infection [5–11]. Two studies that have examined the virus genetic diversity in primary CMV infections have reported infection with a single genotype [12, 13]. However, infection with multiple virus strains has been demonstrated in infants with congenital infections, suggesting that multiple virus strains can be transmitted from the mother to the infant [10, 11]. Although the biologic significance of CMV genetic diversity remains unclear, it has been suggested that increased diversity plays a role in CMV pathogenesis [4, 9].
The gB/MF59 vaccine is a subunit recombinant CMV vaccine based on the sequence of the Towne strain of CMV. Phase 2 trials in seronegative women and adolescent girls demonstrated 43%–50% efficacy in preventing acquisition of primary CMV infection [14, 15]. Utilizing samples from adolescent women who participated in a gB/MF59 vaccine trial and were identified to have acquired primary infection, we sought to determine the genetic diversity of CMV and describe changes in CMV diversity over time.
METHODS
Study Population
The study population was selected from seronegative adolescent girls (12–17 years of age) who participated in a randomized, double-blind, placebo-controlled trial of the CMV gB/MF59 vaccine and acquired primary CMV infection [14]. Primary CMV infection was defined as identification of CMV from urine or blood specimens or seroconversion to nonvaccine antigens. Serum specimens obtained at scheduled clinic visits were tested to identify seroconversion. Subjects who seroconverted were enrolled in a substudy to monitor shedding in urine, saliva, and blood samples obtained monthly for 4 months, then every other month for 8 months. Specimens underwent nucleic acid isolation, and CMV was detected by real-time polymerase chain reaction (PCR) using 2 sets of primers and probes to simultaneously amplify a 68-bp region of the gB (UL55) gene and an 84-bp region of the UL123 exon 4, as described previously [14]. For quantitation, a real-time PCR assay was established using the same primers and probes but using a standard curve generated from a commercially available preparation of quantitated CMV DNA (Advanced Biotechnologies) [14]. A convenience sampling of specimens from 15 participants who had multiple specimens available were selected for detailed analysis of virus genetic diversity. Informed consent was obtained from each participant (<18 years of age) and a parent, and samples from the participants who agreed that their specimens could be used for future studies were included in the present study. Institutional review board approval was obtained from the University of Alabama at Birmingham.
Characterization of CMV Genotypes
The CMV virus diversity was evaluated by genotyping the envelope glycoproteins UL55 (gB), UL73 (gN), and UL75 (gH), and the CMV-encoded cytokine/chemokine receptor homologues US28 and UL144, as previously reported [10, 11] (Supplementary Figures 1–3). Genotyping was performed directly from the clinical samples (urine, saliva, and plasma), which were processed to extract DNA using commercial spin columns (Qiagen) [16]. To reduce the possibility of sequence variation from amplification, PCR amplification of the genes was performed with high-fidelity Taq polymerase. For genotyping of gN (UL73), the complete gN region was amplified with primers gN-Fw (5′ GGC GGT GGT GTG ATG GAG TG) and gN-Rev (5′ AAT AGC CTT TGG TGG TGG TTG C). After an initial 2-minute denaturation at 96°C, the samples underwent 7 cycles of denaturation at 96°C for 30 seconds, annealing at 65°C for 30 seconds, and extension at 72°C for 40 seconds, and the annealing temperature was decreased by 1°C each cycle. The samples were further subjected to 28 cycles with an annealing temperature of 58°C and a final extension step at 72°C for 7 minutes. The variable region in the N terminus of US28 was amplified with the following primers: US28-Fw (5′-GTGAACCGCTCATATAGACC); US28-Rev (5′-GAAACAGGCAGTGAGTAACG-3′); and US28 heminested-Rev (5′-CATCCACAGAGGTAGTGTAC-3′).
Primers for amplification of the entire UL144 gene were as follows: UL144-Fw (5′-CGTATTACAAACCGCGGAGAGGAT); UL144-Rev (5′-CTCAGACACGGTTCCGTAAAGTC); UL144 nested-Fw (5′-CTTCCGGTAGGAGGCATGAAG); and UL144 nested-Rev (5′-GACTTCATCGTACCGTGATC). The conditions for amplification with US28 and UL144 primer sets were 94°C for 5 minutes, followed by 34 cycles of 94°C for 1 minute, 55°C for 1 minute, and 72°C for 1 minute. This was followed by a single extension cycle of 72°C for 7 minutes [6, 10, 11, 17]. The amplified regions of UL73, UL144, and US28 were then cloned into the TOPO TA cloning vector pCR 2.1 (Invitrogen), and up to 10 individual colonies were screened for the presence of the insert [6]. Positive and negative controls were included with each PCR run and through all steps of the cloning reaction. The nucleotide sequences of the inserts were aligned with published sequences from GenBank to assign a genotype using the MAFFT multiple sequence alignment software [18]. The following genotypes were assessed and sequences submitted to GenBank: gN (UL73): 7 known genotypes: 1, 2, 3a, 3b, 4a, 4b, 4c, GenBank accession numbers AF309971, AF309976, AF309980, AF390773, AF309987, AF309997, AF310004; gB (UL55): 4 known genotypes: 1, 2, 3, 4, GenBank accession numbers M60929, FJ527563, M60933, M60926; gH (UL75): 2 known genotypes: 1 and 2, GenBank accession numbers FJ527563 and FJ616285; CMV (US28): 7 known genotypes: a1, a2, a3, b1, b2, c, d, GenBank accession numbers KT726953, KU317610, KT726955, KT726954, KX544839, KT726949, KT726946; UL144: 5 known genotypes (A, B, C, AB, AC), GenBank accession numbers KT726945, KX544837, KP745673, KX544836, AF498090 (Supplementary Figures 1–3).
Genotyping of UL55 (gB) and UL75 (gH) was performed by a genotype-specific real-time PCR assay using the TaqMan platform, which is based on probe hybridization [5, 10, 11]. Primers and probes identified individual gB genotypes at the cleavage site of the gB gene and gH genotypes at the N-terminal region of the gH gene [5]. Plasmid standards for each gB and gH genotype were prepared using the amplified region of interest from our sample repository that contained known gB and gH genotypes. The gene products were cloned into a TOPO/TA vector and the correct sequence was confirmed by sequence analysis. Standards were quantified and serially diluted from 10 to 106 copies, and these were included in each PCR run to generate standard curves. Results were analyzed using the R statistical programming language (version 3.2.5) and R Bioconductor (version 3.2) packages. Mixed infection was defined as the presence of >1 CMV genotype in any of the samples collected at one time point [10, 11].
RESULTS
Table 1 describes the initial duration and magnitude of CMV shedding from each compartment (blood, urine, and saliva) of the 34 subjects who were infected during the study and who enrolled in the shedding substudy. Placebo and vaccine recipients were combined because there was no significant difference in the shedding patterns [14]. Overall, 24 of 32 subjects in the shedding population had a positive PCR result in at least 1 follow-up visit. Shedding was detected in most saliva and urine samples but was only detected in about a third of peripheral blood specimens. Two subjects had CMV detected in only saliva and 1 subject only in urine; the duration (230.8–260.5 days) of shedding was longer, and the magnitude (1.7 × 104 copies/mL to 3.9 × 105 copies/mL) was higher in saliva and urine compared to blood samples. Although the duration of CMV shedding was similar for saliva and urine, the viral load was 10-fold higher in saliva than in urine.
Table 1.
Duration and Magnitude of Cytomegalovirus (CMV) Shedding After Primary CMV Infection
| Parameter | Blood (n = 10) | Urine (n = 22) | Saliva (n = 23) |
|---|---|---|---|
| % of visits positivea | 31.3 ± 23.0 (13, 75) | 68.3 ± 28.7 (13, 100) | 77.8 ± 29.5 (13, 100) |
| Initial durationb, d | 36.9 ± 54.1 (1, 166) | 134.4 ± 128.8 (1, 332) | 77.8 ± 29.5 (13, 100) |
| Overall durationc, d | 65.3 ± 88.6 (1, 267) | 230.8 ±109.1 (1, 332) | 260.5 ± 113.4 (1, 338) |
| Peak magnitude, copies/mL | 2 × 102 ± 3.6 × 102 (16 × 100, 1 × 103) | 1.7 × 104 ± 3.2 × 104 (16 × 100, 1.1 × 105) | 3.9 × 105 ± 1.4 × 106 (14 × 100, 6.4 × 106) |
Data are shown as mean ± standard deviation (min, max). Eight subjects did not have any shedding during the substudy and are not included.
aThe percentage of positive visits is only calculated for subjects with at least 1 positive visit.
bInitial duration is the number of days from the first positive shedding result to the first negative shedding.
cOverall duration is the number of days from the first positive shedding result to the last positive shedding result.
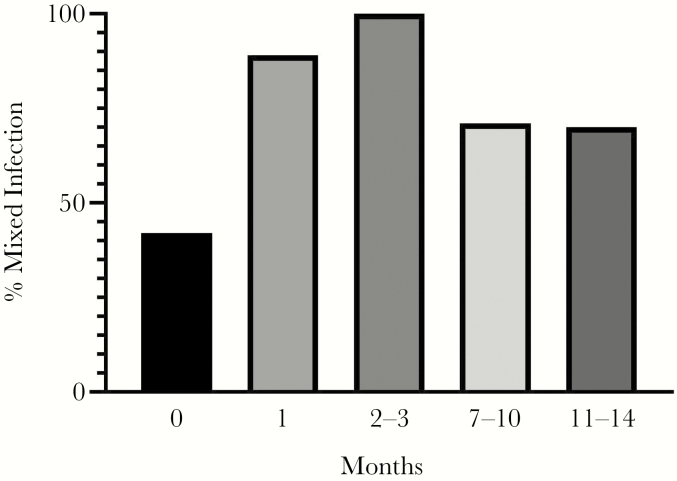
Among the 34 subjects who enrolled in the shedding substudy, 15 study participants were selected for detailed CMV genotyping analysis. The 15 study participants had an average age of 15.1 years, were predominantly non-Hispanic, and were 50% white and 37.5% black or African American (Supplementary Table 1). Urine collected at the time of primary infection was available for testing in 12 subjects. Urine samples collected at 1 and 3 months after primary infection from 2 additional study participants were available for testing. The initial saliva samples were obtained at a median of 1 month (range, 1–3 months) after primary infection (n = 15) and plasma at a median of 2 months (range, 1–2 months) after primary infection (n = 4). The median time for the collection of the final specimen that was genotyped was 10 months (range, 2–14 months), 11 months (range, 8–14 months), and 9.5 months (range, 7–12 months) after primary infection for urine, saliva, and plasma, respectively. Figure 1 demonstrates that at the time of primary infection, almost half of the urine samples (5/12 [42%]) contained multiple genotypes. When samples collected at 1 month after primary infection were analyzed (urine, saliva, and plasma), mixed infection was seen in most (8/9 [89%]). Of the samples collected at the final visit (11–14 months after primary infection), 70% (7/10) had >1 CMV genotype.
Figure 1.
Percentage of subjects with mixed infection at the time of primary infection (month 0, urine) and longitudinally (urine, saliva, and plasma).
The genotyping results of the first sample collected following primary infection by subject and sample type are shown in Table 2. All genotyping results are provided in Supplementary Table 2 (GenBank accession numbers:BankIt2273014 gN_2N1 N583224;BankIt2273014 gN_3aN1 MN583225;BankIt2273014 gN_3aN2 MN583226;BankIt2273014 gN_3bN1 MN583227; BankIt2273014 gN_4aN MN583228;BankIt2273014 gN_4aN2 MN583229;BankIt2273014 gN_4bN1 MN583230;BankIt2273014 gN_4cN1 MN583231). No single genotype predominated in any of the compartments (saliva, urine, or plasma). Specimens from 10 of 15 subjects had different CMV genomic variants in different compartments. Subject 10 had gN genotype 2 in saliva that was not found in urine. Four subjects (subjects 2, 7, 11, and 15) had unique gB genotypes found in either saliva or urine, whereas different gH types were found in different samples from 7 women, and 1 subject had a unique US28 genotype in saliva that was not detected in urine.
Table 2.
Change in Cytomegalovirus Genotypes and Time in Months From First Sample Following Primary Infection to Genotype Change
| Subject | First Genotype | New Genotype | Time to New Genotype, mo |
|---|---|---|---|
| SUB01 | gB 1 | gB 2 | 1 |
| SUB02a | gN 4AN1 | gN 3BN1 | 11 |
| gB 3 | gB 1, 2, 4 | 1 | |
| gH 2 | gH 1 | 12 | |
| SUB03 | gB 2 | gB 1 | 5 |
| US28 A2 | US28 A3 | 5 | |
| SUB05 | gB 4 | gB 2 | 12 |
| SUB08 | gN 1 | gN 3A | 2 |
| gH 1 | gH 2 | 1 | |
| SUB09 | gH 1 | gH 2 | 2 |
| SUB11 | gB 4 | gB 1 | 1 |
| US28 A1 | US28 C | 11 | |
| UL144 A | UL144 B | 11 | |
| SUB14 | gB2 | gB1 | 7 |
| SUB15 | US28 B1 | US28 A1 | 9 |
aCytomegalovirus positive at baseline.
New CMV genotypes within the same sample were observed in more than half (9/15 [60%]) of subjects during the study period (Table 2). The median time for the detection of a new CMV genotype was 5 months (range, 1–12 months). New genotypes were detected in gB for 6 subjects (SUB01, SUB02, SUB03, SUB05, SUB11, and SUB14), gH for 3 subjects (SUB02, SUB08, and SUB09), US28 for 3 subjects (SUB03, SUB11, and SUB15), gN for 2 subjects (SUB02 and SUB08), and UL144 for 1 subject (SUB11).
Among the 15 study subjects, 8 received the gB/MF59 vaccine and 7 received placebo. Urine samples that were obtained at the time of primary infection from 10 participants (6 vaccine and 4 placebo recipients) were analyzed for gB genotyping. Among these, the vaccine strain, or gB1 genotype, was detected in 4 vaccine recipients and 2 placebo recipients (Table 3).
Table 3.
Study Subjects and Their Respective Treatment Arm During the gB/MF59 Vaccine Trial and gB Genotype Detected
| Subject | Treatment | gB (UL55) Genotype |
|---|---|---|
| SUB01 | Vaccine | 1 |
| SUB04 | Vaccine | a |
| SUB05 | Vaccine | 4 |
| SUB07 | Vaccine | 1, 2 |
| SUB11 | Vaccine | 4 |
| SUB12 | Vaccine | 1 |
| SUB13 | Vaccine | 1 |
| SUB14 | Vaccine | a |
| SUB02a | Placebo | 3 |
| SUB03 | Placebo | 2 |
| SUB06 | Placebo | a |
| SUB08 | Placebo | a |
| SUB09 | Placebo | a |
| SUB10 | Placebo | 1 |
| SUB15 | Placebo | 1 |
aCytomegalovirus positive at baseline.
Discussion
CMV has a large genome with many well-described regions that demonstrate high diversity [4, 16]. In the current study, genotyping of 5 of these polymorphic CMV genes, which encode viral envelope glycoproteins that are subject to immune pressure and viral proteins involved in immune evasion, demonstrated that infection with multiple CMV genotypes (mixed infection) is common during primary CMV infection in healthy adolescents. Multiple CMV genotypes were detected in >40% of urine specimens from women at the time of identification of primary CMV infection. When urine, saliva, and plasma specimens obtained within 3 months after primary infection were analyzed, mixed infection was detected in all 15 study subjects. Furthermore, compartmentalization, the presence of distinct viral strains in different compartments from the same individual, was common.
These findings are in contrast to previous studies that have analyzed CMV genetic diversity in immunocompetent individuals with primary CMV infection and found that fewer women shed multiple CMV genotypes [12, 13]. The study by Murthy et al examined samples from a cohort of women who participated in a phase 2 trial evaluating the same candidate vaccine, gB/MF59 [13]. The study population predominantly included African American women with a mean age of 19.6 years. In the Murthy et al study, only 2 of 53 participants were found to have multiple strains in samples from multiple sites of shedding at several time points after primary infection. In contrast, we found mixed infection in all participants with primary infection. This difference is likely due to methodological differences in identifying CMV genotypes. The study by Murthy et al primarily analyzed cultured viral isolates from the study women and, therefore, most of the samples underwent tissue culture propagation prior to genotyping. It is well described that CMV isolates in clinical samples undergo significant genetic changes in cell culture in as few as 2–3 passages [19] and that this process could select out virus strains that are better able replicate in cell cultures. Additionally, in the previous study, the majority of samples underwent direct PCR and Sanger sequencing of PCR amplicons, which could also only detect the dominant genotype [13]. In contrast, we performed genotyping of original urine, saliva, and plasma samples by either real-time PCR (gB and gH) or cloning of PCR gene products (gN, US28, and UL144), providing a higher likelihood for the detection of minor CMV variants. Although genotyping was only performed for the 5 known polymorphic CMV gene regions, all women were found to have multiple CMV strains after primary infection. Because CMV has a large genome with extensive variability within many gene regions and only a limited number of clones were sequenced to determine genotypes of gN, US28, and UL144, our findings likely underestimate the true viral diversity. Therefore, future studies utilizing next-generation sequencing methods are needed to confirm our findings and to describe the true extent of CMV variation in primary infection.
In 10 study women, the appearance of new genotypes was detected in the same sample type at 1–12 months after primary infection. This finding could either be due to infection with new virus strains or secondary to the inability of our assays to detect minor variants early in infection. Over time, these minor variants may become more dominant, as this has been observed in other patient populations [5]. Next-generation sequencing data suggest that interaction with the host exerts positive selective pressure at specific loci involved in immune evasion [20, 21]. The CMV loci analyzed in this study are known targets of the host immune response [1]; thus, the change in predominant genotype seen in our patient population could reflect selection of genotypes in response to host immunity. Alternatively, as reinfections with new virus strains are common in young, healthy individuals [22], the appearance of a new strain over time could reflect acquisition of this strain due to reinfection. However, this is less likely because the time period between the initial sample and subsequent sample with the new viral strain was only 1 month in some of the study subjects. In a recent study, Gantt et al examined early events following oral CMV exposure and infection in young infants [23]. Using a mathematical model, they suggested that transient infections account for the majority of virus shedding events and that oral infection in this setting usually begins with a single virus that infected a small number of cells. In the present study, we analyzed samples from adolescent girls with an established primary infection and were unable to define events prior to the establishment of the infection.
The gBMF59 subunit vaccine was constructed using the Towne strain of CMV, which is a gB1 genotype strain. Among 10 of the study participants with gB genotyping of samples obtained after primary infection, 4 of 6 who received the vaccine were found to be shedding the gB1 genotype within the first 3 months of primary infection, suggesting a lack of genotype-specific protection from the vaccine. Natural immunity to CMV does not provide complete protection against intrauterine transmission, and congenital CMV infection has been shown to occur as a result of nonprimary maternal infection in pregnant women who were seroimmune prior to pregnancy [24–27]. Reinfection with a new viral strain has been shown to lead to congenital CMV, demonstrated by the development of new maternal antibody specificities to gH and/or gB epitopes as well as infant shedding of virus with these newly identified gH and/or gB epitopes [24, 25]. With the recognition of the extent of CMV strain diversity, there has been increased interest in determining whether strain-specific antibody responses play a role in modulating CMV infections/disease. The virus neutralizing activity in sera from CMV-seropositive individuals has been shown to vary against different virus strains [28], suggesting that genetic mutations in the viral envelope glycoproteins that are major immune targets of virus neutralizing antibodies could contribute to evasion of the immune system. CMV gB has distinct regions that have been characterized as targets for neutralizing antibodies, in addition to other envelope glycoproteins. A study in seropositive women followed prospectively for CMV reinfection demonstrated that women with strain-specific antibodies against >1 viral strain were less likely to be reinfected over time [22], suggesting that strain-specific immunity may play an important protective role in CMV infection.
The limitations of our study include the fact that genotyping was only performed in 15 of 34 subjects who acquired primary CMV infection during the vaccine trial. In addition, samples were not available from all time points and testing was only performed on samples with higher copy numbers in which longitudinal samples were available. However, all subjects had mixed infection at the time or shortly after primary infection, and samples were from an equal number of vaccine and placebo recipients. Therefore, it is likely that our genotyping findings are representative of the entire cohort.
In summary, by utilizing direct examination of samples from different sites of shedding, we demonstrate that infection with multiple CMV strains is common after primary CMV infection. Since tissue culture propagation may select for a limited number of virus strains that have better replication capacity in cell culture, analyzing samples directly from the host is necessary to determine the true CMV genetic diversity. Future studies utilizing next-generation sequencing to fully describe CMV genetic diversity are needed to understand the importance of this diversity in primary infection and its impact on immune protection, which is critical for future vaccine efforts.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: Seventh International Congenital Cytomegalovirus Conference and Seventh International Cytomegalovirus Workshop, Birmingham, Alabama, 7–11 April 2019.
Financial support. This work was supported by funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (contract numbers HHSN272200800006C to Cincinnati Children’s Hospital and HHSN272200800013C to Emmes).
Potential conflicts of interest. S. A. R. is a consultant to Merck regarding the development of cytomegalovirus (CMV) vaccines and is a consultant to Roche. D. I. B. and S. B. B. are consultants to Merck and Sanofi Pasteur regarding the development of CMV vaccines. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Britt W. Cytomegalovirus. In: Remington JS, Klein JO, Wilson CB, Baker CJ, eds. Infectious diseases of the fetus and newborn infant. 7th ed Philadelphia: W.B. Saunders Company, 2011:704–53. [Google Scholar]
- 2. Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 2007; 17:355–63. [DOI] [PubMed] [Google Scholar]
- 3. Stratton KR, Durch JS, Lawrence RS, eds. Vaccines for the 21st century: a tool for decision making. Washington, DC: National Academies Press, 2000. [PubMed] [Google Scholar]
- 4. Renzette N, Gibson L, Jensen JD, Kowalik TF. Human cytomegalovirus intrahost evolution—a new avenue for understanding and controlling herpesvirus infections. Curr Opin Virol 2014; 8:109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Görzer I, Kerschner H, Jaksch P, et al. Virus load dynamics of individual CMV-genotypes in lung transplant recipients with mixed-genotype infections. J Med Virol 2008; 80:1405–14. [DOI] [PubMed] [Google Scholar]
- 6. Novak Z, Ross SA, Patro RK, et al. Cytomegalovirus strain diversity in seropositive women. J Clin Microbiol 2008; 46:882–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pignatelli S, Lazzarotto T, Gatto MR, et al. Cytomegalovirus gN genotypes distribution among congenitally infected newborns and their relationship with symptoms at birth and sequelae. Clin Infect Dis 2010; 51:33–41. [DOI] [PubMed] [Google Scholar]
- 8. Rasmussen L, Hong C, Zipeto D, et al. Cytomegalovirus gB genotype distribution differs in human immunodeficiency virus–infected patients and immunocompromised allograft recipients. J Infect Dis 1997; 175:179–84. [DOI] [PubMed] [Google Scholar]
- 9. Arav-Boger R. Strain variation and disease severity in congenital cytomegalovirus infection: in search of a viral marker. Infect Dis Clin North Am 2015; 29:401–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pati SK, Pinninti S, Novak Z, et al. NIDCD CHIMES Study Investigators Genotypic diversity and mixed infection in newborn disease and hearing loss in congenital cytomegalovirus infection. Pediatr Infect Dis J 2013; 32:1050–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ross SA, Novak Z, Pati S, et al. Mixed infection and strain diversity in congenital cytomegalovirus infection. J Infect Dis 2011; 204:1003–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Görzer I, Kerschner H, Redlberger-Fritz M, Puchhammer-Stöckl E. Human cytomegalovirus (HCMV) genotype populations in immunocompetent individuals during primary HCMV infection. J Clin Virol 2010; 48:100–3. [DOI] [PubMed] [Google Scholar]
- 13. Murthy S, Hayward GS, Wheelan S, et al. Detection of a single identical cytomegalovirus (CMV) strain in recently seroconverted young women. PLoS One 2011; 6:e15949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bernstein DI, Munoz FM, Callahan ST, et al. Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: a randomized clinical trial. Vaccine 2016; 34:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med 2009; 360:1191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martí-Carreras J, Maes P. Human cytomegalovirus genomics and transcriptomics through the lens of next-generation sequencing: revision and future challenges. Virus Genes 2019; 55:138–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arav-Boger R, Willoughby RE, Pass RF, et al. Polymorphisms of the cytomegalovirus (CMV)–encoded tumor necrosis factor-alpha and beta-chemokine receptors in congenital CMV disease. J Infect Dis 2002; 186:1057–64. [DOI] [PubMed] [Google Scholar]
- 18. Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 2013; 30:772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murphy E, Yu D, Grimwood J, et al. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc Natl Acad Sci U S A 2003; 100:14976–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Renzette N, Bhattacharjee B, Jensen JD, Gibson L, Kowalik TF. Extensive genome-wide variability of human cytomegalovirus in congenitally infected infants. PLoS Pathog 2011; 7:e1001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sijmons S, Thys K, Mbong Ngwese M, et al. High-throughput analysis of human cytomegalovirus genome diversity highlights the widespread occurrence of gene-disrupting mutations and pervasive recombination. J Virol 2015; 89:7673–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ross SA, Arora N, Novak Z, Fowler KB, Britt WJ, Boppana SB. Cytomegalovirus reinfections in healthy seroimmune women. J Infect Dis 2010; 201:386–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mayer BT, Krantz EM, Swan D, et al. Transient oral human cytomegalovirus infections indicate inefficient viral spread from very few initially infected cells. J Virol 2017; 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med 2001; 344:1366–71. [DOI] [PubMed] [Google Scholar]
- 25. Yamamoto AY, Mussi-Pinhata MM, Boppana SB, et al. Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. Am J Obstet Gynecol 2010; 202:297 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leruez-Ville M, Magny JF, Couderc S, et al. Risk factors for congenital cytomegalovirus infection following primary and nonprimary maternal infection: a prospective neonatal screening study using polymerase chain reaction in saliva. Clin Infect Dis 2017; 65:398–404. [DOI] [PubMed] [Google Scholar]
- 27. Townsend CL, Forsgren M, Ahlfors K, Ivarsson SA, Tookey PA, Peckham CS. Long-term outcomes of congenital cytomegalovirus infection in Sweden and the United Kingdom. Clin Infect Dis 2013; 56:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pötzsch S, Spindler N, Wiegers AK, et al. B cell repertoire analysis identifies new antigenic domains on glycoprotein B of human cytomegalovirus which are target of neutralizing antibodies. PLoS Pathog 2011; 7:e1002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.