Abstract
The present study was designed to investigate the effect of adipose-derived stem cells (ADSCs) on acute graft vs. host disease (aGVHD) and hematopoietic recovery after allogeneic hematopoietic stem cell transplantation. ADSCs, bone marrow-derived stem cells (BMSCs) and fibroblasts were cultured. ADSCs were cocultured with hematopoietic stem/progenitor cells. Then, ADSCs were infused into the aGVHD rat model. The survival of the rats was recorded. Livers and small intestines were obtained from sacrificed rats for pathological examinations. Expression of the Sry gene in recipient rats that survived longer than 21 days was examined by real-time PCR to detect the presence of donor Y chromosome. Expression of serum interferon (INF)-γ and interleukin (IL)-4 was detected by ELISA at 0, 7, 14, 21 and 50 days after transplantation. Transplantation of ADSCs improved the survival of aGVHD rats. Survived ADSCs participated in hematopoietic reconstitution in aGVHD rats. ADSCs decreased aGVHD severity by immunomodulation. ADSCs support the proliferation of hematopoietic stem/progenitor cells in vitro. The present study demonstrated that ADSCs may reduce aGVHD by influencing the balance of IL-4 and INF-γ and can promote long-term hematopoiesis.
Keywords: adipose-derived stem cells, hematopoiesis support, immunomodulation, acute graft vs. host disease, bone marrow stem cells
Introduction
Acute graft vs. host disease (aGVHD) is the most common complication after allogeneic hematopoietic stem cell transplantation (allo-HSCT). It is reported that the incidence of aGVHD has reached 32±3% in several transplantation centers (1), and severe aGVHD is the most prevalent cause of death after transplantation. The first choice of treatment for aGVHD is steroids. When poor clinical outcome is shown, a combination of steroids with immunosuppressive drugs is recommended. Given that some aGVHD cases persist even after treatment with this combination, novel approaches to overcome aGVHD have been explored in recent years.
Mesenchymal stem cells (MSCs) are multipotent cells that can self-renew and differentiate into various somatic lineages. MSCs have also been reported to regulate immunological reactions by secreting soluble cytokines and/or by direct contact with lymphocytes. MSCs were first identified and isolated from bone marrow (2), and were subsequently confirmed to exist in a variety of tissues such as adipose, muscle, tendon, umbilical cord blood and amniotic fluid. Le Blanc et al (3) reported that infusion of bone marrow-derived MSCs (BMSCs) systemically attenuated aGVHD in a mouse model, indicating the therapeutic potential of MSCs in amelioration of aGVHD.
Adipose-derived mesenchymal stem cells (ADSCs), which were first identified by Zuk et al in 2001 (4), share similar biological characteristics and immunological phenotype with BMSCs. It is believed that ADSCs confer more advantages in terms of proliferation and cause reduced damages than BMSCs (5). The present study was designed to ascertain whether ADSCs alleviate the incidence and severity of aGVHD in a rat model. Hemopoiesis after treatment with ADSCs was also observed.
Materials and methods
Animals
Specific-pathogen-free Sprague-Dawley (SD) and Wistar rats (n=10 for each type of rat) were provided by the Animal Center, Xinjiang Medical University, China [license no. SCXK (Xin) 2003-001]. The study was approved by the Ethics Committee of The First Affiliated Hospital of Xinjiang Medical University, China (lot no. 20080701017). The rats were housed in a specific-pathogen-free laboratory as approved by the US Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC; http://www.aaalac.org). Donor rats were male SD rats and recipient rats were female Wistar rats aged 6–8 weeks and weighing 180–210 g. Before sacrifice, all rats were given acidified water containing erythromycin (250 mg/l) and gentamicin (pH 3.0–3.5) for bowel cleansing. Animal experiments were conducted in the Animal Experimental Center of Clinical Research Institute of the First Affiliated Hospital of Xinjiang Medical University. All animal experiments were performed following the US Guidelines for the Use and Management of Laboratory Animals (6). After intraperitoneal anesthesia with 10% chloral hydrate at a dose of 300 mg/kg body weight, the animals were sacrificed by neck dislocation after losing consciousness. No rats developed peritonitis due to the use of chloral hydrate. Then, tissues and blood samples were obtained.
Culture and identification of ADSCs, BMSCs and fibroblasts
After intraperitoneal anesthesia with 10% chloral hydrate at a dose of 300 mg/kg body weight, the rats were sacrificed by neck dislocation. Then, the rats were soaked in 75% ethanol for 15 min. Bilateral inguinal skin was cut, bilateral inguinal fat, femur and tibia were isolated and obtained, and the required cells were obtained according to the experimental method described below.
Bilateral inguinal fat was aseptically obtained, washed with phosphate-buffered saline (PBS, pH 7.4) and cut into small pieces. Following digestion with 0.1% type I collagenase (Worthington Biochemical Corp.) for 30 min, the samples were centrifuged at 1,200 × g for 10 min and the supernatant was discarded. Cells were resuspended in low-glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 100 U/ml penicillin, 100 mg/ml streptomycin and 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.), and plated at a density of 4×104 cells/cm2 in 100-mm culture dishes (Falcon, USA).
BMSCs were harvested from bone marrow in the femur and tibia by flushing with 5 ml low-glucose DMEM using a 21G syringe. Cells were incubated at a density of 6–8×106/ml for 48 h to allow adhesion. When reaching 70–80% confluency, the cells were passaged and BMSCs before the 4th passage were used in subsequent studies.
ADCSs and BMSCs at passage 3 were prepared into a single-cell suspension after trypsinization with 0.25% trypsin. After centrifugation at 1,000 × g for 10 min, the supernatant was removed before washing with PBS twice. Cells (1×106) were bound with monoclonal antibodies (100 µl system, the antibody was 0.25 µg). The antibodies included: CD34-PE (cat. #119307; Biolegend), HCAM-FITC (cat. #203906; Biolegend), CD106-PE (cat. #200403; Biolegend), CD49-d-FITC (cat. #200103; Biolegend), and CD29-PE (cat. #102207; Biolegend). At 4°C, the sample was incubated for 30 min in the dark before flow cytometry using the CytoFLEX V2-B4-R0 Flow Cytometer (C02944; Beckman Coulter). Then, EXPOTM32 MultiCOMP Software (Beckman Coulter, Inc.) was used for data analysis. Adipogenic and osteogenic differentiation of cells was identified by Oil red staining and Alizarin red staining, respectively. Fibroblasts were obtained from rat dermis and cultured according to previously described methods (7).
For Oil red staining, ADSCs and BMSCs in logarithmic growth were mixed with low-glucose DMEM containing 10% FBS, 0.1 µmol/l dexamethasone, 200 µmol/l indometacin, and 0.5 mmol/l 3-isobutyl-1-methylxanthine. After culture for 8–10 days, transparent lipid droplets appeared, and the cells were fixed with 4% paraformaldehyde before washing with PBS twice. Then, the cells were stained with Oil red for 10 min before observation.
For Alizarin red staining, ADSCs and BMSCs in logarithmic growth were mixed with low-glucose DMEM osteogenic induction medium containing 10% FBS, 0.1 µmol/l dexamethasone, 50 µmol/l ascorbic acid, and 10 mmol/l sodium β-glycerophosphate. After culture for 15 days, black sediments appeared, and the cells were fixed with 4% paraformaldehyde before washing with PBS twice. Then, the cells were stained with 1% Alizarin red (pH 4.2) for 3 min.
Coculture of ADSCs with hematopoietic stem/progenitor cells
Bone marrow mononuclear cells (BMMNCs) were obtained by density gradient centrifugation using rat lymphocyte separation medium (1.083 g/cm3). After centrifugation, mononuclear cells were resuspended and centrifuged at 290 × g for 5 min. Then, the cells were collected and cocultured with ADSCs, BMSCs or fibroblasts, respectively, which had been treated with mitomycin C (0.5 µg/ml) for 24 h. The colony-forming ability of non-adherent mononuclear cells cocultured with ADSCs, BMSCs or fibroblasts was determined after 14 and 35 days using methylcellulose colony-forming assay (Stem Cell Technologies, Canada). The number and morphological characteristics of hematopoietic colony-forming units (CFUs) were observed under an inverted microscope (×100 magnification; DMI4000B; Leica Microsystems). Each test was performed 3 times.
Giemsa staining
The cells on the slides were fixed with methanol for 5 min, and Giemsa staining working solution (Sangon) was added onto the slides before incubation at room temperature for 8 min. Then, the slides were washed with distilled water and dried in the air before observation under a microscope.
Infusion of ADSCs in the aGVHD rat model
After sacrifice of the rats, splenic lymphocytes were obtained by grinding the spleen into a single-cell suspension. Then, inguinal adipose tissue was obtained for isolation and culture of ADSCs. The femur of male rats was extracted, the epiphysis was cut off, and the bone marrow cells of all the donors were washed out with complete culture medium. Then, the single-cell suspension of bone marrow cells was formed by passing the cells through 200 meshes of metal mesh. Allogeneic hematopoietic stem cells (HSCs) were obtained. According to previous reports (8,9), infusion of allogeneic HSCs alone is not able to induce aGVHD. Therefore, the present study established the model of aGVHD by infusing a mixture of allogeneic HSCs and spleen lymphocytes. Briefly, after receiving a total of 6 Gy body irradiation, the rats were transplanted with a mixture of BMMNCs (2×108 cells/kg) and spleen cells (3×108/kg) (BM+spleen group) by infusion via caudal vein of the tail.
To observe the suppressive effects of ADSCs on aGVHD, ADSCs were infused at a dose of 1×107/kg, in combination with either the mixture of BMMNCs and spleen lymphocytes (ADSCs+BM+spleen group) or BMMNCs alone (BM group), through the caudal vein of rats at 4–6 h after irradiation. Occurrence of aGVHD was characterized by clinical manifestations such as weight loss, abnormal hunched posture, decreased motion, hair loss and skin ulcers (10). Livers and small intestines were obtained from sacrificed rats for pathological examinations. Life span exceeding 50 days was considered a long-period of survival. Expression of the Sry gene in recipient rats that survived longer than 21 days was examined by real-time PCR to detect the presence of donor Y chromosome. Briefly, the Blood & Cell Culture DNA Midi Kit (cat. no. 13343; Qiagen) was used to extract DNA. Using the primers 5′-GAGGGTTATACTTTGCAGCGTGAA-3′ and 5′-CTGCTGTTTCTGCTGTAGTGGGT-3′, the Sry gene on the Y chromosome of the rats was determined. The reaction system (25 µl) was composed of 12.5 µl PCR mix, 0.5 µl upstream primer, 0.5 µl downstream primer, 1 µl cDNA and 10.5 µl ddH2O. PCR condition consisted of initial denaturation at 95°C for 60 sec; 40 cycles of denaturation at 95°C for 15 sec, annealing at 58°C for 15 sec and elongation at 72°C for 45 sec. The reaction product underwent agarose gel electrophoresis. Simultaneously, expression of serum interferon-γ (IFN-γ; eBioscience; Thermo Fisher Scientific, Inc.) and interleukin-4 (IL-4; eBioscience; Thermo Fisher Scientific, Inc.) was detected by ELISA at 0, 7, 14, 21 and 50 days after transplantation.
Statistical analysis
Data were analyzed using SPSS 13.0 software (IBM, Corp.), and are expressed as means ± standard deviations. For analysis of the survival rate, Kaplan-Meier method and the log-rank method were used. For analysis of IL-4/INF-γ levels and cell colonies, Student's t-test was used for analysis of the data. For analysis of body weight and white blood cell count, one-way ANOVA followed by Newman-Keuls test was utilized. A value of P<0.05 was considered statistically significant.
Results
Transplantation of ADSCs improves the survival of aGVHD rats
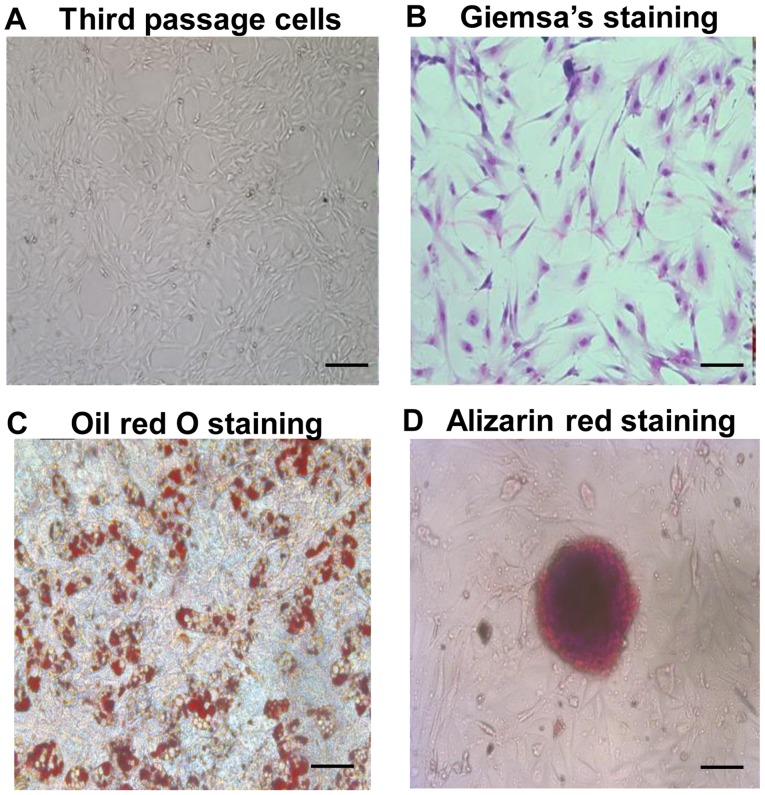
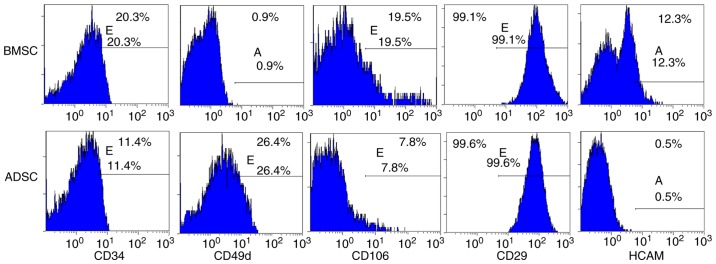
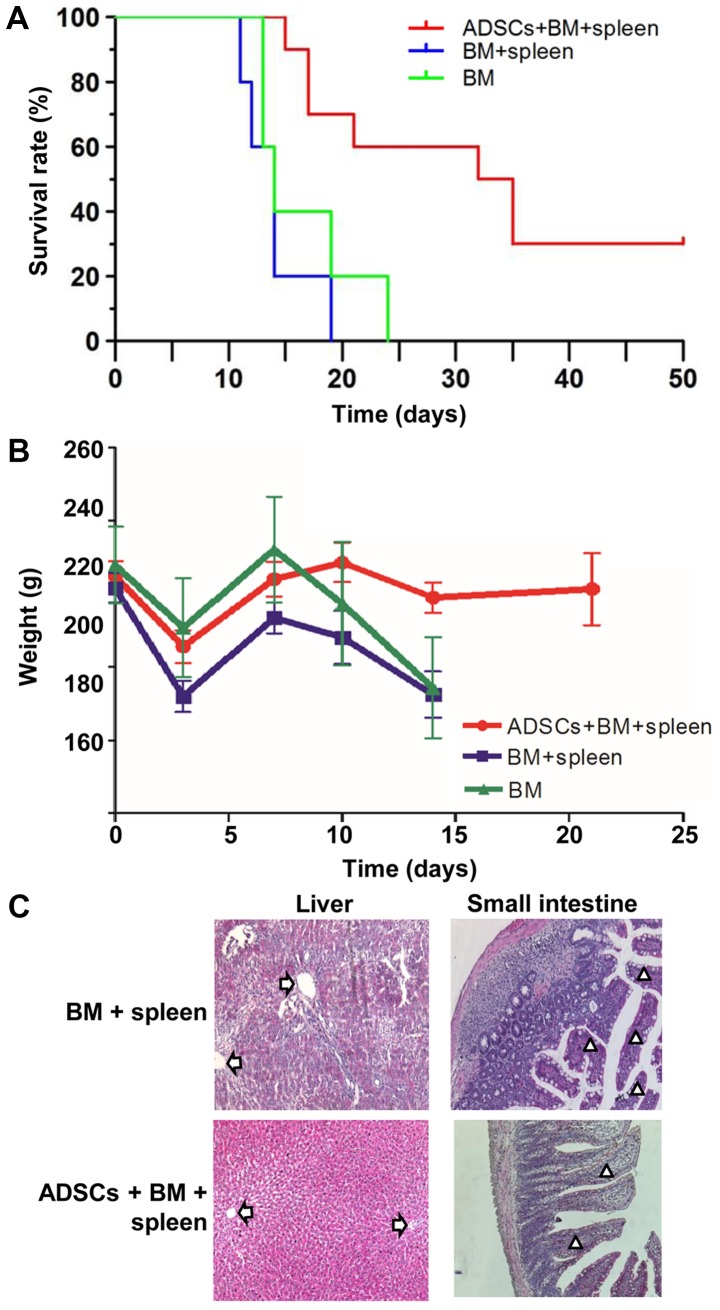
As shown in Fig. 1A and B, ADSCs at passage 3 exhibited a typical spindle fibroblast-like shape. Differentiation potential of ADSCs into adipogenic and osteogenic lineages was determined by Oil red O and Alizarin red staining, respectively (Fig. 1C and D). Flow cytometry revealed characteristic expression of CD markers, including CD34, CD49d, CD29, CD44 (or HCAM) and CD106 (Fig. 2). The median survival time of rats that received a combined infusion of BMMNCs and spleen lymphocytes (BM+spleen group) (14 days) was the same with that of rats that received BMMNCs alone (BM group) (14 days). After transplanting ADSCs into the aGVHD model, the median survival time of rats (ADSCs+BM+spleen group) was significantly extended to 33.5 days, indicating that ADSCs improved survival of the aGVHD rats (Fig. 3A). After transplanting BMMNCs and spleen lymphocytes, the rats showed typical aGVHD manifestations, including hunched arched posture, hair loss and decreased movement range of motion after 14 days. Body weight changes of rats from each group at different time points after transplantation showed that ADSCs significantly reduced the weight loss of the recipient rats on day 14 after transplantation, and no difference between the BM+spleen group and BM group was observed (Fig. 3B). Moreover, pathological changes in liver and intestinal track such as sinusoidal dilation and congestion, periportal inflammation, intestinal epithelial necrosis and submucosal congestion and edema in the aGVHD rats were markedly ameliorated after receiving ADSC transplantation (Fig. 3C). The results suggest that transplantation of ADSCs improves the survival of aGVHD rats.
Figure 1.
Morphology and the differentiation potential of ADSCs at the third passage. (A) Morphology of ADSCs at the third passage observed under an inverted microscope. Scale bar, 200 µm. (B) Giemsa's staining of ADSCs. Scale bar, 200 µm. (C) Oil red O staining of ADSCs on days 5–8 after adipogenic induction. Scale bar, 100 µm. (D) Alizarin red staining of ADSCs on day 14 after osteogenic induction. Scale bar, 200 µm. ADSCs, adipose tissue-derived stem cells.
Figure 2.
Immunophenotype of ADSCs and BMSCs. The third passage cells were treated with FITC- or PE-labeled antibodies, followed by flow cytometry. Mesenchymal cell markers included CD49d, CD106, CD29 and HCAM. ADSCs, adipose tissue-derived stem cells; BMSCs, bone marrow-derived stem cells.
Figure 3.
ADSCs improve the survival of aGVHD rats. (A) Overall survival rates of aGVHD rats in the BM group, BM+spleen group and ADSCs+BM+spleen group. n=10. (B) Weight of the aGVHD model rats in the BM group, BM+spleen group and ADSCs+BM+spleen group. n=10. (C) Morphology of liver and small intestinal tissues of the aGVHD model rats in the BM group, BM+spleen group and ADSCs+BM+spleen group. Magnification, ×100. Arrows indicate portal vein of the liver, and triangles indicate small intestinal villi. ADSCs, adipose-derived stem cells; aGVHD, acute graft vs. host disease; BMMNCs, bone marrow mononuclear cells. Groups: BM, 2×108/kg bone marrow cells were infused; BM+spleen, 2×108/kg bone marrow cells + 3×108/kg spleen cells were infused; ADSCs+BM+spleen, 2×108/kg bone marrow cells + 3×108/kg spleen cells + 1×107/kg ADSCs were infused.
Infusion of ADSCs promotes hematopoietic reconstitution in the aGVHD rats
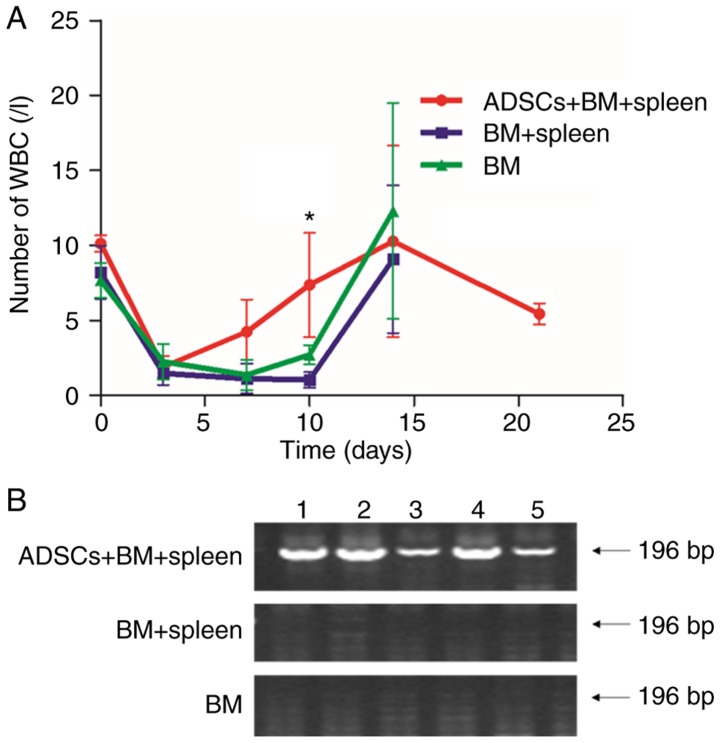
The number of leukocytes in the aGVHD models was observed to decrease from 9×109 to 1×109 g/l on day 3 after irradiation. On day 10 after transplantation, the number of leukocytes in rats that received BMMNCs and a mixture of BMMNCs and lymphocytes decreased to 2.7±0.65 and 1.03±0.51, respectively, being significantly lower than that in the ADSCs+BM+spleen group (7.36±3.47) (P<0.05) (Fig. 4A). To further trace the surviving ADSCs in the recipient rats, the expression of the Sry gene in the bone marrow was determined using RT-PCR. Expression of Sry was detected even on day 14 after transplantation (Fig. 4B). The results indicate that the surviving ADSCs participated in the hematopoietic reconstitution in the aGVHD rats.
Figure 4.
ADSCs promotes hematopoietic reconstitution. (A) Leukocyte count of aGVHD model rats in the BM group, BM+spleen group and ADSCs+BM+spleen group. n=10. *P<0.05. (B) Images of agarose gel electrophoresis of the Sry gene of male Wistar rat as detected by PCR. The numbers 1, 2, 3, 4, and 5 represent the five rats. The expression of the Sry gene was detected in the ADSC group, suggesting that rats in the ADSCs+BM+spleen group reached complete donor engraftment. ADSCs, adipose-derived stem cells; aGVHD, acute graft vs. host disease. Groups: BM, 2×108/kg bone marrow cells were infused; BM+spleen, 2×108/kg bone marrow cells + 3×108/kg spleen cells were infused; ADSCs+BM+spleen, 2×108/kg bone marrow cells + 3×108/kg spleen cells + 1×107/kg ADSCs were infused.
ADSCs decrease aGVHD severity by immunomodulation
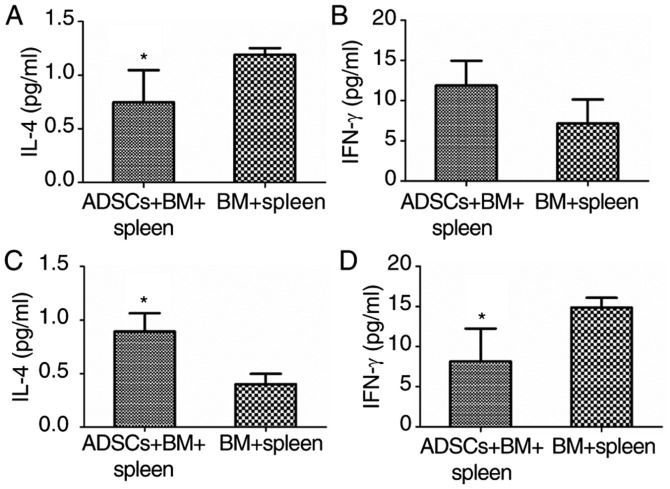
An imbalance in Th1 and Th2 cytokines is suggested to play an important role in the pathogenesis of aGVHD (11). IL-4 and INF-γ can influence the balance of Th1 and Th2. As determined by ELISA analysis, serum concentration of IL-4 in rats that received BMMNCs and spleen lymphocytes (BM+spleen) was 1.19±0.06 µg/ml on day 7. Administration of ADSCs significantly reduced IL-4 concentration to 0.75±0.3 µg/ml (P<0.05) (Fig. 5A), while the concentration of IFN-γ showed no significant change when ADSCs were transplanted on day 7 (Fig. 5B). However, on day 14 after infusion, transplantation of ADSCs resulted in significant increase in IL-4 concentration and a decrease in IFN-γ concentration (P<0.05) (Fig. 5C and D). The results suggest that ADSCs decrease aGVHD severity by immunomodulation.
Figure 5.
Immune-related factor expression during the restoration of aGVHD in rats. (A) IL-4 levels on day 7 (n=4, *P<0.05); (B) IFN-γ levels on day 7 (n=4, *P>0.05); (C) IL-4 levels on day 14 (n=3, *P<0.05) and (D) IFN-γ levels on day 14 (n=3, *P<0.05). ADSCs, adipose-derived stem cells; aGVHD, acute graft vs. host disease; IL-4, interleukin 4; INF-γ, interferon-γ. Groups: BM, 2×108/kg bone marrow cells were infused; BM+spleen, 2×108/kg bone marrow cells + 3×108/kg spleen cells were infused; ADSCs+BM+spleen, 2×108/kg bone marrow cells + 3×108/kg spleen cells + 1×107/kg ADSCs were infused.
ADSCs support the proliferation of hematopoietic stem/progenitor cells in vitro
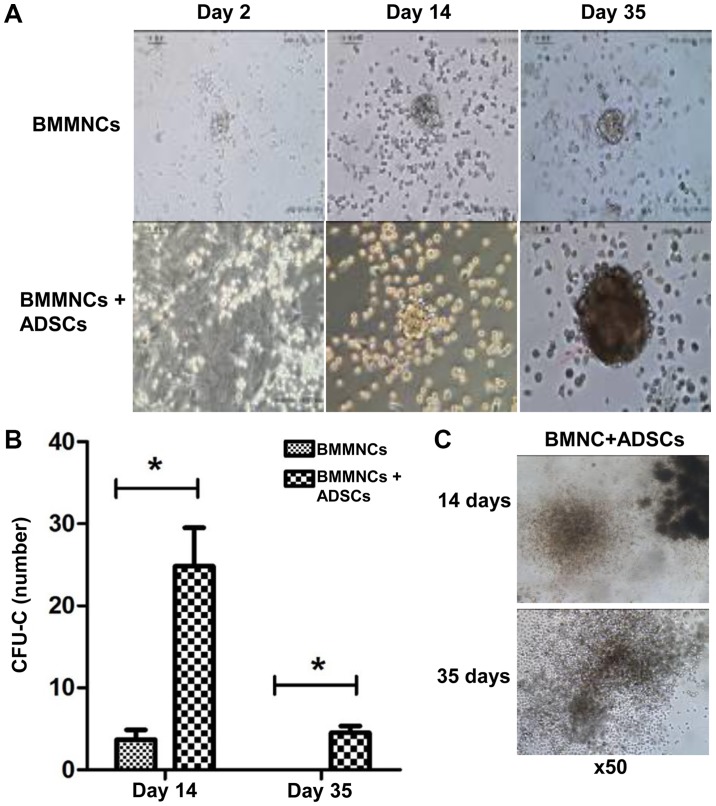
Within the duration of coculture, CFUs of hematopoietic stem/progenitor cells cultured with ADSCs were readily identified, with their sizes gradually growing larger. By contrast, few CFUs were observed when BMMNCs were cultured alone. On day 14 and 35, the number of CFUs of cells supported by ADSCs were significantly higher than that of cells cultured alone, respectively (P<0.05) (Fig. 6A and B). ADSCs were still able to promote the proliferation of BMMNCs on day 35 (Fig. 6C). The results indicate that ADSCs support the proliferation of hematopoietic stem/progenitor cells in vitro.
Figure 6.
Methylcellulose analysis exhibited colony-forming units after coculture of BMMNCs with ADSCs. (A) Changes in cell morphology at 2, 14 and 35 days. Magnification, ×100. (B) With prolonged time, methylcellulose analysis exhibited colony-forming units in the BMMNCs + ADSCs group on days 14 and 35. No colonies were visible in the BMMNCs group (*P<0.05, n=6). (C) Representative CFU-GM colonies from BMMNCs + ADSCs group after 14 and 35 days of coculture. Magnification, ×50. ADSCs, adipose-derived stem cells; BMMNCs, bone marrow mononuclear cells.
Discussion
Research concerning the mechanism of mesenchymal stem cells (MSCs) on hematopoietic support and immune regulation has mainly focused on bone marrow-derived mesenchymal stem cells (BMSCs) and umbilical cord-derived mesenchymal stem cells (UMSCs). Few studies have been conducted on the mechanism of adipose-derived stem cell (ADSC) immune regulation. Moreover, some studies have reported that BMSCs, ADSCs and UMSCs may have various differences in regards to the mechanisms involved in tissue repair, immune regulation and support of hematopoiesis (12–14). It has been documented that acute graft vs. host disease (aGVHD), the main cause of death after transplantation, can be relieved by infusing BMSCs (15). Te Boome et al (16) reported that an increase in circulating immature myeloid dendritic cells is associated with decreased mortality in patients with aGVHD treated with BMSCs in vivo. An increase in serum level of IL-2 and a lower IFN-γ to IL-4 ratio in BMSC-treated patients were observed by Jitschin et al (17). However, BMSCs have failed to significantly increase durable complete remission rates in steroid-refractory aGVHD patients in a phase III, multicenter, randomized controlled trial. Several factors, including MSC sources and manufacturing process, may account for the conflicting results (18). Compared to MSCs from bone marrow, ADSCs are more accessible, with a higher yield at harvest and rapid expansion in long-term expansion, being attractive in regenerative medicine (19–21). The immunological regulatory properties of ADSCs have been well documented. Previous studies (22,23) demonstrated that ADSCs inhibited the proliferation of T lymphocytes in a mixed lymphocyte reaction via the production of PGE2. Yañez et al (24) demonstrated the potential effect of ADSCs in alleviating aGVHD by the regulation of the levels of IFN-γ, IL-12 and TNF-α in vitro. However, whether transplantation of ADSCs reduces aGVHD in vivo remains unclear. In the present study, it was demonstrated the prophylactic effect of ADSCs on aGVHD with improved long-term hematopoiesis by modulating the balance of IL-4 and INF-γ in a rat model.
The experiments in the present study revealed that severe clinical manifestations of aGVHD are observed on day 14 after infusion of BM+spleen cells from male SD rats into lethally irradiated female Wistar rats. With the mixture of ADSCs to BM+spleen cells, it was discovered that aGVHD was significantly alleviated, and the long-term survival rate on day 35 was apparently improved after allogeneic hematopoietic stem cell transplantation. It has been well documented that disturbance in the imbalance between Th1 and Th2 cytokines plays an important role in the pathogenesis of aGVHD (25–27). Furthermore, the present study showed that the serum level of Th1 cytokine IFN-γ was not significantly different, while the serum level of Th2 cytokine IL-4 was lower at the early stage (day 7). However, IFN-γ was decreased and IL-4 was increased significantly at the late stage (day 14) after transplantation in the ADSC group compared with the control group. As the current rat model exhibited aGVHD on day 14, it was speculated that ADSCs participate in the immune regulation and alleviate aGVHD symptoms after transplantation.
The most innovative finding of the present study was the long-term support of ADSCs on hematopoiesis in vivo and in vitro. In particular, in vitro hematopoietic support for 7 weeks was significantly better than that of BMSCs, which has not been previously reported. In the present study, it was demonstrated that the leukocyte number was reconstituted faster, and Sry gene expression was only detected in the ADSC group on day 21, suggesting that ADSCs strongly promote the implantation of the donor hematopoietic stem cells in recipients in vivo. de Barros et al (28) plated CD34+ cells onto spheroid BMSCs and co-cultured them for up to 7 days, and found that BMSCs self-assembled in a 3-dimensional spheroid and formed a microenvironment that was informative for hematopoietic progenitor cells. Arthur et al (29) reported that Twist-1-overexpressing BMSCs exhibited an enhanced capacity in maintaining human CD34+ hematopoietic stem cells (HSCs) in long-term culture-initiating cell (LTC-IC) assays. It was also confirmed that MSCs support the proliferation of hematopoietic stem/progenitor cells in vitro by coculture of human umbilical cord blood mononuclear cells and umbilical cord mesenchymal stem cells for 14 days (30). Few studies have demonstrated that ADSCs support the long-term proliferation of hematopoietic stem/progenitor cells. A previous study (31) confirmed that ADSCs isolated from inguen differentiate into osteoblast cells and adipose cells in vitro. The present study found that ADSCs had similar effects to BMSCs in the support of survival and proliferation of hematopoietic stem/progenitor cells in short-term coculture (<14 days) in vitro. Moreover, the present study also showed that ADSCs support the survival and proliferation of hematopoietic stem/progenitor cells in long-term coculture (35 days), and the partial hematopoietic CFU diameter in the ADSC group was greater than that in the BMSC group, although the CFU counts were similar in both groups. Therefore, the present study demonstrated that ADSCs may also have long-term supportive function on hematopoiesis in vitro. In conclusion, the present study demonstrated preliminarily that rat ADSCs regulated immune responses and reduced the occurrence of aGVHD in a rat allo-HSCT model, mainly through Th1/Th2 cytokine immune adjustment mode. The present study verifies that rat ADSCs can promote the long-term proliferation of hematopoietic stem cells in vitro and prolong survival after bone marrow transplantation in vivo. Further in-depth research concerning these features of ADSCs will be carried out in the future.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- ADSCs
adipose-derived stem cells
- aGVHD
acute graft vs. host disease
- BMSCs
bone marrow-derived stem cells
- MSCs
mesenchymal stem cells
- SD
Sprague-Dawley
- BMMNCs
bone marrow mononuclear cells
- HSCs
hematopoietic stem cells
- IL-4
interleukin 4
- INF-γ
interferon-γ
Funding
The study was supported by the National Natural Science Foundation of China (no. 81460023) and Natural Science Foundation of Xinjiang Uygur Autonomous Region (no. 200821113).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MJ and LC designed and directed the experiment. XB, HW and RZ performed the experiments. XD, NP and HY collected the data and performed the statistical analysis. XB wrote the manuscript. LC and XD reviewed and edited the manuscript. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
Ethical approval for the study was granted from the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (Urumqi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Piemontese S, Ciceri F, Labopin M, Bacigalupo A, Huang H, Santarone S, Gorin NC, Koc Y, Wu D, Beelen D, et al. A survey on unmanipulated haploidentical hematopoietic stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29:1069–1075. doi: 10.1038/leu.2014.336. [DOI] [PubMed] [Google Scholar]
- 2.Murray IR, West CC, Hardy WR, James AW, Park TS, Nguyen A, Tawonsawatruk T, Lazzari L, Soo C, Péault B. Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cell Mol Life Sci. 2014;71:1353–1374. doi: 10.1007/s00018-013-1462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 4.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 5.Mirlashari MJ, Josefsen D, Landsverk K, Hasvold G, Gullestad H, Kvalheim G. Culturing of human adipose derived mesenchymal stem cells (ADSCS) under hypoxic conditions affects production of wound healing cytokines and growth factors. Cytotherapy. 2014;16:S93. doi: 10.1016/j.jcyt.2014.01.346. [DOI] [Google Scholar]
- 6.Institute of Laboratory Animal Resources (US). Committee on Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. US Department of Health and Human Services, Public Health Service, National Institutes of Health. 1986 [Google Scholar]
- 7.Frazier K, Williams S, Kothapalli D, Klapper H, Grotendorst GR. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Dermatol. 1996;107:404–411. doi: 10.1111/1523-1747.ep12363389. [DOI] [PubMed] [Google Scholar]
- 8.Hu J, Yan M, Jiang M, Shi W, Xu Y, Zhang D, Li J. Establishment of a rat model of acute graft-versus-host disease after allogeneic bone marrow transplantation. Chin J Com Med. 2007;17:581–584. [Google Scholar]
- 9.Vogelsang GB, Hess AD, Gordon G, Santos GW. Treatment and prevention of acute graft-versus-host disease with thalidomide in a rat model. Transplantation. 1986;41:644–647. doi: 10.1097/00007890-198605000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Renkonen R, Häyry P. Bone marrow transplantation in the rat. I. Histologic correlations and quantitation of cellular infiltrates in acute graft-versus-host disease. Am J Pathol. 1984;117:462–470. [PMC free article] [PubMed] [Google Scholar]
- 11.Ju XP, Xu B, Xiao ZP, Li JY, Chen L, Lu SQ, Huang ZX. Cytokine expression during acute graft-versus-host disease after allogeneic peripheral stem cell transplantation. Bone Marrow Transplant. 2005;35:1179–1186. doi: 10.1038/sj.bmt.1704972. [DOI] [PubMed] [Google Scholar]
- 12.Dong Y, Zhu T, Xia R, Li Y, Ge X, Zeng Q, Ni J, Li Q. Mechanism of bone marrow mesenchymal stem cells for treating model mice with aplastic anemia. J Clin Rehabil Tissue Eng Res. 2009;13:7138–7142. [Google Scholar]
- 13.Wu KH, Tsai C, Wu HP, Sieber M, Peng CT, Chao YH. Human application of ex vivo expanded umbilical cord-derived mesenchymal stem cells: Enhance hematopoiesis after cord blood transplantation. Cell Transplant. 2013;22:2041–2051. doi: 10.3727/096368912X663533. [DOI] [PubMed] [Google Scholar]
- 14.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 15.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 16.Te Boome LC, Mansilla C, van der Wagen LE, Lindemans CA, Petersen EJ, Spierings E, Thus KA, Westinga K, Plantinga M, Bierings M, et al. Biomarker profiling of steroid-resistant acute GVHD in patients after infusion of mesenchymal stromal cells. Leukemia. 2015;29:1839–1846. doi: 10.1038/leu.2015.89. [DOI] [PubMed] [Google Scholar]
- 17.Jitschin R, Mougiakakos D, Von Bahr L, Völkl S, Moll G, Ringden O, Kiessling R, Linder S, Le Blanc K. Alterations in the cellular immune compartment of patients treated with third-party mesenchymal stromal cells following allogeneic hematopoietic stem cell transplantation. Stem Cells. 2013;31:1715–1725. doi: 10.1002/stem.1386. [DOI] [PubMed] [Google Scholar]
- 18.Galipeau J. The mesenchymal stromal cells dilemma-does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy. 2013;15:2–8. doi: 10.1016/j.jcyt.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Res Ther. 2017;8:145. doi: 10.1186/s13287-017-0598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schäffler A, Büchler C. Concise review: Adipose tissue-derived stromal cells-basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Zhu Y, Li Y, Cao J, Zhang H, Chen M, Wang L, Zhang C. Long-term engraftment of myogenic progenitors from adipose-derived stem cells and muscle regeneration in dystrophic mice. Hum Mol Genet. 2015;24:6029–6040. doi: 10.1093/hmg/ddv316. [DOI] [PubMed] [Google Scholar]
- 22.Bai W, Jiang M, Cui L, Yi S. An experimental study for the immunological properties of human adipose-derived stem cells after expansion. J Tissue Eng Reconstr Sur. 2008;4:312–314. [Google Scholar]
- 23.Cui L, Yin S, Liu W, Li N, Zhang W, Cao Y. Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng. 2007;13:1185–1195. doi: 10.1089/ten.2006.0315. [DOI] [PubMed] [Google Scholar]
- 24.Yañez R, Lamana ML, García-Castro J, Colmenero I, Ramírez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–2591. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 25.Yao Y, Song X, Cheng H, Tang G, Hu X, Zhou H, Wang J. Dysfunction of bone marrow vascular niche in acute graft-versus-host disease after MHC-haploidentical bone marrow transplantation. PLoS One. 2014;9:e104607. doi: 10.1371/journal.pone.0104607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nasef A, Chapel A, Mazurier C, Bouchet S, Lopez M, Mathieu N, Sensebé L, Zhang Y, Gorin NC, Thierry D, Fouillard L. Identification of IL-10 and TGF-beta transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expr. 2007;13:217–226. doi: 10.3727/000000006780666957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Najar M, Rouas R, Raicevic G, Boufker HI, Lewalle P, Meuleman N, Bron D, Toungouz M, Martiat P, Lagneaux L. Mesenchymal stromal cells promote or suppress the proliferation of T lymphocytes from cord blood and peripheral blood: The importance of low cell ratio and role of interleukin-6. Cytotherapy. 2009;11:570–583. doi: 10.1080/14653240903079377. [DOI] [PubMed] [Google Scholar]
- 28.de Barros AP, Takiya CM, Garzoni LR, Leal-Ferreira ML, Dutra HS, Chiarini LB, Meirelles MN, Borojevic R, Rossi MI. Osteoblasts and bone marrow mesenchymal stromal cells control hematopoietic stem cell migration and proliferation in 3D in vitro model. PLoS One. 2010;5:e9093. doi: 10.1371/journal.pone.0009093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arthur A, Cakouros D, Cooper L, Nguyen T, Isenmann S, Zannettino AC, Glackin CA, Gronthos S. Twist-1 enhances bone marrow mesenchymal stromal cell support of hematopoiesis by modulating CXCL12 expression. Stem Cells. 2016;34:504–509. doi: 10.1002/stem.2265. [DOI] [PubMed] [Google Scholar]
- 30.Sun HP, Zhang X, Chen XH, Zhang C, Gao L, Feng YM, Peng XG, Gao L. Human umbilical cord blood-derived stromal cells are superior to human umbilical cord blood-derived mesenchymal stem cells in inducing myeloid lineage differentiation in vitro. Stem Cells Dev. 2012;21:1429–1440. doi: 10.1089/scd.2011.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang R, Bi X, Ma Y, Duan X, Jiang M. Biological characterization of C57 mouse bone marrow mesenchymal stem cells using a whole bone marrow adherent culture technique. Chin J Tissue Eng Res. 2014;18:45–50. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.