Abstract
Objectives:
Deep vein thrombosis (DVT) is a major health-care burden in Europe, but exact estimates are lacking. This study reports results from the PREFER venous thromboembolism (VTE) study concerning health-related quality of life (HrQoL) and mortality of patients with DVT.
Methods:
PREFER VTE was a prospective, observational study, conducted in 7 European countries, designed to provide data concerning treatment patterns, resource utilization, mortality, and QoL. First-time or recurrent patients with DVT were followed at 1, 3, 6, and 12 months. Health-related QoL—as measured by the EuroQoL 5-Dimension 5-Level instrument ( EQ-5D-5L)—was analyzed using Tobit regression with repeated measures, assessing the impact of baseline characteristics stratified by cancer activity. Mortality was analyzed using logistic regression.
Results:
At baseline, patients with DVT had a 0.14 lower EQ-5D-5L index score (0.72 for total sample) compared to the reference UK population (0.85). The EQ-5D-5L index score improved from baseline to 12 months in patients with active cancer (from 0.70 to 0.79) and those without (0.72-0.87); 7.3% died within a year, a 5.2% excess mortality compared to the age- and gender-adfjusted general population. The 12-month mortality rate of DVT varied between 2.9% in the pooled data from Germany, Switzerland, or Austria and 15.4% in Italy. Furthermore, the mortality rate differed between patients with active cancer and those without (42.9% vs 4.7%).
Conclusions:
Deep vein thrombosis is associated with a substantial burden of illness in terms of HrQoL at baseline, which following treatment normalizes after 12 months and has a significant mortality rate. In addition, active cancer has a significant impact on mortality and the HrQoL of patients with DVT.
Keywords: deep vein thrombosis, acute venous thromboembolism, health-related quality of life, mortality, PREFER
Introduction
Acute venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common disorder, with an annual incidence of approximately 1 or 2 cases per 1000 persons.1–3 Patients with VTE have increased morbidity and mortality related not only to VTE but also to cancer, surgical procedures, and other medical conditions.4 Moreover, the burden of VTE may well be underestimated.3
In 2005, the UK Health Select Committee estimated that hospital-acquired VTE alone is responsible for between 25 000 and 32 000 deaths annually in the United Kingdom.5 Given the presence of many comorbidities, the number of potential preventable deaths are uncertain but likely to be substantial. The burden of VTE includes impaired quality of life (QoL), additional costs of treatment, and potentially indirect costs due to days lost from work. As such, effective treatment and prevention is imperative. Current guidelines support the use of anticoagulants that decrease mortality and recurrence rates but are also associated with an increased risk of bleeding that is associated with its own burden. Additionally, traditional treatment may require frequent international normalized ratio measurements often by specialized outpatient services.
An epidemiologic modeling study from 2007 offers valuable insights in the burden of VTE in Europe.3 This study estimated an annual number of symptomatic DVT events of 684 000. The total costs of VTE were estimated at €3 billion per year. To supplement these findings, real-life observational data are worthwhile. The PREFER VTE registry was partly set up to offer such data. This study—as described in Agnelli et al6—was established to assess the real-life acute and mid-term management of patients with VTE, the use of health-care resources, and to provide data to estimate the costs for 12 months treatment following a first-time and/or recurrent VTE diagnosis in hospitals or specialized centers in Europe. With this registry, data were collected about clinical outcomes, treatment satisfaction, and health-related quality of life (HrQoL) as measured by the EuroQoL 5-Dimension 5-Level instrument (EQ-5D-5L). This manuscript aims to offer supplementary insights into the burden of illness for patients with DVT from a European perspective concentrating on mortality and HrQoL. Within this scope, differences per country and differences between patients with and without active cancer are reported and patient characteristics associated with increased mortality and/or morbidity are identified. A separate publication is available dealing with health-care resource use and productivity loss.7
Data and Methods
Setting and Study Population
The PREFER VTE registry was a prospective, observational, multicenter study with a follow-up of 12 months and 3545 consecutive enrolled patients from 381 centers (311 active centers, ie, centers that enrolled at least 1 patient) in 7 European countries (Austria, France, Germany, Italy, Spain, Switzerland, and the United Kingdom) between January 2013 and July 2014. Prior to study commencement, the registry protocol was approved by the responsible ethics committees for the participating countries and the relevant hospital-based institutional review boards. All patients enrolled in the registry provided written informed consent. The outline of the study has been previously described.6 Briefly, patients were eligible to be enrolled into the registry if they were at least 18 years, had a symptomatic, and objectively confirmed first-time or recurrent acute VTE (the index event) defined as either distal or proximal DVT or PE or both. Patients were recruited within 2 weeks of the index event and no exclusion criteria applied. At baseline, patients were assessed through a face-to-face interview in terms of their demography, disease, previous clinical events, risk factors, comorbidities, and presented PE/DVT symptoms, as well as previous treatments. At 1, 3, 6, and 12 months, information regarding the occurrence of clinical event, treatment, resource utilization, HrQoL, and treatment satisfaction during each follow-up interval was measured. The current study concerned patients with DVT, of which 2056 were recruited.
Data Quality Control
The validity of the data entered into the database was assured by training investigators on data collection ensuring a uniform method. Furthermore, a random audit was performed on the centers included in the registry. During these visits, the monitor verified informed consent documentation, performed source data verification against patient’s medical records, and controlled consecutiveness of patient enrolment at site. The data collection comprised 2 different sources. These sources included the hospitals or specialized centers at the time of diagnosis of acute VTE and, as hospital-based investigators do not always see patients in the following year for routine clinical care, patients were also asked to participate in follow-ups by phone, safeguarding the collection of resource consumption data. Information was collected directly from patients during standardized phone calls at 1, 3, 6, and 12 months after baseline. The data entered in the database were checked electronically for completeness and plausibility at the time of data entry and additional validation was performed on data sets.
Analyses and Statistics
Descriptive statistics of baseline measurements were provided, including demographic (age, gender, body mass index [BMI], marital status, and country), clinical factors (with previous VTE event, distal vs proximal, and [un]provoked; note 1), previous clinical event (within 3 years prior to enrollment: myocardial infarction, coronary heart disease, percutaneous coronary intervention, atrial fibrillation, transient ischemic attack, stroke, or bleeding event), risk factors (within past 3 months or ongoing: active cancer, prolonged immobilization, >5 days in bed, varicose veins, history of major surgery or trauma), comorbidities (hypertension, congestive heart failure, vascular disease, dyslipidemia, diabetes, chronic venous insufficiency, renal disease, liver disease, chronic respiratory disease, arthritis, lower extremity paralysis, bone fracture/soft-tissue trauma, alcohol use, smoking history, thrombophilia, and cardiovascular disease), and the presence of DVT symptoms. Treatment at baseline and use of heparin, vitamin K antagonist (VKA), or direct oral anticoagulants (DOACs) are also tabulated. Patients receiving ongoing treatment for cancer are labeled as “active cancer” patients. Country-specific differences are analyzed for which Austria, Switzerland, and Germany are combined into one prespecified region label (DACH). The DACH countries were grouped as patient population, practice patterns and health-care systems were assumed to be similar, and the number of sites for Germany, Austria, and Switzerland were, respectively, 74, 5, and 3. Further details are described elsewhere.8
Health-related quality of life
The analysis of HrQoL was performed using the EQ-5D-5L.9 The EQ-5D-5L data were collected through face-to-face interview at baseline and by telephone interview during the follow-up. The EQ-5D has 5 dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Within each dimension, one may have no problems, slight problems, moderate problems, severe problems, or extreme problems. Based on the individual response to the descriptive system (5 dimensions and their responses), a health state can be defined (eg, 11111 or 21112). For each health state, an index score is assigned, which represents social preference toward each state. The distribution of responses of each dimension and EQ-5D-5L index score at baseline and each follow-up were presented by country and cancer subgroups. The population reference values of EQ-5D-5L index scores, based on the UK EQ-5D-5L valuation study,10 were compared to the DVT population. Subsequently, the association between baseline characteristics and the EQ-5D index score was assessed using a Tobit regression model with repeated measures. The baseline characteristics included age, gender, BMI, previous clinical events, clinical factors, comorbidities, and risk factors. Symptoms at baseline, which were significantly related to HrQoL, were excluded from the analysis. There is an upper limit of one in the EQ-5D-5L index score, and up to 5 assessments for any one patient were made over the 1-year follow-up. As a sensitivity analysis, the impact of VTE recurrence and bleeding events during follow-up and the impact of treatment on the EQ-5D-5L index score were tested.
Mortality
Following enrolment in the registry, mortality data were collected at baseline and at each follow-up through a standardized questionnaire completed by the investigator. The primary outcomes were 12-month mortality rates by country, for patients with and without active cancer. The observed DVT patient mortality rates were compared with the age- and gender-matched mortality rate from the UK general population.11 Separate logistic regression models were used to identify which individual baseline characteristics and DVT symptoms were significantly associated with mortality in both groups after backward stepwise elimination. Additionally, logistic regression models were run after adding the HrQoL data at baseline (EQ-5D-5L index score) as an explanatory variable for survival. Sensitivity analyses addressed differences between countries. Treatment type was not included in the analyses as this was highly correlated with country, the DOACs were not reimbursed in Italy and Spain at the time of the data collection.
Missing data
There was missing information due to lost follow-up (death or incomplete data), at each follow-up. No imputation was conducted for any missing value in the main analysis. However, the difference in baseline characteristics between patients who completed the follow-ups and those who did not were tested, using χ2, rank-sum test, or t test where appropriate. Furthermore, a sensitivity analysis, using the multiple imputation technique to generate values for missing data, was conducted to evaluate the potential impact of missing data. Baseline characteristics and available index scores at different time points were used for imputing missing data.
Results
Patients Characteristics
Among 2056 patients with DVT at baseline (Table 1), mean age was 59.8 and 52.9% were male; 30.3% of patients were from DACH, followed by Italy 28.0%, the United Kingdom 18.2%, Spain 12.0%, and France 11.6%. One-quarter of patients with DVT had previous VTE events, 71.3% were proximal (28.7% were distal DVT), and 27.5% were provoked DVT. Deep vein thrombosis can be provoked (defined by having prolong immobilization, >5 days in bed, or history of major surgery or trauma)—or unprovoked, but accompanied with comorbidities. Of the provoked patients, 5.1% had a known thrombophilia condition at baseline, while this percentage was 8.5% in patients without provoked DVT. The most common comorbidity among the patients with DVT was hypertension (39.7%). One-third (30.3%) of patients had a smoking history. Commonly reported DVT symptoms included pain (82.8%) and swelling (73.4%). In 8.5% of patients, active cancer was diagnosed. Most patients received heparin at baseline (64.9%), followed by VKAs (42.7%). Only 26.8% of patients used DOACs (mostly in France and DACH). Detailed baseline characteristics per country are reported elsewhere.7 Patients with active cancer were significantly older and had a higher proportion of liver disease and history of major surgery or trauma. Patients with active cancer had fewer previous VTE events and reported less alcohol use, cardiovascular disease, prolonged immobilization, and varicose veins. The percent of patients with active cancer differed substantially between countries. In DACH, Spain, and the United Kingdom, less than 5% of the included patients had active cancer, in France 12.2% and in Italy 15.8% of the patients who were included had active cancer. At baseline, patients with active cancer were most often treated with heparin and significantly less (than those without active cancer) with VKAs or DOACs. Direct oral anticoagulants were infrequently used in Italy and Spain at the time of the data collection.7
Table 1.
Patients’ Characteristics at Baseline.
| Active Cancer | Active Cancer | ||||||
|---|---|---|---|---|---|---|---|
| Baseline, % | Total N = 2056 | With, N = 174 | Without, N = 1882 | Baseline, % | Total N = 2056 | With, N = 174 | Without, N = 1882 |
| Age, years, mean (SD) | 59.8 (16.8) | 59.1 (17.0) | 67.9 (11.9)a | ||||
| Male | 52.9 | 52.7 | 55.8 | Comorbidities | |||
| BMI, mean (SD) | 27.8 (5.3) | 27.9 (5.3) | 26.4 (5.1) | Active cancer | 84.6 | 100.0 | 0.0 |
| Highest graduation | Hypertension | 39.7 | 39.5 | 41.4 | |||
| Primary school | 26.7 | 25.6 | 38.5 | Congestive heart failure | 2.8 | 2.8 | 3.5 |
| Secondary school | 47.7 | 48.0 | 44.3 | Vascular disease | 5.5 | 5.3 | 7.5 |
| Above | 20.9 | 21.4 | 15.5 | Dyslipidemia | 17.5 | 17.5 | 17.8 |
| Marital status | Diabetes | 9.7 | 9.5 | 12.6 | |||
| Single | 13.8 | 15.6 | 6.3 | Chronic venous insufficiency | 16.6 | 17.1 | 10.9 |
| Married/living as married | 65.7 | 64.8 | 75.3 | Renal disease | 6 | 5.8 | 8.7 |
| Separated/divorced | 5.7 | 6.0 | 3.5 | Liver disease | 2.6 | 1.9 | 9.8a |
| Widowed | 10.8 | 10.5 | 13.2 | Chronic respiratory disease | 7.6 | 7.7 | 6.3 |
| Other | 1.1 | 1.1 | 1.2 | Arthritis | 8.8 | 9.1 | 5.2 |
| Country | Bone fracture/soft-tissue trauma | 12.4 | 13.0 | 6.3a | |||
| France | 11.6 | 11.1 | 16.7a | Lower extremity paralysis | 1.2 | 1.2 | 1.2 |
| DACH | 30.3 | 31.8 | 14.4 | Thrombophilia | 7.5 | 7.9 | 4.0 |
| Italy | 28.0 | 25.7 | 52.3a | Cardiovascular disease | 12.9 | 12.6 | 15.5 |
| Spain | 12.0 | 12.4 | 6.9 | ||||
| The United Kingdom | 18.2 | 19.0 | 9.8 | Risk factors (within past 3 months or ongoing) | |||
| Previous clinical event (within 3 years prior to enrollment) | Alcohol use | 18.4 | 19.4 | 7.5a | |||
| Myocardial infarction | 3.1 | 2.9 | 4.6 | Smoking history | 30.3 | 30.5 | 27.8 |
| Coronary artery disease | 3.8 | 3.6 | 5.8 | Prolong immobilization | 15.9 | 16.2 | 12.1a |
| Percutaneous coronary intervention | 1.8 | 1.7 | 2.9 | >5 days in bed | 9.9 | 9.6 | 13.9 |
| Atrial fibrillation | 2.6 | 2.5 | 4.1 | Varicose veins | 22.1 | 22.8 | 14.9a |
| Transient ischemic attack | 2 | 2.0 | 2.3 | Major surgery or trauma | 14.4 | 14.1 | 17.2a |
| Stroke | 2.2 | 2.2 | 2.3 | DVT symptoms present | |||
| Bleeding event | 3.6 | 3.3 | 6.9 | Pain | 82.8 | 83.9 | 71.3 |
| Clinical factors | Discoloration | 16.2 | 16.2 | 16.1 | |||
| Previous VTE event | 25.7 | 26.8 | 13.8a | Calf tenderness | 29.4 | 29.6 | 27.0 |
| Proximal | 71.3 | 71.2 | 71.8 | Swelling | 73.4 | 73.2 | 75.3 |
| Provoked | 27.5 | 27.4 | 27.8 | Collateral superficial veins | 7.3 | 7.2 | 8.1 |
| Baseline treatment | Other | 5.8 | 5.5 | 9.2 | |||
| Use of heparin | 64.9 | 83.9 | 63.1a | ||||
| Use of VKA | 42.7 | 17.8 | 45.0a | ||||
| Use of DOACs | 26.8 | 5.7 | 28.7a | ||||
Abbreviations: DACH, Austria, Switzerland, and Germany; DOAC, direct oral anticoagulants; DVT, deep vein thrombosis; SD, standard deviation; VKA, vitamin K antagonists; VTE: venous thromboembolism.
a Difference between the groups with/without active cancer reached statically significant level at P < .05.
Health-Related Quality of Life
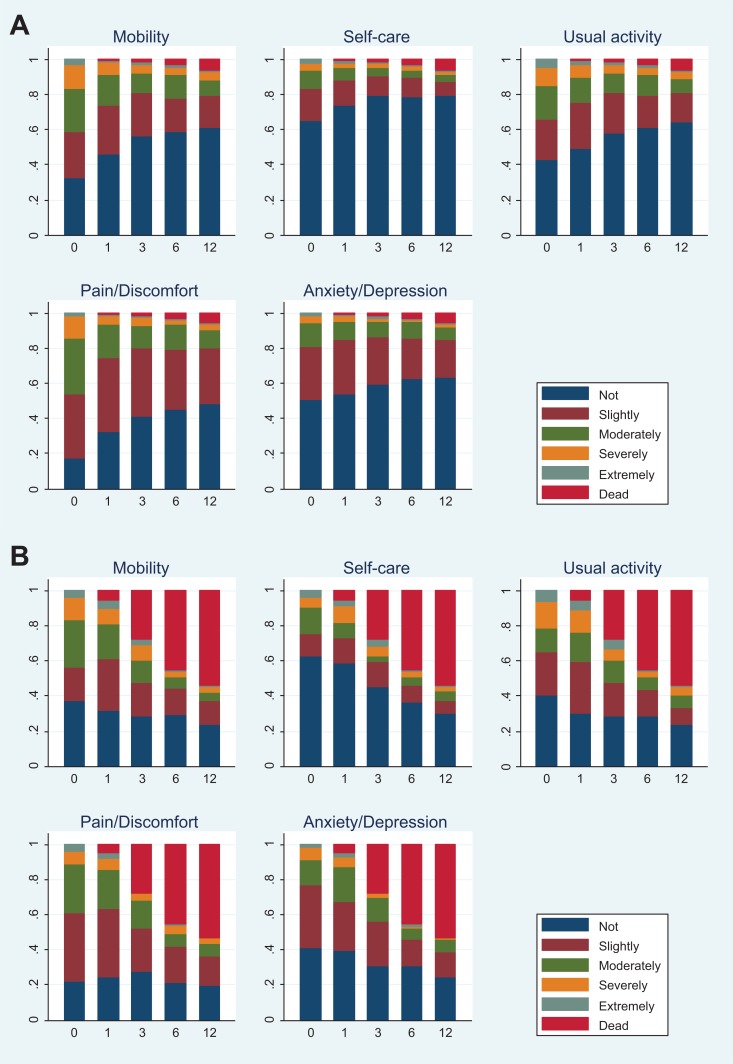
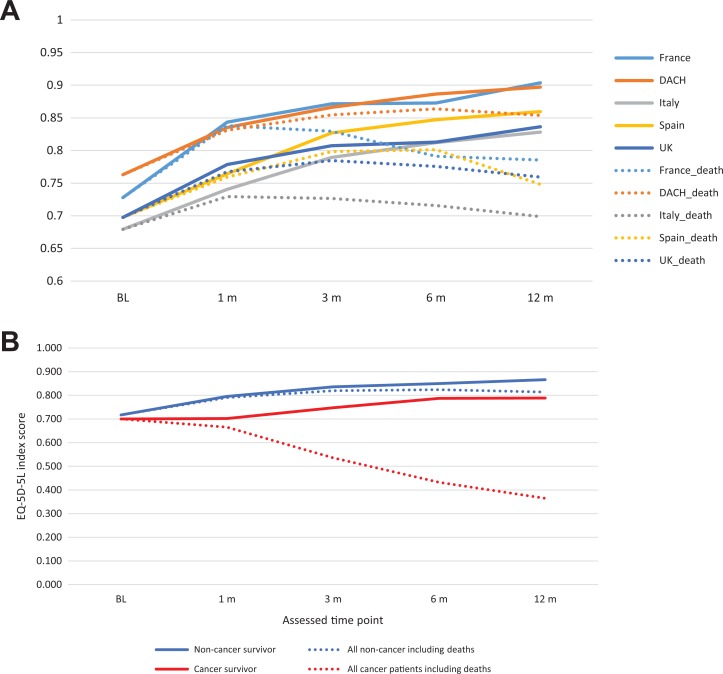
At baseline, 1921 patients completed the HrQoL questionnaire, 1366 at 1 month, 1163 at 3 months, 1013 at 6 months, and 847 patients completed the HrQoL questionnaire at 12 months. Figure 1 presents the distribution of each dimension of EQ-5D-5L and death at baseline and each follow-up for patients with active cancer and those without. Among 5 assessment time points, baseline had the highest proportion of patients reporting problems for each dimension. Furthermore, among the 5 dimensions, self-care was the dimension where patients reported fewest problems, while pain/discomfort had the highest proportion of reported problems. In patients without active cancer, trends showing less problems or indicating less severity of problems over time were seen across dimensions. Findings were similar for patients with active cancer, although less obvious due to the high mortality rate. The mean EQ-5D-5L index score moved from 0.717 at baseline to 0.866 at month 12 in patients without active cancer, whereas in patients with active cancer, the mean index score changed from 0.700 to 0.788 over this time, showing a small increase in EQ-5D-5L index score in both groups (Figure 2). The index score at month 12 of patients without active cancer is similar to that of the average UK population, which based on a recent UK valuation study can be estimated at 0.852.10 When death was scored as 0, a substantial drop in index score over time was observed in patients with active cancer. This is explained by a higher proportion of death occurring in the cancer group.
Figure 1.
A, Distribution of EQ-5D-5L domains and death at baseline and follow-up for patients without active cancer. B, Distribution of EQ-5D-5L domains and death at baseline and follow-up for patients with active cancer.
Figure 2.
A, EQ-5D-5L index score at baseline and follow-up by country. B, EQ-5D-5L index score at baseline and follow-up by patients with and without active cancer.
The Tobit repeated-measures regression on EQ-5D-5L index scores for patients without active cancer, excluding the presence of bleeds and recurrent VTE events, showed significant effects for a selection of factors (Table 2). Male gender and thrombophilia were associated with a higher EQ-5D-5L index score, suggesting a protective effect. Adding a recurrent VTE event and bleed as predictors in the model showed these events have no significant impact on EQ-5D-5L index score. Thus, although the effect of the events on index score is negative, having additional events on top of the index event does not appear to decrease HrQoL in a statistically significant magnitude. When repeating the above regression analyses and adding country as a predictor (the United Kingdom as the reference country), patients from France, DACH, or Italy without active cancer were associated with a significantly higher EQ-5D-5L index score. In other words, after correction for baseline and clinical characteristics (ie, patient case mix), significant differences between countries remain. For patients with active cancer, age, prolonged immobilization and bone fracture/soft-tissue trauma were associated with a lower EQ-5D-5L index score, while male gender and previous VTE events were associated with a higher EQ-5D-5L index score. Country effects were not present for patients with active cancer.
Table 2.
Tobit Regression on EQ-5D-5L Index Score.
| Excluding Recurrent VTE /Bleeding Event (n = 1695, obs = 5638) | Including Recurrent VTE /Bleeding Event (n = 1695, obs = 5365) | |||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | Standard Error | P > z | 95% Confidence Interval | Coefficient | Standard Error | P > z | 95% Confidence Interval | |
| Patient without active cancer | ||||||||
| Constant | 0.982 | 0.036 | 0 | 0.911 to 1.052 | 0.987 | 0.036 | 0 | 0.916 to 1.058 |
| Age | −0.001 | 0.000 | .009 | −0.002 to 0.000 | −0.001 | 0.000 | .007 | −0.002 to 0.000 |
| Male | 0.082 | 0.011 | 0 | 0.061 to 0.103 | 0.080 | 0.011 | 0 | 0.059 to 0.102 |
| BMI | −0.003 | 0.001 | .009 | −0.005 to −0.001 | −0.003 | 0.001 | .009 | −0.005 to −0.001 |
| Previous stroke | −0.098 | 0.038 | .011 | −0.173 to −0.022 | −0.099 | 0.039 | .011 | −0.175 to −0.023 |
| Previous bleed events | −0.070 | 0.030 | .019 | −0.129 to −0.011 | −0.071 | 0.030 | .02 | −0.130 to −0.011 |
| Prolonged immobilization | −0.036 | 0.017 | .031 | −0.068 to −0.003 | −0.034 | 0.017 | .04 | −0.067 to −0.002 |
| >5 days in bed | −0.104 | 0.021 | 0 | −0.145 to −0.063 | −0.100 | 0.021 | 0 | −0.142 to −0.059 |
| Lower extremity paralysis | −0.227 | 0.052 | 0 | −0.330 to −0.124 | −0.229 | 0.053 | 0 | −0.332 to −0.125 |
| Congestive heart failure | −0.114 | 0.034 | .001 | −0.181 to −0.048 | −0.109 | 0.035 | .002 | −0.176 to −0.041 |
| Chronic respiratory disease | −0.055 | 0.021 | .007 | −0.096 to −0.015 | −0.057 | 0.021 | .006 | −0.098 to −0.016 |
| Arthritis | −0.084 | 0.019 | 0 | −0.121 to −0.047 | −0.083 | 0.019 | 0 | −0.120 to −0.046 |
| Bone fracture/soft-tissue trauma | −0.075 | 0.016 | 0 | −0.106 to −0.043 | −0.074 | 0.016 | 0 | −0.106 to −0.042 |
| Smoking history | −0.031 | 0.012 | .009 | −0.054 to −0.008 | −0.033 | 0.012 | .006 | −0.057 to −0.010 |
| Thrombophilia | 0.048 | 0.019 | .014 | 0.010 to 0.086 | 0.047 | 0.020 | .016 | 0.009 to 0.086 |
| Proximal VTE event | −0.048 | 0.012 | 0 | −0.072 to −0.024 | −0.048 | 0.012 | 0 | −0.072 to −0.024 |
| Recurrent VTE event during follow-up | Not included | −0.008 | 0.032 | .811 | −0.070 to 0.055 | |||
| Bleeding event during follow-up | Not included | −0.013 | 0.027 | .634 | −0.065 to 0.040 | |||
Abbreviations: BMI, body mass index; Obs.: number of observations of index scores (per patient could contribute multiple, up to 5, observations); VTE, venous thromboembolism.
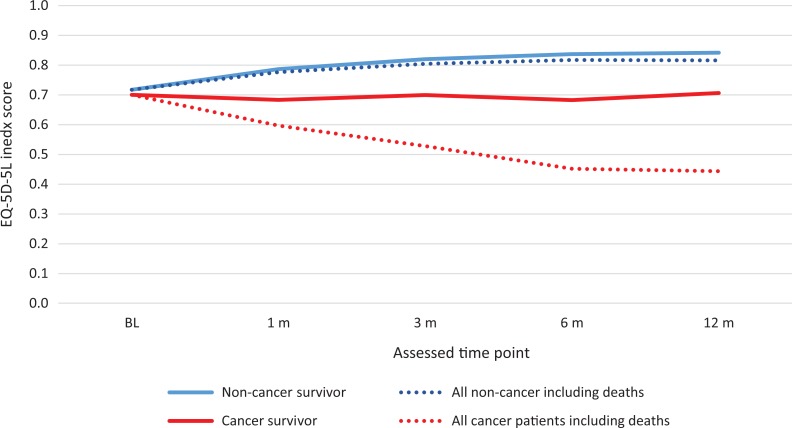
The results of sensitivity analyses, addressing potential selection bias, were mostly aligned with the main results. As shown in Appendix Figure 1, after the imputation, the trend of improvement over time was less obvious compared to that in the main analysis (without imputation, Figure 2B). The same set of significant covariates was observed in the Tobit regression using the imputed data.
Missing Data
Patients who did not complete EQ-5D-5L at 12 months follow-up were younger, mostly British, and with less dyslipidemia, chronic venous insufficiency, and thrombophilia, but more smoking history.
Mortality
The mortality rate at 12 months for patients with DVT was 7.3% (128/1766 patients with mortality information). The mortality rate differed among countries, 6.7% (14/210) in France, 2.9% (16/554) in DACH, 15.4% (75/487) in Italy, 6.1% (14/228) in Spain, and 3.1% (9/287) in the United Kingdom (P value ≤.0001). Moreover, there was a clear distinction between cancer subgroup, as 43.0% with active cancer and 3.8% without active cancer died within a year. In comparison, the age- and gender-adjusted (reflecting DVT study population) annual mortality rate of a UK general population was estimated at 2.1%.
Table 3 presents the cause of death at each follow-up time point for all patients with DVT and by cancer subgroup. Among all observed deaths, only 3.1% were VTE related. This proportion is almost similar in the cancer subgroups. A difference between the 2 groups was observed in cardiovascular death, which was more common in patients without active cancer (11.5% vs 3.0%).
Table 3.
Mortality Over Time.
| BL | F1 | F3 | F6 | F12 | |
|---|---|---|---|---|---|
| All | |||||
| Total | 2056 | 2034 | 1899 | 1833 | 1766 |
| Death, n (%) | 8 (0.4) | 29 (1.4) | 70 (3.7) | 100 (5.5) | 128 (7.3) |
| Reason for death | |||||
| Missing/unknown | – | 7 (24.1) | 14 (20.0) | 20 (20.0) | 23 (18.0) |
| VTE-related death | 1 (12.5) | 1 (3.4) | 2 (2.9) | 3 (3.0) | 4 (3.1) |
| Cardiovascular death | – | 2 (6.9) | 4 (5.7) | 7 (7.0) | 9 (7.0) |
| Other | 7 (87.5) | 19 (65.5) | 50 (71.4) | 70 (70.0) | 92 (71.9) |
| Patients without active cancer | |||||
| Total | 1882 | 1862 | 1736 | 1673 | 1610 |
| Death, n (%) | 5 (0.3)% | 15 (0.8) | 29 (1.7) | 40 (2.4) | 61 (3.8) |
| Reason for death | |||||
| Missing/unknown | – | 3 (20.0) | 6 (20.7) | 7 (17.5) | 10 (16.4) |
| VTE-related death | 1 (20.0)% | 1 (6.7) | 2 (6.9) | 2 (5.0) | 2 (3.3) |
| Cardiovascular death | – | 2 (13.3) | 3 (10.3) | 5 (12.5) | 7 (11.5) |
| Other | 4 (80.0)% | 9 (60.0) | 18 (62.1) | 26 (65.0) | 42 (68.9) |
| Patients with active cancer | |||||
| Total | 174 | 172 | 163 | 160 | 156 |
| Death, n (%) | 3 (1.7) | 14 (8.1) | 41 (25.2) | 60 (37.5) | 67 (43.0) |
| Reason for death | |||||
| Missing/unknown | – | 4 (28.6) | 8 (19.5) | 14 (23.3) | 13 (19.4) |
| VTE-related death | – | – | – | 1 (1.7) | 2 (3.0) |
| Cardiovascular death | – | – | 1 (2.4) | 2 (3.3) | 2 (3.0) |
| Other | 3 (100.0) | 10 (71.4) | 32 (78.0) | 44 (73.3) | 50 (74.6) |
Abbreviations: BL, baseline; F1, 1 month follow-up; F3, 3 months follow-up; F6, 6 months follow-up; F12, 12 months follow-up.
Table 4 presents the results from 2 models analyzing mortality as a function of patient characteristics with a breakdown in patients with active cancer and those without, including and excluding HrQoL as an explanatory variable. In patients without active cancer, increasing age, decreasing BMI, more than 5 days in bed, major surgery, varicose veins, previous atrial fibrillation, liver disease, smoking history, and alcohol use showed a significant association with all-cause mortality. Most effects are positive, having an association with higher mortality (odds ratio [OR] > 1). Body mass index, major surgery, varicose veins, and alcohol use show protective effects (lower mortality, OR < 1). In patients with active cancer, BMI, varicose veins, and liver disease were significant predictors. Health-related QoL index score showed a significant association with mortality in both cancer groups without major changes in the effects of the other variables. No country effects were seen in any models when repeating the above regression analyses with the addition of this as a predictor (the United Kingdom as the reference country).
Table 4.
Logistic Regression on 12-Month Mortality Rate.
| Baseline Characteristics, n = 1502 | Baseline Characteristics and EQ-5D-5L Index Score, n = 1469 | |||||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio | Standard Error | P Value | 95% Confidence Interval | Odds Ratio | Standard Error | P Value | 95% Confidence Interval | |
| Patients without active cancer | ||||||||
| Constant | 0.012 | 0.015 | 0 | 0.001-0.134 | 0.059 | 0.073 | .022 | 0.005-0.664 |
| Age | 1.063 | 0.012 | 0 | 1.041-1.086 | 1.066 | 0.012 | 0 | 1.043-1.089 |
| Body mass index | 0.894 | 0.032 | .002 | 0.833-0.959 | 0.878 | 0.031 | 0 | 0.820-0.940 |
| >5 days in bed | 2.426 | 0.871 | .014 | 1.200-4.905 | ||||
| Major surgery | 0.261 | 0.171 | .04 | 0.072-0.944 | 0.281 | 0.180 | .047 | 0.080-0.985 |
| Varicose veins | 0.333 | 0.137 | .007 | 0.149-0.745 | 0.351 | 0.145 | .011 | 0.156-0.789 |
| Previous atrial fibrillation | 4.462 | 2.179 | .002 | 1.713-11.622 | 3.820 | 1.907 | .007 | 1.436-10.163 |
| Liver disease | 4.644 | 2.856 | .013 | 1.392-15.499 | 4.186 | 2.540 | .018 | 1.274-13.751 |
| Smoking history | 2.279 | 0.687 | .006 | 1.262-4.113 | 2.042 | 0.619 | .019 | 1.127-3.700 |
| Alcohol use | 0.348 | 0.177 | .038 | 0.128-0.945 | ||||
| EQ-5D-5L index score | Not included | 0.161 | 0.080 | 0 | 0.061-0.427 | |||
Discussion
This study aimed to contribute to current scientific knowledge regarding the burden of DVT in Europe, based on real-world data as collected in PREFER. The EQ-5D-5L index score was 0.136 lower (0.716 at baseline) in patients with DVT than that in the reference UK population (0.852). Following treatments, the HrQoL normalizes over 12 months, particularly noticeably in patients with nonactive cancer (0.866). The mortality results show substantial differences in cancer subgroups, 43.0% for patients with active cancer and 3.8% for those without at 12 months. Both groups had excess mortalities, higher than that of the age- and gender-adjusted UK mortality.
Utility
Overall, patients had the worst index scores at baseline (after the index event) and the scores gradually improved over time. Furthermore, larger differences are seen when evaluating patients with and without active cancer. The observed improvement of the index score was based on the alive patient population who participated in the follow-up. With the inclusion of patients who died (with a score of 0), the average index score decreased. One must note that patients who were lost to follow-up usually tend to be less healthy with lower EQ-5D-5L index scores and so results should be interpreted with caution. As demonstrated in Appendix Figure 1, after including the imputed missing values, the improvement over time was relative limited, particularly for survivors with cancer.
The analyses of HrQoL and patient characteristics included some unexpected results. For patients without active cancer, there is a suggestion that thrombophilia significantly improves HrQoL, which may be due to unobserved interaction, confounding, or possibly the patients’ interest in general health and preventive therapies. For patients with active cancer, previous VTE event was significantly associated with a higher EQ-5D-5L index score. Tobit repeated-measures regression model results will be less robust because of the small sample size of the active cancer group, incurring wider confidence intervals for the predictors included in the models. When including country as a predictor for EQ-5D-5L index score, France, DACH, and Italy (vs the United Kingdom) were found to be significant predictors of improved EQ-5D-5L index score. Thus, even after correcting for case mix, differences between countries are found. This implies that there may be other factors contributing to this difference in EQ-5D-5L index score that were not included in the current analysis.
To the authors’ best knowledge, the current study is the first reporting EQ-5D-5L data in patients with DVT outside clinical trials. A few studies examined HrQoL of patients with DVT using EQ-5D 3 level.12–14 Utne et al showed that EQ-5D-3L index score (0.72) after a mean follow-up time of 5 years in 254 patients with previous DVT was lower than age- and sex-matched “buddy” controls. The index score was also significantly lower than age- and sex-adjusted population norms.12 Marvig et al13 evaluated newly diagnosed patients with VTE and found similarly to our study an improvement of the index score over time after evaluating complete cases only at baseline and 3 months follow-up. The authors also reported between-country differences in HrQoL of patients with VTE. Their study suggested various factors that might explain the country variation, such as different response style, reference levels, external factors, and cultural differences.13 However, comparisons of our study with those in the literature should be undertaken with caution due to differences in the populations and the measurement tools used. Utne et al focused on long-term effects of DVT and Marvig et al evaluated newly diagnosed patients with VTE up to 3 months. Furthermore, our study used EQ-5D-5L, which is not comparable with the 3L version.
Mortality
A wide range of 1-year mortality rates was observed across countries (2.9% in DACH to 15.4% in Italy). Several factors may have contributed to this variation. First, in Italy and Spain, only inpatients were recruited, whereas in France, DACH, and the United Kingdom, both inpatients and outpatients were included. We found considerably more patients with active cancer in Italy and France (15.8% and 12.2%, respectively). In other words, countries may have included a dissimilar “mix” of patients. Second, there is variation in disease management across countries. For instance, not all DOACs were available in Spain and Italy at the time of data collection. The use of DOACs in the United Kingdom was also low (19.25%) in comparison to France and DACH (42.0% and 54.4%, respectively). However, when controlling for case mix (age, gender, BMI, previous clinical events, clinical factors, comorbidities, risk factors, and DVT symptoms at baseline, as well as EQ-5D-5L index score), the regression results showed that country variation was not a significant predictor for survival in patients with or without active cancer. This suggests that the higher mortality in Italy versus the United Kingdom can mostly be explained by differences in patient characteristics.
The study also showed a substantial difference in mortality between patients with and without active cancer. For patients without active cancer (91.5% of the total DVT sample), the mortality rate at 12 months was 4.7%. This still implies an excess mortality of 2.6% compared to the age- and gender-adjusted general population. Regression results of this subgroup showed that high BMI, major surgery, varicose veins, and alcohol use had a protective effect on the 12-month mortality rate. One could argue that these predictors are present in a relatively healthy group and that short-term mortality in these patients is lower compared to more severe comorbidities and risk factors. As for the patients with active cancer (8.5% of the DVT sample), 43% died within a year. As in the PREFER registry, patients with VTE cancer with a poor prognosis were less likely to be included, the current estimate can be considered as conservative. A recent trial analysis in patients with cancer with DVT (with or without PE) reported a 23.9% mortality rate at 6 months in patients with standard therapy.15 However, most mortality rates reported in recent randomized controlled trials for DOACs tend to be lower, which is likely related to the selection of relatively healthy patients.
Our study reported a mortality rate of 7.3% at 12 months, which is lower than the numbers reported in other population cohort studies with long-term follow-up. A nationwide population-based cohort study in Denmark following first-time VTE diagnosed patients over the period of 30 years reported a 31- to 364-day mortality rate of 13% for patients with DVT.16 The study also reported that mortality after DVT remained fairly constant over the past 3 decades. A Norwegian population-based study of 740 patients with a first-time VTE reported 1-year mortality rate of 21%.17 Martinez et al18 reported a mortality rate of 21.6% after 1 year for patients with VTE, using the UK Clinical Practice Research Datalink with Hospital Episodes Statistics. In addition, Cohen et al19 reported 64.5 % mortality after 1 year for patients with DVT with active cancer based on the same UK data set. The fact that the current study includes both first-time diagnosed and recurrent patients with DVT may contribute to the observed differences. The 2-week recruitment window might also result in recruiting less severe patients.
Strengths and Limitations
The PREFER in VTE registry provides a rich data source of epidemiology, management, and outcomes of VTE based on a large sample with frequent timings of measurements in a real-world setting. It is one of largest prospective disease registries in VTE. The PREFER in VTE collected data from 7 European countries, providing much-needed data in the light of current scarcity of data for the region. Furthermore, the design of PREFER—including both proximal and distal DVT—allowed all types of patients with DVT to be recruited, providing a source of heterogeneous data in a real-world setting. Similarly, the PREFER registry was conducted at time when DOACs availability varied among countries, offering a unique opportunity of understanding the potential impact of DOACs.
The main limitation of the study is missing data, which is not a unique problem to this registry. There are many possible approaches to impute missing values; however, in this study, the multiple imputation method was employed and resulted in a similar finding as that of the main analysis, particularly the factors determining the HrQoL. It is unclear to what extent the approach to include subsequent patients who fulfilled the inclusion criteria may have given a higher likelihood to include relatively mild, returning patients thus underestimating the impact of DVT on mortality and HrQoL. The causes of death were not adjudicated by a centralized committee and this is also a limitation.
Conclusion
The HrQoL in patients with DVT at baseline are below age-adjusted UK estimates. The mortality rates are higher than the average mortality rates in the general UK population. Even after distinguishing between patients with DVT with and without active cancer, an excess in the burden of illness, as measured by HrQoL and mortality in patients with DVT compared to the general population, was found. Following treatments, the HrQoL normalizes over 12 months, particularly noticeably in patients with nonactive cancer.
Appendix A
Figure A1.
Imputed 5-level EQ-5D instrument index score at baseline and follow-up by patients without active cancer and with active cancer.
Note
Provoked deep vein thrombosis was defined as having prolong immobilization, >5 days in bed, or history of major surgery or trauma.
Authors' Note: P. Mismetti is also affiliated with CHU Saint-Etienne, Hospital Nord, Saint Etienne Cedex 2, France.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: L.H. Chuang, S. Kroep, and B. van Hout have served as consultants for Daiichi-Sankyo; A. Cohen, M. Monreal, S. Willich, P. Mismetti, A. Gitt, R. Bauersachs, and G. Agnelli have received honoraria from Daiichi-Sankyo for participating in the advisory committee; P Gumbs is an employee of Daiichi-Sankyo Europe GmbH. A. Cohen serves an advisor to the National Institute for Health and Care Excellence (NICE) and to the Charity Lifeblood.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Daiichi Sankyo.
ORCID iD: S. Kroep  https://orcid.org/0000-0002-1005-8690
https://orcid.org/0000-0002-1005-8690
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
| Country | Name/Place | Date |
|---|---|---|
| Germany | Rhineland Palatinate | 11.01.2013 |
| Austria | Österreichische Agentur für Gesundheit und Ernährungssicherheit GmbH | 08.07.2013 |
| Switzerland | Basel Region Ethics Committee | 10.04.2013 |
| France | Comité consultatif sur le traitement de l’information en matière de
recherche dans le domaine de la santé Commission Nationale de l’Informatique et des Libertés Conseil National de l’Ordre des Médecins |
21.02.2013 15.04.2013 22.01.2013 |
| Italy | Perugia Hospital Ethics Committee | 24.01.2013 |
| Spain | Asturia Hospital Ethics Committee Agencia Española de Medicamentos y Productos Sanitarios |
27.03.2013 10.04.2013 |
| The United Kingdom | NRES Committee–Camden & Islington | 10.04.2013 |
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1. Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008;29(18):2276–2315. [DOI] [PubMed] [Google Scholar]
- 2. National Heart Lung And Blood Institute. Pulmonary embolism. 2011. https://www.nhlbi.nih.gov/health-topics/venous-thromboembolism.
- 3. Cohen AT, Agnelli G, Anderson FA, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98(4):756–764. [DOI] [PubMed] [Google Scholar]
- 4. Spencer F, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med. 2006;21(7):722–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The Prevention of Venous Thromboembolism in Hospitalised Patients. Second Report of Session 2004-05 https://publications.parliament.uk/pa/cm200405/cm.select/cmhealth/99/99.pdf.
- 6. Agnelli G, Gitt AK, Bauersachs R, et al. The management of acute venous thromboembolism in clinical practice—study rationale and protocol of the European prefer in VTE registry. Thromb J. 2015;13(7):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chuang LH, Van Hout B, Cohen AT, et al. Deep-vein thrombosis in Europe—burden of illness in relationship to healthcare resource utilization and return to work. Thromb Res. 2018;170:165–174. [DOI] [PubMed] [Google Scholar]
- 8. Cohen AT, Gitt AK, Bauersachs R, et al. The management of acute venous thromboembolism in clinical practice. Results from the European prefer in VTE registry. Thromb Haemost. 2017;117(7):1326–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life. 2011;20(10):1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Devlin N, Shah K, Feng Y, et al. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ. 2018;27(1):7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Office for National Statistics: UK Mortality Rate. 2013. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths.
- 12. Utne KK, Tavoly M, Wik HS, et al. Health-related quality of life after deep vein thrombosis. Springerplus. 2016;5(1):1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marvig CL, Verhoef TI, De Boer A, et al. EU-PACT consortium: quality of life in patients with venous thromboembolism and atrial fibrillation treated with coumarin anticoagulants. Thromb Res. 2015;136(1):69–75. [DOI] [PubMed] [Google Scholar]
- 14. Hedner E, Carlsson J, Kulich KR, et al. An instrument for measuring health-related quality of life in patients with deep venous thrombosis (DVT): Development and Validation of Deep Venous Thrombosis Quality of Life (DVTQOL) Questionnaire. Health Qual Life Outcom. 2004;23(2):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Doormaal FF, Cohen AT, Davidson BL, et al. Idraparinux versus standard therapy in the treatment of deep venous thrombosis in cancer patients: a subgroup analysis of the Van Gogh DVT trial. Thromb Haemost. 2010;104(1):86–91. [DOI] [PubMed] [Google Scholar]
- 16. Søgaard K, Schmidt M, Pedersen L, et al. 30-Year mortality following venous thromboembolism: a population-based cohort study. Circulation. 2014;130(10):829–836. [DOI] [PubMed] [Google Scholar]
- 17. Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrøm J. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost. 2007;5(4):692–699. [DOI] [PubMed] [Google Scholar]
- 18. Martinez C, Cohen AT, Bamber L, Rietbrock S. Epidemiology of first and recurrent venous thromboembolism: a population-based cohort study in patients without active cancer. Thromb Haemost. 2014;112(2):255–263. [DOI] [PubMed] [Google Scholar]
- 19. Cohen AT, Catholing A, Rietbrock S, et al. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. A population-based cohort study. Thromb Haemost. 2017;117(1): 57–65. [DOI] [PubMed] [Google Scholar]