Abstract
Objective:
Being physically active has broad health benefits for people with osteoarthritis (OA), including pain relief. Increasing physical activity requires reducing time in other behaviors within a fixed 24-hour day. We examined the potential benefits in relation to pain from trading time in one type of wake or sleep behavior for another.
Method:
In this cross-sectional study, we used isotemporal logistic regression models to examine the estimated effect on pain from replacing time in one behavior with equal time in another, controlling for sociodemographic and health factors. Stratified analysis was conducted by the report of restless sleep. Sleep and wake behaviors [sedentary behavior, light physical activity (PA), moderate PA] were monitored by accelerometer in a pilot study of 185 Osteoarthritis Initiative participants. Outcomes were bodily pain interference and knee pain.
Results:
Moderate PA substituted for an equivalent time in sleep or other types of wake behaviors was most strongly associated with lower odds of pain (bodily pain interference odds reduced 21%−25%, knee pain odds reduced 17%−20% per 10-minute exchange). These beneficial associations were particularly pronounced in individuals without restless sleep, but not in those with restless sleep, especially for bodily pain interference.
Conclusion:
Interventions promoting moderate physical activities may be most beneficial to address pain among people with or at high risk for knee OA. In addition to encouraging moderate-intensity physical activity, pain management strategies may also include the identification and treatment of sleep problems.
Keywords: isotemporal substitution, pain, physical activity, sleep, sedentary behavior, OA
INTRODUCTION
Osteoarthritis (OA) is the most prevalent form of arthritis, affecting 27 million community-dwelling Americans.(1) OA has a profound impact on an individual’s health and well-being. It is a leading cause of disability and functional limitation.(2) Pain is a hallmark of OA and is the primary reason OA patients seek medical services including joint replacement surgery.(3)
Evidence-based OA management guidelines recommend regular physical activity regardless of disease severity.(4) Regular physical activity is widely accepted as an essential strategy to control pain.(4–6) The effect of exercise on OA pain is comparable to that of simple analgesics and oral non-steroid anti-inflammatory drugs with far fewer side effects.(7) Large longitudinal studies among adults with or at risk for knee OA from the Osteoarthritis Initiative study have shown that even light intensity physical activity was protective against incident functional limitation and disability.(8, 9)
In contrast, prolonged sedentary time independent of physical activity has been distinctively associated with deleterious health outcomes including obesity, a known risk factor for OA and arthritis-related pain.(3, 10, 11) Older adults with musculoskeletal pain were more likely to be sedentary than those without pain.(12)
In addition to sedentary behavior, sleep disturbances such as sleep latency, insomnia, and insufficient sleep are common among people with OA.(13–15) It has been shown that pain explained a large portion of the variance of the relationship between arthritis and sleep problems.(16) While pain can lead to sleep problems, several studies have also indicated that sleep problems are associated with enhanced pain sensitivity in healthy individuals (17–19) and increased pain severity among individuals with chronic pain conditions, including OA (15, 20). Thus, addressing sleep may provide additional benefits in reducing pain.
Within a 24-hr day, increased time spent in one type of wake or sleep activity requires reduced time in other types of activity. For example, if time spent in sleep and light intensity activity is unchanged, an increase in moderate-to-vigorous activity time must lead to a reduction in the same amount of time spent in sedentary behavior. Although different types of activity (e.g., sleep, sedentary behavior, light intensity, moderate-to-vigorous intensity) are known to have a distinct influence on health outcomes, little is known about the substitution effects of time spent in one activity type/intensity for another in relation to pain among individuals with OA.
In this study, we applied isotemporal substitution methods to observational data to investigate the associations in relation to pain from trading time in one type of wake activity or sleep for another among people with or at high risk for knee OA. In addition, we examined how the presence of restless sleep influences the isotemporal substitution associations. These relationships may provide insight into potential benefits of changes in different types or intensities of activity or sleep to better manage pain among people with OA.
METHOD
This cross-sectional study examined data from the Physical Activity and Sleep Monitoring Pilot Study of the Osteoarthritis Initiative (OAI) at the 96-month follow-up visit (2012–2014). The parent OAI is a multi-center longitudinal prospective observational study of the risk factors and natural history of knee OA.(21) The OAI recruited 4796 men and women aged 45–79 years with or at high risk for knee OA at enrollment (2004–2006) from four study sites: Baltimore, MD; Columbus, OH; Pittsburgh, PA; and Pawtucket, RI. An OAI accelerometer physical activity ancillary substudy was conducted among a subset of participants at OAI 48-month and 72-month visits, which included 599 participants from Ohio State University (OSU). For the Physical Activity and Sleep Monitoring Pilot Study conducted at the 96-month OAI visit at OSU, a stratified random sample of 211 participants was selected based on presence or absence of knee OA, obesity, and pain to promote generalization across these key characteristics. Out of 211 invited, 187 (89%) consented to participate in the pilot study. The analysis was further restricted to 185 persons with complete data (see flow chart in the appendix). All data were from the OAI 96-month follow-up unless noted elsewhere.
Pilot study participants were instructed to simultaneously wear two accelerometers (ActiGraph GT3X, Pensacola, FL, USA), one on the non-dominant wrist and the other at the waist on an elastic band worn above the right hip for 24 hours over 7 days except for shower or water activities. Participants returned the accelerometers to Northwestern University, where data were downloaded using the manufacturer’s software (ActiLife version 6.13.3) and checked for valid recording.
Sleep Duration, Sedentary Behavior, and Physical Activity
Sleep duration was determined using established protocols.(22, 23) Wrist-worn accelerometers were programmed to collect activity and ambient light data in 60-sec epochs. Participants were required to keep a sleep diary (bedtime, wake time) for nighttime sleep. Upon return of the accelerometers and sleep diary, data were transmitted electronically to the central reading center at Northwestern University for scoring. Each participant’s data was evaluated by a scorer who used a standardized protocol (22, 23) that assessed ambient light intensity and physical activity level from the accelerometer and self-report sleep diary information to determine the time in bed and time out of bed. The Cole-Kripke algorithm(24) was applied to identify actual sleep minutes between time in bed and time out of bed.
Physical activity (PA) and sedentary behavior during wake time were measured by the waist-worn accelerometer. We applied non-wear algorithms to define non-wear periods during non-sleep hours as ≥90 minutes of zero vertical axis activity counts allowing for 2 minutes of interruption with 30-minute upstream and downstream screening for artifactual movements.(25) The analysis was restricted to 3–7 days with complete sleep and valid PA monitoring (non-sleep wear time ≥10 hours); this requirement was met by all participants. Furthermore, only 1% (13 out of 1233) of the monitoring days were excluded due to non-sleep wear time <10 hours. Thresholds used by the National Cancer Institute (NCI) on a minute-by-minute basis were applied to waist-worn accelerometer vertical axis activity counts to identify sedentary behavior and physical activity over non-sleep wear time: (1) sedentary behavior 0–99 count/minute, (2) light physical activity (light PA) 100–2019 count/minute, and (3) moderate-to-vigorous PA ≥2020 count/minute. (26) Because vigorous-intensity PA was very rare in this sample (median 0 minutes/day), hereafter the term moderate PA is used to indicate moderate-to-vigorous PA.
Covariates
Covariates included sociodemographic factors (age, sex, race, and income) and health factors (body mass index, comorbidity, knee OA severity, high depressive symptoms, pain catastrophizing, and presence of restless sleep). Individuals were classified as African-American, White, or other race based on self-report. Annual income was classified as ≥$50k, <$50k or not reported. BMI was calculated from measured height and weight [weight (kg)/height (m2)]. We used OAI 72-month or 48-month BMI as a proxy for missing 96-month BMI (n=6, 3%). Obesity was defined as BMI ≥30. Comorbidity was ascertained from the OAI 72-month Charlson index.(27) Radiographic knee OA severity at person-level was evaluated using the worse Kellgren-Lawrence grade from “fixed-flexion” knee radiography protocol (28) between the two knees. Presence of radiographic knee OA was identified by a Kellgren-Lawrence grade ≥2 in one or both knees at the OAI 96-month or an earlier visit. (29)
Restless sleep was evaluated by a question from the Center for Epidemiologic Studies Depression Scale (CES-D) instrument, which asks participants “how often sleep was restless” in the past week. Candidate responses to the question include 1) rarely: rarely or none of the time (less than 1 day), 2) some: some or a little of the time (1–2 days), 3) moderate/much: occasionally or a moderate amount of time (3–4 days), and 4) most: most or all of the time (5–7 days). Presence of restless sleep was ascertained as any report of restless sleep during the past week.
CES-D scores were re-calculated excluding the restless sleep question and included the remaining 19 questions from the full 20-item scale. High depressive symptoms were identified as a CES-D score ≥16.(30) Pain catastrophizing was measured by Catastrophizing Subscale of the OAI Coping Strategies Questionnaire; scores range from 0–6 with higher scores indicating greater frequency of pain catastrophizing.
Outcomes
Pain outcomes separately assessed the presence of bodily pain interference and knee pain. Bodily pain interference refers to the impact of perceived global pain measurement on work function. Because OAI participants were either with or at high risk for knee OA, we also examined knee pain as an outcome.
Short Form Health Survey (SF-12) bodily pain was assessed by the question of “how much did pain interfere with your normal work (including both work outside the home and housework)” during the past four weeks ascertained from a 5-point Likert scale from “not at all” to “extremely”. The raw score was transformed to a score on a 0–100 scale, with 100 indicating no pain. The presence of bodily pain interference was defined as transformed SF-12 bodily pain scale<100.
Knee-specific pain was assessed using the Western Ontario and McMaster University Osteoarthritis Index (WOMAC).(31) Participants were asked to rate pain in each knee on a 5-point Likert scale during five activities (walking, climbing stairs, in bed, sit or lie down, and standing) in the past 7 days. The WOMAC pain score ranges from 0 to 20 with the higher numbers representing worse symptoms. We defined the presence of knee pain as any pain (e.g. WOMAC pain score >0) reported in one or both knees.
Statistical analysis
Isotemporal substitution methods were used to investigate the effects of trading time mathematically in one type of activity for another while holding time in other activities constant.(32, 33) In each isotemporal model, except for the type of activity (e.g., sedentary behavior) considered to be replaced, all other types of behaviors (i.e., sleep duration, light PA and moderate PA) and total daily time were entered into the model simultaneously. Total daily time was the summation of sleep duration, time spent in sedentary behavior, light, and moderate PA. Logistic regression was conducted separately for each pain outcome to assess the substitution relationship of activity replacement. Odds ratios (ORs) and 95% confidence intervals (CIs) for a 10-minute replacement were presented, adjusting for potential confounders (age, sex, race, income, obesity, comorbidity, K/L grade, high depressive symptoms, pain catastrophizing, and restless sleep). While the choice of 10-minute replacement time is arbitrary, this interval has practical application towards a behavior change for patients and clinicians, and it is the interval used in federal physical activity guidelines for assessing bouted periods of physical activity.(26)
Isotemporal models assume a linear relationship between exposure variables and the modeled outcome. Current sleep literature suggests that short and long sleep time, typically considered as ≤6 h and ≥9 h per day respectively, are both linked to poor health outcomes.(34) To test nonlinearity, a quadratic relationship between sleep duration and pain outcomes was included in the isotemporal models but was not found to be significant, supporting the linear assumption.
Sleep disturbance, obesity, and OA severity are potentially related to physical activity and pain.(3, 15, 35) To examine whether self-reported restless sleep, obesity, or OA status modified the isotemporal substitution association with pain, we conducted sensitivity analyses, testing interactions with wake/sleep behavior adjusted for age and sex; BMI was included in the restless sleep and OA status interaction models.
Sensitivity analyses repeated the isotemporal substitution models using high depressive symptoms based on the raw CESD scale, instead of the re-calculated score excluding the restless sleep item. To examine the possible influence from pain medication, further sensitivity analyses were conducted controlling for knee pain medications.
All analyses were performed using SAS version 9.4 (Cary, NC). Statistical testing was conducted at a two-sided 5% significance level.
RESULTS
Clinical characteristics.
This sample with or at high risk for knee OA had a mean age of 67 years, 50% were female, 60% had radiographic knee OA in at least one knee, and 41% were obese (Table 1). About three in five participants reported the presence of bodily pain interference (58%, 108 persons) or knee pain (63%, 116 persons). Eighty-four individuals reported both bodily pain interference and knee pain presence. On average, accelerometer monitoring showed these 185 participants spent about 7 hours in sleep, 11 hours in sedentary behavior, 4.5 hours in light PA, and only 14 minutes in moderate PA per day. Among these participants, 64% achieved 7–8 hours’ sleep/day, an additional 29% had 6 hours/day or less sleep time, and 8% had more than 8 hours’ sleep.
Table 1.
Characteristics of the 185 Adults from the Physical Activity and Sleep Monitoring Pilot Study of the Osteoarthritis Initiative (OAI) (2012–2014).
| Overall (n=185) | Restless Sleepa | ||
|---|---|---|---|
| % or mean (SD) | No (n=73) % or mean (SD) |
Yes (n=112) % or mean (SD) |
|
| Sociodemographic factors | |||
| Age | 67.1 (8.5) | 67.8 (8.4) | 66.6 (8.6) |
| Female | 49.7 | 48.0 | 50.9 |
| White | 94.1 | 95.9 | 92.9 |
| Income >= $50k | 70.8 | 72.6 | 69.6 |
| Health factors | |||
| Obeseb | 40.5 | 43.8 | 38.4 |
| Presence of comorbidity | 30.3 | 31.5 | 29.5 |
| Kellgren-Lawrence grade | |||
| 0 | 21.6 | 21.9 | 21.4 |
| 1 | 18.9 | 19.2 | 18.8 |
| 2 | 31.4 | 27.4 | 33.9 |
| 3 | 21.1 | 26.0 | 17.9 |
| 4 | 7.0 | 5.5 | 8.0 |
| OA presencec | 59.5 | 58.9 | 59.8 |
| High depressive symptomsd | 6.0 | 4.1 | 7.1 |
| Pain catastrophizing scale | 0.5 (1.0) | 0.3 (0.9) | 0.6 (1.1) |
| Wake and sleep activities (minutes/day) | |||
| Sleep duration | 422.3 (58.6) | 411.2 (55.6) | 429.6 (59.7) |
| Sedentary | 660.9 (88.9) | 666.8 (89.9) | 657.0 (88.5) |
| Light PA | 272.6 (79.7) | 278.2 (82.8) | 269.0 (77.8) |
| Moderate PA | 13.6 (17.5) | 15.4 (19.8) | 12.4 (15.7) |
| Outcomes | |||
| Presence of bodily pain interference | 58.4 | 41.1 | 69.6 |
| Presence of knee pain | 62.7 | 54.8 | 67.9 |
Self-report of restless sleep during the past week.
Body Mass Index ≥30
Kellgren-Lawrence grade ≥ 2
High Depressive symptoms as defined by modified Center for Epidemiological Studies Depression Scale ≥16
Approximately two out of five participants had no restless sleep in the past week. Compared to people who had no restless sleep, those reporting restless sleep were similar in sociodemographics but more likely to report high depressive symptoms (7% vs. 4%), pain catastrophizing (0.6 vs. 0.3), bodily pain interference (70% vs. 41%) and knee pain (68% vs. 55%) (Table 1).
Bodily pain interference.
The presence of bodily pain interference was reported by 58% of participants. Initial isotemporal analyses examined the relationship between bodily pain interference and the exchange of time spent in target behaviors (physical activity, sedentary behavior, or sleep) controlling for sociodemographics and health factors, including the presence of restless sleep. As shown in Figure 1, moderate PA substituting for sleep, sedentary behavior, or light PA had the strongest isotemporal associations, with a 21%−25% lower odds of bodily pain interference. The relationships of moderate PA substitution for sedentary behavior or light PA were statistically significant (sedentary behavior: adjusted OR=0.74, 95% CI=[0.57, 0.95]; light PA: adjusted OR=0.75, 95% CI=[0.57, 0.98] for 10 minutes/day exchange respectively). No relationship was found between bodily pain interference and reallocating sedentary time to either sleep or light PA or reallocating sleep time to light PA.
Figure 1. Isotemporal substitution models for bodily pain interference with work reallocating time in sleep, sedentary behavior (SB), or light intensity physical activity (PA) to moderate PA, light PA, or sleep among full sample* and stratified by self-reported restless sleep **.
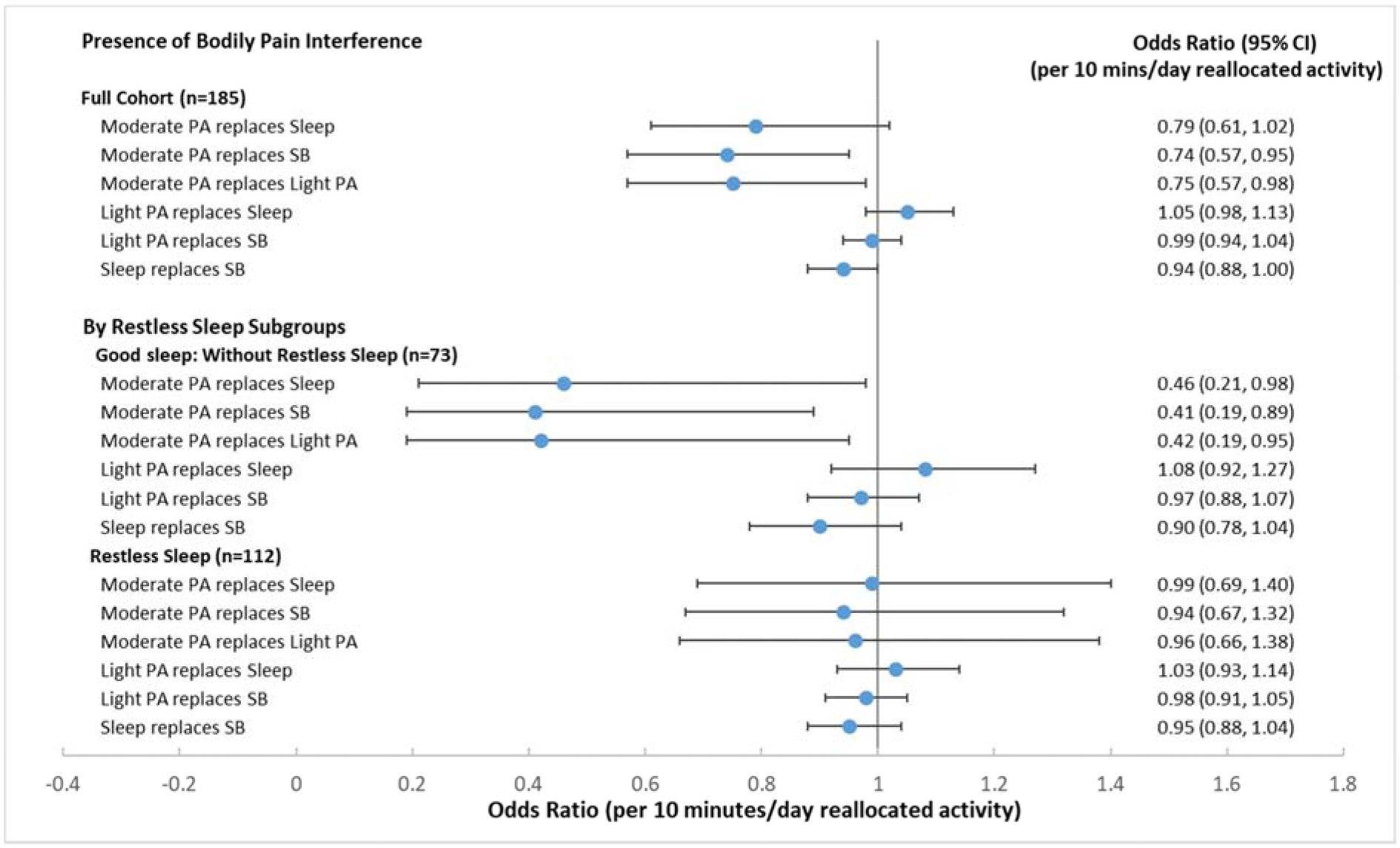
* Full sample models were adjusted for age, sex, race, income, obesity, comorbidity, Kellgren-Lawrence grade, high depressive symptoms, pain catastrophizing, and restless sleep.
** Stratified models were adjusted for age, sex, race, income, obesity, comorbidity, Kellgren-Lawrence grade, high depressive symptoms, and pain catastrophizing.
Sensitivity analyses tested for interactions between wake/sleep factors with restless sleep, obesity or OA status in relation to bodily pain interference. Only interactions between moderate PA replacement and restless sleep reached or nearly achieved statistical significance. Therefore subgroup analyses were conducted among people with and without restless sleep.
Subgroup analysis (Figure 1) for bodily pain interference showed strong and statistically significant beneficial associations of reallocating time from other types of activity to moderate PA for people without restless sleep, but not for those who reported restless sleep. Among people with no restless sleep, substituting another type of activity with moderate PA was associated with statistically significantly reduced odds in the presence of bodily pain interference (sleep: adjusted OR=0.46, 95% CI=[0.21, 0.98]; sedentary behavior: adjusted OR=0.41, 95% CI=[0.19, 0.89]; light PA: adjusted OR=0.42, 95% CI=[0.19, 0.95], respectively for 10 minute/day exchange). No such findings were seen in those with restless sleep.
Knee pain.
The presence of pain in one or both knees was reported by 63% of participants. Initial isotemporal analyses summarized in Figure 2 indicated the strongest behavior exchanges related to lower odds of knee pain was substituting moderate PA for sleep, for sedentary behavior, or for light PA. None of the time reallocations were statistically significantly related to reduced odds of knee pain; however, moderate PA substituted for sleep (adjusted OR=0.80, 95% CI=[0.63, 1.00] for 10 minutes/day exchange) had borderline statistical significance. No relationship was observed between other types of time exchange and knee pain.
Figure 2. Isotemporal substitution models for presence of knee pain reallocating time in sleep, sedentary behavior (SB), or light intensity physical activity (PA) to moderate PA, light PA, or sleep among full sample* and stratified by self-reported restless sleep**.
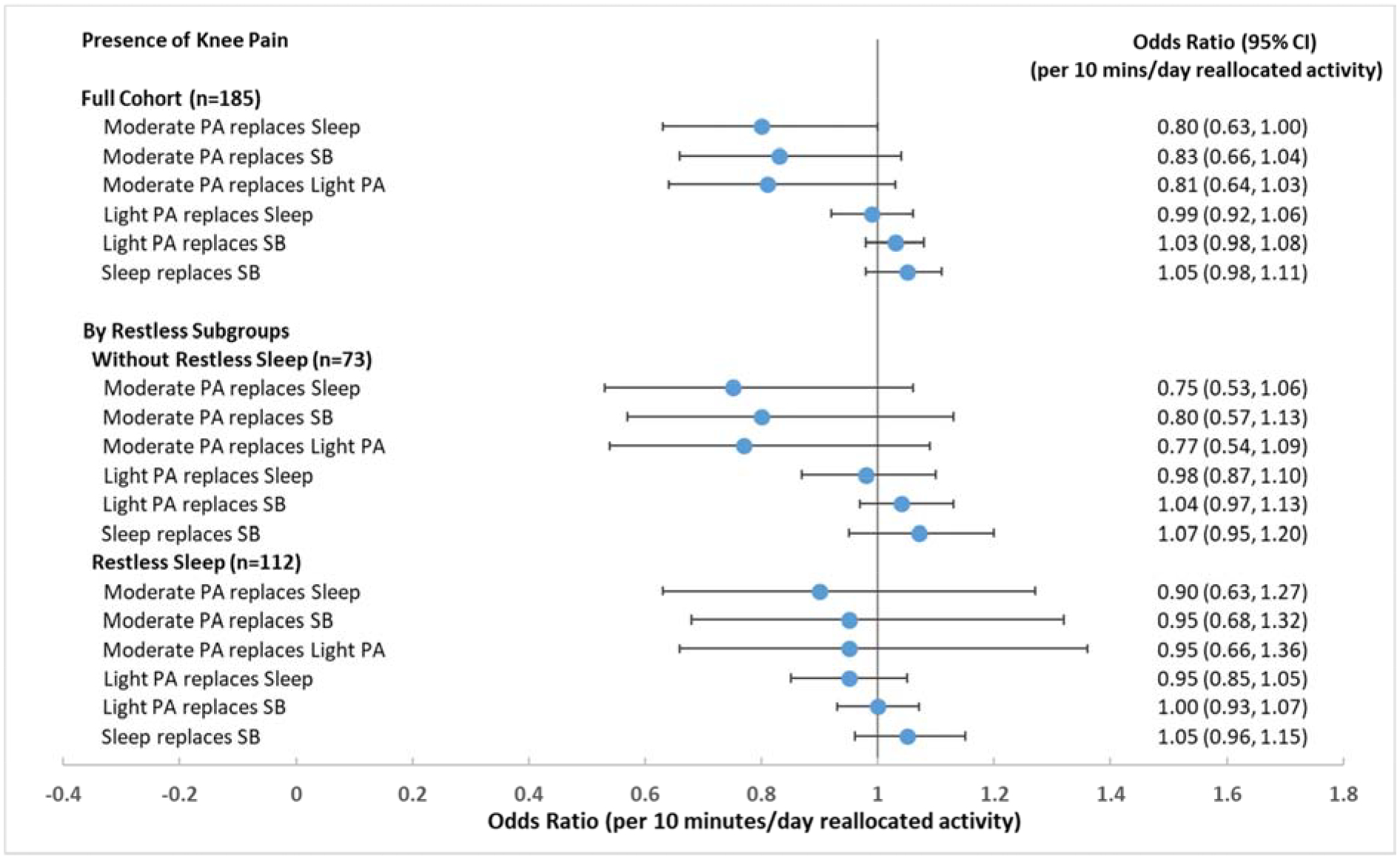
* Full sample models were adjusted for age, sex, race, income, obesity, comorbidity, Kellgren-Lawrence grade, high depressive symptoms, pain catastrophizing, and restless sleep.
** Stratified models were adjusted for age, sex, race, income, obesity, comorbidity, Kellgren-Lawrence grade, high depressive symptoms, and pain catastrophizing.
Sensitivity analyses testing for interactions between wake/sleep factors with restless sleep, obesity or OA status did not find any statistically significant interactions related to knee pain. However, to be consistent with bodily pain interference analysis, we performed subgroup analysis by restless sleep. Subgroup analysis for knee pain in Figure 2 showed that reallocating time from other types of activity to moderate PA may have stronger benefits for people without restless sleep than for people with restless sleep. However, none of these isotemporal substitution relationships reached statistical significance in either restless sleep subgroup.
Sensitivity analyses were done to define high depressive symptoms using the raw CESD scale, which included the restless sleep item. Our findings remained the same. Additional analyses included knee pain medications as a covariate found similar results.
DISCUSSION
This study investigated the interplay of sleep, sedentary behavior and active behaviors with pain among people with or at high risk for knee OA. For this purpose, we applied isotemporal substitution analysis to understand the potential benefits in regard to pain of reallocating time spent in one type of sleep or wake behavior (sleep, sedentary, light PA, or moderate PA) to another. Moderate PA substituted for an equivalent time in other types of behaviors was most strongly associated with lower odds of pain. The potential benefit in pain associated with reallocating moderate PA time was strongest among persons who did not report any restless sleep during the past week.
Previous isotemporal studies in other study populations have found that substituting other types of activity with moderate PA is more strongly related to favorable health outcomes than other activity exchanges. Studies in general populations (32, 36, 37) showed reallocating sedentary time to moderate PA rather than light PA or sleep was associated with stronger cardiovascular benefits.(36). Replacing 30 minutes in TV viewing with 30 minutes in jogging/running or brisk walking per day was associated with twice the potential weight loss compared to substituting TV time with slow walking.(32) Similarly, replacing sedentary behavior with moderate PA but not light PA was linked with less frequent chronic musculoskeletal pain.(37) In rheumatic disease populations, substituting sedentary behavior with moderate PA or light PA was protective against reduction in mobility. Increasing moderate PA held greater potential benefit than light PA in adults with high risk of knee OA. Specifically, a 60-minute replacement of light PA for sedentary time was needed to achieve the equivalent mobility risk reduction associated with a 5-minute replacement of moderate PA for sedentary time.(8) In line with these findings, our study demonstrated that reallocating time from other wake and sleep activity to moderate PA may be most beneficial to reduce the odds of pain.
There are several reasons why moderate PA may be more beneficial than other types of activity in relation to pain. First, a study of fibromyalgia patients showed that higher intensity level of physical activity may modify central pain processing, reducing sensitivity to experimentally induced pain.(38) Second, the literature shows higher intensity physical activity was associated with improved psychological health, such as less depression(33, 39), which may improve pain tolerance. Finally, improved muscle strength and movement coordination through higher intensity PA may provide greater joint stability and shock absorption, thus potentially alleviating pain during functional activities.(40, 41)
Our findings further revealed that the strength of association between replacing time spent in sleep, sedentary time, or light PA with moderate PA and pain outcomes depended on patient-reported restless sleep. Specifically, the potential benefits of replacing sleep or other activities with moderate PA on bodily pain interference were stronger among people not reporting restless sleep. Intuitively, this observation could be explained if people with restless sleep slept less than people without restless sleep. However, this was not the case. In fact, people with restless sleep slept 18 minutes/day more on average than those who did not report restless sleep. This observation highlights two important points: 1) sleep duration alone may not represent sleep quality, and 2) restless sleep as a component of sleep quality may have a stronger relation to pain than sleep duration.
The underlying mechanisms linking restless sleep to the association between physical activity and pain are not known. However, data from previous studies suggest that sleep problems may modify the relationship between physical activity and pain through their effect on psychosocial factors and pain processing. Several studies have suggested that sleep problems are associated with psychosocial problems (e.g., catastrophizing, anxiety) and abnormalities in the central nervous system (CNS) pain processing.(42, 43) People who report restless sleep may be more likely to have psychosocial problems and abnormalities in CNS pain processing. The impact of these factors on pain may overwhelm the benefits of increasing physical activity. Consistent with this hypothesis, individuals with restless sleep were more likely to report high levels of depressive symptoms as well as catastrophizing in this study. On the other hand, it is plausible that restful sleep associated with better physical and psychological well-being may amplify the positive association between moderate PA and pain outcomes. In light of this interesting finding, PA recommendations or interventions that are designed for pain management should be mindful of sleep quality and consider incorporating education on sleep hygiene to maximize the pain-moderating benefits of moderate PA. Future studies including assessments of other psychosocial factors (e.g., anxiety), as well as measures of CNS pain dysregulation, will be key to furthering our understanding of these complex relationships.
Engaging in physical activity, reducing sedentary behavior, and improving sleep quality are important public health strategies to promote good health. The health consequences of each type of behavior are distinct, but within a 24-hour day increased time in one behavior necessarily decreases time in another. Traditional methods to address this synergistic relationship, such as regression models, measure incremental effects of an activity, but do not address from which activity the time displacement occurs, and do not account for the fixed time frame.(33) Isotemporal substitution methods mathematically describe time displacement effects accounting for the constraint of total time. This method has been widely applied to investigate potential benefits of reallocating time spent in other activities to physical activity in different populations and a broad spectrum of health outcomes including cardiovascular disease, weight change, mental health, diabetes, function, and mortality.(8, 32, 33, 36, 44–48)
To our knowledge, no prior OA study has investigated the interrelationship between objective measures of sedentary behavior, physical activity, and sleep in relation to health benefits. Few isotemporal studies on physical activity considered the full 24-hour behavioral spectrum including sleep.(33, 36, 45–48) These studies examined sleep largely relied on memory recall of sleep duration, therefore were subject to memory bias. For instance, a sleep study of children and adolescents relying on time diaries alone have found that participants overestimated their sleep time by roughly one hour.(49) In contrast, our study objectively measured the full 24-hour spectrum of wake and sleep behaviors via accelerometer monitoring. The use of a standardized algorithm to assess sleep time utilizing accelerometer data combined with a sleep diary strengthens the validity of our study results over other studies which solely relied on self-report.
Strengths of this study included a 24-hour spectrum of wake and sleep measures by accelerometer monitoring and sleep diary, sleep assessment based on established protocols, and isotemporal substitution analytic methods. Several limitations of our study should be noted. We recognize the potentially insufficient statistical power of this small sample size and the observational nature of this study are limitations of this study. Larger confirmatory intervention studies are needed. The study is cross-sectional and causation cannot be inferred from these observational data. The association between moderate PA and pain is likely to be bi-directional. A decrease in pain could allow a person to engage in more or higher intensity physical activity. Our assessment of restless sleep disturbance was based on a single question on presence versus absence of restless sleep, thereby providing little nuance regarding the severity or the different manifestations of sleep problems (e.g., insomnias, parasomnias, excessive somnolence, etc.). However, even this simple question was able to identify individuals who may be more likely to benefit from substituting moderate PA for sleep, sedentary behavior or light physical activity. Also, accelerometers were not worn during water activities, and it is recognized that accelerometers may underestimate activities with minimal vertical acceleration/deceleration such as cycling. However historical diary data from OAI participants indicated that minimal time was spent in water and cycling activities (interquartile range 0 to 3.4 minutes/day).(50) Finally, it is recognized odds ratios reported in this study should not be interpreted as relative risks.
In conclusion, replacing time spent in sedentary behavior or light physical activity with moderate physical activity was associated with significant reductions in odds of bodily pain interference among a population of adults with or at high risk for osteoarthritis. Among persons without restless sleep problem, replacing sleep and all other types of wake behavior with moderate physical activity was strongly associated with bodily pain interference. However, no isotemporal associations were observed among persons reporting restless sleep. These results inform future research and practice that in addition to promoting moderate PA participation, restless sleep may be an important factor to address in managing pain among people with chronic knee symptoms. Larger confirmatory intervention studies are necessary to document the potential effects of moderate PA substitution and sleep problem identification/treatment on pain interference and knee pain.
Acknowledgments
The authors would like to thank Sukyoung Sophie Suh for her support to this study.
Role of the funding source
This work was supported in part by National Institute for Arthritis and Musculoskeletal Diseases (grant no. R01-AR054155 and P60-AR064464). All authors report no disclosures or conflicts of interest.
APPENDIX
Figure.
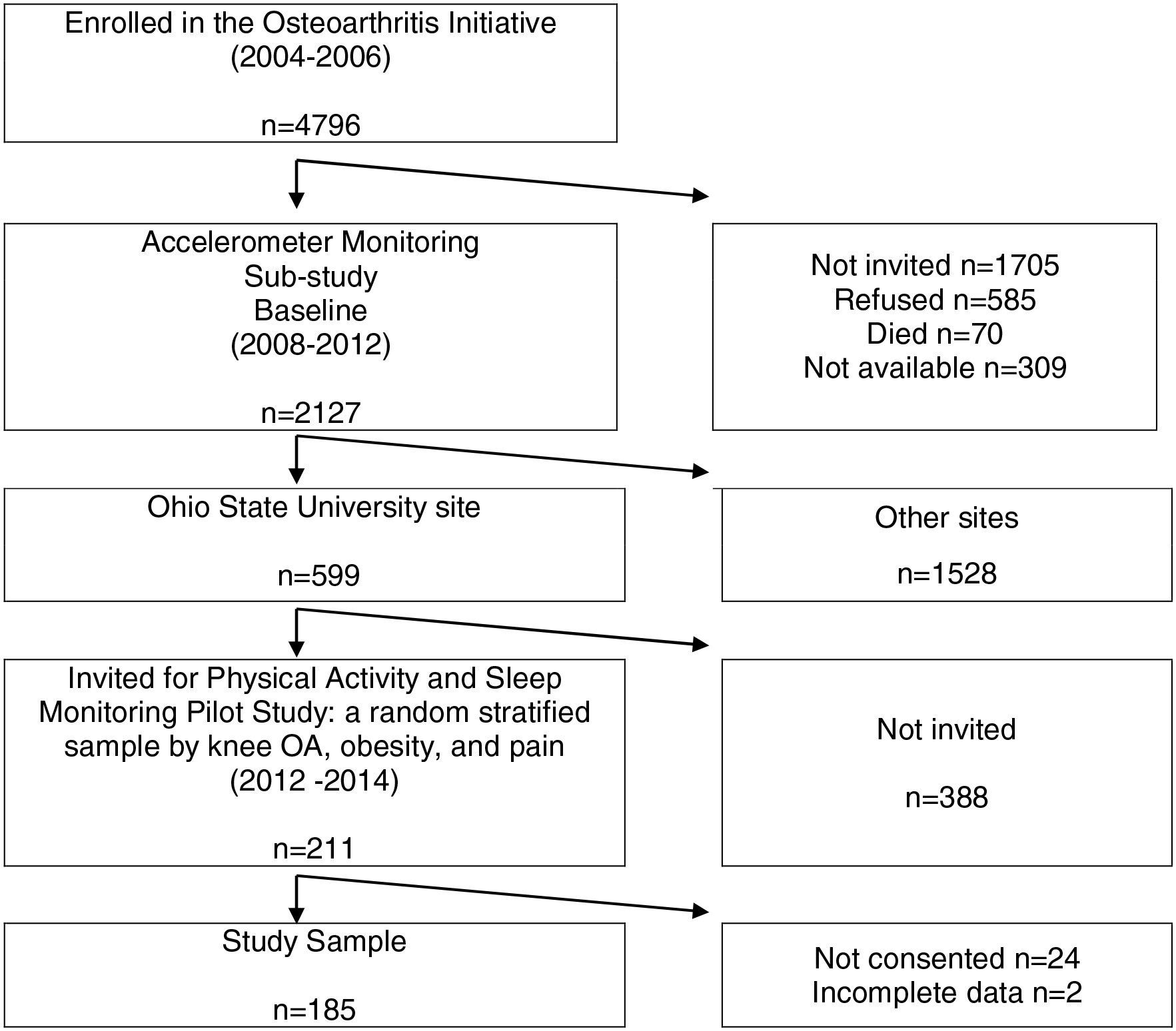
Flow Chart of the Analytic Sample from the Physical Activity and Sleep Monitoring Pilot Study of the Osteoarthritis Initiative (OAI) (2012–2014)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
All authors report no disclosures or conflicts of interest.
REFERENCE
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hootman JM, Helmick CG, Barbour KE, Theis KA, Boring MA. Updated Projected Prevalence of Self-Reported Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation Among US Adults, 2015–2040. Arthritis Rheumatol. 2016;68(7):1582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2013;39(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137–62. [DOI] [PubMed] [Google Scholar]
- 5.Uthman OA, van der Windt DA, Jordan JL, Dziedzic KS, Healey EL, Peat GM, et al. Exercise for lower limb osteoarthritis: systematic review incorporating trial sequential analysis and network meta-analysis. BMJ. 2013;347:f5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fransen M, McConnell S. Land-based exercise for osteoarthritis of the knee: a metaanalysis of randomized controlled trials. J Rheumatol. 2009;36(6):1109–17. [DOI] [PubMed] [Google Scholar]
- 7.Bennell KL, Dobson F, Hinman RS. Exercise in osteoarthritis: moving from prescription to adherence. Best Pract Res Clin Rheumatol. 2014;28(1):93–117. [DOI] [PubMed] [Google Scholar]
- 8.White DK, Lee J, Song J, Chang RW, Dunlop D. Potential Functional Benefit From Light Intensity Physical Activity in Knee Osteoarthritis. Am J Prev Med 2017;53(5):689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunlop DD, Song J, Semanik PA, Sharma L, Bathon JM, Eaton CB, et al. Relation of physical activity time to incident disability in community dwelling adults with or at risk of knee arthritis: prospective cohort study. BMJ. 2014;348:g2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bankoski A, Harris TB, McClain JJ, Brychta RJ, Caserotti P, Chen KY, et al. Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care. 2011;34(2):497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorp AA, Owen N, Neuhaus M, Dunstan DW. Sedentary behaviors and subsequent health outcomes in adults a systematic review of longitudinal studies, 1996–2011. Am J Prev Med. 2011;41(2):207–15. [DOI] [PubMed] [Google Scholar]
- 12.Peeters G, Edwards KL, Brown WJ, Barker AL, Arden N, Redmond AC, et al. Potential effect modifiers of the association between physical activity patterns and joint symptoms in middle aged women. Arthritis Care Res (Hoboken). 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen KD, Renner JB, Devellis B, Helmick CG, Jordan JM. Osteoarthritis and sleep: the Johnston County Osteoarthritis Project. J Rheumatol. 2008;35(6):1102–7. [PMC free article] [PubMed] [Google Scholar]
- 14.Wilcox S, Der Ananian C, Abbott J, Vrazel J, Ramsey C, Sharpe PA, et al. Perceived exercise barriers, enablers, and benefits among exercising and nonexercising adults with arthritis: results from a qualitative study. Arthritis Rheum. 2006;55(4):616–27. [DOI] [PubMed] [Google Scholar]
- 15.Parmelee PA, Tighe CA, Dautovich ND. Sleep disturbance in osteoarthritis: linkages with pain, disability, and depressive symptoms. Arthritis Care Res (Hoboken). 2015;67(3):358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Power JD, Perruccio AV, Badley EM. Pain as a mediator of sleep problems in arthritis and other chronic conditions. Arthritis Rheum. 2005;53(6):911–9. [DOI] [PubMed] [Google Scholar]
- 17.Campbell CM, Bounds SC, Simango MB, Witmer KR, Campbell JN, Edwards RR, et al. Self-reported sleep duration associated with distraction analgesia, hyperemia, and secondary hyperalgesia in the heat-capsaicin nociceptive model. Eur J Pain. 2011;15(6):561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30(4):494–505. [DOI] [PubMed] [Google Scholar]
- 19.Salwen JK, Smith MT, Finan PH. Mid-Treatment Sleep Duration Predicts Clinically Significant Knee Osteoarthritis Pain reduction at 6 months: Effects From a Behavioral Sleep Medicine Clinical Trial. Sleep. 2017;40(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasaki E, Tsuda E, Yamamoto Y, Maeda S, Inoue R, Chiba D, et al. Nocturnal knee pain increases with the severity of knee osteoarthritis, disturbing patient sleep quality. Arthritis Care Res (Hoboken). 2014;66(7):1027–32. [DOI] [PubMed] [Google Scholar]
- 21.Nevitt MC, Felson DT, Lester G. The Osteoarthritis Initiative Protocol for the Cohort Study. Available from https://oai.epi-ucsf.org/datarelease/docs/StudyDesignProtocol.pdf.; 2006.
- 22.Patel SR, Weng J, Rueschman M, Dudley KA, Loredo JS, Mossavar-Rahmani Y, et al. Reproducibility of a Standardized Actigraphy Scoring Algorithm for Sleep in a US Hispanic/Latino Population. Sleep. 2015;38(9):1497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;19(7):1374–81. [DOI] [PubMed] [Google Scholar]
- 24.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15(5):461–9. [DOI] [PubMed] [Google Scholar]
- 25.Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. [DOI] [PubMed] [Google Scholar]
- 27.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. [DOI] [PubMed] [Google Scholar]
- 28.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32(3):128–32. [DOI] [PubMed] [Google Scholar]
- 29.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pandya R, Metz L, Patten SB. Predictive value of the CES-D in detecting depression among candidates for disease-modifying multiple sclerosis treatment. Psychosomatics. 2005;46(2):131–4. [DOI] [PubMed] [Google Scholar]
- 31.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee 12. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 32.Mekary RA, Willett WC, Hu FB, Ding EL. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am J Epidemiol. 2009;170(4):519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mekary RA, Lucas M, Pan A, Okereke OI, Willett WC, Hu FB, et al. Isotemporal substitution analysis for physical activity, television watching, and risk of depression. Am J Epidemiol. 2013;178(3):474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee MS, Shin JS, Lee J, Lee YJ, Kim MR, Park KB, et al. The association between mental health, chronic disease and sleep duration in Koreans: a cross-sectional study. BMC Public Health. 2015;15:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith MT, Quartana PJ, Okonkwo RM, Nasir A. Mechanisms by which sleep disturbance contributes to osteoarthritis pain: a conceptual model. Curr Pain Headache Rep. 2009;13(6):447–54. [DOI] [PubMed] [Google Scholar]
- 36.Buman MP, Winkler EA, Kurka JM, Hekler EB, Baldwin CM, Owen N, et al. Reallocating time to sleep, sedentary behaviors, or active behaviors: associations with cardiovascular disease risk biomarkers, NHANES 2005–2006. Am J Epidemiol. 2014;179(3):323–34. [DOI] [PubMed] [Google Scholar]
- 37.Ryan CG, Wellburn S, McDonough S, Martin DJ, Batterham AM. The association between displacement of sedentary time and chronic musculoskeletal pain: an isotemporal substitution analysis. Physiotherapy. 2017;103(4):471–7. [DOI] [PubMed] [Google Scholar]
- 38.Ellingson LD, Shields MR, Stegner AJ, Cook DB. Physical activity, sustained sedentary behavior, and pain modulation in women with fibromyalgia. J Pain. 2012;13(2):195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28(1):1–8. [DOI] [PubMed] [Google Scholar]
- 40.Bartholdy C, Juhl C, Christensen R, Lund H, Zhang W, Henriksen M. The role of muscle strengthening in exercise therapy for knee osteoarthritis: A systematic review and meta-regression analysis of randomized trials. Semin Arthritis Rheum. 2017;47(1):9–21. [DOI] [PubMed] [Google Scholar]
- 41.Lange AK, Vanwanseele B, Fiatarone Singh MA. Strength training for treatment of osteoarthritis of the knee: a systematic review. Arthritis Rheum. 2008;59(10):1488–94. [DOI] [PubMed] [Google Scholar]
- 42.Campbell CM, Buenaver LF, Finan P, Bounds SC, Redding M, McCauley L, et al. Sleep, Pain Catastrophizing, and Central Sensitization in Knee Osteoarthritis Patients With and Without Insomnia. Arthritis Care Res (Hoboken). 2015;67(10):1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lerman SF, Finan PH, Smith MT, Haythornthwaite JA. Psychological interventions that target sleep reduce pain catastrophizing in knee osteoarthritis. Pain. 2017;158(11):2189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamer M, Stamatakis E, Steptoe A. Effects of substituting sedentary time with physical activity on metabolic risk. Med Sci Sports Exerc. 2014;46(10):1946–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fanning J, Porter G, Awick EA, Ehlers DK, Roberts SA, Cooke G, et al. Replacing sedentary time with sleep, light, or moderate-to-vigorous physical activity: effects on self-regulation and executive functioning. J Behav Med. 2017;40(2):332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knaeps S, De Baere S, Bourgois J, Mertens E, Charlier R, Lefevre J. Substituting Sedentary Time With Light and Moderate to Vigorous Physical Activity is Associated With Better Cardiometabolic Health. J Phys Act Health. 2017:1–7. [DOI] [PubMed] [Google Scholar]
- 47.Stamatakis E, Rogers K, Ding D, Berrigan D, Chau J, Hamer M, et al. All-cause mortality effects of replacing sedentary time with physical activity and sleeping using an isotemporal substitution model: a prospective study of 201,129 mid-aged and older adults. Int J Behav Nutr Phys Act. 2015;12:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Del Pozo-Cruz B, Gant N, Del Pozo-Cruz J, Maddison R. Relationships between sleep duration, physical activity and body mass index in young New Zealanders: An isotemporal substitution analysis. PLoS One. 2017;12(9):e0184472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goodwin JL, Silva GE, Kaemingk KL, Sherrill DL, Morgan WJ, Quan SF. Comparison between reported and recorded total sleep time and sleep latency in 6- to 11-year-old children: the Tucson Children’s Assessment of Sleep Apnea Study (TuCASA). Sleep Breath. 2007;11(2):85–92. [DOI] [PubMed] [Google Scholar]
- 50.Song J, Hochberg MC, Chang RW, Hootman JM, Manheim LM, Lee J, et al. Racial and ethnic differences in physical activity guidelines attainment among people at high risk of or having knee osteoarthritis. Arthritis Care Res (Hoboken). 2013;65(2):195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]


