Abstract
Norepinephrine is considered as a potential alternative for blood pressure stabilization during spinal anesthesia for cesarean delivery, as it maintains a better maternal heart rate and cardiac output compared with phenylephrine. However, its use as a bolus dose for hypotension treatment remains largely unexplored. Therefore, the present study investigated the ED50 and ED95 of norepinephrine as a bolus for maternal hypotension during cesarean delivery. In the present prospective trial, 42 patients were enrolled for elective delivery under spinal anesthesia. The dose of norepinephrine was decided by the up-and-down sequential allocation method (UDM) with an initial dose of 0.075 µg/kg and a 0.025 µg/kg increment. The 42 patients received a bolus of norepinephrine when systolic blood pressure fell to <80% of baseline. The ED50 was calculated by the sequential method and the probit regression model. The ED95 was then calculated using the probit regression model. The ED50 of norepinephrine, which was determined by the UDM, was 0.067 µg/kg (95% CI, 0.056–0.081). The probit regression model calculated an ED50 of 0.072 µg/kg (95% CI, 0.056–0.088) and an ED95 of 0.121 µg/kg (95% CI, 0.1–0.207). In summary, the present results suggested the ED50 of a bolus norepinephrine for preventing hypotension in elective CD is 0.067 µg/kg (95% CI, 0.056–0.081), with an ED95 of 0.121 µg/kg (95% CI, 0.1–0.207).
Keywords: norepinephrine, spinal anesthesia, cesarean section
Introduction
Spinal anesthesia is a widely used anesthetic method for cesarean delivery (CD), and is associated with a high incidence of maternal hypotension, at 70–80% (1). Persistent hypotension can lead to maternal nausea and vomiting, as well as fetal acidosis owing to reduced uterine blood flow (2). Therefore, common vasopressors such as ephedrine, phenylephrine and norepinephrine are recommended to decrease the occurrence of hypotension (3). However, controversies exist regarding the choice and use of vasopressors (3). Compared with ephedrine, phenylephrine is associated with improved fetal acid-base status (4) and is considered a better choice for hypotension prevention during cesarean delivery (5,6). However, phenylephrine has a potential risk of maternal bradycardia, with significant effects on maternal heart rate (HR) and cardiac output (CO) (7). Norepinephrine has pharmacologic characteristics that make it a potential alternative to phenylephrine for blood pressure stabilization during spinal anesthesia (8). In comparison with phenylephrine, norepinephrine is a potent α-adrenergic receptor agonist that can also excite β-adrenergic receptors (3). A recent study demonstrated that norepinephrine may be a useful vasopressor for stabilizing blood pressure with fewer side effects, such as reducing HR and CO (9). However, there are limited studies on the use of norepinephrine for treating hypotension during spinal anesthesia, and few studies have reported its application in obstetric patients. Norepinephrine infusion has been used to prevent hypotension during spinal anesthesia (10) but the use of this drug as a bolus has not been fully investigated.
Although the ED90 of norepinephrine that prevents hypotension during spinal anesthesia has been assessed (11), the use of ED50 and ED95 for treating hypotension has not been completely defined. The objective of the present study was to determine the intravenous bolus dose of norepinephrine in treating maternal hypotension in 50% (ED50) and 95% (ED95) of puerperas undergoing spinal anesthesia. The secondary outcomes of the present study included maternal anesthetic block level, hemodynamic changes, bradycardia, adverse effects, umbilical arterial blood gases, Apgar scores and the ratio of hypotensive patients to hypertensive patients following treatment.
Patients and methods
Patient characteristics
A total of 42 patients were enrolled into the present study between 3rd November 2018 and 31st December 2018. The present study was conducted in Jiaxing Maternity and Child Healthcare Hospital (Jiaxing, China), and informed consent was provided by each participating patient. Inclusion criteria were elective cesarean delivery under spinal anesthesia, healthy singleton full-term pregnancy beyond 37 weeks of gestation, American Society of Anesthesiologists physical status I or II (12), weight of 50–100 kg, height of 150–180 cm, and fasting for >6 h. Exclusion criteria were hypertension, cardiovascular diseases, preeclampsia, anisorrhythmia, diabetes mellitus, spinal cord malformation, abnormal fetus and patient refusal. The included puerperas received different doses of norepinephrine (0.025, 0.050, 0.075, 0.100 and 0.125 µg/kg) to stabilize blood pressure. The present prospective, double-blinded study was approved by the Ethical Committee of Jiaxing Maternity and Child Healthcare Hospital, and was registered in the Chinese Clinical Registry Center (registration no. ChiCTR1800018474).
Patient monitoring
No patients received premedication. Non-invasive systolic blood pressure (SBP), diastolic blood pressure (DBP) and HR were recorded in the supine position on the morning of the surgery. Baseline systolic arterial BP was the average of three continuous measurements at 1 min intervals using an automated device for non-invasive BP assessment on the morning of surgery. In the operating room, patients underwent continuous standard monitoring throughout the whole surgery, including electrocardiography, non-invasive blood pressure measurement and pulse oximetry on a patient monitor system (B650; GE Healthcare Finland Oy). A 18G intravenous catheter cannula was inserted into a right forearm vein and infused with Ringer's lactate solution at 10 ml/kg within 20 min of infusion, followed by a maintained dose of 20 ml/min. The patients were placed in the left lateral position. Spinal anesthesia was performed using a 16G epidural puncture needle, with a 25G Whitacre needle at the Lumbar 3–4 interspace. Upon entry into the subarachnoid space and noticing the cerebrospinal fluid flowing out, 0.5% bupivacaine, which was obtained by mixing 2 ml 0.75% bupivacaine (Shanghai Hefeng Pharmaceutical Co., Ltd.) with 1 ml sterile saline, and was administered intrathecally at the rate of 0.1 ml/sec. The dose of bupivacaine is referred to in the Harten dose table, and was adjusted according to the height and weight of a patient (13). Then, the patient was placed in the supine position with left uterine displacement by tilting the bed to the left by 15 (14). Oxygen was administered via a mask at 5 l/min. The spinal sensory block level was evaluated by pinprick and confirmed to be within Thoracic (T) 4–6. If not, the case was excluded from the current study.
Treatment preparation
Prior to treatment, the anesthesiologist assistant, who was not involved in patient management or data acquisition, prepared the study bolus dose by adding a measured volume of 2 mg norepinephrine (Changzhou Yuanda Pharmaceutical Chemical Co. Ltd.) to 500 ml of physiological saline. The patient and the attending anesthetic manager were blinded to group allocation. Based on patient weight, the corresponding dose of the drug was determined by the assistant anesthesiologist. The anesthetic manager who collected the data was blinded to the drug and its dose. After delivery, 5U oxytocin (Nanjing Xinbai Pharmaceutical Co., Ltd.) was administered slowly by intravenous infusion, and an additional 5U oxytocin was injected into the uterine muscle. Meanwhile, the pediatric nurse, who was unaware of the study, assessed Apgar scores (15) at 1 and 5 min, including activity, pulse, grimace, appearance and respiration. Arterial blood specimens were collected from the clamped umbilical cord for immediate blood gas analysis using a blood gas analyzer (COBAS B123; Roche Diagnostics).
Treatment regime
Previous studies have suggested that a 100 µg dose of phenylephrine approximates an 8 µg dose of norepinephrine (16), though a dose 100 µg of phenylephrine is a suitable dosage (17,18), and the use of a 6 µg bolus norepinephrine appears sufficient to prevent hypotension (11). In the present trial, the dosage of norepinephrine was decided by the up-and-down sequential allocation method with an initial dose of 0.075 µg/kg and a 0.025 µg/kg gradient. For the dose finding study by the up-and-down method, the data distribution was unknown and non-independent (19–21). As required by the sequential approach, the dose level for the next patient was determined by the response of the preceding patient. In the present study, the first patient received a dose of 0.075 µg/kg, which was thought to be closest to the estimated dose. If the first patient showed a positive response, the second patient would be exposed to a lower dose (0.05 µg/kg). In case of a negative response by the first patient at 0.075 µg/kg, the second patient would be exposed to a next higher dose (0.1 µg/kg). Successive patients were assigned respective doses similarly based on the responses of the preceding participants.
In the present study the norepinephrine regimen was started immediately after intrathecal injection. According to our standard practice, SBP was assessed every min, beginning immediately after intrathecal injection until delivery. The study drug was administered manually by the attending anesthetic manager whenever SBP was <80% of baseline, to maintain SBP within the 95% baseline value. Hypotension was defined as SBP <80% of baseline, and hypertension as a 20% increase from baseline. In case SBP returned to within the 95% baseline value within 1 min after administration, treatment was considered to be successful. If SBP was still <80% of baseline, 6 mg ephedrine was administered. Bradycardia, which is a heart rate below 50 beats per minute, was treated with 0.5 mg atropine.
SBP, DBP and HR were recorded at baseline and 1 min after administration. The times of norepinephrine injection before delivery and throughout the surgery were recorded. Maternal infusion volume, blood loss, urine output and adverse effects such as nausea, vomiting, bradycardia, hypertension and hypotension were also recorded. In addition, fetal heart rates before and after spinal anesthesia, Apgar scores at 1 and 5 min, and umbilical arterial blood gases were assessed.
Statistical analysis
In the present UDM study, data distribution was non-independent and unknown, which prevents the development of theoretical rules for determining the necessary sample size from an accurate estimation of the ED50 (21). Simulation studies suggested that a stopping rule with 20–40 patients would provide stable estimates of the study dose for most realistic cases (21). The present study required a sample size of 42 patients for the stopping rule. The data were used to calculate the ED50 with 95% CI, using the up-and-down method. Data were further analyzed using the probit regression model to calculate the ED50 and ED95. The sequential method was performed according to the following formula: ED50=lg−1ΣrlgC/ΣrC, where C is the dose, r is the number of injections. The standard error was calculated as SlgED50=d {Σ[p(1-p)/(r-1)]}1/2, where p is the effective rate. The 95%CI was determined for the ED50 obtained by the sequential method as lg−1(lgED50±1.96SlgED50) (22). Data analysis was performed using the SPSS 20.0 software (IBM Corp.).
Results
Patient recruitment
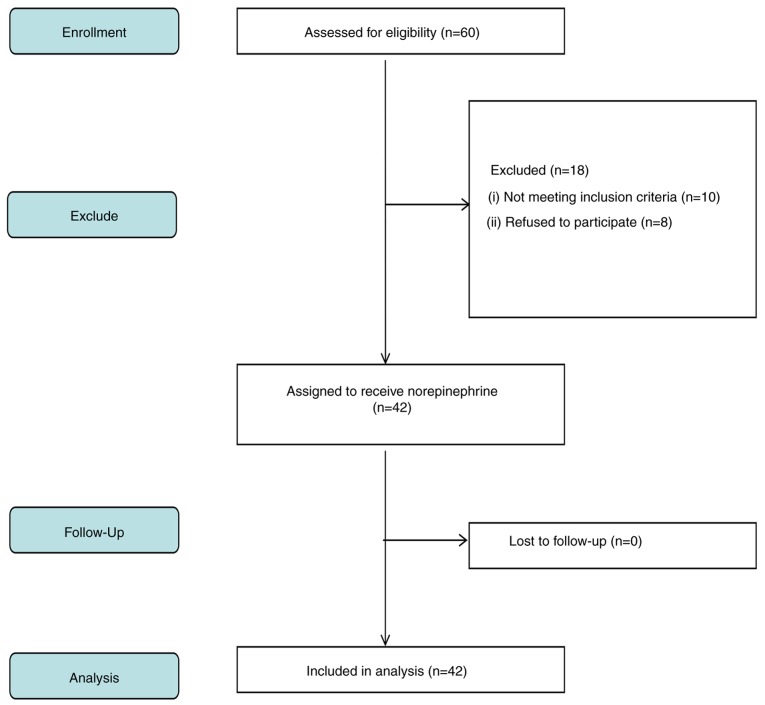
A total of 60 patients undergoing elective cesarean delivery under spinal anesthesia were recruited in the present study, and 42 were included in the final analysis. Fig. 1 indicates a flow diagram detailing patient enrolment. The demographics of the mothers are presented in Table I, with parameters presented as mean ± SD or number. The mean age of the participants was 30.4±4.3 years, the mean body weight was 70.5±8.4 kg. The anesthesia block level was between T4 and T6.
Figure 1.
Flowchart of patient recruitment.
Table I.
Demographic data and surgical characteristics. Data are presented as mean ± SD.
| Characteristic | Index |
|---|---|
| Age, years | 30.4±4.3 |
| Weight, kg | 70.5±8.4 |
| Height, cm | 159.2±6.3 |
| Gestation, weeks | 38.0±1.4 |
| SBP at baseline, mmHg | 120.4±11.4 |
| DBP at baseline, mmHg | 71.5±6.1 |
| HR at baseline, beats/min | 77.8±11.2 |
| Block level (T) | T5(T4 to T6) |
SD, standard deviation; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate.
Neonatal measurements
Umbilical arterial blood gas data after delivery are presented as mean ± SD (Table II). Umbilical artery base excess values ranged from −7.5 to −0.4 mEq/l. The pH values ranged from 7.23–7.34. All pH values were within the normal range, and no neonate experienced fetal acidosis defined as pH <7.2, which is the lower limit of normal (data not shown) (23). Values in blood gas analysis of the umbilical artery fluctuated within the normal range without obvious abnormality. Apgar scores at 1 and 5 min were eight or above for all cases. No neonates required intubation or ventilation.
Table II.
Neonatal umbilical artery outcomes.
| Parameters | Index |
|---|---|
| PO2, mm Hg | 21.4±4.8 |
| PCO2, mm Hg | 46.4±4.1 |
| pH | 7.3±0.1 |
| HCO3−, mmol/l | 24.6±2.5 |
| Base excess, mEq/l | −3.2±2.0 |
| Apgar score at 1 min | 8.7±0.5 |
| Apgar score at 5 min | 8.8±0.4 |
Data are presented as mean ± SD. SD, standard deviation.
Maternal outcomes
Side effects observed after treatment included nausea, vomiting, bradycardia, hypertension and hypotension (Table III). Of the eight patients (19.1%) who developed nausea, six received a dose <0.1 µg/kg, and one case (2.4%) had vomiting symptoms. Of the 19 patients (45.2%) who developed hypotension, 12 received bolus norepinephrine doses <0.075 µg/kg (data not shown). Table IV indicates the observed response rates for various norepinephrine dose levels. A total of 21 participants reported the norepinephrine dosage to be effective, and 21 considered their received dosage to be ineffective.
Table III.
Maternal outcomes.
| Parameter | Index |
|---|---|
| Nausea | 8 (19.1%) |
| Vomiting | 1 (2.4%) |
| Bradycardia | 2 (4.8%) |
| Hypertension | 2 (4.8%) |
| Hypotension | 19 (45.2%) |
| Transfusion volume, ml | 871.4±74.2 |
| Bleeding volume, ml | 260.7±59.0 |
| Urine volume, ml | 104.3±15.3 |
Data are presented as number (%) or mean ± SD. SD, standard deviation.
Table IV.
Observed response rate.
| Groups | Assigned dose, µg/kg | Effective | Total number | Effective rate |
|---|---|---|---|---|
| NE 1 | 0.025 | 0 | 3 | 0.00 |
| NE 2 | 0.050 | 3 | 12 | 0.3 |
| NE 3 | 0.075 | 9 | 16 | 0.6 |
| NE 4 | 0.100 | 7 | 9 | 0.8 |
| NE 5 | 0.125 | 2 | 2 | 1.00 |
NE, norepinephrine.
Norepinephrine dose response
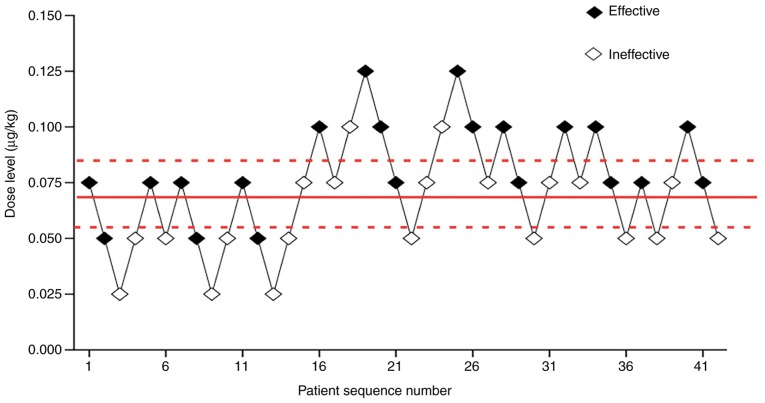
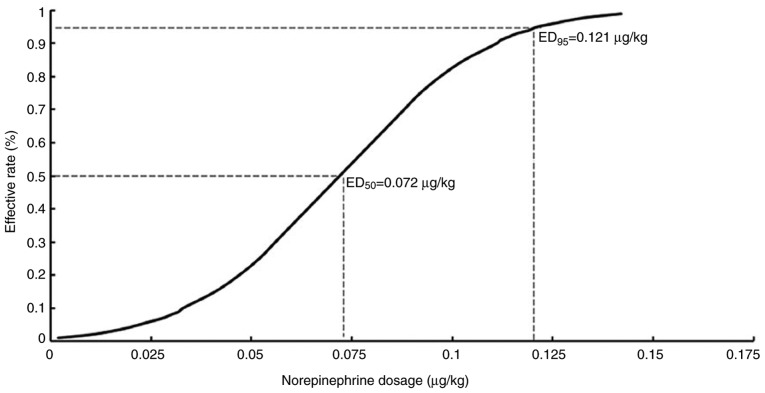
Effective and ineffective responses were evaluated at various norepinephrine dose levels, including 0.025, 0.05, 0.075, 0.1 and 0.125 µg/kg. Fig. 2 showed each dose, anesthetic result and subsequent dosing. The ED50 of norepinephrine determined by the up-and-down sequential allocation method was 0.067 µg/kg (Fig. 2; 95% CI, 0.056–0.081). In addition, effective and ineffective responses to norepinephrine were evaluated by the probit regression model. The ED50 of norepinephrine was 0.072 µg/kg (95% CI, 0.056–0.088) and the ED95 identified by the probit regression model was 0.121 µg/kg (Fig. 3; 95% CI, 0.1–0.207).
Figure 2.
Sequential test diagram. Scatter plot showing effective, represented by a solid dot, and ineffective, represented by an open dot, doses for all 42 participants. Median effective dosage was 0.067 µg/kg (95% CI, 0.056–0.081).
Figure 3.
Norepinephrine dose-effective probability curve. The dose-response curve identified the relationship between the norepinephrine dosage in the patients and the proportion of patients reporting the effectiveness (defined as the SBP returned to within 95% of the baseline value). The ED50 and ED95 were estimated using probit regression model. SBP, systolic blood pressure.
Discussion
The present study determined the weight-based dose of norepinephrine given as a bolus to treat the first episode of hypotension in patients undergoing spinal anesthesia for elective CD. The present results suggested that ED50 values for norepinephrine were 0.067 µg/kg (95% CI, 0.056–0.081) and 0.072 µg/kg (95% CI, 0.056–0.088) as assessed by the up-and-down method and the probit regression model, respectively. The ED95 calculated by the probit regression model was 0.121 µg/kg (95% CI, 0.1–0.207). The ED50 measured by the up-and-down method was close to that obtained by the probit regression model. No significant adverse effects were identified in the present study; however, it is possible that the low incidence of side effects was due to the small sample size. For the dosage determination study by the up-and-down method, data distribution was unknown and non-independent. As required by the sequential approach, the dose for the subsequent patient was determined by the response of the preceding patient (21). Using the sequential approach to calculate median effective dose, once six pairs of reversal of sequence was achieved, it was possible to consider the sample size as adequate (20,24). The present study had >6 pairs of reversal of sequence, so the sample size was sufficient. In addition, previous simulation studies suggest that including ≥20–40 patients will provide stable estimates of the target dose for most realistic scenarios (19). The present study required a sample size of 42 patients for the stopping rule.
A previous study reported an ED90 preventing hypotension for norepinephrine of 6 µg (11), however the weight based ED50 and ED95 of norepinephrine have not been widely investigated in patients. Therefore, the present study assessed the ED50 and ED95 of norepinephrine. Understanding the ED50 of a drug is important as it is located in the most sensitive portion of the dose-response curve, and small adjustments are expected to have significant increases in therapeutic response (25). In addition, determining the ED50 can potentially limit the total number of patients enrolled in a clinical trial, which is important when there is limited published information related to the side effects of a drug. The present study used the up-and-down method to determine the effective dose of norepinephrine, when the starting dose approximates the therapeutic scope for the drug, which resulted in the identification of the ED50 in a limited number of patients. A limitation of the up-and-down method is the inability to accurately determine the ED95 (26); therefore, the probit regression model was used for this purpose.
Ngan Kee et al (16) conducted a random-allocation, graded dose-response study of norepinephrine and phenylephrine and the authors estimated the ED50 (dose yielding a 50% response) of norepinephrine at 10 µg (95% CI, 6 to 17 µg), which was different from the results of the present study. With dose-response analysis, the value represents the dose that results in responses of 50% magnitude, which differs from the same term in the more traditional quantal dose-response methodology (27). In the present study, the intrathecal anesthetic dose was 11 mg hyperbaric bupivacaine 0.5% w/v and 15 µg fentanyl, while the dose of bupivacaine was 10 mg bupivacaine (2 ml 0.75% bupivacaine). The magnitude of response is measured as the percentage of full restoration of SBP to baseline (16), which is different to the present study where SBP returning to within 95% of the baseline value was satisfactory. The ED50 calculated was much higher in the study by Ngan Kee et al (16) than the ED50 values for norepinephrine in the present study. This variation could be attributed to the differing study designs. Compared with the random-allocation graded dose-response study, the present study used the sequential method to collect cases, with the advantage of obtaining the effective dose with a fewer number of patients. Differences in patient populations could have also affected the study results.
A previous study has suggested that the incidence of maternal hypotension is 50–80% depending upon the position of the patient, the rate of spinal anesthetic agent injected, intravenous fluid loading and whether the women is laboring or has associated morbidity, including pregnancy induced hypertension (28). Anesthesiologists are required to be careful since hypotension is common during cesarean delivery under spinal anesthesia (28). Commonly used vasopressors include ephedrine, phenylephrine and norepinephrine (3). Ephedrine has been previously considered the most appropriate vasopressor during spinal anesthesia (28). However, ephedrine is associated with decreased umbilical artery pH compared with other vasopressors such as phenylephrine (29). In addition, ephedrine does not completely prevent hypotension, nausea and vomiting and fetal acidosis (9). On the contrary, it may cause reactive hypertension in some patients (30). Phenylephrine is more effective for venoconstriction than arterial constriction, and predictably increases blood pressure by elevating both systemic vascular resistance and preload (28). In addition, due to its minimal β-2 receptor activity, phenylephrine does not cause tachycardia, and instead induces reflex bradycardia with increasing blood pressure (28).
Ali Elnabtity and Selim (3) compared ephedrine and norepinephrine, and found that the latter is associated with reduced numbers of hypotension and hypertension episodes as well as decreased frequencies of bradycardia and tachycardia, while maintaining maternal blood pressure and uterine artery blood flow during cesarean delivery. Furthermore, the study also found that the number of norepinephrine boluses used during spinal anesthesia is lower compared with that of ephedrine. Norepinephrine does not readily cross the placental barrier, because of the ability of the placenta to degrade catecholamines (31). However, it is a mild β-adrenergic and a potent α-adrenergic receptor agonist (7,8). Hence, norepinephrine may be a more appropriate choice for stabilizing maternal blood pressure with fewer adverse effects on HR and cardiac output in the setting of elective cesarean delivery under spinal anesthesia.
Although prophylactic infusion is the recommended method for treating spinal hypotension to reduce hemodynamic fluctuation and maternal side effects, it may be associated with a higher incidence of reactive hypertension (32). Doherty et al (33) reported that no significant difference could be found in maintaining baseline maternal CO and providing BP stability between both options of an infusion regimen and a bolus dose of phenylephrine in elective cesarean delivery under spinal anesthesia. Compared with the bolus regimen, the infusion regimen required a higher total dose of phenylephrine to maintain maternal arterial blood pressure at baseline during the pre-delivery period (33). Administration of the bolus regimen resulted in a smaller reduction in baseline SBP in the initial minutes after intrathecal injection. The bolus regimen allows faster delivery of an effective dose of phenylephrine recovering maternal vascular resistance rapidly during the establishment of spinal blockade. Of note, many anesthesiologists may favor bolus doses of vasopressors, while being prepared for repeated doses rather than selecting infusion initially during spinal anesthesia (34). Therefore, the present study selected a bolus dose of norepinephrine, which may be familiar to the majority of anesthesiologists. To the best of our knowledge, no previous study has precisely determined the ED50 and ED95 of weight-based norepinephrine as a bolus in the setting of elective cesarean delivery under spinal anesthesia. Furthermore, using norepinephrine as a bolus to treat hypotension during cesarean delivery is not well understood.
Chen et al (10) performed a randomized double-blinded controlled study of 120 patients for elective section delivery under spinal anesthesia; the patients treated by infusion were assigned to four groups, and administered saline or norepinephrine at 5, 10 and 15 µg/kg/h, respectively. Onwochei et al (11) carried out a prospective, double-blind sequential allocation dose-finding study, using the biased coin up-and-down design. In the latter trial, 40 pregnant women received a set intermittent norepinephrine bolus of 3, 4, 5, 6, 7 or 8 µg, when systolic blood pressure fell below 100% of baseline. Vallejo et al (14) conducted an open-label randomized controlled clinical trial including 85 patients undergoing spinal anesthesia for elective cesarean delivery, who were randomized to the phenylephrine (0.1 µg/kg/min) and norepinephrine (0.05 µg/kg/min) fixed-rate infusion groups. The present results suggested the ED50 and ED95 of norepinephrine were 0.067 µg/kg (95% CI, 0.056–0.081) and 0.121 µg/kg (95% CI, 0.1–0.207), respectively, during cesarean delivery, corroborating the previous studies.
The issue of tissue injury caused by bolus norepinephrine through peripheral venous catheters has become the main concern in the field (35). Previous studies have reported complications associated with norepinephrine administered through peripheral venous catheters, e.g. skin necrosis, with a 3.6% complication rate in 55 patients administered the vasopressor (36). However, a recent retrospective trial in neonates using vasopressors via peripheral venous catheters found no complications (37). In another prospective study, 55 patients received vasopressors via peripheral venous catheters, and the rate of complications (5.45%) was very low, with no significant morbidity (38). In the present study, the highest single bolus dose of norepinephrine was 0.125 µg/kg, and was associated with no complications.
However, the present study does have limitations that need to be mentioned. First, the present study, which was based on treatment for the first episode of hypotension following spinal anesthesia, was not extended until the end of surgery; responses to subsequent episodes of hypotension could be different. Secondly, the individual sensitivity to vasoactive drugs differs, which may impact the results. Thirdly, all participants were from the same geographic area. Finally, the present study had no control group.
In conclusion, the present results indicated that the ED50 values of a single bolus of norepinephrine for preventing hypotension in elective CD were 0.067 µg/kg (95% CI, 0.056–0.081) and 0.072 µg/kg (95% CI, 0.056–0.088) by the up-and-down method and the probit regression model, respectively. The ED95 obtained by the probit regression model was 0.121 µg/kg (95% CI, 0.1–0.207).
Acknowledgements
Not applicable.
Funding
The current study was supported, in part, by grants from the Natural Science Foundation of Zhejiang Province (grant no. LY17H090019), the Science and Technology Project of Jiaxing City (grant no. 2018AY32012), Medical Scientific Research Foundation of Zhejiang Province, China (grant no. 2020358554) the Medical and Health General Research Program of Zhejiang Province (grant no. 2019KY687), the Construction Project of Anesthesiology Discipline Special Disease Center in Zhejiang North Region (grant no. 201524), the Key Medical Subjects Established by Zhejiang Province and Jiaxing City Jointly, Pain Medicine (grant no. 2019-ss-ttyx), and the Construction Project of Key Laboratory of Nerve and Pain Medicine in Jiaxing City.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
TW and QH conducted the majority of the experiments and wrote the manuscript. WZ and JZ conceived and designed the study. HN and RY performed the data analysis. QL and LX collected the data and MY coordinated and supervised the experiments. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethical Committee of Jiaxing Maternity and Child Healthcare Hospital (Zhejiang, China) and registered in the Chinese Clinical Registry Center (registration no. ChiCTR1800018474). All of the participants who participated in the study provided written informed consent at the time of enrolment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Mercier FJ, Augè M, Hoffmann C, Fischer C, Gouez AL. Maternal hypotension during spinal anesthesia for caesarean delivery. Minerva Anestesiol. 2013;79:62–73. [PubMed] [Google Scholar]
- 2.Antoine C, Young BK. Fetal lactic acidosis with epidural anesthesia. Am J Obstet Gynecol. 1982;142:55–59. doi: 10.1016/S0002-9378(16)32284-0. [DOI] [PubMed] [Google Scholar]
- 3.Ali Elnabtity AM, Selim MF. Norepinephrine versus ephedrine to maintain arterial blood pressure during spinal anesthesia for cesarean delivery: A prospective double-blinded trial. Anesth Essays Res. 2018;12:92–97. doi: 10.4103/aer.AER_204_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee A, Kee WD, Gin T. A quantitative, systematic review of randomized controlled trials of ephedrine versus phenylephrine for the management of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg. 2002;94:920–926. doi: 10.1097/00000539-200204000-00028. [DOI] [PubMed] [Google Scholar]
- 5.Ngan Kee WD, Lee A, Khaw KS, Ng FF, Karmakar MK, Gin T. A randomized double-blinded comparison of phenylephrine and ephedrine infusion combinations to maintain blood pressure during spinal anesthesia for cesarean delivery: The effects on fetal acid-base status and hemodynamic control. Anesth Analg. 2008;107:1295–1302. doi: 10.1213/ane.0b013e31818065bc. [DOI] [PubMed] [Google Scholar]
- 6.Ngan Kee WD, Khaw KS, Tam YH, Ng FF, Lee SW. Performance of a closed-loop feedback computer-controlled infusion system for maintaining blood pressure during spinal anaesthesia for caesarean section: A randomized controlled comparison of norepinephrine versus phenylephrine. J Clin Monit Comput. 2017;31:617–623. doi: 10.1007/s10877-016-9883-z. [DOI] [PubMed] [Google Scholar]
- 7.Stewart A, Fernando R, McDonald S, Hignett R, Jones T, Columb M. The dose-dependent effects of phenylephrine for elective cesarean delivery under spinal anesthesia. Anesth Analg. 2010;111:1230–1237. doi: 10.1213/ANE.0b013e3181f2eae1. [DOI] [PubMed] [Google Scholar]
- 8.Ngan Kee WD, Lee SW, Ng FF, Tan PE, Khaw KS. Randomized double-blinded comparison of norepinephrine and phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery. Anesthesiology. 2015;122:736–745. doi: 10.1097/ALN.0000000000000601. [DOI] [PubMed] [Google Scholar]
- 9.Chooi C, Cox JJ, Lumb RS, Middleton P, Chemali M, Emmett RS, Simmons SW, Cyna AM. Techniques for preventing hypotension during spinal anaesthesia for caesarean section. Cochrane Database Syst Rev. 2017;8:CD002251. doi: 10.1002/14651858.CD002251.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen D, Qi X, Huang X, Xu Y, Qiu F, Yan Y, Li Y. Efficacy and safety of different norepinephrine regimens for prevention of spinal hypotension in cesarean section: A randomized trial. Biomed Res Int. 2018;2018:2708175. doi: 10.1155/2018/2708175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onwochei DN, Ngan Kee WD, Fung L, Downey K, Ye XY, Carvalho JC. Norepinephrine intermittent intravenous boluses to prevent hypotension during spinal anesthesia for cesarean delivery: A sequential allocation dose-finding study. Anesth Analg. 2017;125:212–218. doi: 10.1213/ANE.0000000000001846. [DOI] [PubMed] [Google Scholar]
- 12.Doyle DJ, Garmon EH. StatPearls Publishing LLC; Treasure Island, FL: 2019. American Society of Anesthesiologists Classification (ASA Class) [PubMed] [Google Scholar]
- 13.Harten JM, Boyne I, Hannah P, Varveris D, Brown A. Effects of a height and weight adjusted dose of local anaesthetic for spinal anaesthesia for elective Caesarean section. Anaesthesia. 2005;60:348–353. doi: 10.1111/j.1365-2044.2005.04113.x. [DOI] [PubMed] [Google Scholar]
- 14.Vallejo MC, Attaallah AF, Elzamzamy OM, Cifarelli DT, Phelps AL, Hobbs GR, Shapiro RE, Ranganathan P. An open-label randomized controlled clinical trial for comparison of continuous phenylephrine versus norepinephrine infusion in prevention of spinal hypotension during cesarean delivery. Int J Obstet Anesth. 2017;29:18–25. doi: 10.1016/j.ijoa.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Apgar V. A proposal for a new method of evaluation of the newborn infant. Anesthesia Analgesia. 1953;32:250–259. doi: 10.1213/00000539-195301000-00041. [DOI] [PubMed] [Google Scholar]
- 16.Ngan Kee WD. A Random-allocation graded dose-response study of norepinephrine and phenylephrine for treating hypotension during spinal anesthesia for cesarean delivery. Anesthesiology. 2017;127:934–941. doi: 10.1097/ALN.0000000000001880. [DOI] [PubMed] [Google Scholar]
- 17.Ngan Kee WD, Khaw KS, Ng FF. Comparison of phenylephrine infusion regimens for maintaining maternal blood pressure during spinal anaesthesia for Caesarean section. Br J Anaesth. 2004;92:469–474. doi: 10.1093/bja/aeh088. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Huang Y, Diao M, Li H, Ma Y, Lin X, Zhou J. Determination of the 90% effective dose (ED90) of phenylephrine for hypotension during elective cesarean delivery using a continual reassessment method. Eur J Obstet Gynecol Reprod Biol. 2015;194:136–140. doi: 10.1016/j.ejogrb.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Stylianou M, Flournoy N. Dose finding using the biased coin up-and-down design and isotonic regression. Biometracs. 2002;58:171–177. doi: 10.1111/j.0006-341X.2002.00171.x. [DOI] [PubMed] [Google Scholar]
- 20.Dixon WJ. Staircase bioassay: The up-and-down method. Neurosci Biobehav Rev. 1991;15:47–50. doi: 10.1016/S0149-7634(05)80090-9. [DOI] [PubMed] [Google Scholar]
- 21.Pace NL, Stylianou MP. Advances in and limitations of up-and-down methodology: A précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology. 2007;107:144–152. doi: 10.1097/01.anes.0000267514.42592.2a. [DOI] [PubMed] [Google Scholar]
- 22.Dixon WJ. The up-and-down method from small samples. J Am Stat Assoc. 1965;60:967–978. doi: 10.1080/01621459.1965.10480843. [DOI] [Google Scholar]
- 23.Miller JM, Jr, Bernard M, Brown HL, St Pierre JJ, Gaber HA. Umbilical cord blood gases for term healthy newborns. Am J Perinatol. 1990;7:157–159. doi: 10.1055/s-2007-999470. [DOI] [PubMed] [Google Scholar]
- 24.Sell A, Olkkola KT, Jalonen J, Aantaa R. Minimum effective local anaesthetic dose of isobaric levobupivacaine and ropivacaine administered via a spinal catheter for hip replacement surgery. Br J Anaesth. 2005;94:239–242. doi: 10.1093/bja/aei015. [DOI] [PubMed] [Google Scholar]
- 25.Lynde GC. Determination of ED50 of hydromorphone for postoperative analgesia following cesarean delivery. Int J Obstet Anesth. 2016;28:17–21. doi: 10.1016/j.ijoa.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Johnston DF, Sondekoppam RV, Giffin R, Litchfield R, Ganapathy S. Determination of ED50 and ED95 of 0.5% Ropivacaine in adductor canal block to produce Quadriceps weakness: A dose-finding study. Reg Anesth Pain Med. 2017;42:731–736. doi: 10.1097/AAP.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 27.Tallarida RJ. Chapman and Hall/CRC; New York, NY: 2000. Drug Synergism and Dose-Effect Data Analysis; pp. 21–39. [Google Scholar]
- 28.Macarthur A, Riley ET. Obstetric anesthesia controversies Vasopressor choice for postspinal hypotension during cesarean delivery. Int Anesthesiol Clin. 2007;45:115–132. doi: 10.1097/AIA.0b013e31802b8d53. [DOI] [PubMed] [Google Scholar]
- 29.Thomas D, Robson S, Redfern N, Hughes D, Boys R. Randomized trial of bolus phenylephrine or ephedrine for maintenance of arterial pressure during spinal anaesthesia for Caesarean section. Br J Anaesth. 1996;76:61–65. doi: 10.1093/bja/76.1.61. [DOI] [PubMed] [Google Scholar]
- 30.Ngan Kee WD, Khaw KS, Lee BB, Lau TK, Gin T. A dose-response study of prophylactic intravenous ephedrine for the prevention of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg. 2000;90:1390–1395. doi: 10.1097/00000539-200006000-00024. [DOI] [PubMed] [Google Scholar]
- 31.Puolakka J, Kauppila A, Tuimala R, Jouppila R, Vuori J. The effect of parturition on umbilical blood plasma levels of norepinephrine. Obstet Gynecol. 1983;61:19–21. [PubMed] [Google Scholar]
- 32.Kinsella SM, Carvalho B, Dyer RA, Fernando R, McDonnell N, Mercier FJ, Palanisamy A, Sia AT, Van de Velde M, Vercueil A, Consensus Statement Collaborators International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anaesthesia. Anaesthesia. 2018;73:71–92. doi: 10.1111/anae.14080. [DOI] [PubMed] [Google Scholar]
- 33.Doherty A, Ohashi Y, Downey K, Carvalho JC. Phenylephrine infusion versus bolus regimens during cesarean delivery under spinal anesthesia: A double-blind randomized clinical trial to assess hemodynamic changes. Anesth Analg. 2012;115:1343–1350. doi: 10.1213/ANE.0b013e31826ac3db. [DOI] [PubMed] [Google Scholar]
- 34.Langesæter E, Rosseland LA, Stubhaug A. A randomized, double-blind comparison of low-dose versus high-dose spinal anesthesia with intravenous phenylephrine or placebo infusion. Anesthesiology. 2008;109:856–863. doi: 10.1097/ALN.0b013e31818a401f. [DOI] [PubMed] [Google Scholar]
- 35.Smiley RM. More perfect? Int J Obstet Anesth. 2017;29:1–4. doi: 10.1016/j.ijoa.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Greenwald HP, Gootnick A, Luger NM, King JA. Tissue necrosis following subcutaneous infiltration with nor-epinephrine; Report of two cases. N Engl J Med. 1952;246:252–253. doi: 10.1056/NEJM195202142460704. [DOI] [PubMed] [Google Scholar]
- 37.Turner DA, Kleinman ME. The use of vasoactive agents via peripheral intravenous access during transport of critically III infants and children. Pediatr Emerg Care. 2010;26:563–566. doi: 10.1097/PEC.0b013e3181ea71e1. [DOI] [PubMed] [Google Scholar]
- 38.Medlej K, Kazzi AA, El Hajj Chehade A, Saad Eldine M, Chami A, Bachir R, Zebian D, Abou Dagher G. Complications from administration of vasopressors through peripheral venous catheters: An observational study. J Emerg Med. 2018;54:47–53. doi: 10.1016/j.jemermed.2017.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.