Abstract
Objectives:
To verify quantitative differences of the mandibular cortical and trabecular bone between patients with multiple myeloma (MM) under bisphosphonate (BP) therapy and a control group never exposed to BP.
Methods:
Clinical and demographic characteristics were collected through medical records and interviews. Mandibular cortical thickness (MCT) and fractal dimension (FD) were measured on cone beam computed tomography (CBCT) images, on the molar region, in both groups. Additionally, FD was measured on periapical digital intraoral radiography and results were compared to CBCT measurements.
Results:
There were 33 patients with MM under BP therapy and 28 controls, with no significant differences in gender and age between groups. Pamidronate was used by all MM patients, either associated or not to other types of BP. The median MCT was higher in MM group exposed to BP (5.20 mm) than in controls (3.50 mm, p < 0.001). There were no significant differences in the median FD between patients in the MM group and controls, on CBCT (0.95 vs 0.90, p = 0.814) and periapical digital intraoral radiography (0.98 vs 0.96, p = 0.963), respectively, even when more than one type of BP was used.
Conclusions:
The MCT represents an useful tool in the detection of bone dimensional changes caused by BP, in patients with MM. Additional studies are necessary to improve the knowledge on the quantitative evaluation of trabecular jaw bone, in individuals with MM, under BP therapy.
Keywords: multiple myeloma, cone beam computed tomography, fractals, bisphosphonates, jaw
Introduction
Multiple myeloma (MM) is a cancer of plasma cells, in which clonal cells expand in the bone marrow and cause osteolytic bone lesions.1 Antiresorptive therapy is often used to control the bone damage, pain and skeletal events observed in individuals with MM.2–6 Bisphosphonates (BP) are potent medications mainly used to reduce the skeletal morbidity rate, improve the quality of life and increase survival rates in these patients.2,3 The main adverse BP effects in MM patients are renal damage and medication-related osteonecrosis of the jaws (MRONJ).2,3 If BP bone alterations could be quantified in the jaw bone, maybe the detection of patients in risk for MRONJ could be predicted.
Studies on local, systemic and genetic risk factors have contributed to the knowledge of the development and severity of MRONJ, in individuals using BP.7 Subjective evaluations of radiographic imaging aspects of patients with MRONJ have described thickening of the lamina dura, sclerosis, osteolysis, narrowing of the mandibular canal, persisting alveolar sockets, increased density, and bone sequestration.6,8–19 Moreover, an abnormal increase of tracer uptake has been observed in the bone scintigraphy of more than 60% of patients taking BP who subsequently developed MRONJ.20 These characteristics are useful to identify bony changes in MRONJ cases, but none of them are pathognomonic. Qualitative bone aspects have been reported in different populations of patients with MRONJ,6,8–10,12–23 but studies evaluating the bone of patients exposed to BP without MRONJ are scarce.11,24–26
Bisphosphonate therapy leads to alterations in bone microarchitecture.27 An increase in the measurements of trabecular and cortical bones have been reported through different techniques, in the imaging of patients with MRONJ.8,12,21–24 Quantitative analyses have also shown higher mandibular cortical thickness (MCT) and fractal dimension (FD) in patients exposed to BP without MRONJ, when compared to controls non-exposed to BP.23–25,28 The detection of local dimensional changes may represent a tool in the evaluation of the bone exposure to BP.
Studies with homogeneous populations are needed to confirm if bone dimensional changes represent a valuable measurement for the detection of BP changes in the jaw bones. Thus, the aim of this study was to evaluate quantitatively the trabecular and the cortical jaw bones of MM patients exposed to BP, and compare them to a control group not exposed to BP.
Methods and materials
This is a blinded case control study in which cortical and trabecular bone measurements of patients with MM exposed to BP were compared to controls not exposed to BP.
Selection and description of participants
Patients with MM under BP therapy, attending the Haematology Clinic of the Clementino Fraga Filho University Hospital at the Universidade Federal do Rio de Janeiro were enrolled in the study. Patients with MM were excluded if they had no history of BP intake; had less than two doses of BP; if presented other gammopathies or hematological conditions (solitary plasmacytoma, monoclonal gammopathy, polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes syndrome); endocrinopathies; or if not willing to participate in study protocol.
Individuals not presenting MM and not exposed to BP (oral or endovenous) were included in the control group. They were recruited from the implant candidate patients who were scheduled for a cone beam computer tomography (CBCT) exam, at the Oral Radiology Clinic of the Dental School of the Universidade Federal do Rio de Janeiro. Controls were excluded if they presented diseases that affect bone remodeling, exposed to BP for osteopenia/osteoporosis, or if refused to participate in the study.
The procedures followed in the study were in accordance with the ethical standards of the institution committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 1983, and were approved under protocol number 693.402. All individuals signed a consent form.
Sample size
Sample size calculation for the cortical thickness was based on the measurements of the first 21 studied subjects (15 MM patients and 6 controls). The cutoff point for the low and high values of the MCT and FD measurements were established as the values of the 75th percentile, of the control group. The high MCT was defined as ≥3.82; and the high FD in the CBCT and periapical digital intraoral radiography (PDIR) were both ≥1.04.
High MCT was observed in 71% of MM patients using BP and 28% of controls. The required sample size of 40 individuals was calculated, with equal proportions between groups, considering 5% of α error and power of 80%. Sample size was also calculated for the FD evaluation, on the same subjects and conditions, and there were 62.5% of MM patients and 27.3% controls with high FD; and the required sample size was 30 individuals for each group.
Data collection
Clinical data of MM patients were collected from medical records. Information about sociodemographic characteristics of subjects from both groups was collected through an interview. The CBCT and PDIR images were obtained for all subject in the study, and further anonymized for analysis.
Cortical and trabecular mandibular bone were measured on CBCT imaging, and trabecular bone was also measured on PDIR. The cortical bone was measured as the MCT and the trabecular bone measurement was obtained through FD. A blinded and trained dentist performed all the measurements. The reliability varied from good to excellent, and was assessed through intra- and interrater evaluations, using the intraclass correlation coefficient (ICC), in the first 21 measurements. The intrarater ICC were 0.90 [95% confidence interval (CI) 0.84–0.94] for MCT; 0.92 (95% CI 0.79–0.94) for FD on CBCT; and, 0.89 (95% CI 0.80–0.93) for FD on PDIR. The interrater reliabilities for MCT and FD were 0.98 (95% CI 0.88–0.99) and 0.78 (95% CI 0.68–0.97) respectively, and were performed by two investigators, for each type of measurement in 10 unidentified cases, before final evaluations of cases. The intrarater reliability was performed within 10 days interval, and was considered excellent. After this, all the measurements in the study were performed by the main investigator with unidentified images.
The CBCT exams were obtained in a KODAK 9500® digital scanner (Carestream Health, Rochester), following standard settings. All images have been acquired with 90 kV, 10 mA, 10.8 s, voxel of 0.2 mm, FOV 15 × 9 cm, and have been archived in DICOM format.
A PDIR of the mandibular molar area, on the right side was obtained during the same appointment. The images were acquired through standard methods using electronic digital radiography equipment (Dabi Atlante, Ribeirão Preto, Brazil), with 70 KV, 10 mA, 0.2 s of exposition in a phosphor plate digital detector. The images were then obtained with an EXPRESSTM imaging plate system (KaVo Kerr, Joinville, Brazil), using the CLINIVIEWTM software (KaVo Kerr, Tuusula, Finland).
Quantitative analysis
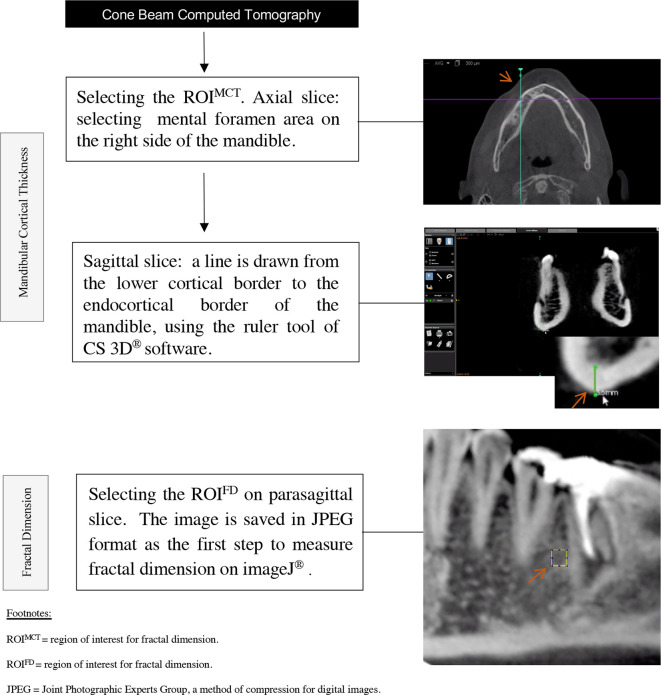
For MCT evaluation, standard images with 0.3 mm reconstructed slice thickness were analysed in the CS 3D® imaging software, v. 3.1.9 (Carestream Health, Rochester). Based on a previous study, the region of interest for the MCT (ROIMCT) was selected below the mental foramen, on the right side of the mandible,24 which was obtained in the axial plane reconstructed in the area of mental foramen opening. A maximum zoom was applied in the sagittal slice, and a line was drawn from the lower cortical border to the endocortical border, perpendicular to the ground. The ruler tool was selected on the software and the inferior MCT was measured below of the mental foramen (Figure 1).
Figure 1.
Flowchart of study methodology. ROI, region of interest.
For the FD evaluation, the image was refined in a CS 3D® imaging software. The sagittal line was rotated to the right side until it became parallel to the inferior border of the mandible. Then, both the sagittal and coronal slices were positioned in a way that they crossed on the mental foramen, resulting in a parasagittal slice. A maximum zoom was applied, and the image was archived in JPEG format (Figure 1).
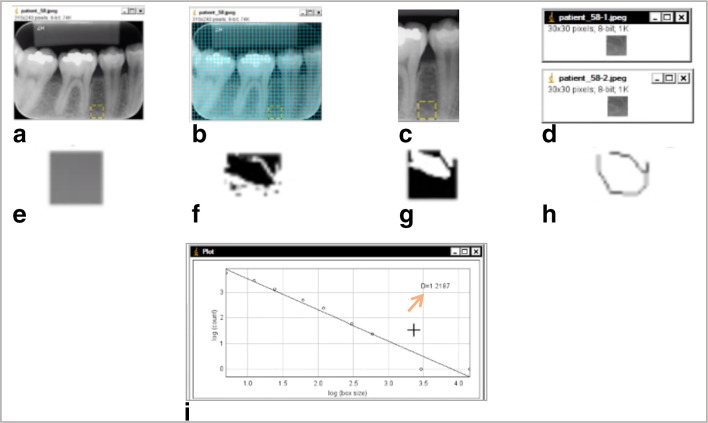
The FD calculation was performed according to pre-established protocol,8 in the ImageJ software.29 All CBCT and PDIR were evaluated in the software and a region of interest for fractal dimension (ROIFD) of 30 × 30 pixels was selected. The ROIFD was placed between the roots of teeth 45 and 46, avoiding anatomical structures, such as lamina dura, and the cortical of the mandible canal. Gridlines (GD) with size of 80 pixels2 were used to guarantee the fidelity of the ROIFD position. The ROIFD was placed one GD above the upper cortical of the mandible canal. If tooth 45 was absent, the ROIFD would be positioned three GD mesially from the mesial lamina dura of tooth 46. If tooth 46 was absent, the ROIFD would be positioned three GD distally from the distal lamina dura of tooth 45. If both teeth 45 and 46 were absent, the ROIFD would be positioned three GD distally to the mental foramen and three GD above the upper cortical of the mandible canal. After selecting the ROIFD, the GD were removed from the image and the selected ROIFD was duplicated. Then, the first ROIFD was blurred with a Gaussian filter (sigma 35). The blurred image was subtracted from the second duplicated image (original ROIFD) and a gray value of 128 was applied to the resultant image. At this point, the resultant image was turned into a binary image, and, the regions that represent trabecular bone were set to black and marrow spaces were set to white. The image was eroded and dilated to reduce the noise. After dilation, the image was skeletonized and was used for FD analysis, which was calculated by the box-counting method. The widths of the square boxes were 2, 3, 4, 6, 8, 12, 16, 32 and 64 pixels.10 Figure 2 shows the methodology steps used for the FD calculation.
Figure 2.
Methodology steps used to obtain the fractal dimension measurement on the ImageJ® software through periapical radiograph: a. ROIFD (30 × 30 pixels) was selected; b. Gridlines were applied for sizing and positioning of the ROIFD; c. Gridlines were removed; d. Duplication of the ROIFD; e. Blurred image with Gaussian filter (σ 35) ; f. Binarized image; g. Inverted image; Hh Skeletonized image; i. Graph showing results from box-counting command. The number on the exposed on the top right is the ROIFD (arrow).
Statistics analysis
Data were stored and analyzed using the statistical software SPSS v. 17.0 (SPSS Inc., Chicago, IL). Descriptive data were reported as the absolute frequency and percentage for categorical variables, and the median, with minimum and maximum values, was used for the continuous variables. For the comparison of the two groups, the obtained data were analyzed using the χ2 test for categorical variables and Mann–Whitney test for continuous variables. The Spearman coefficient was applied to verify the correlation between FD obtained on CBCT and on PDIR, and the correlation of MCT and FD with the respective cumulative doses. The significance level value was set at 0.05.
Results
A total of 74 individuals were enrolled in the study. No one refused to participate. In the MM group, individuals were excluded because they did not receive BP therapy (n = 8), or they presented one of the following conditions: solitary plasmacytoma (n = 1); polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes syndrome (n = 1); or, monoclonal gammopathy of undetermined significance (n = 1). In the control group, two individuals were excluded because they had been exposed to BP for osteoporosis treatment. Thus, 61 subjects were included in the study: 33 MM patients exposed to BP and 28 controls not exposed to BP. Sociodemographic and clinical characteristics of subjects in the study are shown in Table 1. The type of BP, cumulative doses and therapy duration of patients with MM are exhibited in Table 2.
Table 1.
Demographic and clinical characteristics of the studied population
| Characteristic | Multiple Myeloma | Controls |
|---|---|---|
| n = 33 | n = 28 | |
| Gender | ||
| Male | 17 (60.7%) | 14 (50%) |
| Female | 14 (39.3%) | 14 (50%) |
| Age (median[max-min]) | 60 (42–83) | 57 (36–74) |
| Tobacco | ||
| Never used | 21 (63.6%) | 16 (57.1%) |
| Light smoker (<20 cigarretes) | - | 02 (7.1%) |
| Heavy smoker (>20 cigarretes) | 01 (0.33%) | 01 (3.5%) |
| Ex-smoker | 11 (33.3%) | 09 (32.1%) |
| Education | ||
| Illiterate | 01 (0.33%) | - |
| Elementary School | 17 (51.5%) | 09 (32.1%) |
| High-School | 10 (30.3%) | 11 (39.2%) |
| Graduation | 05 (15.1%) | 08 (28.5%) |
| Other medical conditionsa | ||
| Cardiovascular | 20 (60.6%) | - |
| Diabetes | 07 (21.2%) | 11 (39.2%) |
| Hepatophaties | 01 (3.00%) | 06 (21.4%) |
| Thyroid disease | 03 (9.09%) | - |
| Renal disease | 07 (21.2%) | 01 (3.5%) |
| Medicationsa | ||
| Anti platelet/anticoagulant | 09 (27.2%) | 02 (7.1%) |
| Antihistamine | 13 (39.4%) | - |
| Antimicrobial | 17 (51.5%) | - |
| Antifungal | 05 (15.1%) | - |
| Antilipidemic | 01 (3.00%) | 07 (25.0%) |
| Bortezomib | 02 (3.20%) | - |
| Corticosteroids | 30 (90.9%) | - |
| Cyclophosphamide | 12 (19.3%) | - |
| Cardiovascular | 24 (72.7%) | 06 (21.4%) |
| Etoposide | 01 (1.60%) | - |
| Hormones | 03 (9.09%) | - |
| Melphalan | 06 (9.60%) | - |
| Thalidomide | 18 (29.0%) | - |
| Tranquilizer | 04 (12.1%) | - |
| Vitamins/supplements | 06 (18.1%) | - |
p-value non-significant for all variables.
Some patients presented more than one medical conditions or were taking more than one medications.
Table 2.
Median cumulative doses and duration of bisphosphonate therapy for the 33 studied patients with multiple mieloma
| Pamidronate | Zoledronate | Clodronate | |
|---|---|---|---|
| Number of patientsa | 33 (100%) | 04 (12.1%) | 03 (9.1%) |
| Cumulative dosisb (mg) | 993 (60–9.000) | 66 (52–120) | 15000 (4.500–43.500) |
| Durationb (months) | 13 (2–100) | 16 (13–30) | 10 (3–29) |
Four individuals had history of pamidronate and zoledronate exposition, and three individuals had history of pamidronate and clodronate exposition.
Median (minimum–maximum).mg=milligrams.
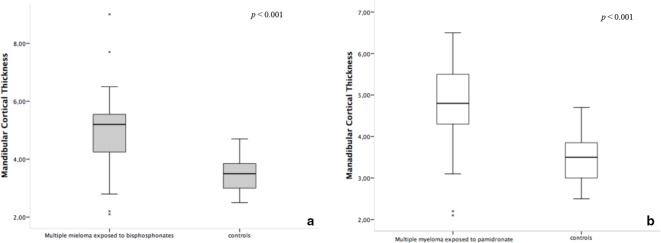
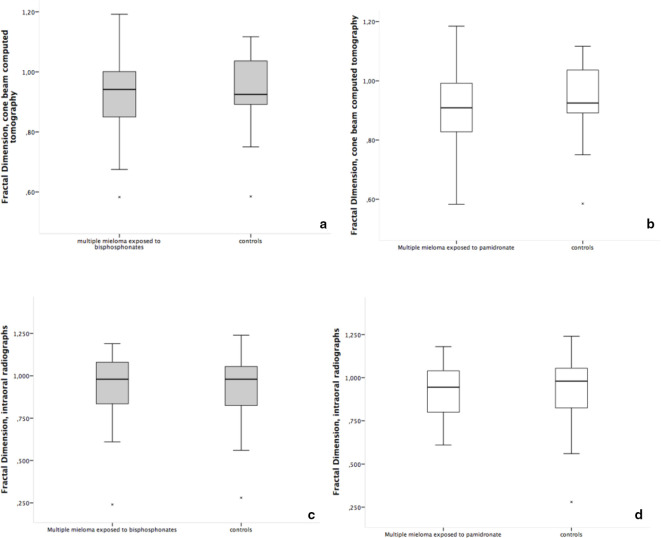
The trabecular and cortical mandibular bone measurements of the studied patients with MM using BP and controls are shown in Table 3. The median MCT on CBCT was higher in individuals with MM exposed to BP than in controls (p < 0.001). However, there were no statistical differences in the trabecular bone measured by FD between groups, neither in the CBCT (p = 0.814), nor in the PDIR measurements (p = 0.963). The correlation between FD measurements taken from CBCT and PDIR showed a strong similarity (Spearman’s ρ = 1.00, p = 0.02). When MCT and FD results were graded in high and low, similar results were found as for the continuous data analyses.
Table 3.
Measurements of the cortical and trabecular bone of the studied population
| Measurement of quantitative analysis | MM exposed to BP (N = 33) |
Controls (N = 28) |
p-value | |
|---|---|---|---|---|
| Cortical bone thickness (mm) |
MCT, CBCT median (min-max) |
5.20 (2.10–9.00) |
3.50 (2.50–4.70) |
<0.001* |
| Trabecular bonea |
FD, intraoral radiograph median (min-max) |
0.98 (0.24–1.19) |
0.96 (0.28–1.20) |
0.963 |
|
FD, CBCT median (min-max) |
0.95 (0.58–1.19) |
0.90 (0.59–1.12) |
0.814 | |
MM, multiple myeloma; BP, bisphosphonates; CBCT, cone beam computer tomography; MCT, mandibular cortical thickness; FD, fractal dimension; min-max, minimum-maximum; mm, millimeters
One individual with MM and two controls were excluded from FD measurement because imaging did not show enough amount of trabecular bone for the measurement, in the region of interest, due to missing teeth.
Since most of the individuals with MM in the study were using only pamidronate, a separated evaluation of these individuals and controls was performed. Figures 3 and 4 show box-plot graphs for MCT and FD measurements, respectively, for the whole group (individuals exposed to pamidronate with or without association with zoledronate or clodronate) (Figure 3A and B) and for the individuals exposed only to pamidronate (Figure 4B and D), comparing to controls. The separated analysis of pamidronate exposed group, showed similar results as the whole group, when compared to controls. The median MCT for the pamidronate group was higher than the median from controls (Table 3). The median FD of the pamidronate exposed group did not present significant differences from controls, neither for the CBCT, nor for the PDIR measurements (Table 3).
Figure 3.
Box-plot graphs of the mandibular cortical thickness measurement on cone beam computed tomography of individuals exposed to all types of bisphosphonates vs controls (a), and individuals exposed only to pamidronate vs controls (b).
Figure 4.
Box-plot graphs of the fractal dimension (trabecular bone) measurement on cone beam computed tomography (a, b) and periapical intraoral radiograph (c, d). Figures a and c show individuals exposed to all types of bisphosphonates vs controls, and figures b and d show individuals exposed only to pamidronate vs controls.
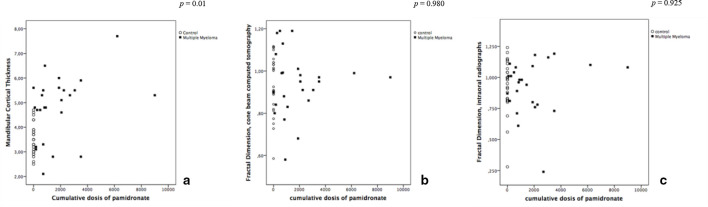
Figure 5 displays the pattern in the distribution of the MCT and FD measurements according to the cumulative doses of pamidronate. A correlation between the MCT taken from CBCT and the cumulative doses of pamidronate was observed (Spearman’s ρ = 0.51, p < 0.01), but this correlation was not observed for FD, neither on CBCT (Spearman’s ρ = 0.04, p = 0.980) or on PDIR imaging (Spearman’s ρ = 0.13, p = 0.925). Figure 6 shows the box-plot graphs comparing the MCT and the FD measurements according to the type of BP intake. There was a difference between the MCT of controls and patients with MM using different types of BP (p < 0.001), but not among the FD (p = 0.876 on the CBCT and p = 0.838 on the PDIR) between groups.
Figure 5.
Dispersion graphs showing the relationship between the cumulative doses of pamidronate and the measurements of the mandibular cortical thickness (a), and trabecular bone (b, c) measurements.
Figure 6.
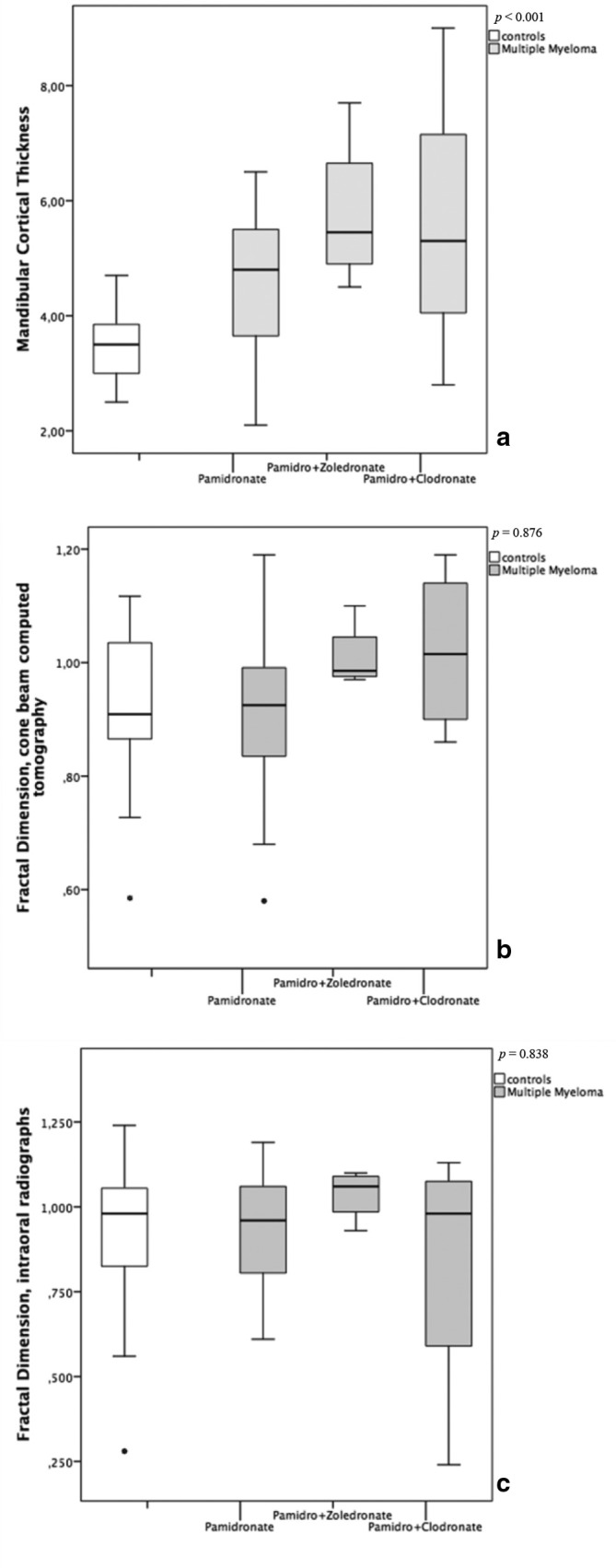
Box-plot graphs comparing the mandibular cortical thickness (a) and fractal dimension measurements (b, c) with different types of bisphosphonate intake.
Discussion
In this case–control study, the effects of BP were measured in the cortical and trabecular bone of individuals with MM exposed to endovenous BP. Previous studies have quantitatively assessed bony architecture of patients with MRONJ.8,12,21–23,25 Only one study has evaluated patients using BP without MRONJ,24 but the sample was heterogeneous. In the present study, a homogeneous sample of patients with MM using BP, but not presenting MRONJ has been evaluated. Furthermore, the evaluations have been performed with the use of free access public domain software,29 and the protocol used a small ROI, which may be reproduced in both patients with or without teeth, with good reliability.
The individuals with MM exposed to BP presented higher MCT when compared to controls not exposed to BP. This significant result is in agreement with other studies which have shown that MCT is an useful tool for the detection of bone alterations caused by BP.24 Bone quantitative evaluation as a result of BP exposition has been studied in mixed populations, in which MM patients have sometimes been included.8,12,21–23,27,28
Bisphosphonates induce the suppression of bone turn over and reduce remodeling rates, mainly in the intracortical region of the bone.27 Therefore, the higher MCT resulting from the BP exposure in the lower jaw could be explained by the reduction of the high remodeling rate of the mandible, potentialized by BP deposition in the cortical region. Interestingly, Spearman correlation coefficient showed a clear influence of higher cumulative doses of pamidronate on the increase of the cortical bone thickness. The correlation between MCT and the cumulative doses has also been reported in the literature, among patients taking zolendronate.11,24 Figure 5 shows that only few patients under BP therapy had high cumulative doses of pamidronate. We do not know the impact of these data on developing quantitative measures of bisphosphonate exposure if the studied population had longer periods of BP treatment.
A previous study using FD on CBCT8 has reported trabecular bone alterations caused by BP in patients with MRONJ. An increase in the trabecular bone measurements was also expected in the present study, but no significant differences were observed in FD between groups. Trabecular bone alterations may not have been evidenced because of the small ROI, or the overlap of anatomical structures (roots of teeth, lamina dura and the cortical of the mandible canal) may have prevented a possible equivocal measurement. The evaluated area was elected to the study because it has been demonstrated to present more quantitative bone alterations induced by BP's, comparing to other sites of the jaws.8,22,24
Some studies using panoramic radiographs have not detected differences in FD between osteoporotic bone exposed to BP and controls either.10,25 Maybe an extended period of BP exposition is needed, in order to observe trabecular bone alterations in MM patients. Indeed, BP are not evenly distributed into the skeletal sites, and show different timing for attaching to cortical and trabecular bone.30–32 In the present study, nearly 20% of the MM patients were taking BP for less than 6 months (data not shown). However, FD seems to show an increase, and then a tendency of stabilize over time in MM patients exposed to pamidronate, even though there were no statistical differences between the groups (Figure 5).
Some particularities of MM bone disease must be considered. The bone destruction caused by MM increases marrow spaces between the bone trabeculae, as a result of plasma cell clone proliferation. Even under treatment, MM bone lesions never heal.4 Thus, the measurements in the study may be attributed both to bone changes of MM, as well as to the BP therapy. The BP action on the osteocytes increase the trabecular density, but not necessarily reduce the spaces between the trabeculae.2,4,33 Although osteoclasts and osteoblasts participate actively in the process of bone remodeling, the plasma cell clones of MM destroy the osteocytes, which correspond to 90% of the bone cells, and are important signaling factors in the bone remodeling process.34 Once osteocytes are destroyed, the process of bone remodeling is compromised, contributing to bone damage.
Other particularities of this population related to the clinical complications and medical management of these patients may also have an impact on bone pathophysiology (Table 1). Associated comorbidities like renal disease may impact the bone turnover. The use of some pharmacological agents like corticosteroids could decrease bone mineralization. Other medications used in cancer treatment, like thalidomide and bortezomib, present antiangiogenic properties.
The remodeling rate in the mandible is 20 times higher than in others bones.33 Systemic markers may not appropriately estimate the activity of remodeling in specific sites, as the jawbones.30–32 The C-terminal crosslinking telopeptide test seems to show relative accuracy in sensibility for the identification of low or high remodeling activity, but no specificity in determination of the bone sites.31,35 Thus, local bone measurements could represent a more accurate tool to evaluate the bone exposed to BP at risk for the development MRONJ. In this study, we detected BP exposure differences in the MCT, but we could not detect risk for MRONJ, for there were no individuals presenting the condition in the studied population.
All individuals with MM in this study were exposed to pamidronate, but 21.2% of them had also a history of zoledronate or clodronate intake (Table 2). Although nitrogenous BP have similar mechanism of action, each type of BP has a different pattern of deposition in the body skeleton.32 The accumulation of more than one type of BP could potentially boost their effects on bone, with increase of cortical and trabecular jawbone measurements in patients exposed to the medication, as shown on the CBCT graphs (Figure 6), even though it has not reached statistical significance for FD.
Dimensional changes of the jaw bone exposed to BP (with or without MRONJ) have been reported in populations with different types of diseases.8,12,21–24 Different techniques like MCT,22,24 FD,8,23 bone density12,21 and histomorphometry36 have been used for this purpose. All studies have shown some kind of BP induced changes on bone. Interestingly, dimensional effects of BP in the jawbone have been studied mainly in the lower jaw,8,12,21–24 because of its anatomical properties. The deposition of BP occurs more in the mandible than in any other bone sites.33 Moreover, the mandible is affected in 70% of the patients with MRONJ,37 and it is easier to avoid anatomical structures and accidents during bone evaluation of the mandible, when compared to the maxilla.
Imaging methods as CBCT12,21,22 and panoramic radiograph24,25 have been used for the measurement the jawbone exposed to BP. In this study, the PDIR was also obtained to evaluate the trabecular bone through FD, and has shown similar results when compared with the bone evaluation in CBCT images (Figure 4). Indeed, CBCT and PDIR measurements have shown similar results in bone exposed to BP in another study, and authors suggest that they could be reproduced in individuals with MRONJ.17 Although the CBCT provides accurate three-dimensional reconstructions, this imaging method emits a high dose of radiation when compared to two-dimensional tests, in addition to requiring higher costs. When compared to other tomographic methods, the dose of radiation of CBCT is lower and the image more accurate for evaluation of the bone tissues of the head and neck region.
This study has some limitations. The comparison with a third group of MM patients without BP exposition would be interesting. But the great majority of the patients in the hospital were using BP. They were oftenly diagnosed in other centers, with hypercalcemia. In these cases, BP was administrated urgently, before they were referred to the University Hospital. Thus, the paucity of patients with MM free of BP exposition impaired this evaluation. Thus, one can not exclude the effect of MM on MCT given there is no control group of MM patients without BP intake.
In conclusion, MCT measurement may represent a potential useful tool in the detection of cortical jaw bone changes caused by BP, in patients with MM. Longitudinal studies will be useful to show the increase of MCT overtime, and are necessary to indicate the point of MCT which represents a major risk of MRONJ development, in the bone exposed to BP. Additionally, studies on BP bone mechanism are necessary to improve information about the trabecular jaw bone evaluation.
Footnotes
Funding: Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ – JCNE E-26/103.046/2012), grants to Dr. Sandra R. Torres. The investigator Édila F. Feitosa is under the scholarship program (BN10 E-26/200.542/2018), from the same institution.
Contributor Information
Édila Figuerêdo Feitosa, Email: edilaff@yahoo.com.br.
Mariana Monteiro Vasconcellos, Email: maryvasconcellos1@hotmail.com.
Roberto José Pessoa Magalhães, Email: robmag@hucff.ufrj.br.
Andrea Castro Domingos-Vieira, Email: andreacastrodomingos@gmail.com.
Maria Augusta Visconti, Email: gutavisconti@odonto.ufrj.br.
Fabio Ribeiro Guedes, Email: fabiorguedes@gmail.com.
Angelo Maiolino, Email: angelomaiolino@gmail.com.
Sandra Regina Torres, Email: sandratorres@ufrj.br.
REFERENCES
- 1.Mosebach J, Thierjung H, Schlemmer H-P, Delorme S. Multiple Myeloma Guidelines and Their Recent Updates: Implications for Imaging. Fortschr Röntgenstr 2019; 28. doi: 10.1055/a-0897-3966 [DOI] [PubMed] [Google Scholar]
- 2.Teixeira S, Branco L, Fernandes MH, Costa-Rodrigues J. Bisphosphonates and cancer: a relationship beyond the antiresorptive effects. Mini Rev Med Chem 2019; 19: 988–98. doi: 10.2174/1389557519666190424163044 [DOI] [PubMed] [Google Scholar]
- 3.Berenson JR, Hillner BE, Kyle RA, Anderson K, Lipton A, Yee GC, et al. American Society of clinical oncology clinical practice guidelines: the role of bisphosphonates in multiple myeloma. JCO 2002; 20: 3719–36. doi: 10.1200/JCO.2002.06.037 [DOI] [PubMed] [Google Scholar]
- 4.Hillengass J, Usmani S, Rajkumar SV, Durie BGM, Mateos M-V, Lonial S, et al. International myeloma Working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol 2019; 20: e302–12. doi: 10.1016/S1470-2045(19)30309-2 [DOI] [PubMed] [Google Scholar]
- 5.Hungria VTM, Maiolino A, Martinez G, Duarte GO, Bittencourt R, Peters L, et al. Observational study of multiple myeloma in Latin America. Ann Hematol 2017; 96: 65–72. doi: 10.1007/s00277-016-2866-9 [DOI] [PubMed] [Google Scholar]
- 6.Roodman GD. Skeletal imaging and management of bone disease. Hematology 2008; 2008: 313–9. doi: 10.1182/asheducation-2008.1.313 [DOI] [PubMed] [Google Scholar]
- 7.Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the Jaw—2014 update. Journal of Oral and Maxillofacial Surgery 2014; 72: 1938–56. doi: 10.1016/j.joms.2014.04.031 [DOI] [PubMed] [Google Scholar]
- 8.Torres SR, Chen CSK, Leroux BG, Lee PP, Hollender LG, Schubert MM. Fractal dimension evaluation of cone beam computed tomography in patients with Bisphosphonate-associated osteonecrosis. Dentomaxillofac Radiol 2011; 40: 501–5. doi: 10.1259/dmfr/14636637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treister NS, Friedland B, Woo S-B. Use of cone-beam computerized tomography for evaluation of Bisphosphonate-associated osteonecrosis of the jaws. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 2010; 109: 753–64. doi: 10.1016/j.tripleo.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 10.Sindeaux R, Figueiredo PTdeS, de Melo NS, Guimarães ATB, Lazarte L, Pereira FB, et al. Fractal dimension and mandibular cortical width in normal and osteoporotic men and women. Maturitas 2014; 77: 142–8. doi: 10.1016/j.maturitas.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 11.Rocha GCMA, Jaguar GC, Moreira CR, Neves EG, Fonseca FP, Pedreira EN. Radiographic evaluation of maxillofacial region in oncology patients treated with bisphosphonates. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 114(5 Suppl): S19–25. doi: 10.1016/j.tripleo.2011.08.016 [DOI] [PubMed] [Google Scholar]
- 12.Guggenberger R, Koral E, Zemann W, Jacobsen C, Andreisek G, Metzler P. Cone beam computed tomography for diagnosis of bisphosphonate-related osteonecrosis of the jaw: evaluation of quantitative and qualitative image parameters. Skeletal Radiol 2014; 43: 1669–78. doi: 10.1007/s00256-014-1951-1 [DOI] [PubMed] [Google Scholar]
- 13.Cardoso CL, Barros CA, Curra C, Fernandes LMPdaSR, Franzolin SdeOB, Júnior JSF, et al. Radiographic findings in patients with medication-related osteonecrosis of the jaw. Int J Dent 2017; 2017: 1–6. doi: 10.1155/2017/3190301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianchi SD, Scoletta M, Cassione FB, Migliaretti G, Mozzati M. Computerized tomographic findings in Bisphosphonate-associated osteonecrosis of the jaw in patients with cancer. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 2007; 104: 249–58. doi: 10.1016/j.tripleo.2007.01.040 [DOI] [PubMed] [Google Scholar]
- 15.Phal PM, Myall RWT, Assael LA, Weissman JL. Imaging findings of Bisphosphonate-associated osteonecrosis of the jaws. American Journal of Neuroradiology 2007; 28: 1139–45. doi: 10.3174/ajnr.A0518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barragan-Adjemian C, Lausten L, Ang DB, Johnson M, Katz J, Bonewald LF. Bisphosphonate-Related osteonecrosis of the jaw: model and diagnosis with cone beam computerized tomography. Cells Tissues Organs 2009; 189(1–4): 284–8. doi: 10.1159/000151451 [DOI] [PubMed] [Google Scholar]
- 17.Olutayo J, Agbaje JO, Jacobs R, Verhaeghe V, Velde FV, Vinckier F. Bisphosphonate-Related osteonecrosis of the jaw bone: radiological pattern and the potential role of CBCT in early diagnosis. J Oral Maxillofac Res 2010; 1: e3. doi: 10.5037/jomr.2010.1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutchinson M, O'Ryan F, Chavez V, Lathon PV, Sanchez G, Hatcher DC, et al. Radiographic findings in bisphosphonate-treated patients with stage 0 disease in the absence of bone exposure. Journal of Oral and Maxillofacial Surgery 2010; 68: 2232–40. doi: 10.1016/j.joms.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 19.Treister N, Sheehy N, Bae EH, Friedland B, Lerman M, Woo S. Dental panoramic radiographic evaluation in Bisphosphonate-associated osteonecrosis of the jaws. Oral Dis 2009; 15: 88–92. doi: 10.1111/j.1601-0825.2008.01494.x [DOI] [PubMed] [Google Scholar]
- 20.O'Ryan FS, Khoury S, Liao W, Han MM, Hui RL, Baer D, et al. Intravenous bisphosphonate-related osteonecrosis of the jaw: bone scintigraphy as an early indicator. Journal of Oral and Maxillofacial Surgery 2009; 67: 1363–72. doi: 10.1016/j.joms.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 21.Metzler P, Zemann W, Lübbers H-T, Guggenberger R, Lüssi A, Obwegeser JA, et al. Bone mineral density measurements performed by cone-beam computed tomography in the bisphosphonate-related osteonecrosis-affected jaw. Oral Radiol 2012; 28: 101–8. doi: 10.1007/s11282-012-0093-1 [DOI] [Google Scholar]
- 22.Torres SR, Chen CSK, Leroux BG, Lee PP, Hollender LG, Santos ECA, et al. Mandibular cortical bone evaluation on cone beam computed tomography images of patients with bisphosphonate-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 113: 695–703. doi: 10.1016/j.oooo.2011.11.011 [DOI] [PubMed] [Google Scholar]
- 23.Şahin O, Odabaşı O, Demiralp Kemal Özgür, Kurşun-Çakmak Emine Şebnem, Aliyev T. Comparison of findings of radiographic and fractal dimension analyses on panoramic radiographs of patients with early-stage and advanced-stage medication-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol 2019; 128: 78–86. doi: 10.1016/j.oooo.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 24.Torres SR, Chen CSK, Leroux BG, Lee PP, Hollender LG, Lloid M, et al. Mandibular inferior cortical bone thickness on panoramic radiographs in patients using bisphosphonates. Oral Surg Oral Med Oral Pathol Oral Radiol 2015; 119: 584–92. doi: 10.1016/j.oooo.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demiralp KÖ, Kurşun-Çakmak EŞ, Bayrak S, Akbulut N, Atakan C, Orhan K. Trabecular structure designation using fractal analysis technique on panoramic radiographs of patients with bisphosphonate intake: a preliminary study. Oral Radiol 2019; 35: 23–8. doi: 10.1007/s11282-018-0321-4 [DOI] [PubMed] [Google Scholar]
- 26.Faria KM, Ribeiro ACP, Brandão TB, Silva WG, Lopes MA, Pereira J, et al. Radiographic patterns of multiple myeloma in the jawbones of patients treated with intravenous bisphosphonates. The Journal of the American Dental Association 2018; 149: 382–91. doi: 10.1016/j.adaj.2017.12.028 [DOI] [PubMed] [Google Scholar]
- 27.Allen MR, Burr DB. Bisphosphonate effects on bone turnover, microdamage, and mechanical properties: What we think we know and what we know that we don't know. Bone 2011; 49: 56–65. doi: 10.1016/j.bone.2010.10.159 [DOI] [PubMed] [Google Scholar]
- 28.Koo C-H, Lee J-H. Evaluation of mandibular cortical bone ratio on computed tomography images in patients taking bisphosphonates. Maxillofac Plast Reconstr Surg 2018; 40: 17. doi: 10.1186/s40902-018-0153-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ImageJ Image Processing and analysis in Java [homepage on the Internet. Wayne Rasbanad (USA: National institute of Health; [version 1.47v; updated 2013 july 8]. http://rsbweb.nih.gov/ij/. [Google Scholar]
- 30.Allen MR, Turek JJ, Phipps RJ, Burr DB. Greater magnitude of turnover suppression occurs earlier after treatment initiation with risedronate than alendronate. Bone 2011; 49: 128–32. doi: 10.1016/j.bone.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 31.Dal Prá KJ, Lemos CAA, Okamoto R, Soubhia AMP, Pellizzer EP. Efficacy of the C-terminal telopeptide test in predicting the development of bisphosphonate-related osteonecrosis of the jaw: a systematic review. Int J Oral Maxillofac Surg 2017; 46: 151–6. doi: 10.1016/j.ijom.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 32.Allen MR, Kubek DJ, Burr DB. Cancer treatment dosing regimens of zoledronic acid result in near-complete suppression of mandible intracortical bone remodeling in beagle dogs. J Bone Miner Res 2010; 25: 98–105. doi: 10.1359/jbmr.090713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.RUSSELL RGG, Xia Z, Dunford JE, Oppermann U, Kwaasi A, Hulley PA, et al. Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci 2007; 1117: 209–57. doi: 10.1196/annals.1402.089 [DOI] [PubMed] [Google Scholar]
- 34.Florencio-Silva R, Sasso GRdaS, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int 2015; 2015: 1–17. doi: 10.1155/2015/421746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enciso R, Keaton J, Saleh N, Ahmadieh A, Clark GT, Sedghizadeh PP. Assessing the utility of serum C-telopeptide cross-link of type 1 collagen as a predictor of bisphosphonate-related osteonecrosis of the jaw. The Journal of the American Dental Association 2016; 147: 551–60. doi: 10.1016/j.adaj.2016.02.011 [DOI] [PubMed] [Google Scholar]
- 36.Bedogni A, Saia G, Bettini G, Tronchet A, Totola A, Bedogni G, et al. Osteomalacia: the missing link in the pathogenesis of bisphosphonate-related osteonecrosis of the jaws? Oncologist 2012; 17: 1114–9. doi: 10.1634/theoncologist.2012-0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saad F, Brown JE, Van Poznak C, Ibrahim T, Stemmer SM, Stopeck AT, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol 2012; 23: 1341–7. doi: 10.1093/annonc/mdr435 [DOI] [PubMed] [Google Scholar]