Fig. 2.
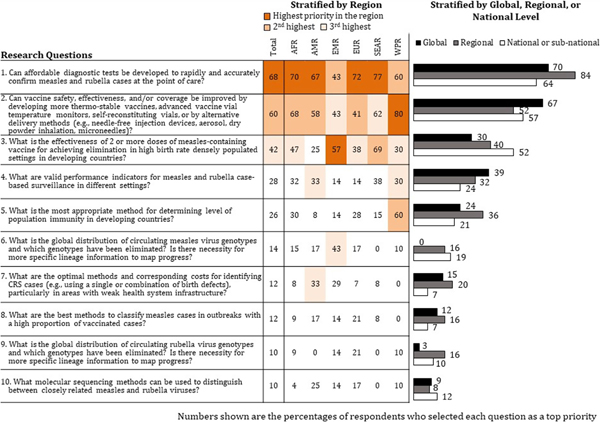
Surveillance, vaccine delivery, and laboratory testing research priorities, Measles & Rubella Initiative web-based survey, 2016 (n = 144). Note: Other surveillance, vaccine delivery, and laboratory testing research questions that were selected by fewer respondents as a high priority: What are the technical requirements and epidemiologic utility of developing serologic assays to differentiate immunity acquired from exposure to wild-type viruses and immunity acquired from exposure to vaccine strains? (9%); Can tests be developed to accurately measure neutralizing antibodies to measles and rubella viruses, and provide results faster than the plaque reduction neutralization assay (PRNT)? (8%).
