Abstract
Myocardial ischemia-reperfusion (I/R) injury is the oxidative stress and inflammatory response that occurs when a tissue is reperfused following a prolonged period of ischemic injury. Growing evidence has demonstrated that microRNAs (miRs) are essential in the development of myocardial I/R injury. Salidroside, a phenylpropanoid glycoside isolated from a traditional Chinese medicinal plant, Rhodiola rosea, possesses multiple pharmacological functions and protects against myocardial I/R injury in vitro and in vivo. However, the role of miRs in the cardioprotective effects of salidroside against myocardial I/R injury has not been studied, to the best of our knowledge. In the present study, the role of miR21 in the underlying mechanism of salidroside-induced protection against oxidative stress and inflammatory injuries in hypoxia/reoxygenation (H/R)-treated H9c2 cardiomyocytes was determined. The cell viability was assessed with an MTT assay. Lactate dehydrogenase (LDH) release, caspase-3 activity, malondialdehyde (MDA) level, superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities were determined by commercial kits. Cell apoptosis was measured by flow cytometry. Intracellular reactive oxygen species (ROS) generation was monitored by DCFH-DA. The miR-21 level was quantified by reverse transcription-quantitative (RT-q)PCR. The interleukin (IL)-6, IL-1β and tumor necrosis factor (TNF)-α levels were measured by RT-qPCR and ELISA. The results showed that salidroside pretreatment significantly increased cell viability and decreased the release of LDH, accompanied by an increase in miR-21 expression in H/R-treated H9c2 cells and a miR-21 inhibitor reversed these effects. In addition, the miR-21 inhibitor also abrogated the inhibition of salidroside on H/R-induced increases in apoptosis and caspase-3 activity in H9c2 cells. Salidroside mitigated H/R-induced oxidative stress as illustrated by the downregulation of ROS generation and MDA level and increased the activities of the antioxidant enzymes, SOD and GSH-Px, all of which were abrogated in cells transfected with the miR-21 inhibitor. Salidroside induced a decrease in the expression and levels of the pro-inflammatory cytokines, IL-6, IL-1β and TNF-α, which were prevented by the miR-21 inhibitor. Together, these results provide evidence of the beneficial effects of salidroside against myocardial I/R injury by reducing myocardial oxidative stress and inflammation which are enhanced by increasing miR-21 expression.
Keywords: microRNA-21, salidroside, myocardial ischemia-reperfusion injury, oxidative stress, inflammation
Introduction
Myocardial ischemia/reperfusion (I/R) injury exacerbates tissue injuries during reperfusion following prolonged myocardial ischemia and is a significant clinical problem associated with procedures such as angioplasty, thrombolysis and coronary bypass surgery (1–3). The underlying mechanism of myocardial I/R injury is complicated and unclear, and this may be associated with a number of factors, including overproduction of reactive oxygen species (ROS), apoptosis, mitochondrial dysfunction, intracellular calcium overload and inflammation (4–6). Although an emerging number of innovative approaches to protect cardiac tissue against myocardial I/R injury are under preclinical and clinical investigation; at present, there are no effective therapeutic strategies to reduce or protect against I/R injury (7,8). Therefore, compounds with antioxidant or anti-inflammatory properties may prove valuable for treating myocardial I/R injury.
Salidroside is isolated from Rhodiola rosea, which is used as a herbal medicine used to mitigate high altitude sickness and protect erythrocytes against oxidative stress injury (9). Salidroside has been reported to possess a wide range of pharmacological properties, including anti-inflammatory, antioxidative, anti-asthmatic and cardioprotective effects (10,11). It has previously been reported that salidroside may possess therapeutic value against myocardial I/R injury (12,13). Chang et al (14) demonstrated that salidroside protects against myocardial injury under I/R conditions by regulating energy metabolism homeostasis and inflammation. A previous study also demonstrated that salidroside exhibited its cardioprotective effects against myocardial I/R injury in rats by inhibiting apoptosis and inflammation (15). However, the underlying protective molecular mechanisms of salidroside during H/R injury remains unknown.
MicroRNAs (miRs) are a class of endogenous, small non-coding single-stranded RNAs ~22 nucleotides in length, which participate in the regulation of multiple physiological and pathophysiological processes (16). miR-21 expression is endogenously high in cardiomyocytes, vascular endothelial cells, cardiac fibroblasts and vascular smooth muscle cells (17). Although, the physiological functions of miR-21 in cardiovascular diseases have not been fully determined, the contribution of miR-21 in various cardiovascular diseases, including heart failure, myocardial infarction, myocardial fibrosis and atherosclerosis are being uncovered (18–20). Studies have shown that miR-21 is involved in numerous pathophysiological processes associated with myocardial I/R injury (21–23). MiR-21 effectively reduced the level of myocardial apoptosis and the release of inflammatory factors induced by myocardial I/R injury in rats (21). In addition, miR-21 expression was decreased in myocardial I/R injury and restoring miR-21 expression levels attenuated myocardial I/R injury (21,24,25). Therefore, miR-21 may serve as a novel biomarker of myocardial I/R injury and may be a promising therapeutic target.
In the present study, the protective effects of salidroside on oxidative stress and inflammatory injuries in an in vitro model of myocardial I/R injury was examined. The key targets and signaling pathways associated with salidroside during I/R were explored to elucidate the mechanism by which miR-21 mediated the cardioprotective effects of salidroside. The results of the present study may improve understanding of the pharmacological mechanisms of salidroside and may also provide additional evidence of the clinical value of combining traditional Chinese medicines treatment with agents which upregulate the effects of miR-21 or its downstream targets for preventing and treating myocardial I/R injury.
Materials and methods
Cell culture and hypoxia/reoxygenation (H/R) model
H9c2 rat derived cardiomyocytes (American Type Culture Collection; ATCC) were cultured in high glucose Dulbecco's modified Eagle's medium (DMEM; HyClone; GE Healthcare Life Sciences) supplemented with 10% heat-inactivated fetal bovine syndrome (Gibco; Thermo Fisher Scientific, Inc.) with a penicillin-streptomycin solution (100×; Beyotime Institute of Biotechnology). Cells were seeded in a humidified atmosphere containing 5% CO2 at 37°C (Thermo Fisher Scientific, Inc.). To mimic myocardial I/R injury in vitro, the H9c2 cells were exposed to an H/R environment as described previously (26). H9c2 cells at 80–90% confluence were incubated with serum-free DMEM and placed in an anaerobic chamber that was supplied with a gas mixture containing 94% N2, 5% CO2, and 1% O2 for 6 h to induce hypoxia. Subsequently, the cells were provided with the normal medium and placed in an incubator with 95% O2 and 5% CO2 for 12 h to allow reoxygenation. The control plates were incubated with 95% O2/5% CO2 at 37°C for 18 h. In the drug treatment experiments, H9c2 cells were pretreated with salidroside (10 µM) for 1 h and were maintained under hypoxia for 6 h, followed by reoxygenation for 12 h. In the transfected cells, H9c2 cells were transfected with miR-21 inhibitor or negative control inhibitors for 6 h before salidroside (10 µM) pre-treatment for 1 h. Subsequently, the H9c2 cells were exposed to the H/R environment as described above.
Transfection of a miR-21 inhibitor
A miR-21 inhibitor and the negative control (NC) inhibitor were purchased from Shanghai GenePharma Co., Ltd with the following sequences: miR-21 inhibitor, 5′-UCAACAUCAGUCUGAUAAGCUA-3′; Negative control, 5′-CAGUACUUUUGUGUAGUACAA-3′. Cells were transfected with the miR-21 inhibitor (100 nM) or NC inhibitor (100 nM) using Lipofectamine® RNAi Max (Thermo Fisher Scientific, Inc.) with Opti-MEM Reduced Serum Medium (Gibco; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocols. A total of 6 h after transfection, the medium was replaced with fresh DMEM. The transfection efficiency was determined using reverse transcription-quantitative (RT-q)PCR 48 h after transfection.
RT-qPCR
Total RNA from cells was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc) according to the manufacturer's protocol. The cDNA was reverse transcribed using Prime Script™ RT master mix according to the manufacturer's protocol (Takara Biotechnology Co., Ltd.). The temperature protocol was: 37°C for 15 min; followed by 85°C for 5 sec and 4°C for 20 sec. RT-qPCR was performed on an ABI 7300 thermocycler (Applied Biosystems; Thermo Fisher Scientific, Inc.) and the expression of miR-21 was quantified using SYBR Green I fluorescent dye (Takara Biotechnology Co., Ltd.). The miR-21 expression levels were normalized to the expression of U6. The thermocycling conditions were: 95°C for 2 min; followed by 40 cycles of 95°C for 15 sec and 58°C for 20 sec. The expression levels of interleukin (IL)-6, IL-1β and tumor necrosis factor (TNF)-α were normalized to the expression of GAPDH. The thermocycling conditions were: 95°C for 2 min; followed by 40 cycles of 95°C for 5 sec, 56°C for 20 sec; and 72°C for 25 sec, 65°C for 5 sec, and 95°C for 50 sec. The relative levels of miR-21 or IL-6, IL-1β and TNF-α was calculated using the comparative 2−ΔΔCq method (27) normalized to U6 or GAPDH, respectively. The sequences of the primers used were presented in Table I.
Table I.
Primer sequences.
| Genes | Sequence 5′-3′ |
|---|---|
| miR-21, forward | GCCGCTAGCTTATCAGACTGATGT |
| miR-21, reverse | GTGCAGGGTCCGAGGT |
| U6, forward | CTCGCTTCGGCAGCACA |
| U6, reverse | AACGCTTCACGAATTTGCGT |
| IL-6, forward | GCCAGAGTCATTCAGAGCAAT |
| IL-6, reverse | CTTGGTCCTTAGCCACTCCT |
| TNF-α, forward | CACCACGCTCTTCTGTCTACTG |
| TNF-α, reverse | GCTACGGGCTTGTCACTCG |
| GAPDH, forward | CGCTAACATCAAATGGGGTG |
| GAPDH, reverse | TTGCTGACAATCTTGAGGGAG |
miR, microRNA; TNF-α, tumor necrosis factor; IL, interleukin.
MTT assay
Cell viability was measured using an MTT Cell Proliferation and Cytotoxicity assay kit (Beyotime Institute of Biotechnology) according to manufacturer's protocols. Briefly, H9c2 cells were cultured in 96-well plates at a density of 1×104 cells/well. Following the treatment as described above, MTT reagent at a final concentration of 0.5 mg/ml (10 µl) was added to each well and incubated at 37°C for 4 h. The formazan crystals were subsequently dissolved using DMSO (100 µl/well). The absorbance was measured at 570 nm using a microplate spectrophotometer (BioTek China).
Measurement of lactate dehydrogenase (LDH) release, malondialdehyde (MDA) levels and superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activity
Following treatment as described above, the cells were collected and centrifuged at 3,000 × g for 10 min at 4°C, and the supernatant was stored at −80°C. The LDH release levels, MDA levels and SOD and GSH-Px activity were detected using a LDH cytotoxicity kit (Beyotime Institute of Biotechnology), and MDA assay kit (Nanjing Jiancheng Institute of Bioengineering Institute), an SOD assay kit (Beijing Solarbio Science & Technology Co., Ltd.) and a GSH-Px assay kit (Beijing Solarbio Science & Technology Co., Ltd.), respectively. All assays were performed strictly according to the manufacturer's protocol.
Detection of apoptosis
Cell apoptosis was measured using an Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining kit (BD Bioscience; Becton, Dickinson and Company) according to manufacturer's protocol. Following treatment, H9c2 cells (1×106) were collected, washed with cold PBS and resuspended in 500 µl 1× binding buffer followed by Annexin V-FITC (5 µl) and PI (10 µl). The mixture was placed in the dark for 15 min at room temperature and analyzed using a flow cytometer (Beckman Coulter) and Kaluza software 2.1.1 (Beckman Coulter, Inc.). Each experiment was performed at least three times.
Measurement of caspase-3 activity
The activity of caspase-3 was determined using a caspase-3 colorimetric assay kit (RayBiotech, Inc.) according to the manufacturer's protocol. H9c2 cells were seeded in 6-well plates at a density of 5×105 cells/well and treated as mentioned above. Subsequently, the cells were collected and lysed using a cell lysis buffer included in the kit followed by centrifuging at 10,000 × g for 5 min at 4°C. The protein concentration was measured using a bicinchoninic acid assay kit (Beyotime Institute of Biotechnology). The caspase-3 substrate Ac-DEVD-pNA (0.2 mM) was added to the lysis buffer and incubated at 37°C for 2 h. The relative fluorescence of each well was measured using a fluorescence plate reader at 450 nm within 30 min.
Measurement of intracellular ROS
Intracellular ROS generation was monitored using the Reactive Oxygen Species assay kit (Beyotime Institute of Biotechnology) which uses the fluorescent probe DCFH-DA to detect ROS. DCFH-DA is converted to DCFH by intracellular esterases, which are subsequently converted to the fluorescent DCF by ROS. After treatment, H9c2 cells were incubated with DCFH-DA (10 µM) at 37°C for 20 min in the dark. Subsequently, the cells were washed twice with PBS and analyzed using a flow cytometer (Beckman Coulter, Inc.) with excitation at 488 nm and emission at 525 nm. The data were viewed in FlowJo 10 (Tree Star, Inc.).
ELISA
The IL-6 (cat. no. M6000B), IL-1β (cat. no. RLB00) and TNF-α (cat. no. RTA00) levels in the culture supernatant were analyzed using commercially available high sensitivity ELISA kits (R&D Systems Europe, Ltd.) according to manufacturer's protocol. A microplate reader measured the optical density at 450 nm and the data were used to calculate the concentrations of various cytokines based on the standard curves. The values were determined in three independent experiments.
Statistical analysis
All data are expressed as the mean ± standard deviation of at least three independent experiments and statistical analyses were performed using Graphpad 6.0 (GraphPad Software, Inc.). The statistical significance of differences between multiple groups was estimated using a one-way analysis of variance followed by a Dunnett's post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Salidroside inhibits H/R injury in H9c2 cell
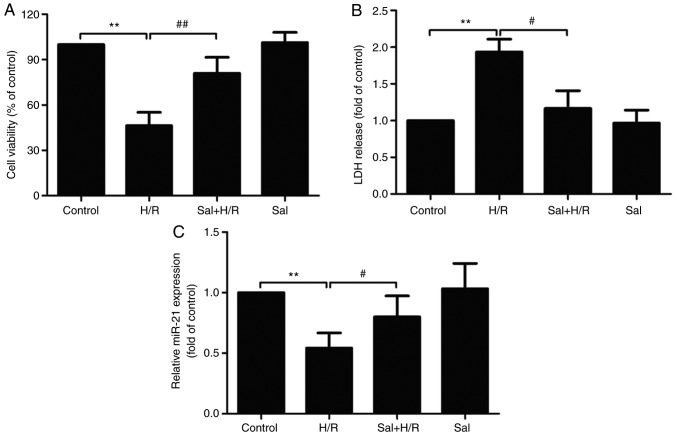
The potential effects of salidroside on H/R-induced H9c2 cell injury were first determined. The result of the MTT assay demonstrated that H/R treatment significantly reduced H9c2 cell viability, which was reduced by salidroside pretreatment (P<0.01; Fig. 1A). Salidroside reduced H/R-induced H9c2 cell death, which was further confirmed by the LDH assay. H/R resulted in a significant increase in LDH release in H9c2 cells (P<0.01), whereas salidroside pretreatment significantly attenuated the increase in LDH release (P<0.05; Fig. 1B). Furthermore, the effects of salidroside on miR-21 expression levels in the presence or absence of H/R were determined. RT-qPCR results showed that, compared with the control group, the expression of miR-21 was significantly decreased in the H/R group (P<0.01), whereas, in comparison with the H/R group, the expression of miR-21 in the salidroside + H/R co-treatment group was significantly increased (P<0.05; Fig. 1C). Together, these results suggest that salidroside reduces or prevents H/R-induced injury in H9c2 cells and miR-21 expression was increased.
Figure 1.
Effect of Sal on H/R-induced H9c2 cell injury and miR-21 expression. H9c2 cells were pretreated with Sal and maintained under hypoxia for 6 h, followed by 12 h of reoxygenation. (A) Cell viability was detected using an MTT assay. (B) An LDH release assay was performed using a commercial kit. (C) miR-21 expression was analyzed using reverse transcription-quantitative PCR. U6 was used as an internal control. Data are presented as the mean ± standard deviation of three experiments. **P<0.01 vs. the control group; #P<0.05 and ##P<0.01 vs. the H/R group. H/R, hypoxia/reoxygenation; miR-21, microRNA-21; Sal, salidroside; LDH, lactate dehydrogenase.
miR-21 inhibitor suppresses the salidroside-induced protective effects in H9c2 cells
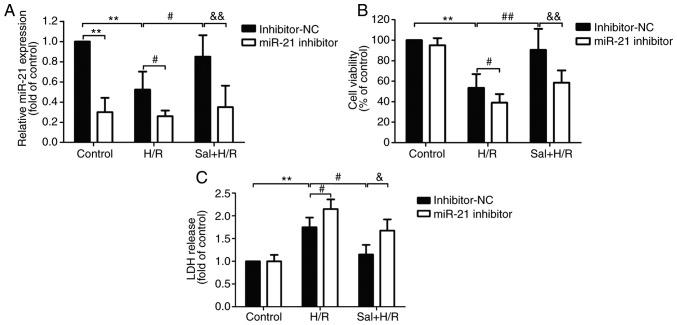
To determine whether miR-21 conferred cardioprotective effects when salidroside was used to protect against H/R injury, H9c2 cells were transfected with miR-21 inhibitor or inhibitor-NC and the transfection efficiency was confirmed by RT-qPCR. The results demonstrated that compared with the inhibitor-NC transfection group, the expression levels of miR-21 were significantly decreased in the cells transfected with miR-21 inhibitor following H/R or salidroside + H/R (P<0.05). There were no significant differences in the cells transfected with miR-21 inhibitor + control, miR-21 inhibitor + H/R and miR-21 inhibitor + salidroside + H/R group (Fig. 2A). Therefore, transfection with the miR-21 inhibitor further aggravated H/R-induced cell viability and reversed the protective effects of salidroside on cell viability in H9c2 cells (Fig. 2B). In addition, measurement of LDH release showed that transfection with a miR-21 inhibitor significantly increased the release of LDH compared with transfection with inhibitor-NC in H9c2 cells pretreated with H/R alone or salidroside + H/R group (Fig. 2C). These results suggest that miR-21 mediates the protective effects of salidroside against H/R injury in H9c2 cells.
Figure 2.
Effects of miR-21 inhibitor on cell viability and LDH release in H9c2 cells subjected to H/R injury in the presence or absence of Sal. H9c2 cells were transfected with either an miR-21 inhibitor or inhibitor-NC. Following transfection, cells were pretreated with Sal, and were subsequently maintained under hypoxia for 6 h, followed by 12 h of reoxygenation. (A) Cell viability was detected using an MTT assay. (B) An LDH release assay was conducted using a commercial kit. (C) miR-21 expression was analyzed using reverse transcription-quantitative PCR. U6 was used as an internal control. Data are presented as the mean ± standard deviation of three experiments. **P<0.01 vs. the control group; #P<0.05, ##P<0.01 vs. the H/R group; &P<0.05 and &&P<0.01 vs. the Sal + H/R control group. H/R, hypoxia/reoxygenation; miR-21, microRNA-21; Sal, salidroside; NC, negative control; LDH, lactate dehydrogenase.
miR-21 inhibitor reduces the salidroside-induced inhibition of apoptosis
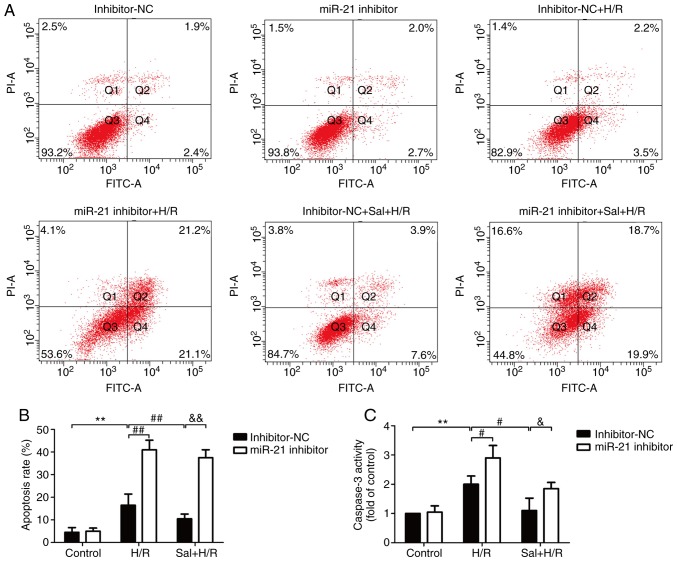
The effects of salidroside on apoptosis in H/R-treated H9c2 cells and the role of miR-21 in this process were determined. Flow cytometry results showed that salidroside pretreatment reversed the H/R-induced increase in apoptosis in the cells transfected with the inhibitor-NC, whereas this effect was blocked by transfection with the miR-21 inhibitor (Fig. 3A and B). Notably, the miR-21 inhibitor also further exacerbated H/R-induced apoptosis and no significant difference in apoptosis rate was observed between the H/R and the salidroside + H/R groups when transfected with miR-21. In the H9c2 cells transfected with the inhibitor-NC, compared with the H/R group, the caspase-3 activity was significantly reduced compared with the salidroside + H/R treated cells (P<0.05; Fig. 3C). However, the protective effect of salidroside was reversed by transfection with the miR-21 inhibitor. These data suggest that salidroside inhibited H/R-induced apoptosis by increasing miR-21 expression in H9c2 cells.
Figure 3.
Effects of the miR-21 inhibitor on apoptosis in H9c2 cells subjected to H/R in the presence or absence of salidroside. H9c2 cells were transfected with either a miR-21 inhibitor or inhibitor-NC. Following transfection, cells were pretreated with salidroside, and were subsequently maintained under hypoxia for 6 h, followed by 12 h of reoxygenation. (A) Apoptotic cell rate was assessed by staining cells with Annexin V- FITC/PI followed by flow cytometry. (B) Quantitative analysis of flow cytometry results. (C) Caspase-3 activity was determined using a caspase-3 colorimetric assay kit. Data are presented as the mean ± standard deviation of three experiments. **P<0.01 vs. the control group; #P<0.05 and ##P<0.01 vs. the H/R group; &P<0.05 and &&P<0.01 vs. the Sal + H/R control group. H/R, hypoxia/reoxygenation; miR-21, microRNA-21; PI, propidium iodide; Sal, salidroside; NC, negative control; FITC, fluorescein isothiocyanate.
Salidroside decreases oxidative stress via miR-21 in H9c2 cells
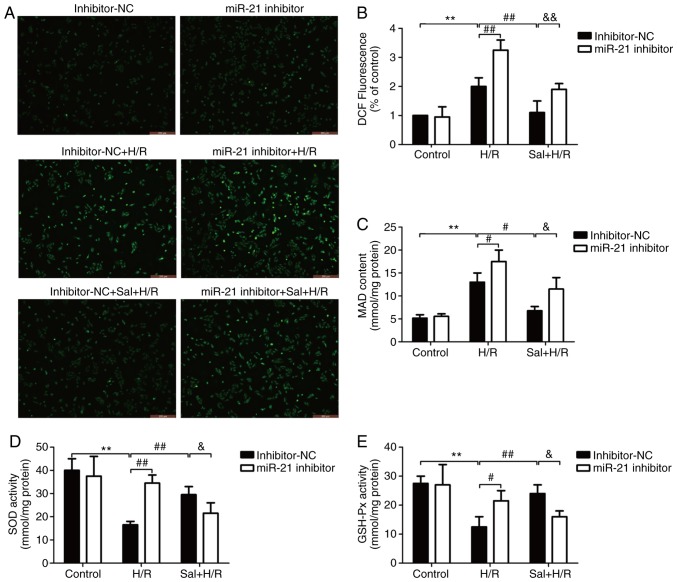
The effect of salidroside on oxidative stress in H/R-treated H9c2 cells and the role of miR-21 in this process were determined. As shown in Fig. 4, ROS generation (Fig. 4A and B) and MDA levels (Fig. 4C) were significantly increased in the H/R-treated cells compared with the control cells (P<0.01), and salidroside pretreatment significantly decreased the levels of these molecules (P<0.05). However, miR-21 downregulation, by transfecting cells with the miR-21 inhibitor, abrogated the protective effects of salidroside on oxidative stress in H/R-treated H9c2 cells. Notably, in the miR-21 inhibitor group, salidroside had no effect on the generation of ROS (Fig. 4A and B) and MDA levels (Fig. 4C) under H/R compared with H/R alone group. The enzymatic activities of SOD (Fig. 4D) and GSH-Px (Fig. 4E) were significantly reduced in the cells treated with H/R compared with the control group (P<0.01). The observed decreases were reversed when cells were treated with salidroside, and the protective effects were abrogated by transfection with the miR-21 inhibitor in the salidroside + H/R treated cells. Similarly, salidroside did not significantly alter the activity of SOD and GSH-Px when the cells were transfected with the miR-21 inhibitor compared with H/R alone group. Thus, miR-21 contributed to the protection of salidroside against H/R-induced oxidative stress injury in H9c2 cells.
Figure 4.
Effect of the miR-21 inhibitor on oxidative stress in H9c2 cells subjected to H/R injury in the presence or absence of Sal. H9c2 cells were transfected with either a miR-21 inhibitor or inhibitor-NC. Following transfection, cells were pretreated with Sal, and were subsequently maintained under hypoxia for 6 h, followed by 12 h of reoxygenation. (A) Production of ROS was observed using a DCFH-DA fluorescent probe under a fluorescence microscope. Scale bar, 100 µm. (B) ROS levels were determined by flow cytometry. (C) MDA levels were analyzed using an MDA assay kit. (D) SOD activity was measured using an SOD assay kit. (E) GHS-Px activity was determined using a GSH-Px assay kit. Data are presented as the mean ± standard deviation of three experiments. **P<0.01 vs. the control group; #P<0.05 and ##P<0.01 vs. the H/R group; &P<0.05 and &&P<0.01 vs. the Sal + H/R control group. H/R, hypoxia/reoxygenation; miR-21, microRNA-21; MDA, malondialdehyde; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; NC, negative control; Sal, salidroside.
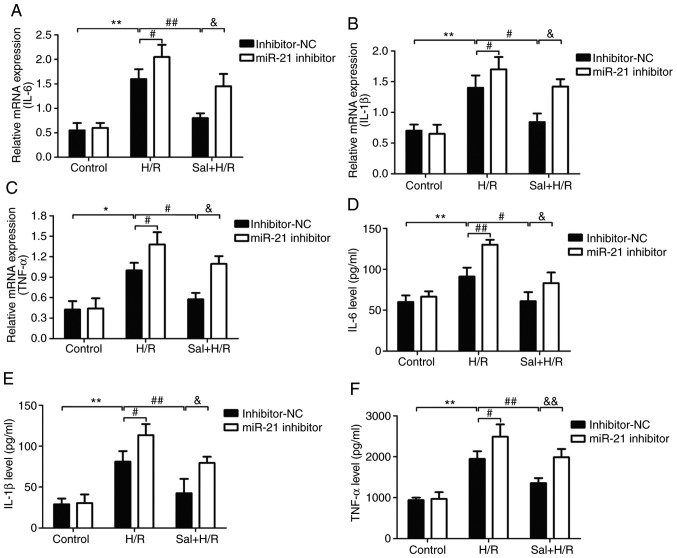
miR-21 inhibitor abrogates the protective effects of salidroside to the inflammatory response
The effect of salidroside on the inflammatory response and the role of miR-21 in these processes were determined. The levels of the inflammatory markers including IL-6, IL-1β, and TNF-α in H9c2 cells or culture supernatant were measured by RT-qPCR and ELISA. As shown in Fig. 5, ELISA analysis showed that H/R treatment significantly upregulated the mRNA expression levels of IL-6 (P<0.01; Fig. 5A), IL-1β (P<0.01; Fig. 5B) and TNF-α (P<0.01; Fig. 5C), and these effects were reversed by salidroside in cells transfected with the inhibitor-NC. Transfection with miR-21 inhibitor prevented the salidroside-induced downregulation of IL-6, IL-1β and TNF-α levels. In addition, the mRNA expression levels of IL-6 (Fig. 5D), IL-1β (Fig. 5E) and TNF-α (Fig. 5F) were also increased in the H/R group compared with the control group, whereas the expression of the inflammatory markers were decreased in the salidroside + H/R group compared with the H/R group. Transfection with the miR-21 inhibitor significantly attenuated the effects of the salidroside-induced decrease in IL-6, IL-1β and TNF-α mRNA expression (P<0.05). Additionally, transfection with the miR-21 inhibitor further exacerbated the H/R-induced increase in the levels of IL-6, IL-1β and TNF-α, and the mRNA expression levels of IL-1β and TNF-α mRNA (P<0.05). Taken together, the data above demonstrated that miR-21 acts as an anti-inflammatory molecule which regulates the effects of salidroside in the H/R-induced inflammatory response.
Figure 5.
Effect of the miR-21 inhibitor on inflammatory factors in H9c2 cells subjected to H/R injury in the presence or absence of Sal. H9c2 cells were transfected with either a miR-21 inhibitor or inhibitor-NC. Following transfection, cells were pretreated with Sal, and were subsequently maintained under hypoxia for 6 h, followed by 12 h of reoxygenation. mRNA expression levels of (A) IL-6, (B) IL-1β and (C) TNF-α were determined using RT-qPCR and normalized to GAPDH. The levels of (D) IL-6, (E) IL-1β and (F) TNF-α in the culture supernatant were detected by western blot analysis. Data are presented as the mean ± standard deviation of three experiments. *P<0.05 and **P<0.01 vs. the control group; #P<0.05 and ##P<0.01 vs. the H/R group; &P<0.05 and &&P<0.01 vs. the Sal + H/R control group. H/R, hypoxia/reoxygenation; miR-21, microRNA-21; IL, interleukin; TNF-α, tumor necrosis factor-α; NC, negative control; Sal, salidroside.
Discussion
In the present study, the effects of salidroside on H/R injury in H9c2 cells and the role of miR-21 in this process was assessed. The results demonstrated that salidroside attenuated H/R-induced cytotoxicity and apoptosis, and decreased oxidative stress and the inflammatory response. Additionally, the results suggested that miR-21 mediated the protective effects of salidroside in H/R-induced injury in H9c2 cells. These findings suggest that salidroside may be an effective means of reducing myocardial I/R injury and enhancing miR-21 levels may additionally reduce myocardial I/R injury, highlighting a potentially novel therapeutic approach.
Salidroside is a major active ingredient obtained from the medicinal plant Rhodiola rosea and has various pharmacological properties, including antioxidant (28), anti-inflammatory (29) and cardioprotective effects (26). To date, numerous studies have demonstrated the protective effects of salidroside on myocardial injury (30), myocardial hypoxia (31) and myocardial I/R injury (13). However, the underlying molecular mechanisms of salidroside action remained unclear. Consistent with these studies, the data of the present study also confirmed that salidroside pretreatment attenuated the H/R-induced cytotoxicity and apoptosis, inhibiting myocardial I/R injury. Notably, emerging evidence has shown that myocardial I/R injury leads to decreased levels of miR-21 and overexpression of miR-21 is able to effectively inhibit myocardial apoptosis and the inflammatory response, protecting the myocardium from I/R injury (21,22,32). However, the role of miR-21 in the cardioprotective effects of salidroside has not been reported previously, to the best of our knowledge. In present study, it was demonstrated, for the first time, that salidroside reversed the H/R-induced downregulation of miR-21, and inhibition of miR-21 abrogated the effects of salidroside treatment in the H/R model of injury in H9c2 cells. These results suggest that miR-21 mediates the protective effects of salidroside in myocardial I/R injury.
Myocardial oxidative stress is a major initiator of the pathological process of cardiac remodeling following I/R (4). Accumulating evidence has shown that ROS are the major initiators of myocardial damage in myocardial I/R injury and the attenuation of oxidative stress in myocardial cell has been demonstrated to improve myocardial function following ischemia (4,33). It has been demonstrated that salidroside may suppress oxidative stress-induced endothelial dysfunction, cardiomyocyte injury and necrosis, and cerebral ischemia/reperfusion injury, through decreasing excessive ROS generation and improving mitochondrial function (34–37). However, the effect of salidroside in myocardial I/R injury-induced oxidative stress has not been studied. In the present study, salidroside pretreatment reduced the H/R-induced increase in production of ROS and the levels of MDA, suggesting that salidroside reduced oxidative stress during H/R in H9c2 cells. Oxidative stress occurs when there is an imbalance between ROS production and the antioxidant defense systems in cells, such that the latter becomes overwhelmed (38). SOD and GSH are the primary cellular defense mechanisms against oxidative stress injury and are the major intracellular redox buffers in several cell types (39,40). In the present study, salidroside improved the antioxidant defense system in H/R-treated H9c2 cells as shown by the increase in the activities of SOD and GSH-Px. Numerous studies have demonstrated the ability of miR-21s to preserve an efficient antioxidant response induced by multiple injuries (41,42). In the present study, it was demonstrated that inhibition of miR-21 abolished the protective effects of salidroside on oxidative stress in H/R-treated H9c2 cells. These results suggest that miR-21 may contribute to the protective effect of salidroside against H/R-induced oxidative stress in H9c2 cells.
The inflammatory response is another crucial component in the initiation and exacerbation of myocardial I/R injury (43,44). I/R increases the local or systemic release of proinflammatory cytokines, including TNF-α, IL-1β and IL-6, and further increases cardiac injury, and inhibition of the I/R-induced inflammatory response alleviates myocardial I/R injury (45,46). It has been previously hypothesized that salidroside may exhibit a protective effect on myocardial injury by inhibiting the inflammatory response (11,14,47). In vitro and in vivo experiments have demonstrated that miR-21 regulated the production of various important pro-inflammatory cytokines secreted during I/R injury (48,49). However, the role of miR-21 in the protective effects mediated by salidroside during the inflammatory response induced by myocardial I/R injury have not been extensively studied. In the present study, salidroside reversed the H/R-induced increases in expression and activity of IL-6, IL-1β and TNF-α in H9c2 cells, thus inhibiting the inflammatory response during H/R, and these effects were abolished by the miR-21 inhibitor. These results suggest that miR-21 mediates the salidroside-induced inhibition of the inflammatory response in H/R-treated H9c2 cells.
In conclusion, the present study demonstrated that salidroside pretreatment mitigated damage of cardiomyocytes after H/R-stimulation of H9c2 cells. The cardioprotective effects of salidroside may be attributed to its ability of suppressing myocardial oxidative stress and inflammation in vitro, through enhancing miR-21 expression. Therefore, the results of the present study suggest that salidroside may be a potential therapeutic agent for the treatment of myocardial I/R injury and modulation of miR-21 levels may provide a new strategic option for limiting damage following a cardiovascular event. However, the molecular mechanism by which miR-21 protects cultured H9c2 cells against oxidative stress and inflammation induced by H/R requires further elucidation.
Acknowledgements
Not applicable.
Funding
The present study was funded by the China Aerospace Science and Technology Group Corporation Medical and Health Research Project (grant no. 2017-LCYL-003).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the author on reasonable request.
Authors' contribution
BL and HW designed the study and carried out the experiment. ML, NJ, and JL performed the analysis. MZ participated in the design of the study. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 2.Humphries KH, Izadnegahdar M, Sedlak T, Saw J, Johnston N, Schenck-Gustafsson K, Shah RU, Regitz-Zagrosek V, Grewal J, Vaccarino V, et al. Sex differences in cardiovascular disease-impact on care and outcomes. Front Neuroendocrinol. 2017;46:46–70. doi: 10.1016/j.yfrne.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu C, Li T, Jiang S, Yang Z, Lv J, Yi W, Yang Y, Fang M. AMP-activated protein kinase sparks the fire of cardioprotection against myocardial ischemia and cardiac ageing. Ageing Res Rev. 2018;47:168–175. doi: 10.1016/j.arr.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Shvedova M, Anfinogenova Y, Atochina-Vasserman EN, Schepetkin IA, Atochin DN. c-Jun N-terminal kinases (JNKs) in myocardial and cerebral ischemia/reperfusion injury. Front Pharmacol. 2018;9:715. doi: 10.3389/fphar.2018.00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu LM, Di WC, Dong X, Li Z, Zhang Y, Xue XD, Xu YL, Zhang J, Xiao X, Han JS, et al. Melatonin protects diabetic heart against ischemia-reperfusion injury, role of membrane receptor-dependent cGMP-PKG activation. Biochim Biophys Acta Mol Basis Dis. 2018;1864:563–578. doi: 10.1016/j.bbadis.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 6.Sun MS, Jin H, Sun X, Huang S, Zhang FL, Guo ZN, Yang Y. Free radical damage in ischemia-reperfusion injury: An obstacle in acute ischemic stroke after revascularization therapy. Oxid Med Cell Longev. 2018;2018:3804979. doi: 10.1155/2018/3804979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González-Montero J, Brito R, Gajardo AI, Rodrigo R. Myocardial reperfusion injury and oxidative stress: Therapeutic opportunities. World J Cardiol. 2018;10:74–86. doi: 10.4330/wjc.v10.i9.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang CF. Clinical manifestations and basic mechanisms of myocardial ischemia/reperfusion injury. Ci Ji Yi Xue Za Zhi. 2018;30:209–215. doi: 10.4103/tcmj.tcmj_33_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kucinskaite A, Briedis V, Savickas A. Experimental analysis of therapeutic properties of Rhodiola rosea L. and its possible application in medicine. Medicina (Kaunas) 2004;40:614–619. [PubMed] [Google Scholar]
- 10.Chang X, Luo F, Jiang W, Zhu L, Gao J, He H, Wei T, Gong S, Yan T. Protective activity of salidroside against ethanol-induced gastric ulcer via the MAPK/NF-κB pathway in vivo and in vitro. Int Immunopharmacol. 2015;28:604–615. doi: 10.1016/j.intimp.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 11.Zhu L, Wei T, Chang X, He H, Gao J, Wen Z, Yan T. Effects of salidroside on myocardial injury in vivo in vitro via regulation of Nox/NF-κB/AP1 Pathway. Inflammation. 2015;38:1589–1598. doi: 10.1007/s10753-015-0134-0. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Liu P, Feng X, Ma C. Salidroside suppressing LPS-induced myocardial injury by inhibiting ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J Cell Mol Med. 2017;21:3178–3189. doi: 10.1111/jcmm.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun MY, Ma DS, Zhao S, Wang L, Ma CY, Bai Y. Salidroside mitigates hypoxia/reoxygenation injury by alleviating endoplasmic reticulum stress induced apoptosis in H9c2 cardiomyocytes. Mol Med Rep. 2018;18:3760–3768. doi: 10.3892/mmr.2018.9403. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Chang X, Zhang K, Zhou R, Luo F, Zhu L, Gao J, He H, Wei T, Yan T, Ma C. Cardioprotective effects of salidroside on myocardial ischemia-reperfusion injury in coronary artery occlusion-induced rats and Langendorff-perfused rat hearts. Int J Cardiol. 2016;215:532–544. doi: 10.1016/j.ijcard.2016.04.108. [DOI] [PubMed] [Google Scholar]
- 15.Zhu L, Wei T, Gao J, Chang X, He H, Luo F, Zhou R, Ma C, Liu Y, Yan T. The cardioprotective effect of salidroside against myocardial ischemia reperfusion injury in rats by inhibiting apoptosis and inflammation. Apoptosis. 2015;20:1433–1443. doi: 10.1007/s10495-015-1174-5. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Wang DZ. microRNAs in cardiovascular development. J Mol Cell Cardiol. 2012;52:949–957. doi: 10.1016/j.yjmcc.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng Y, Zhang C. MicroRNA-21 in cardiovascular disease. J Cardiovasc Transl Res. 2010;3:251–255. doi: 10.1007/s12265-010-9169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oyama Y, Bartman CM, Gile J, Eckle T. Circadian MicroRNAs in Cardioprotection. Curr Pharm Des. 2017;23:3723–3730. doi: 10.2174/1381612823666170707165319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panagal M, Biruntha M, Vidhyavathi RM, Sivagurunathan P, Senthilkumar SR, Sekar D. Dissecting the role of miR-21 in different types of stroke. Gene. 2019;681:69–72. doi: 10.1016/j.gene.2018.09.048. [DOI] [PubMed] [Google Scholar]
- 20.Pordzik J, Pisarz K, De Rosa S, Jones AD, Eyileten C, Indolfi C, Malek L, Postula M. The potential role of platelet-related micrornas in the development of cardiovascular events in high-risk populations, including diabetic patients: A review. Front Endocrinol (Lausanne) 2018;9:74. doi: 10.3389/fendo.2018.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan YQ, Li J, Li XW, Li YC, Li J, Lin JF. Effect of miR-21/TLR4/NF-κB pathway on myocardial apoptosis in rats with myocardial ischemia-reperfusion. Eur Rev Med Pharmacol Sci. 2018;22:7928–7937. doi: 10.26355/eurrev_201811_16420. [DOI] [PubMed] [Google Scholar]
- 22.Tong Z, Tang Y, Jiang B, Wu Y, Liu Y, Li Y, Xiao X. Phosphorylation of nucleolin is indispensable to upregulate miR-21 and inhibit apoptosis in cardiomyocytes. J Cell Physiol. 2019;234:4044–4053. doi: 10.1002/jcp.27191. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, Kriegel AJ, Jiao X, Liu H, Bai X, Olson J, Liang M, Ding X. miR-21 in ischemia/reperfusion injury: A double-edged sword? Physiol Genomics. 2014;46:789–797. doi: 10.1152/physiolgenomics.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu K, Ma L, Zhou F, Yang Y, Hu HB, Wang L, Zhong L. Identification of microRNAs related to myocardial ischemic reperfusion injury. J Cell Physiol. 2018;234:11380–11390. doi: 10.1002/jcp.27795. [DOI] [PubMed] [Google Scholar]
- 25.Ye Y, Perez-Polo JR, Qian J, Birnbaum Y. The role of microRNA in modulating myocardial ischemia-reperfusion injury. Physiol Genomics. 2011;43:534–542. doi: 10.1152/physiolgenomics.00130.2010. [DOI] [PubMed] [Google Scholar]
- 26.Cheng J, Wu Q, Lv R, Huang L, Xu B, Wang X, Chen A, He F. MicroRNA-449a inhibition protects H9C2 cells against hypoxia/reoxygenation-induced injury by targeting the notch-1 signaling pathway. Cell Physiol Biochem. 2018;46:2587–2600. doi: 10.1159/000489686. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Gao J, He H, Jiang W, Chang X, Zhu L, Luo F, Zhou R, Ma C, Yan T. Salidroside ameliorates cognitive impairment in a d-galactose-induced rat model of Alzheimer's disease. Behav Brain Res. 2015;293:27–33. doi: 10.1016/j.bbr.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Xiao L, Zhu L, Hu M, Wang Q, Yan T. The effect of synthetic salidroside on cytokines and airway inflammation of asthma induced by diisocyanate (TDI) in mice by regulating GATA3/T-bet. Inflammation. 2015;38:697–704. doi: 10.1007/s10753-014-9979-x. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Xu P, Wang Y, Liu H, Zhou Y, Cao X. The protection of salidroside of the heart against acute exhaustive injury and molecular mechanism in rat. Oxid Med Cell Longev. 2013;2013:507832. doi: 10.1155/2013/507832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu ZW, Chen X, Jin XH, Meng XY, Zhou X, Fan FX, Mao SY, Wang Y, Zhang WC, Shan NN, et al. SILAC-based proteomic analysis reveals that salidroside antagonizes cobalt chloride-induced hypoxic effects by restoring the tricarboxylic acid cycle in cardiomyocytes. J Proteomics. 2016;130:211–220. doi: 10.1016/j.jprot.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 32.Han Q, Zhang HY, Zhong BL, Zhang B, Chen H. Antiapoptotic effect of recombinant HMGB1 A-box protein via regulation of microRNA-21 in myocardial ischemia-reperfusion injury model in rats. DNA Cell Biol. 2016;35:192–202. doi: 10.1089/dna.2015.3003. [DOI] [PubMed] [Google Scholar]
- 33.Francis A, Baynosa R. Ischaemia-reperfusion injury and hyperbaric oxygen pathways: A review of cellular mechanisms. Diving Hyperb Med. 2017;47:110–117. doi: 10.28920/dhm47.2.110-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han J, Xiao Q, Lin YH, Zheng ZZ, He ZD, Hu J, Chen LD. Neuroprotective effects of salidroside on focal cerebral ischemia/reperfusion injury involve the nuclear erythroid 2-related factor 2 pathway. Neural Regen Res. 2015;10:1989–96. doi: 10.4103/1673-5374.172317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xing SS, Li J, Chen L, Yang YF, He PL, Li J, Yang J. Salidroside attenuates endothelial cellular senescence via decreasing the expression of inflammatory cytokines and increasing the expression of SIRT3. Mech Ageing Dev. 2018;175:1–6. doi: 10.1016/j.mad.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Zhong Z, Han J, Zhang J, Xiao Q, Hu J, Chen L. Pharmacological activities, mechanisms of action, and safety of salidroside in the central nervous system. Drug Des Devel Ther. 2018;12:1479–1489. doi: 10.2147/DDDT.S160776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Y, Zhang YJ, Liu WW, Shi AW, Gu N. Salidroside Suppresses HUVECs cell injury induced by oxidative stress through activating the Nrf2 signaling pathway. Molecules. 2016;21(pii):E1033. doi: 10.3390/molecules21081033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juránek I, Bezek S. Controversy of free radical hypothesis: Reactive oxygen species-cause or consequence of tissue injury? Gen Physiol Biophys. 2005;24:263–278. [PubMed] [Google Scholar]
- 39.Sena CM, Leandro A, Azul L, Seiça R, Perry G. Vascular oxidative stress: Impact and therapeutic approaches. Front Physiol. 2018;9:1668. doi: 10.3389/fphys.2018.01668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng J, Huang N, Huang S, Li L, Ling Z, Jin S, Huang A, Lin K, Zou X. Effect of miR-21 down-regulated by H2O2 on osteogenic differentiation of MC3T3-E1 cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018;32:276–284. doi: 10.7507/1002-1892.201707030. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi B, Wang Y, Zhao R, Long X, Deng W, Wang Z. Bone marrow mesenchymal stem cell-derived exosomal miR-21 protects C-kit+ cardiac stem cells from oxidative injury through the PTEN/PI3K/Akt axis. PLoS One. 2018;13:e0191616. doi: 10.1371/journal.pone.0191616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ong SB, Hernández-Reséndiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA, Hausenloy DJ. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. 2018;186:73–87. doi: 10.1016/j.pharmthera.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, Hu W, Lu C, Ma Z, Jiang S, Gu C, Acuña-Castroviejo D, Yang Y. Targeting NLRP3 (nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3) inflammasome in cardiovascular disorders. Arterioscler Thromb Vasc Biol. 2018;38:2765–2779. doi: 10.1161/ATVBAHA.118.311916. [DOI] [PubMed] [Google Scholar]
- 45.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia/Reperfusion. Compr Physiol. 2016;7:113–170. doi: 10.1002/cphy.c160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu T, Qin G, Jiang W, Zhao Y, Xu Y, Lv X. 6-gingerol protects heart by suppressing myocardial ischemia/reperfusion induced inflammation via the PI3K/Akt-dependent mechanism in rats. Evid Based Complement Alternat Med. 2018;2018:6209679. doi: 10.1155/2018/6209679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He H, Chang X, Gao J, Zhu L, Miao M, Yan T. Salidroside mitigates sepsis-induced myocarditis in rats by regulating IGF-1/PI3K/Akt/GSK-3β Signaling. Inflammation. 2015;38:2178–2184. doi: 10.1007/s10753-015-0200-7. [DOI] [PubMed] [Google Scholar]
- 48.Song N, Zhang T, Xu X, Lu Z, Yu X, Fang Y, Hu J, Jia P, Teng J, Ding X. miR-21 protects against ischemia/reperfusion-induced acute kidney injury by preventing epithelial cell apoptosis and inhibiting dendritic cell maturation. Front Physiol. 2018;9:790. doi: 10.3389/fphys.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang W, Shu L. Upregulation of miR-21 by ghrelin ameliorates ischemia/reperfusion-induced acute kidney injury by inhibiting inflammation and cell apoptosis. DNA Cell Biol. 2016;35:417–25. doi: 10.1089/dna.2016.3231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the author on reasonable request.