Abstract
Resveratrol (Res) is a natural compound that possesses anti-inflammatory properties. However, the protective molecular mechanisms of Res against lipopolysaccharide (LPS)-induced inflammation have not been fully studied. In the present study, RAW264.7 cells were stimulated with LPS in the presence or absence of Res, and the subsequent modifications to the LPS-induced signaling pathways caused by Res treatment were examined. It was identified that Res decreased the mRNA levels of Toll-like receptor 4 (TLR4), myeloid differentiation primary response protein MyD88, TIR domain-containing adapter molecule 2, which suggested that Res may inhibit the activation of the TLR4 signaling pathway. It suppressed the expression levels of total and phosphorylated TLR4, NF-κB inhibitor, p38 mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase, extracellular signal-regulated kinase 1/2 and interferon (IFN) regulatory factor 3 (IRF3) proteins. Following treatment with Res or specific inhibitors, the production of pro-inflammatory mediators including tumor necrosis factor-α, interleukin (IL)-6, IL-8 and IFN-β were decreased and the expression of anti-inflammatory mediator IL-10 was increased. These results suggested that Res may inhibit the signaling cascades of NF-κB, MAPKs and IRF3, which modulate pro-inflammatory cytokines. In conclusion, Res exhibited a therapeutic effect on LPS-induced inflammation through suppression of the TLR4-NF-κB/MAPKs/IRF3 signaling cascades.
Keywords: inflammation, resveratrol, lipopolysaccharides, nuclear factor-κB, mitogen-activated protein kinases, interferon regulatory factor 3
Introduction
Inflammation is a response of tissues to chemical and mechanical injury or infection, which is usually caused by various bacteria (1). The inflammatory response or chronic infections may cause significant damage to the host, including rheumatoid arthritis and psoriasis. Lipopolysaccharide (LPS), a component of the outer membrane of gram-negative bacteria, initiates a number of major cellular responses that serve critical roles in the pathogenesis of inflammatory responses (2). LPS may lead to an acute inflammatory response towards pathogens. Bacterial LPS has been extensively used to establish an inflammatory model as it stimulates the release of inflammatory cytokines including interleukin (IL)-8, IL-6 and IL-1β in various cell types (3,4).
Toll-like receptor 4 (TLR4) is the cell-surface receptor for LPS. Regulation of TLR4 activation involves glycosylphosphatidylinositol (GPI)-anchored monocyte differentiation antigen CD14 (CD14), lymphocyte antigen 96 (MD-2), and the lipopolysaccharide-binding protein (LBP). LBP binds to the lipid A moiety of LPS and transfers LPS to CD14, which guarantees and optimizes signaling through the TLR4/MD-2 complex (5). A total of 2 signaling pathways are initiated by TLR4 activation; one leads to the activation of NF-κB and mitogen-activated protein kinases (MAPKs) through the recruitment and activation of myeloid differentiation primary response protein MyD88 (MyD88) and Toll/interleukin-1 receptor domain-containing adapter protein (TIRAP). The other pathway is modulated by TIR domain-containing adapter molecule 2 (TRAM) and TIR domain-containing adaptor molecule 1 (TRIF), requiring the internalization of TLR4, which activates IκB kinase and interferon (IFN) regulatory factor 3 (IRF3), leading to the induction of type 1 IFN genes (6). These cascaded transcriptional reactions induce robust expressions of thousands of genes, finally regulating the release of inflammatory cytokines and anti-inflammatory factors. Therefore, the TLR4/NF-κB/MAPKs pathways are considered as some of the primary signaling pathways involved in inflammatory response (7).
Resveratrol (3,4′, 5-Trihydroxy-trans-stilbene; Res), a type of natural phytoalexin polyphenol with marked biological effects, is present in a number of plants. It has been suggested that Res has a number of therapeutic properties, including antioxidant, cardio-protective, antiviral, anti-aging and anti-inflammatory effects (8). At present, Res is present in food, medicine and health care products. One of the primary ways that Res exerts its anti-inflammatory activity is regulation of a number of signaling pathways. It has been suggested that Res may inhibit the NF-κB activation induced by TLR4-mediated signaling (9). In addition, another important anti-inflammatory action of Res it the suppression of LPS-induced TNF receptor-associated factor 6 (TRAF6) expression and ubiquitination, consequently attenuating the LPS-induced TLR4-TRAF6, MAPK and Akt pathways (10). Previous data suggests that Res inhibits the inflammation via regulating the NF-κB, MAPKs, TLR4 and AKT signaling pathways; however, to the best of our knowledge, the combined evaluation of all these pathways following Res treatment has not been performed. Therefore, the aim of present study was to evaluate the association between the anti-inflammatory effect of Res and the production of inflammatory factors and finally to reveal the protective mechanism of Res in LPS-induced inflammation.
Materials and methods
Reagents
Res was purchased from Beijing Solarbio Science and Technology Co., Ltd. SP600125 (cat. no. HY-12041), BAY11-7082 (cat. no. HY-13453) and SB203580 (cat. no. HY-10256) were purchased from MedChemExpress. LPS (Escherichia coli 055:B5; cat. no. L2880) and L-glutamine (cat. no. G3126) were purchased from Sigma-Aldrich; Merck KGaA The mice mononuclear macrophage RAW264.7 cell line was obtained from the China Center for Type Culture Collection. Fetal bovine serum was purchased from Beijing TransGen Biotech Co., Ltd. The Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular Technologies. Dulbecco's modified Eagle medium (DMEM) and PBS were purchased from HyClone; GE Healthcare Life Sciences. Revert Aid first-strand cDNA synthesis kit was purchased from Thermo Fisher Scientific, Inc. The total protein extraction kit for cultured cells was purchased from Wuhan Boster Biological Technology, Ltd. (cat. no. AR0103). The BCA kit was purchased from Beijing Solarbio Science and Technology Co., Ltd. (cat. no. PC0020). IQ SYBR Supermix extraction reagent and TRIzol® reagent were purchased from Bio-Rad Laboratories, Inc. Mouse tumor necrosis factor-α (TNF-α; cat. no. ml002095), IL-6 (cat. no. ml063159) IL-8 (cat. no. ml058632), IL-10 (cat. no. ml002285) and IFN-β (cat. no. ml063095) ELISA kits were purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd. Anti-β-actin (cat. no. 12620), anti-NF-κB inhibitor (IκBα; cat. no. 4814), extracellular signal-regulated kinase (ERK; cat. no. 4695), p38 MAPK (cat. no. 8690), phosphorylated (p)-IκBα (cat. no. 2859), p-p38 MAPK (cat. no. 4511) and p-ERK (cat. no. 4370) antibodies were purchased from Cell Signaling Technology, Inc. Stress-activated kinases (SAPK)/c-Jun N-terminal kinase (JNK; cat. no. ab179461), phospho-SAPK/JNK (cat. no. ab76572), IRF3 (cat. no. ab76493), phospho-IRF3 (cat. no. ab68481) and TLR4 (cat. no. ab13556) antibodies were purchased from Abcam. Secondary antibody for goat anti-rabbit and anti-mouse immunoglobulin (IgG) horseradish peroxidase (HRP) were acquired from BIOSS (cat. nos. BS-0293G and BS-0296G).
Cell culture
RAW264.7 cells were cultured in endotoxin-tested DMEM with 10% fetal calf serum (FCS) supplied by Beijing Transgen Biotech Co., Ltd. in the presence of 5% CO2 at 37°C. Prior to treatment, cells were incubated overnight at 37°C.
Cell viability assay
CCK-8 assays to measure the viability of cells. Briefly, the cells were cultured into 96-wells plate with a density of 5×103 cells/well and incubated overnight in the presence of 5% CO2 at 37°C. Then the cells were washed with fresh 1% FCS and treated with or without LPS (625, 1,500, 2,500, 5,000 and 10,000 ng/ml), Res (6.25, 12.5, 25, 50 and 100 µM), BAY11-7082 (the inhibitor of NF-κB; 2.5, 5, 10, 20 and 40 µM), SP600125 (the broad-spectrum inhibitor of JNK; 2.5, 5, 10, 20 and 40 µM) or SB203580 (the inhibitor of p38 MAPK; 2.5, 5, 10, 20 and 40 µM) for 24 h. The cells were washed with PBS. In each well, 10 µl CCK-8 solution was added and incubated for 1 h at 37°C. The absorbance was measured at 450 nm. The cell viability was calculated and represented graphically.
Stimulation conditions of LPS and Res concentration
RAW264.7 cells in 6-well (5×105 cells/ml) plates were pretreated with 62.5–500 ng/ml LPS for 12 and 24 h. The TNF-α and IL-6 levels were measured using ELISAs. To investigate the optimal Res concentration against LPS-induced inflammation, cells were pretreated with 6.25–25 µM of Res for 2 h followed by the stimulation with LPS (500 ng/ml) for 12 h. The TNF-α and IL-6 levels were analyzed using ELISA.
Western blot analysis
RAW264.7 cells in 6-well (5×105 cells/ml) plates were pretreated with different concentrations of Res (6.25, 12.5 and 25 µM) for 2 h prior to the addition of 500 ng/ml LPS for 12 h. After incubation for 12 h, the total proteins were extracted following the instructions of protein extraction kit. Protein concentrations were measured by the BCA Protein Assay kit. Following this, equal amounts of protein (25 µg) from each sample were heated to 95°C for 5 min with 4X Protein SDS-PAGE Loading Buffer and then separated by SDS-PAGE (30% gel). Proteins were transferred onto polyvinylidene fluoride membranes. Following blocking for 1 h with 5% skim milk at room temperature, the membranes were incubated with primary antibodies against β-actin, IκBα, p-IκBα, p38 MAPK, p-p38 MAPK, IRF3, p-IRF3, ERK1/2, p-ERK1/2, JNK, p-JNK overnight at 4°C. Following washing 3 times with TBST buffer, the membranes were incubated with the aforementioned secondary antibodies for 1 h at room temperature. The electrochemiluminescence kit purchased from Beijing Solarbio Science & Technology Co., Ltd. (cat. no. PE0010) was used to detect the bands. ImageJ v1.8.0 software (National Institutes of Health) and GraphPad Prism v.6 software (GraphPad Software, Inc.) were used to perform the densitometric analysis.
Cytokine assays
RAW264.7 cells in 6-well (5×105 cells/ml) plates were pretreated with Res (25 µM), SP600125 (10 µM), SB203580 (20 µM) or BAY11-7082 (10 µM) for 2 h prior to the addition of 500 ng/ml LPS for 12 h. Then, the supernatants were collected by centrifugation at 0.12 × g for 10 min at 4°C. Levels of TNF-α, IL-6, IL-10, IL-8 and IFN-β were measured by ELISA kits.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RAW264.7 cells in 6-well (5×105 cells/ml) plates were pretreated with Res (25 µM), SP600125 (10 µM), SB203580 (20 µM) or BAY11-7082 (10 µM) for 2 h prior to adding 500 ng/ml LPS for 12 h. Then, the supernatants were harvested at 0.12 × g for 10 min at 4°C. The total RNA of RAW264.7 cells samples were extracted using TRIzol® according to the manufacturer's protocol. Then, RNA quality was determined by measuring the 260/280 ratio. Samples measuring >2.0 were considered to be of sufficient quality for further analysis. A total of ~1.5 µg total RNA was reverse transcribed to cDNA following the Revert Aid first-strand cDNA synthesis kit. The primer sequences are presented in Table I. RT-qPCR was used to estimate the mRNA expression levels using the IQ SYBR Supermix extraction reagent. Quantification cycle (Cq) values were recorded and the relative expression level of target genes was calculated using the 2−ΔΔCq method (7,11).
Table I.
Reverse transcription-quantitative polymerase chain reaction primer sequences.
| Gene | Peimer sequences |
|---|---|
| TNF-α | F: 5′-CCCTCACACTCAGATCATCTTCT-3′ |
| R: 5′-GCTACGACGTGGGCTACAG-3′ | |
| IL-6 | F: 5′-TAGTCCTTCCTACCCCAATTTCC-3′ |
| R: 5′-TTGGTCCTTAGCCACTCCTTC-3′ | |
| IL-8 | F: 5′-TGTGGGAGGCTGTGTTTGTA-3′ |
| R: 5′-ACGAGACCAGGAGAAACAGG-3′ | |
| IFN-β | F: 5′-CAGCTCCAAGAAAGGACGAAC-3′ |
| R: 5′-GGCAGTGTAACTCTTCTGCAT-3′ | |
| IL-10 | F: 5′-GCTCTTACTGACTGGCATGAG-3′ |
| R: 5′-CGCAGCTCTAGGAGCATGTG-3′ | |
| MyD88 | F: 5′-ACTCGCAGTTTGTTGGATG-3′ |
| R: 5′-CACCTGTAAAGGCTTCTCG-3′ | |
| TRAM | F: 5′-AGCCAGAAAGCAATAAGC-3′ |
| R: 5′-CAAACCCAAAGAACCAAG-3′ | |
| TLR4 | F: 5′-GGACTCTGATCATGGCACTG-3′ |
| R: 5′-CTGATCCATGCATTGGTAGGT-3′ | |
| GAPDH | F: 5′-GGTGAAGGTCGGTGTGAACG-3′ |
| R: 5′-CTCGCTCCTGGAAGATGGTG-3′ |
TNF-α, tumor necrosis factor-α; Il, interleukin; MyD88, myeloid differentiation primary response MyD88; TRAM, TIR domain-containing adapter molecule 2; TLR4, Toll-like receptor 4; F, forward; R, reverse.
Effects of Res on LPS-induced signaling pathway
RAW264.7 cells in 6-well (5×105 cells/ml) plates were pretreated with Res (25 µM), SB203580 (20 µM), SP600125 (10 µM) or BAY11-7082 (10 µM) for 2 h prior to the addition of 500 ng/ml LPS for 12 h. The phosphorylation of IκBα, p38 MAPK and JNK in the in the presence of specific inhibitors or Res were measured.
To measure the expression of TLR4, RAW264.7 cells in 6-well (5×105 cells/ml) plates were pretreated with different concentrations of Res (6.25, 12.5 or 25 µM) for 2 h prior to the addition of 500 ng/ml LPS for 12 h. The expression of TLR4 was measured.
Statistical analysis
The data were expressed as the mean ± standard deviation. The SPSS 17.0 software was used for data analysis. GraphPad Prism v.6 software (GraphPad Software, Inc.) was used to analyze the cytokine concentrations, mRNA expression levels and protein densitometry data. Comparisons between the control and experimental groups were made using a one way analysis of variance followed by a Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Cell viability
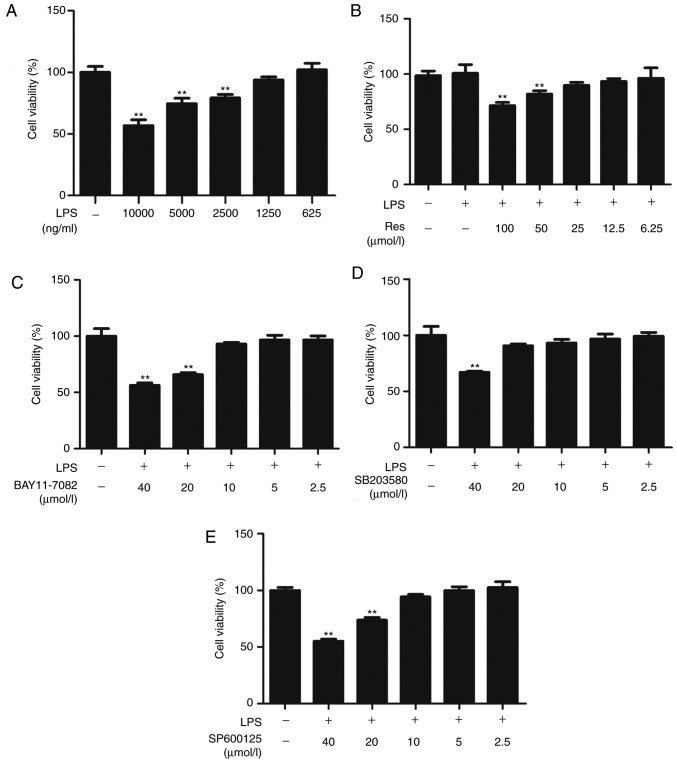
Res and inhibitors were examined for toxicity against RAW264.7 cells in the absence or presence of LPS for 24 h. The maximum non-toxic concentrations of LPS, Res, BAY11-7082, SP600125 and SB203580 were 1250 ng/ml, 25, 10, 10 and 20 µM, respectively (Fig. 1). Therefore, in subsequent experiments, cells were treated with the different compounds at nontoxic concentrations.
Figure 1.
Effect of Res on RAW264.7 cell viability. (A) Cells viability following treatment with different concentrations of LPS. (B) Cells viability following treatment with different concentrations of Res. (C) The viability of LPS-treated RAW264.7 cells in the absence or presence of BAY11-7082. (D) The viability of LPS-treated RAW264.7 cells in the absence or presence of SB203580. (E) The viability of LPS-treated RAW264.7 cells in the absence or presence of SP600125. Data are expressed as the mean ± standard error of the mean (n=3). **P<0.01 vs. control. Cell viability was calculated as the proportion of viable cells as compared with the control. Res, resveratrol; LPS, lipopolysaccharide.
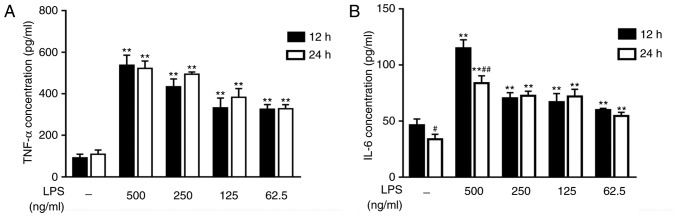
Appropriate stimulus conditions
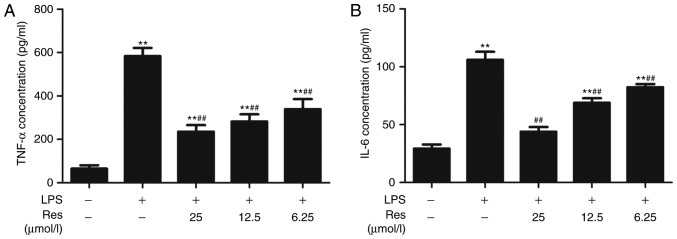
To explore the appropriate stimulus conditions, RAW264.7 cells were treated with different concentrations of LPS for 12 or 24 h. ELISA was used to measure the concentrations of TNF-α and IL-6, which are two inflammatory cytokines. The results indicated that RAW264.7 cells, under stimulation with LPS, expressed increased TNF-α and IL-6 levels compared with the untreated cells. With the increase of LPS concentration, the expression levels of TNF-α and IL-6 were increased (Fig. 2A and B). In terms of treatment time intervals, there was no significant difference observed in the contents of TNF-α between the groups (Fig. 2A). These results revealed that LPS stimulated TNF-α and IL-6 expression, and suggested that adding 500 ng/ml LPS to the cells and culturing for 12 h is an appropriate cell model of LPS-induced inflammation. Res inhibited the secretion of TNF-α and IL-6 (Fig. 3A and B) and effective dose was between 6.25–25 µM.
Figure 2.
Effects of different concentrations of on LPS-induced inflammatory mediator expression. (A) TNF-α and (B) IL-6 secretion levels were analyzed by ELISA. Data are presented as the mean ± SEM (n=3). **P<0.01 vs. control; #P<0.05 and ##P<0.01 vs. 12 h. LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6.
Figure 3.
Effects of different concentrations of Res on LPS-induced inflammatory mediator expression. (A) TNF-α and (B) IL-6 secretion levels were analyzed by ELISA. Data are presented as the mean ± standard error of the mean (n=3). **P<0.01 vs. control; ##P<0.01 vs. LPS. Res, resveratrol; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6.
Effects of Res on LPS-induced NF-κB signaling pathway
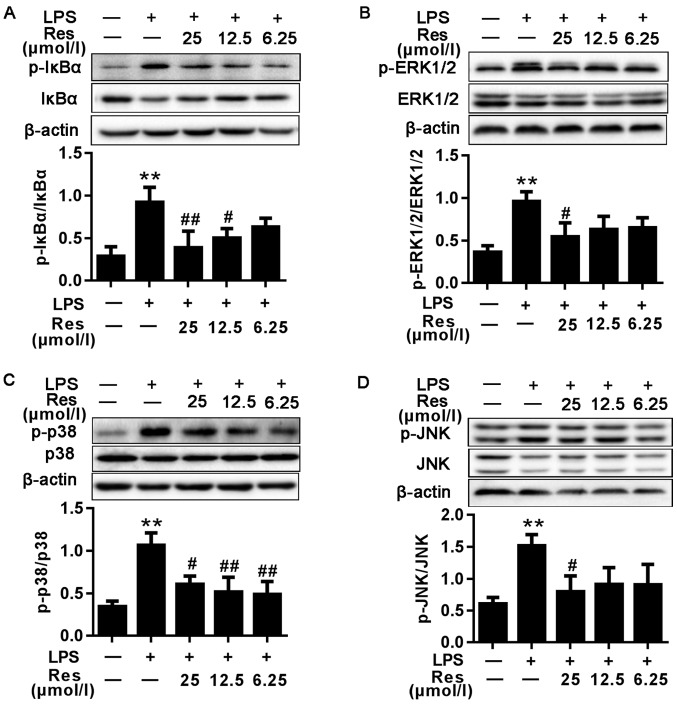
The IκB/NF-κB signaling pathway has been suggested to regulate a number of the genes involved in the inflammatory response and the production of inflammatory cytokines and pro-inflammatory enzymes (2,12). To investigate whether Res inhibited the NF-κB signaling pathway, the effects of Res on IkB phosphorylation were first investigated. As demonstrated in Fig. 4A, the protein level of the p-IκBα in the LPS group was increased compared with that in the blank control group. Compared with the untreated cells, LPS-induced phosphorylation of IκB was significantly decreased in the Res-treated cells in a concentration-dependent manner.
Figure 4.
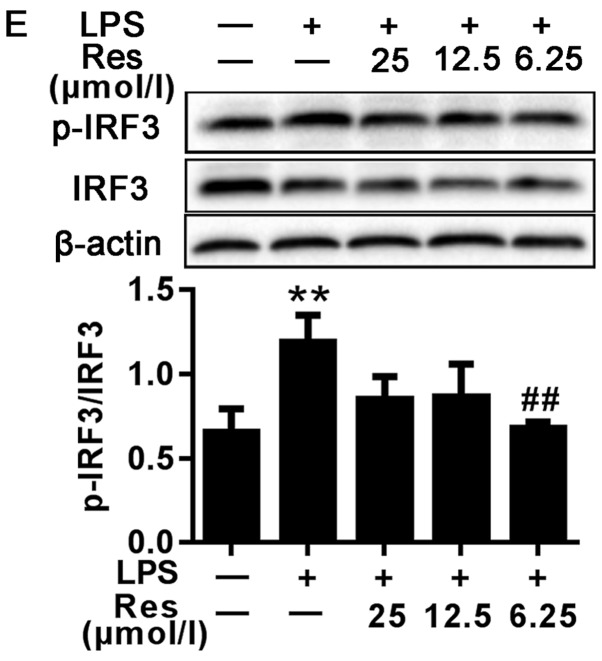
Effects of different concentrations of Res on LPS-induced NF-κB, MAPKs and IRF3 signaling pathway. Protein samples were analyzed by western blot analysis with specific antibodies. (A) The expression levels of the total and phosphorylated proteins associated with the NF-κB signaling pathway. (B-E) The expression levels of the total and phosphorylated proteins associated with the (B-D) MAPKs and (E) IRF3 signaling pathways. Data are presented as the mean ± standard error of the mean (n=3). **P<0.01 vs. control; #P<0.05 and ##P<0.01 vs. LPS. Res, resveratrol; LPS, lipopolysaccharide; MAPKs, mitogen-activated protein kinases; IκBα, NF-κB inhibitor; ERK1/2, extracellular signal-regulated kinase; p38, p38 MAPK; JNK, c-Jun N-terminal kinase; p-, phosphorylated; IRF3, interferon regulatory factor 3.
Effects of Res on LPS-induced MAPKs induction
The MAPK pathway serves a key role in the LPS-induced inflammatory (7). Therefore, whether Res inhibited inflammation through the MAPK pathway was determined. As indicated in Fig. 4B-D, LPS was able to stimulate the phosphorylation of ERK, p38 MAPK and JNK, whereas the phosphorylation of these proteins was inhibited by pretreatment with Res.
Effects of Res on LPS-induced IRF3 signaling pathway
IRF3 has been demonstrated to regulate cell proliferation, apoptosis, inflammation, innate immune responses and insulin resistance (13–16). It can be activated by LPS. TRIF-mediated signaling in response to LPS challenge may activate IRF3, resulting in the production of IFN-β, IP-10 and other IRF-3-dependent genes (17). As shown in Fig. 4E, compared with the blank group, the phosphorylated protein level of IRF3 in the LPS-only group was increased. However, following treatment with Res, the expression levels of the phosphorylated proteins were decreased compared with the LPS-only group.
Res inhibits the LPS-induced pro-inflammatory cytokines production
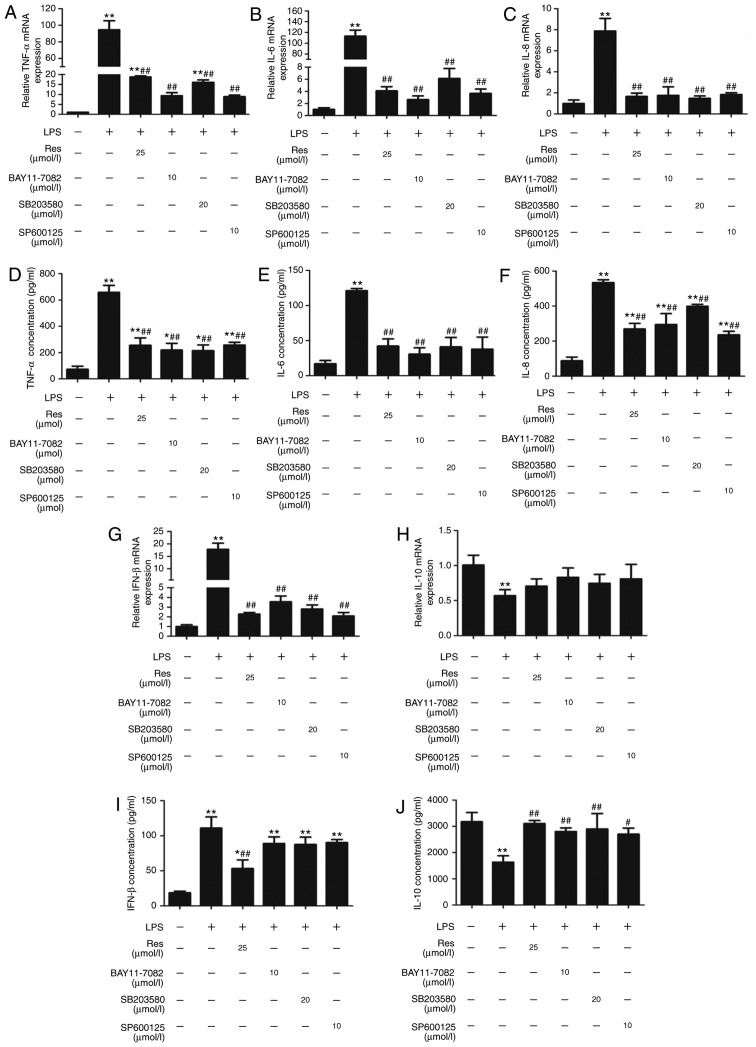
The pro-inflammatory cytokines IL-1β, TNF- α, IL-6, IL-8 and IL-10 serve pivotal roles in inflammation progression as a result of monocyte activation (18). To explore the effect of Res on the expression levels of these inflammatory mediators, LPS-stimulated cells were treated with Res (25 µM) and inhibitors including SP600125 (10 µM), BAY11-7082 (10 µM), SB203580 (20 µM) for 2 h. Then, the mRNA levels and serum concentrations of TNF-α, IL-6, IL-8, IFN-β and IL-10 were detected by RT-qPCR and ELISA, respectively. Res and the pathway-specific inhibitors successfully inhibited the expression and secretion of TNF-α, IL-6, IL-8 and IFN-β, which were increased by LPS treatment (Fig. 5A-G and I). By contrast, the level of IL-10 was decreased following LPS-stimulation, and was recovered following Res treatment (Fig. 5H and J).
Figure 5.
Effect of Res on the production and gene expression of pro-inflammatory cytokines in LPS-stimulated RAW264.7 cells. The RNA and secreted protein levels of proinflammatory cytokines including (A and D) TNF-α, (B and E) IL-6, (C and F) IL-8, (G and I) IFN-β and (H and J) IL-10, were analyzed by reverse transcription-quantitative polymerase chain reaction and ELISA. Data are presented as the mean ± standard error of the mean (n=3). *P<0.05 and **P<0.01 vs. control; #P<0.05 and ##P<0.01 vs. LPS. Res, resveratrol; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-α; IL, interleukin; IFN-β, interferon-β.
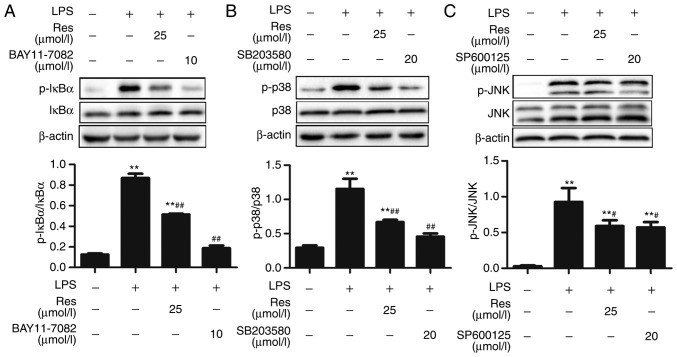
Res serves an anti-inflammatory effect through the MAPK and NF-κB pathway. The above experiments investigated whether Res had an impact on the expression of phosphorylated proteins including IκBα, p38 MAPK, JNK, ERK1/2 and IRF3. The results revealed that Res inhibited IκBα, p38 MAPK and JNK activation through the MAPK and NF-κB pathways. In order to reveal the protective mechanism of Res on LPS-induced RAW264.7 cells inflammation, the RAW264.7 cells were pretreated with 25 µM Res and inhibitors, including SP600125 (10 µM), BAY11-7082 (10 µM) and SB203580 (20 µM) for 2 h, followed by treatment with LPS for 12 h.
For IκBα, the expression level of its phosphorylated form in the LPS group was increased compared with the control group, but it was significantly decreased following treatment with Res. Similar results were obtained when Res was replaced by BAY11-7082, which is a selective inhibitor of NF-κB kinase (Fig. 6A). There was an increased expression of p-p38 MAPK in the LPS-treated group as compared with the control group. However, when RAW264.7 cells were pretreated with SB203580, a specific p38 MAPK signaling inhibitor, this increase was markedly inhibited, which was similar to the effects of Res treatment (Fig. 6B). Similar results were obtained when the inhibitor was replaced by SP600125, which is a broad-spectrum inhibitor of JNK (Fig. 6C).
Figure 6.
Res inhibits LPS-induced NF-κB and MAPKs activation. (A) The expression of IκBα and p-IκBα in the NF-κB signaling pathway. The expression levels of (B) p38 and p-p38 proteins in the p38 signaling pathway and (C) JNK and p-JNK proteins in the JNK signaling pathway. Data are presented as the mean ± standard error of the mean (n=3). **P<0.01 vs. control; #P<0.05 and ##P<0.01 vs. LPS. Res, resveratrol; LPS, lipopolysaccharide; MAPKs, mitogen-activated protein kinases; IκBα, NF-κB inhibitor; JNK, c-Jun N-terminal kinase; p-, phosphorylated.
TLR4 is the target protein of Res
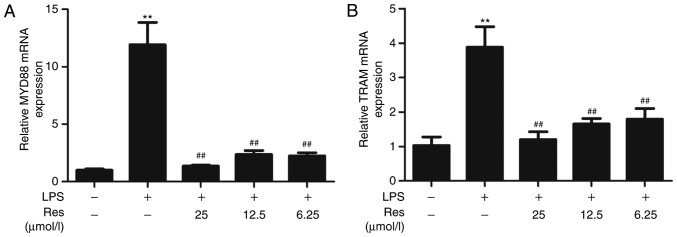
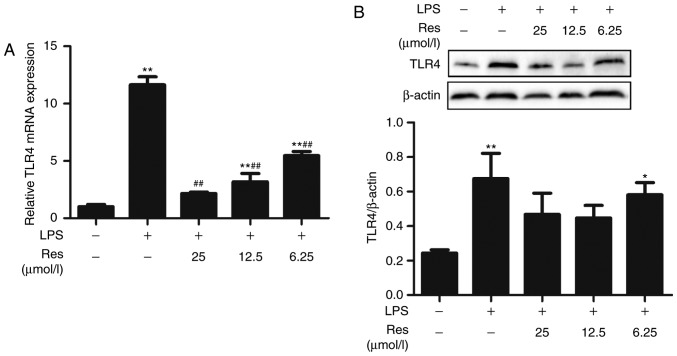
MYD88 and TRAM are two upstream proteins of the TLR4 pathway. Whether Res affected the TLR4 pathway through the MYD88 or TRIF-dependent pathways is not known. Therefore, the effects of Res on the transcription levels of TRAM, MYD88 and TLR4 and the expression of TLR4 protein were investigated. Res exhibited an inhibitory effect on the transcription levels of TRAM and MYD88 and the expression level of TLR4 protein (Figs. 7 and 8). The results of the current study indicated that Res suppressed the release of TNF-α, IL-6, IL-8 and IFN-β and increased the release of IL-10 through inhibiting the TLR4-NF-κB/MAPKs/IRF3 signaling pathway (Fig. 9).
Figure 7.
Res inhibits LPS-induced inflammatory via MyD88- and TRIF-dependent pathways. The mRNA levels of (A) MYD88 and (B) TRAM were measured by reverse transcription-quantitative polymerase chain reaction. Data are presented as the mean ± standard error of the mean (n=3). **P<0.01 vs. control; ##P<0.01 vs. LPS. Res, resveratrol; LPS, lipopolysaccharide; MyD88; TRIF.
Figure 8.
Res inhibits LPS-induced inflammatory via inhibition of the expression of TLR4. (A) mRNA levels of TLR4 were measured by reverse transcription-quantitative polymerase chain reaction. (B) The expression levels of TLR4 protein were analyzed by western blot analysis with specific antibodies. Data are presented as the mean ± standard error of the mean (n=3). *P<0.05 and **P<0.01 vs. control; ##P<0.01 vs. LPS. Res, resveratrol; LPS, lipopolysaccharide; TLR4, Toll-like receptor 4.
Figure 9.
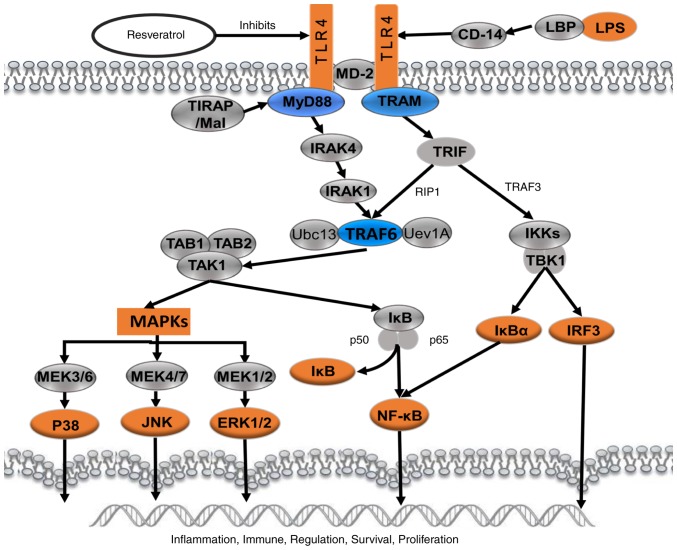
Schematic presentation of resveratrol-mediated effect on LPS-induced inflammation through suppressing the signaling cascades of TLR4-NF-κB/MAPKs/IRF3. LPS, lipopolysaccharide; TLR4, Toll-like receptor 4; MD-2, lymphocyte antigen 96; MAPKs, mitogen-activated protein kinases; IRF3, IRF3, interferon regulatory factor 3; CD14, monocyte differentiation antigen CD14; LBP, lipopolysaccharide binding protein; TIRAP, Toll/interleukin-1 receptor domain-containing adapter protein; MyD88, myeloid differentiation primary response MyD88; TRAM, TIR domain-containing adapter molecule 2; IRAK4, IL-1 receptor-associated kinase 4; TRIF, TIR domain-containing adapter molecule 1; RIP1, receptor-interacting serine/threonine-protein kinase 1; TRAF, TNF receptor-associated factor 6; TAB, transforming growth factor-β-activated kinase 1-binding protein; TAK1, mitogen-activated protein kinase kinase kinase 7; Ubc13, ubiquitin-conjugating enzyme E2 13; Uev1A, ubiquitin-conjugating enzyme E2 variant 1A; IKK, IκB kinase; TBK1, serine/threonine-protein kinase TBK1; IκBα, NF-κB inhibitor; p50, nuclear factor NF-κB p105 subunit; p65, transcription factor p65; MEK, mitogen-activated protein kinase kinase; JNK, c-Jun N-terminal kinase; ERK, extracellular signal-regulated kinase.
Discussion
Inflammation, which presents with the classical features of swelling, redness, heat and often pain is a key defense response to injury, tissue ischemia, autoimmune responses or infectious agents. Inflammation is also a major contributing factor to the damage observed in autoimmune diseases (19). It can induct or activate the production of the inflammatory mediators such as kinins, cyclooxygenases and cytokines. The mouse macrophage RAW264.7 cell line is a type of monocyte macrophage in mice with leukemia, which is commonly used in biological experiments investigating inflammation. For example, a previous study used RAW264.7 cells to examine the mechanism of how mono- (2-ethylhexyl) phthalate aggravates inflammatory response (20). In the present study, an inflammatory model was successfully established using LPS stimulation in RAW264.7 cells. LPS was demonstrated to promote the secretion of TNF-α and IL-6 in a dose-dependent manner (Fig. 2). TNF-α is the earliest endogenous mediator of an inflammatory reaction, and IL-6 is a major pro-inflammatory cytokine that serves an important role in the acute-phase response of inflammation (1). These inflammatory factors can be used as markers of the degree of inflammation.
Firstly, overactivation of the NF-κB and MAPKs pathways were observed in LPS-induced inflammation (7,21). NF-κB, a critical regulator of cytokine production, cell activation and proliferation, serves an important role in regulating inflammation and immune responses to extracellular stimulus (22). The present study identified that LPS-induced inflammation was associated with the activation of the NF-κB pathway, likely involving the disruption of the interaction with IκBα. In addition, Res was demonstrated to decrease IκBα overexpression in the present study. These results were similar to those of our previous study, which revealed that Res mitigated LPS-mediated acute inflammation in rats by inhibiting the TLR4/NF-κBp65/MAPKs signaling cascade (7). MAPKs, including p38 MAPK, ERK and JNK, are members of a ubiquitous protein serine/threonine kinase family responsible for signal transduction in eukaryotic organisms (23). It has been well established that MAPK activation is implicated in the production of LPS-stimulated inflammatory mediators (24–26). MAPKs are a group of signaling molecules that appear to serve key roles in inflammatory processes (27). In the present study, the results demonstrated that LPS-induced inflammation stimulated the phosphorylation of JNK, ERK and p38 MAPK, suggesting that MAPKs are involved in LPS-induced inflammation. It was also revealed that Res treatment efficiently downregulated the LPS-induced expression levels of p38 MAPK, JNK and ERK1/2 in RAW264.7 cells. In addition, it was identified that the IRF3 pathway was overactivated and the levels of IFN-β were significantly increased in the LPS group compared with that of the control group. The levels of p-IRF3 were decreased when treated with Res. These results suggested that Res may relieve LPS-induced inflammation through inhibiting the activation of the TLR4-NF-κB/MAPKs/IRF3 signaling pathway and downregulating the phosphorylation of IκBα, p38 MAPK, JNK, IRF3 and ERK1/2. A number of previous studies have indicated that cytokines including IL-1, IL-12, IFN-β and IL-10 serve important roles in the process of inflammatory diseases (28). Inhibition the production of inflammatory cytokines and their mediators serves as a key mechanism in the control of inflammation.
In the present study, the production of the pro-inflammatory cytokines including TNF-α, IL-6, IL-8 and IFN-β was significantly inhibited by Res. In addition, the LPS-stimulated mRNA expression levels of TNF-α, IL-6, IL-8 and IFN-β were also decreased by Res treatment, suggesting that Res suppressed the production of TNF-α, IL-6, IL-12, IFN-β and IL-1β through the downregulation of their gene expression.
Concomitantly, Res markedly increased the release of IL-10 and its mRNA expression level in LPS-stimulated RAW264.7 cells. TNF-α is a pro-inflammatory cytokine that exerts multiple biological effects. NF-κB signaling may regulate the transcription of certain inflammatory genes, including inducible nitric oxide synthase, prostaglandin G/H synthase 2, TNF-α, IL-1β and IL-6. The pathogenesis of a number of inflammatory processes are associated with the pathological stimuli and dysregulation of the NF-κB pathway (29). The MAPKs signaling pathway primarily regulates the production of IL-12 through the p38 MAPK and SAPK/JNK signaling pathways (30), and ERK1/2 negatively regulates the signaling pathways leading to IL-12 synthesis (31,32). The expression of IFN-β may be induced by the phosphorylation of IRF. A number of gram-positive pathogenic bacteria activate IFN-β production in immune cells (33). Previous data indicated that IRF3 or IRF7 function complementarily to induce the expression of IFN-β (34). IL-10 is considered primarily an inhibitory cytokine, which is crucial in maintaining an adequate balance between inflammatory and immunopathological responses (35). The results from the present study indicated that Res elicits its protective effect against LPS-induced inflammation through regulating the production of the cytokines that correspond with the NF-κB, MAPK and IRF3 signal pathways. Consistent with these data, similar results have indicated that Res inhibited the LPS-induced production of IL-1β and TNF-α (36,37). The NF-κB, MAPK and IRF3 pathways may be involved in the process of Res-based alleviation of LPS-induced inflammation (38–40). Furthermore, Res regulated the secretion of inflammatory factors through inhibiting the associated NF-κB, MAPK and IRF3 signaling pathways.
For further confirmation of the regulation of Res on these signaling pathways, the inhibitors of JNK, NF-κB and p38 MAPK were used and it was identified that Res has a similar effect as these inhibitors.
The TLR4-NF-κB/MAPK pathways are considered to be pivotal in the inflammatory response (41–43). TLR4 can be activated by LPS. Then, the TLR4/MD-2 complex can transmit the signal to two pairs of adaptor proteins such as TIRAP/MyD88 and TRAM/TRIF. MyD88 recruits the IL-1 receptor-associated kinase (IRAK)4 and IRAK2 (or IRAK1) kinases (44–46), which leads to the activation of NF-κB and MAPK and the production of cytokines. Conversely, TRIF promotes the secretion of type I IFN and IFN-inducible chemokines, such as IL-10, through stimulating the IRF3 transcription factor (47). In the present study, Res was demonstrated to inhibit the mRNA expression of MYD88 and TRAM in LPS-stimulated RAW264.7 cells, suggesting that Res alleviates inflammation via inhibiting the MYD88- and TRIF-dependent pathways, which are 2 upstream proteins in the TLR4 pathway. TRAM and MYD88, or their upstream protein, may be the target protein of Res that was responsible for the inhibition of the LPS-induced inflammation observed in the present study; it was also identified that Res inhibited TLR4 mRNA transcription and protein expression in LPS-stimulated RAW264.7 cells, suggesting that Res inhibited inflammation through downregulating TLR4 expression.
In conclusion, Res is a potential anti-inflammatory agent. The anti-inflammatory mechanisms of Res attribute to a suppression of the release of TNF-α, IL-6, IL-8 and IFN-β and an increase in the release of IL-10 through inhibiting the TLR4-NF-κB/MAPKs/IRF3 signaling pathways. TLR4 may be the target protein of Res, which inhibited LPS-induced inflammation in RAW264.7 cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key research and development projects of Sichuan science and Technology Department (grant no. 8ZDYF3246), the Agricultural Technology Research and Development Project of Chengdu (grant no. 2015-NY02-00266-NC) and Sichuan Veterinary Medicine and Drug Innovation Group of China Agricultural Research System (CARS-SVDIP).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. WT, XC, XS and ZY designed the experiments, wrote the manuscript and prepared the figures. YC performed part of the experiments. RJ, YZ, LL, CH, XL and LY analyzed the experimental results. GY, CL and JL analyzed part of the data. All authors reviewed the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hu K, Yang Y, Tu Q, Luo Y, Ma R. Alpinetin inhibits LPS-induced inflammatory mediator response by activating PPAR-γ in THP-1-derived macrophages. Eur J Pharmacol. 2013;721:96–102. doi: 10.1016/j.ejphar.2013.09.049. [DOI] [PubMed] [Google Scholar]
- 2.Wang YP, Wu Y, Li LY, Zheng J, Liu RG, Zhou JP, Yuan SY, Shang Y, Yao SL. Aspirin-triggered lipoxin A 4 attenuates LPS-induced pro-inflammatory responses by inhibiting activation of NF-κB and MAPKs in BV-2 microglial cells. J Neuroinflammation. 2011;8:95. doi: 10.1186/1742-2094-8-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng F, Lowell CA. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr and Lyn. J Exp Med. 1997;185:1661–1670. doi: 10.1084/jem.185.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou S, Chen G, Qi M, El-Assaad F, Wang Y, Dong S, Chen L, Yu DS, Weaver JC, Beretov J, et al. Gram negative bacterial inflammation ameliorated by the plasma protein beta 2-glycoprotein I. Sci Rep. 2016;6:33656. doi: 10.1038/srep33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garay-Malpartida HM, Mourão RF, Mantovani M, Santos IA, Sogayar MC, Goldberg AC. Toll-like receptor 4 (TLR4) expression in human and murine pancreatic beta-cells affects cell viability and insulin homeostasis. BMC Immunol. 2011;12:18. doi: 10.1186/1471-2172-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JJ, Sears DD. TLR4 and insulin resistance. Gastroenterol Res Pract. 2010;2010(pii):212563. doi: 10.1155/2010/212563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang G, Hu Z, Fu Q, Song X, Cui Q, Jia R, Zou Y, He C, Li L, Yin Z. Resveratrol mitigates lipopolysaccharide-mediated acute inflammation in rats by inhibiting the TLR4/NF-κBp65/MAPKs signaling cascade. Sci Rep. 2017;7:45006. doi: 10.1038/srep45006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holthoff JH, Woodling KA, Doerge DR, Burns ST, Hinson JA, Mayeux PR. Resveratrol, a dietary polyphenolic phytoalexin, is a functional scavenger of peroxynitrite. Biochem Pharmacol. 2010;80:1260–1265. doi: 10.1016/j.bcp.2010.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Youn HS, Lee JY, Fitzgerald KA, Young HA, Akira S, Hwang DH. Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: Molecular targets are TBK1 and RIP1 in TRIF complex. J Immunol. 2005;175:3339–3346. doi: 10.4049/jimmunol.175.5.3339. [DOI] [PubMed] [Google Scholar]
- 10.Jakus PB, Kalman N, Antus C, Radnai B, Tucsek Z, Gallyas F, Jr, Sumegi B, Veres B. TRAF6 is functional in inhibition of TLR4-mediated NF-κB activation by resveratrol. J Nutr Biochem. 2013;24:819–823. doi: 10.1016/j.jnutbio.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Tseng CK, Lin CK, Chang HW, Wu YH, Yen FL, Chang FR, Chen WC, Yeh CC, Lee JC. Aqueous extract of Gracilaria tenuistipitata suppresses LPS-induced NF-κB and MAPK activation in RAW264.7 and rat peritoneal macrophages and exerts hepatoprotective effects on carbon tetrachloride-treated rat. PLoS One. 2014;9:e86557. doi: 10.1371/journal.pone.0086557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian WL, Jiang ZX, Wang F, Guo R, Tang P, Huang YM, Sun L. IRF3 is involved in human acute myeloid leukemia through regulating the expression of miR-155. Biochem Biophys Res Commun. 2016;478:1130–1135. doi: 10.1016/j.bbrc.2016.08.080. [DOI] [PubMed] [Google Scholar]
- 14.Guinn Z, Lampe AT, Brown DM, Petro TM. Significant role for IRF3 in both T cell and APC effector functions during T cell responses. Cell Immunol. 2016;310:141–149. doi: 10.1016/j.cellimm.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumari M, Wang X, Lantier L, Lyubetskaya A, Eguchi J, Kang S, Tenen D, Roh HC, Kong X, Kazak L, et al. IRF3 promotes adipose inflammation and insulin resistance and represses browning. J Clin Invest. 2016;126:2839–2854. doi: 10.1172/JCI86080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen PG, Guan YJ, Zha GM, Jiao XQ, Zhu HS, Zhang CY, Wang YY, Li HP. Swine IRF3/IRF7 attenuates inflammatory responses through TLR4 signaling pathway. Oncotarget. 2017;8:61958–61968. doi: 10.18632/oncotarget.18740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajaiah R, Perkins DJ, Ireland DD, Vogel SN. CD14 dependence of TLR4 endocytosis and TRIF signaling displays ligand specificity and is dissociable in endotoxin tolerance. Proc Natl Acad Sci USA. 2015;112:8391–8396. doi: 10.1073/pnas.1424980112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pham TH, Kim MS, Le MQ, Song YS, Bak Y, Ryu HW, Oh SR, Yoon DY. Fargesin exerts anti-inflammatory effects in THP-1 monocytes by suppressing PKC-dependent AP-1 and NF-ĸB signaling. Phytomedicine. 2017;24:96–103. doi: 10.1016/j.phymed.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147(Suppl 1):S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park MH, Gutiérrez-García AK, Choudhury M. Mono-(2-ethylhexyl) phthalate aggravates inflammatory response via sirtuin regulation and inflammasome activation in RAW264.7 cells. Chem Res Toxicol. 2019;32:935–942. doi: 10.1021/acs.chemrestox.9b00101. [DOI] [PubMed] [Google Scholar]
- 21.Zhao X, Cui Q, Fu Q, Song X, Jia R, Yang Y, Zou Y, Li L, He C, Liang X, et al. Antiviral properties of resveratrol against pseudorabies virus are associated with the inhibition of IκB kinase activation. Sci Rep. 2017;7:8782. doi: 10.1038/s41598-017-09365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Liu H, Xu QS, Du YG, Xu J. Chitosan oligosaccharides block LPS-induced O-GlcNAcylation of NF-κB and endothelial inflammatory response. Carbohydr Polym. 2014;99:568–578. doi: 10.1016/j.carbpol.2013.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li D, Hu J, Wang T, Zhang X, Liu L, Wang H, Wu Y, Xu D, Wen F. Silymarin attenuates cigarette smoke extract-induced inflammation via simultaneous inhibition of autophagy and ERK/p38 MAPK pathway in human bronchial epithelial cells. Sci Rep. 2016;6:37751. doi: 10.1038/srep37751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci. 2004;117:4619–4628. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]
- 25.Renda T, Baraldo S, Pelaia G, Bazzan E, Turato G, Papi A, Maestrelli P, Maselli R, Vatrella A, Fabbri LM, et al. Increased activation of p38 MAPK in COPD. Eur Respir J. 2008;31:62–69. doi: 10.1183/09031936.00036707. [DOI] [PubMed] [Google Scholar]
- 26.Gaffey K, Reynolds S, Plumb J, Kaur M, Singh D. Increased phosphorylated p38 mitogen-activated protein kinase in COPD lungs. Eur Respir J. 2013;42:28–41. doi: 10.1183/09031936.00170711. [DOI] [PubMed] [Google Scholar]
- 27.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Tambuyzer BR, Ponsaerts P, Nouwen EJ. Microglia: Gatekeepers of central nervous system immunology. J Leukoc Biol. 2009;85:352–370. doi: 10.1189/jlb.0608385. [DOI] [PubMed] [Google Scholar]
- 29.Veres B, Radnai B, Gallyas F, Jr, Varbiro G, Berente Z, Osz E, Sumegi B. Regulation of kinase cascades and transcription factors by a poly(ADP-ribose) polymerase-1 inhibitor, 4-hydroxyquinazoline, in lipopolysaccharide-induced inflammation in mice. J Pharmacol Exp Ther. 2004;310:247–255. doi: 10.1124/jpet.104.065151. [DOI] [PubMed] [Google Scholar]
- 30.Kim L, Del Rio L, Butcher BA, Mogensen TH, Paludan SR, Flavell RA, Denkers EY. p38 MAPK autophosphorylation drives macrophage IL-12 production during intracellular infection. J Immunol. 2005;174:4178–4184. doi: 10.4049/jimmunol.174.7.4178. [DOI] [PubMed] [Google Scholar]
- 31.Feng GJ, Goodridge HS, Harnett MM, Wei XQ, Nikolaev AV, Higson AP, Liew FY. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J Immunol. 1999;163:6403–6412. [PubMed] [Google Scholar]
- 32.Ropert C, Almeida IC, Closel M, Travassos LR, Ferguson MA, Cohen P, Gazzinelli RT. Requirement of mitogen-activated protein kinases and IκB phosphorylation for induction of proinflammatory cytokines synthesis by macrophages indicates functional similarity of receptors triggered by glycosylphosphatidylinositol anchors from parasitic protozoa and bacterial lipopolysaccharide. J Immunol. 2001;166:3423–3331. doi: 10.4049/jimmunol.166.5.3423. [DOI] [PubMed] [Google Scholar]
- 33.Monroe KM, McWhirter SM, Vance RE. Induction of type I interferons by bacteria. Cell Microbiol. 2010;12:881–890. doi: 10.1111/j.1462-5822.2010.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss G, Maaetoft-Udsen K, Stifter SA, Hertzog P, Goriely S, Thomsen AR, Paludan SR, Frøkiær H. MyD88 drives the IFN-β response to Lactobacillus acidophilus in dendritic cells through a mechanism involving IRF1, IRF3 and IRF7. J Immunol. 2012;189:2860–2868. doi: 10.4049/jimmunol.1103491. [DOI] [PubMed] [Google Scholar]
- 35.Cavalcanti YV, Brelaz MC, Neves JK, Ferraz JC, Pereira VR. Role of TNF-alpha, IFN-gamma and IL-10 in the development of pulmonary tuberculosis. Pulm Med. 2012;2012:745483. doi: 10.1155/2012/745483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Byun EB, Sung NY, Park JN, Yang MS, Park SH, Byun EH. Gamma-irradiated resveratrol negatively regulates LPS-induced MAPK and NF-κB signaling through TLR4 in macrophages. Int Immunopharmacol. 2015;25:249–259. doi: 10.1016/j.intimp.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Zong Y, Sun L, Liu B, Deng YS, Zhan D, Chen YL, He Y, Liu J, Zhang ZJ, Sun J, Lu D. Resveratrol inhibits LPS-induced MAPKs activation via activation of the phosphatidylinositol 3-kinase pathway in murine RAW264.7 macrophage cells. PLoS One. 2012;7:e44107. doi: 10.1371/journal.pone.0044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vartanian KB, Stevens SL, Marsh BJ, Williams-Karnesky R, Lessov NS, Stenzel-Poore MP. LPS preconditioning redirects TLR signaling following stroke: TRIF-IRF3 plays a seminal role in mediating tolerance to ischemic injury. J Neuroinflammation. 2011;8:140. doi: 10.1186/1742-2094-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang G, Chang CC, Yang Y, Yuan L, Xu L, Ho CT, Li S. Resveratrol alleviates rheumatoid arthritis via reducing ROS and inflammation, inhibiting MAPK signaling pathways and suppressing angiogenesis. J Agric Food Chem. 2018;66:12953–12960. doi: 10.1021/acs.jafc.8b05047. [DOI] [PubMed] [Google Scholar]
- 40.Chiang MC, Nicol CJ, Cheng YC. Resveratrol activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against amyloid-beta-induced inflammation and oxidative stress. Neurochem Int. 2018;115:1–10. doi: 10.1016/j.neuint.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Santos SH, Andrade JM, Fernandes LR, Sinisterra RD, Sousa FB, Feltenberger JD, Alvarez-Leite JI, Santos RA. Oral angiotensin-(1–7) prevented obesity and hepatic inflammation by inhibition of resistin/TLR4/MAPK/NF-κB in rats fed with high-fat diet. Peptides. 2013;46:47–52. doi: 10.1016/j.peptides.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Meng Z, Yan C, Deng Q, Gao DF, Niu XL. Curcumin inhibits LPS-induced inflammation in rat vascular smooth muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways. Acta Pharmacol Sin. 2013;34:901–911. doi: 10.1038/aps.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang C, Lin G, Wan W, Li X, Zeng B, Yang B, Huang C. Resveratrol, a polyphenol phytoalexin, protects cardiomyocytes against anoxia/reoxygenation injury via the TLR4/NF-κB signaling pathway. Int J Mol Med. 2012;29:557–563. doi: 10.3892/ijmm.2012.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cochet F, Peri F. The role of carbohydrates in the lipopolysaccharide (LPS)/Toll-like receptor 4 (TLR4) signalling. Int J Mol Sci. 2017;18:E2318. doi: 10.3390/ijms18112318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1443. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 46.Ulevitch RJ, Tobias PS. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr Opin Immunol. 1999;11:19–22. doi: 10.1016/S0952-7915(99)80004-1. [DOI] [PubMed] [Google Scholar]
- 47.Jiang Q, Akashi S, Miyake K, Petty HR. Cutting edge: Lipopolysaccharide induces physical proximity between CD14 and Toll-like receptor 4 (TLR4) prior to nuclear translocation of NF-kappa. J Immunol. 2000;165:3541–3544. doi: 10.4049/jimmunol.165.7.3541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.