Abstract
The extensive arsenal of bioactive molecules secreted by mesenchymal stem cells, also known as the “secretome”, has demonstrated considerable therapeutic benefit in regenerative medicine. The secretome as an investigative treatment has enabled researchers to replicate the anti-inflammatory, angiogenic, and trophic effects of stem cells without the need for the cells themselves. This review will discuss the rationale, mechanisms, and current understanding surrounding the mesenchymal stem cell secretome in urology. We will review recent pre-clinical studies utilizing the secretome for stress incontinence, renal disease, bladder dysfunction, and erectile dysfunction. Finally, we will describe proteomic methods used to characterize the secretome and provide insight into future perspectives regarding stem cells and their secretions.
Keywords: mesenchymal stem cells, conditioned culture media, secretome, regenerative medicine, urology
1. Introduction
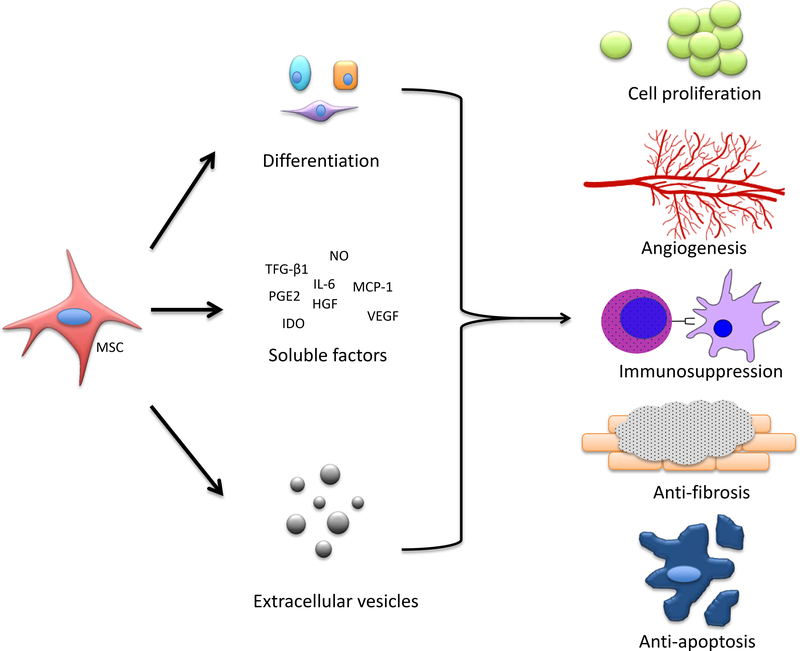
Recent innovations in stem cell research have unlocked the potential for therapies in regenerative urology. Stem cells are a unique cell population due to their ability to self-renew, proliferate, and differentiate into multiple terminal cell types. They possess unique anti-fibrotic, angiogenic, and anti-apoptotic properties, which may benefit urologic diseases for which pharmacologic or surgical therapies are lacking. To date, stem cells are classified into 4 main categories. Embryonic stem cells (ESCs), which are derived from a human blastocyst, represent the most undifferentiated form1. ESCs have the greatest therapeutic potential because they can infinitely self-renew and theoretically differentiate into any type of human cell. However, their use is limited due to ethical concerns, potential allogenicity, and concern for tumor oncogenesis2. Amniotic fluid stem cells (AFSCs) are isolated from the amniotic fluid or placental membrane of a developing fetus3. They possess an intermediate proliferation and differentiation potential, between that of embryonic and adult stem cells4.. Recently, researchers determined that differentiated, somatic cells could be “reprogrammed” into a pluripotent state 5. These induced pluripotent stem cells (IPSCs) possess the differentiation potential of ESCs, without the necessity of utilizing an embryo. However, due to concerns of oncogenesis and the genetic stability of IPSCs, their application for regenerative urology requires further investigation6. Adult stem cells (ASCs), also known as somatic stem cells, are tissue-specific progenitor cells and are the most limited along the spectrum of differentiation. ASCs reside amongst differentiated cells all over the body, and they possess the ability to repair damage and restore function locally7. There are two unique populations of ASCs that challenge these conventional characteristics. Hematopoietic stem cells (HSCs) are myeloid and lymphoid precursors that originate in the bone marrow and may traverse the circulatory system in their path to become mature blood cells8. Autologous bone marrow transplantation, currently used to treat a variety of hematologic diseases such as leukemia, utilizes HSCs as its source of blood progenitor cells9. Mesenchymal stem cells (MSCs), another class of mature stem cells found in the bone marrow, have been thoroughly investigated for their tissue regenerative capabilities. Originally thought to engraft or differentiate into injured host tissue, it is well known that MSCs secrete soluble factors through a paracrine mechanism and this provides the therapeutic regenerative effect 10–13. Utilizing proteomic approaches, researchers have begun to characterize the active cytokines secreted by stem cells. There is increasing evidence that some of these molecules may be contained within extracellular vesicles called exosomes or microvesicles 14–17. This review will discuss the current role and future directions of stem cells and their secretions in urologic disease.
2. Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are a unique subset of ASCs, first described by Friedenstein in 197418. Unlike tissue-specific ASCs, MSCs possess the distinct ability to differentiate into endodermal, mesodermal, and ectodermal lineages19. These properties are comparable to those of ESCs, but without the ethical problems or oncogenic potential. Classically derived from the bone marrow stroma, MSCs have also been isolated from muscle, fat, skin, cartilage, bone, fallopian tissue, umbilical cord blood, and menstrual blood20,21. In fact, they are found in almost all well-vascularized tissues and are phenotypically related to perivascular cells, or pericytes22. Although MSCs isolated from different tissues may contain different surface markers and possess differential gene expression profiles, they all have considerable proliferative ability and multi-lineage differentiation potential23,24.
Much of the clinical urologic literature has focused on MSCs derived from muscle tissue, or muscle-derived stem cells (MDSCs)21. In fact, the first major clinical trials investigating the use of stem cells for stress urinary incontinence (SUI) utilized peri-urethral injections of autologous MDSCs25–27. Although MDSCs are more accessible than bone-marrow derived MSCs, the harvest process requires an invasive biopsy followed by a time-consuming cell expansion process28. Another, more abundant source of MSCs can be found in adipose tissue. Adipose-derived stem cells (ADSCs) can be obtained using excess fatty tissue left over from liposuction procedures and clonally expanded for a high yield of stem cells16. Researchers continue to find novel sources of stem cells, including urine, which may have interesting urologic applications (Box 1 )29.
Box 1: Urine-derived stem cells.
Urine- derived stem cells (UDSCs) offer an exciting alternative to traditional autologous sources of mesenchymal stem cells (MSCs) such as muscle or adipose tissue, which require invasive procedures and can cause donor- site morbidity. Zhang and colleagues142 discovered that a population of cells isolated from urine exhibit MSC- like features and possess trilineage differentiation capacity143. These cells express MSC and/or pericyte markers and are probably derived from the glomerular parietal epithelium144. UDSCs might offer an advantage over other multipotent stem cells for urological applications given that they can be easily and safely obtained from a urine sample, exhibit robust proliferative capacity and can differentiate into urothelial cells with higher efficiency143,145. Moreover, UDSCs can be effectively manipulated to become induced pluripotent stem cells (iPSCs), which have broad therapeutic applications such as personalized regenerative medicine or as vectors for gene delivery36.
Similar to MSCs, the regenerative properties of UDSCs have shown promise in animal models of urological disease. In a rat model of diabetic erectile dysfunction (ED), intracavernous injections of UDSCs or UDSCs transfected with fibroblast growth factor 2 (FGF2; a potent angiogenic protein) upregulated the expression of pro- angiogenic factors, such as endothelial nitric oxide synthase (eNOS) and vascular endothelial growth factor (VEGF), and smooth muscle markers in penile tissues146. FGF2 transfection increased the expression of these factors compared with UDSC treatment only. In addition, UDSC- treated animals exhibited improved functional erectile responses without detection of cells at the injection sites 4 weeks after injection, and FGF2 transfection also markedly enhanced these responses. These findings support the paracrine hypothesis of stem cell action discussed in this Review. Indeed, exosomes secreted by UDSCs decreased urine microalbuminuria, reduced tubular epithelial damage and enhanced endothelial cell proliferation when injected intravenously in a rat model of diabetic nephropathy147. Thus, UDSCs might be a desirable alternative for urological pathologies owing to their ease of harvest, proliferation potential and phenotypic similarity to the urinary system.
2a. MSC Mechanisms of Action
The mechanism by which bone-marrow derived MSCs localize to sites of injury is termed “homing”30. In response to chemical signals release by damaged cells, MSCs travel from the bone marrow, through the circulatory system, and localize to the target tissue. Although the mechanism is still being discerned, it is thought to involve an interaction between homing cytokines and their respective receptors on the cell surface of MSCs. For example, stromal-derived factor 1 (SDF-1, also known as CXCL12), a cytokine expressed by certain cells after injury, has been shown to attract MSCs via its receptor found on the MSC cell surface, CXCR431,32. A study by Lin et al. induced SUI in female rats with vaginal dilation and bilateral ovarectomy, followed by injection of labeled autologous ADSCs either directly into the urethra or intravenously via the tail vein33. One month after treatment, both stem cell groups had significantly improved voiding patterns compared to controls. On histological examination, labeled ADSCs in the tail vein group were found in the urethras of injured animals, along with overexpression of SDF-1.
A multitude of other cytokine receptors that influence homing have been found on MSCs, as well as their counterpart ligands, including platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), and tumor necrosis factor-α (TNF-α)34,35. After reaching their destination in the blood stream, MSCs must also transmigrate through endothelial cells to reach their target. They achieve this through adhesion molecules such as selectins and integrins36.
Once they reach their target tissue, MSCs act through several inter-related mechanisms. Classically, it was thought that stem cells restored function by transdifferentiation into different cell types or even integration into injured cells. Indeed, several pre-clinical studies in the urologic literature support this finding. Chermansky and colleagues induced intrinsic sphincter deficiency (ISD) in female rats using electrocauterization37. Labeled autologous MDSCs were injected peri-urethrally 1 week after injury. Rats treated with stem cells had significant increases in leak point pressure (LPP) compared with rats treated with vehicle. Six weeks after injury LPP was comparable to that of non-injured rats. Histological analysis after 4 weeks showed intact striated muscle in the stem cell group, compared to disrupted muscle fibers in the control group. Furthermore, labeled stem cells had integrated with the muscle fibers in the urethra. In a similar experiment, labeled MDSCs were injected into the detrusor muscle of rats subjected to bladder cryoinjury38. Two weeks after injury, treated animals had improved bladder contractility compared to controls. Up to 8 weeks after injury, the MDSCs survived and expressed α-smooth muscle actin (α-SMA), suggesting the stem cells had differentiated into smooth muscle lineages.
In contrast to these studies, other investigators have demonstrated that the therapeutic effects of MSCs may persist despite disappearance or limited numbers of cells. Sadeghi and colleagues administered labeled human MSCs either peri-urethrally or intravenously to rats immediately after vaginal distension. While treatment groups had comparable LPPs to non-injured controls after 2 weeks, the stem cells could not be visualized after 4 days10. Many studies outside of the urologic literature confirm this finding. In a mouse model of myocardial infarction, systemically-injected MSCs improved cardiac function and fibrosis. However, no evidence of MSCs was observed in the heart tissue 3 weeks after injury39. In another study, rats received intravenous (IV) MSC injections 1 week after middle cerebral artery occlusion40. Treated rats had significantly improved neurological outcomes and less fibrosis compared with controls up to 4 months after injury, with only a negligible amount of MSCs in the brain parenchyma. Interestingly, stem cells have been found to exert their effects despite being distant from their target organ. Shabbir and colleagues injected MSCs into the hamstring muscles of hamsters with congestive heart failure13. Treated animals had improved cardiac function and attenuation of pathological fibrosis. While MSCs did not migrate outside of the hamstring, there were increases in circulating levels of known trophic factors secreted by MSCs.
Taken together, these data clarify the complex mechanism of stem cell action. While MSCs home to sites of injury in response to cytokine gradients, and a small percentage may engraft, differentiate, and integrate with host tissues, their therapeutic benefit cannot be explained by these mechanisms alone. Recently, the body of evidence has bolstered the paracrine hypothesis of stem cell action, which postulates that stem cells exert a significant therapeutic effect by secreting bioactive factors – the “secretome” – with anti-fibrotic, angiogenic, and anti-apoptotic properties.
3. The MSC Secretome
The MSC secretome consists of a complex array of soluble molecules, such as growth factors, cytokines, hormones, and lipid mediators, which together create a microenvironment suitable for cellular regeneration41. There is a growing body of evidence that some of these molecules may be packaged in cell-derived exosomes or extracellular vesicles, enabling more efficient remote communication and targeting14–17. There are several reasons why the MSC secretome is advantageous for regenerative urology over traditional cell-based therapies. Use of the acellular secretome may circumvent issues with tumorigenicity, immuno-reactivity, and maldifferentiation associated with cell therapy42–45. In addition, secretome therapy may allow for cheaper and more efficient development of off-the-shelf treatments, compared to the expansion and maintenance of individualized clonal cell populations. Finally, once the MSC secretome is better characterized, its active components could potentially be customized and tailored to the disease process of interest.
3a. Immune and Inflammatory Modulation
The body’s immune system is crucial to fighting pathogens and repairing damage following tissue injury. However, at the extreme end of the spectrum, the body’s defenses can cause more harm than good. Examples of this include septic shock or pathologic remodeling of the heart following myocardial infarction. MSCs may play a role in subduing such inflammatory responses by (1) switching activated macrophages to an anti-inflammatory phenotype 46,47, (2) inhibiting the activation of NK cells 48, (3) suppressing dendritic cell maturation and function 49 (4) and modulating the balance between TH1/TH17 (pro-inflammatory) and TH2/Treg (anti-inflammatory) T-lymphocyte pathways50–52. The immunosuppressive effect of MSCs is thought to be mediated by secretion of soluble factors such as transforming growth factor-β1 (TGF-β1), prostaglandin-E2 (PGE2), nitric oxide (NO), interleukin-6 (IL-6), and indoleamine-pyrrole 2,3-dioxygenase (IDO) 47,53–55. The influence of these pathways by MSCs or their secretions may be an interesting target for future therapies for urological pathologies related to immune dysregulation, such as interstitial cystitis or chronic prostatitis 56,57.
Humans generally heal by fibrosis (generating scars) in response to tissue injury, rather than by regeneration as do some other eukaryotic organisms58. Human possess some of these regenerative abilities in utero, but they are lost in adult life with a few notable exceptions59. For example, adult liver and epidermis both demonstrate regenerative potential, thought to be due to stem cells residing in these tissues, which may either differentiate to replace injured cells or secrete factors which enhance healing60,61. Similarly, MSCs, through their paracrine secretions, could play a role in shifting tissue healing in other organs after injury or surgery towards a regenerative, rather than fibrotic, process. To this end, it has been shown that MSC conditioned culture medium (CCM) enhances cutaneous wound healing in mice through trophic and anti-inflammatory cytokines, inducing the proliferation of keratinocytes and endothelial cells to the area of injury62. Thus, treatment with MSC CCM after trauma or surgery may facilitate regeneration and attenuate fibrosis and scarring.
Inflammation also plays a role in the fibrotic response of the kidney after ischemic injury, ultimately resulting in chronic kidney disease. One of the major mechanisms involved is the epithelial-mesenchymal transition (EMT) of proximal tubular cells, mediated by the pro-inflammatory cytokine TGF-β63. In an in vitro model of EMT using a human proximal tubular cell line (HK2), incubation with MSC CCM inhibited the morphological changes associated with EMT63. Likewise, in a subtotal nephrectomy model of chronic kidney disease in rats, Semedo et al. showed that IV injections of MSCs improved renal function and significantly reduced fibrosis and glomerulosclerosis in treated animals64. By analyzing mRNA expression in the kidney, the investigators demonstrated that MSC-treated animals upregulated certain anti-inflammatory cytokines, such as HO-1 and HGF, while downregulating pro-inflammatory molecules such as IL-6 and TNF-α64. These data, along with numerous experiments involving other tissues and organ systems establish that MSCs secrete factors that can suppress inflammation systemically in response to injury65–67. This has implications for the future treatment of urological diseases associated with fibrosis, such as urinary tract stricture and retroperitoneal fibrosis.
3b. Angiogenesis
Angiogenesis, the formation of new blood vessels from existing ones, is crucial to tissue regeneration and viability by providing a source of oxygen and nutrients to injured tissue. A major player involved in angiogenesis is vascular endothelial growth factor (VEGF)68. MSC conditioned media contains a significant amount of VEGF, along with other pro-angiogenic cytokines such as basic fibroblast growth factor (bFGF), placental growth factor (PGF), and monocyte chemoattractant protein-1 (MCP-1, also known as CCL2)69. MSC CCM enhances endothelial cell proliferation in vitro through these cytokines, and their effect is partially inhibited by anti-VEGF or anti-bFGF antibodies69. When MSCs were injected intramuscularly in a mouse model of hind limb ischemia, blood flow, collateral formation, and functional outcomes improved without MSC incorporation into tissues. The deleterious effects of ischemia persisted with local injection of MSC control media, not conditioned by MSCs,, suggesting the therapeutic effect of MSCs occurs via a paracrine pathway that can be reproduced by providing the secretions only70.
These vasculogenic properties of the MSC secretome contribute to the recovery of renal function after acute kidney injury. Togel and colleagues showed that through VEGF and other cytokines, MSC CCM stimulates the proliferation of aortic endothelial cells in culture, an effect which may be enhanced by hypoxia71. In addition, intra-arterial injections of MSCs after 60 minute bilateral renal hilum clamp were performed. MSCs homed to the kidney and there was rare engraftment into peritubular capillaries (<1 cell/whole kidney section). In addition, areas of the kidney with MSCs showed less apoptosis than areas without stem cells71. Unfortunately, the angiogenic potential of MSCs may also be harnessed by cancer cells to enable them to flourish. When cultured with MSCs or MSC CCM, the human prostate cancer cell line DU145 exhibited significant growth compared to fibroblast co-culture72. MSC CCM co-cultured with DU145 cells formed capillary tubes, an indicator of angiogenesis72. This effect was also seen in vivo when DU145 and MSCs were injected into nude mice. In addition, the cross-sectional area of blood vessels was increased by MSC injection.
3c. Anti-apoptosis
Data from a wide variety of pathologies indicates that MSCs secrete active factors that aid in cytoprotection and prevent apoptosis, or cell death. This benefit likely stems from the aforementioned immune and angiogenic effects, but also through direct cytoprotection. Takahashi et al. detected platelet-derived growth factor (PDGF) and insulin-like growth factor-1 (IGF-1), along with other common cytokines, in the supernatant of MSCs73. Using TUNEL assays, they showed that these cytokines inhibited apoptosis of cardiomyocytes in vitro. In a rat model of myocardial ischemia, intraperitoneal (IP) plus intramyocardial injections of MSC supernatant improved contractile function, while intramyocardial injections alone did not73. The authors speculate that this could be from the additional dose of cytokines provided by IP injections, or that the intramyocardial injections of cytokines actually harmed the cardiac muscle through anti-inflammatory effects. Using transwell experiments, Li and colleagues found that MSCs secrete factors which prevent apoptosis of alveolar macrophages74. Specifically, MSCs decreased the expression of the pro-apoptotic proteins caspase-3 and Bax, while increasing levels of the anti-apoptotic protein Bcl-274. In a rat model of erectile dysfunction (ED) following bilateral cavernous nerve ablation, Fall et al. demonstrated that local injection of bone marrow mononuclear cells (which contain MSCs) partially restored erectile responses75. Furthermore, cell therapy increased angiogenic responses with elevation in nitric oxide synthase, and significantly reduced the number of apoptotic cells in the erectile tissue.
In animal models of cytotoxic toxic kidney injury, the apoptosis of renal tubular epithelial cells plays a major role in permanent renal dysfunction76. Imberti and colleagues found that co-culture of proximal tubular epithelial cells (PTEC) with MSCs prevents the rapid cell death usually seen when exposed to cisplatin77. In addition, they demonstrated that this effect was facilitated by the anti-apoptotic cytokine IGF-1, which was highly expressed by the MSCs, and blocking IGF-1 attenuated the protective effect77. In another model of acute renal failure caused by cisplatin in immunodeficient NOD-SCID mice, IV MSC injections prevented renal failure and preserved the integrity of the tubular epithelium78. Using TUNEL assays, the researchers demonstrated that animals treated with stem cells had significantly fewer apoptotic cells. As with other studies, the authors demonstrated very low levels of labeled MSCs in the renal tissue, suggesting a paracrine effect. Remarkably, the mice treated with MSCs demonstrated significantly lower mortality than salinetreated animals, offering hope that the stem cells’ renotropic effect could eventually be used to benefit humans with renal failure.
4. The MSC Secretome in Regenerative Urology
4a. Stress Urinary Incontinence
Stress urinary incontinence afflicts up to 25% of American women and accounts for nearly 12 billion annual dollars in health care costs79. While surgical therapy for SUI is effective, it is not without complications and only serves as a symptomatic treatment80. Stem cells and their secretome offer a reproducible, safe therapy which targets the pathophysiology behind incontinence (Table 1).
Table 1.
Selected pre-clinical studies of secretome treatment for urological diseases
| Study | Pathology | Stem Cell Type | Study Design | Major Conclusions |
|---|---|---|---|---|
| Dissaranan et al., 2014 | SUI | MSCs, CCM | IV MSCs or peri-urethral CCM were administered after vaginal dilation. One week after injury, LPP and EUS EMG were measured. | LPP was significantly improved in both treatment groups, demonstrating that local injection of CCM provided a similar benefit to systemic cell therapy |
| Deng et al., 2015 | SUI | MSCs, CCM | IV MSC or IP CCM were administered following vaginal distension and PN crush. Three weeks after injury, LPP and PN sensory branch potentials were measured. | LPP was significantly improved in both treatment groups, suggesting systemic administration of acellular secretome has similar efficacy to cell therapy |
| Bi et al., 2007 | AKI | MSCs, CCM | MSCs or CCM were administered systemically after cisplatin-induced AKI in rats. | Both MSC and CCM treatment improved renal function and histology following acute renal injury when administered systemically. |
| Van Koppen et al., 2012 | CKD | CCM | In a sub-total nephrectomy model of CKD, rats were intravenously injected with CCM or non-conditioned media. Renal function and histology were analyzed 6 weeks after treatment. | Treatment with CCM improved GFR significantly compared to control. CCM-treated rats had less tubular damage. CCM was effective in reversing chronic kidney damage. |
| Da Silva et al., 2015 | CKD | MSCs, CCM | In a unilateral ureteral obstruction model of CKD, rats were administered IV MSC or CCM. Inflammatory cytokines and renal histology were analyzed. | In both treatment groups, levels of inflammatory cytokines were reduced. Histological analysis showed decrease fibrosis and apoptosis in both groups. |
| Zhang et al., | DBD | ADSCs | DBD was induced in rats using a high fat diet and streptozocin. ADSCs were injected in the detrusor or via tail vein. Conscious cystometry was performed 1 month later to assess bladder function. | 60% of rats receiving tail vein injections and 40% of rats receiving intra-detrusor injections demonstrated bladder dysfunction, compared to 100% in the control group. Only a fraction of injected ADSCs remained in the bladder, suggesting a paracrine effect. |
| Song et al., 2013 | OAB | MSCs | Intra-detrusor (human) MSCs or IV solifenacin were administered to rats with OAB induced by urethral ligation. Cystometry was performed at 2 and 4 weeks. | Bladder parameters improved with MSC treatment and at 4 weeks surpassed those of the anti-muscarinic group. This effect occurs without engraftment of human MSCs. |
| Albersen et al., 2010 | ED | ADSCs, ADSC lysate | Intracavernosal ADSCs or ADSC lysate were injected in a bilateral cavernosal nerve injury ED model. Erectile function was measured 4 weeks later by assessing intracavernosal pressure | Animals in both treatment groups had improved erectile function. In the stem cell group, only a small fraction of cells were observed in the cavernosal tissue after 1 month, suggesting a paracrine effect. |
| Sun et al., 2012 | ED | MSC, CCM | Intracavernosal injections of MSCs or CCM were administered to rats with diabetes-induced ED. Erectile function was measured 4 weeks later by assessing intracavernosal pressure. | Both treatment groups experienced partial restoration of erectile function. Immunohistochemistry demonstrated increased staining of nNOS and NF fibers. |
SUI: stress urinary incontinence, MSC: mesenchymal stem cells, CCM: conditioned culture media, LPP: leak point pressure, EUS: external urethral sphincter, EMG: electromyography, IV: intravenous, IP: intraparenchymal, PN: pudendal nerve, AKI: acute kidney injury, GFR: glomerular filtration rate, DBD: diabetic bladder dysfunction, OAB: overactive bladder, ED: erectile dysfunction, nNOS: nitric oxide synthase, NF: neurofilament
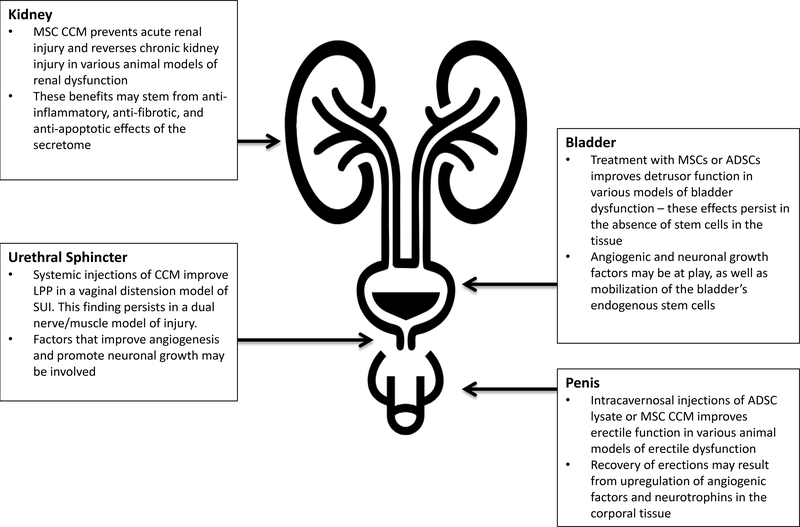
In a study by Dissaranan et al., stimulated childbirth injury was produced in rats using serial vaginal dilation11. Following injury, animals were given either GFP-labeled IV MSCs or peri-urethral CCM. One week after injury, leak point pressure (LPP) and external urethral sphincter electromyography (EUS EMG) were measured. Labeled MSCs preferentially homed to the urethra, vagina, and spleen of injured animals compared to sham-injured animals. LPP was significantly improved in MSC and CCM-treated animals compared to controls, although EUS function as measured by EMG was not improved. Rats treated with MSCs or CCM both demonstrated increased elastin fiber density in peri-urethral smooth muscles compared to control animals. This study demonstrated that the MSC CCM provided a therapeutic benefit in the absence of the stem cells themselves. However, one criticism of the study could be the comparison of IV MSC (systemic) therapy with peri-urethral (local) CCM injections.
In a similar study, the same group demonstrated that systemic administration of MSC CCM restored urethral function after simulated childbirth injury81. For this study, the investigators developed a dual-injury model consisting of vaginal distension and pudendal nerve crush. This model theoretically mimics human childbirth injury more closely as it elicits multiple mechanisms of injury (nerve and muscle). Treatment groups consisted of IV MSCs or IP CCM given 1 hour after injury. Three weeks later, LPP was significantly decreased with control treatment, but rats receiving MSCs or CCM had LPPs which were not significantly different from sham-injured rats. In rats receiving MSCs, stem cells were undetectable in the urethra or vagina after 3 weeks. Like the previous experiment, both stem cell treatment and the acellular secretome treatments were associated with increased peri-urethral elastin fiber density in injured animals. In the latter experiment, pudendal nerve morphology and sensory branch potentials were also preserved with MSC or CCM treatment, suggesting that factors secreted by MSCs can have different therapeutic effects in different microenvironments and injury paradigms. Angiogenic molecules such as VEGF are known to be secreted by MSCs and may counteract the hypoxic microenvironment involved in the pathophysiology of childbirth-induced SUI70. In addition, trophic factors secreted by MSCs could reverse the peri-urethral smooth muscle damage and pudendal nerve dysfunction associated with SUI73,82. While its exact mechanisms require further investigation, these studies demonstrate that the acellular secretions of stem cells restore urethral and nerve function in a rodent SUI model. Several clinical trials have shown local injections of stem cells to be effective in human stress incontinence25–27. Future clinical studies should seek to demonstrate the efficacy of the secretome, possibly administered systemically, for treatment, or even prophylaxis of SUI.
4b. Renal Disease
Chronic kidney disease (CKD) is a global health burden. In the United States, its prevalence approaches 15%, and the number of individuals with end-stage renal disease (ESRD) increases steadily83. Though dialysis is readily available, it is a morbid state with a mortality rate of nearly 200 per 1000 patient-years84. Kidney transplantation, the gold standard of cure for ESRD, leaves over 80,000 patients on the waiting list each year in the US83. MSCs and their secretome offer a regenerative solution that may benefit the millions of patients suffering from chronic renal dysfunction (Table 1).
Several studies have shown MSCs are effective in preventing loss of function following renal insult64,71,78,85. However, in most of these studies, incorporation or differentiation of MSCs into the native tissue were rare, suggesting a paracrine mechanism of action. For example, in a bilateral renal pedicle clamp model of acute kidney injury (AKI) in rats, Tögel and colleagues showed that intra-carotid administration of MSCs significantly improved renal function 48 hours after ischemia, even though administered cells were undetectable in the kidney after 24 hours86. Nonetheless, the kidneys of MSC-treated animals had reduced expression of the pro-inflammatory cytokines IL-1β, TNF-α, and IFN-γ and increased expression of IL-10, an anti-inflammatory cytokine. At the same time, there was upregulation of renotrophic growth factors and anti-apoptotic proteins in the treated kidneys. The brief time interval between cell treatment and renal functional recovery and the absence of MSCs after 24 hours lead us to reject the hypothesis that the injected cells differentiate and replace injured host tissue.
It seems more likely that the therapeutic benefit of MSCs stems from the immediate secretion of regenerative factors that may activate innate repair mechanisms. In a cisplatin-induced model of AKI in mice, IP and IV injections of MSCs improved renal function, animal survival, and histological parameters compared to controls.87 Like the previous study, the authors demonstrated no transplanted cells resided in kidney tubules after 24 hours while the therapeutic benefits lasted up to 6 days. These effects persisted when only MSC CCM was administered, confirming that renal recovery after AKI does not depend on the physical presence of stem cells. Though blood urea nitrogen was significantly improved after treatment with MSC CCM, the authors did not report the changes in creatinine; therefore, it is difficult to make a direct comparison to the MSC-treated groups. However, it is noteworthy that the paracrine factors secreted by MSCs initiated rapid onset renal protection in two distinct (ischemic versus cytotoxic) models of acute kidney injury. We therefore conclude that the regenerative effects of stem cell secretome involve divergent pathways and may even stem from the host cells themselves.
It is well established that AKI and CKD are closely related. Acute renal insult accelerates progression to CKD, while CKD predisposes patients to AKI88. With this in mind, Van Koppen et al. hypothesized that MSC CCM could also influence renal recovery in a rat sub-total nephrectomy model of CKD89. After establishment of CKD, rats were intravenously injected with MSC CCM or non-conditioned media (NCM) twice daily for 4 days. Six weeks after treatment, CCM-treated rats had significantly higher glomerular filtration rates (GFR) than animals injected with NCM. Histological analysis showed kidneys treated with control media, not conditioned by stem cells, had developed less glomerulosclerosis and tubular damage. These results are congruent with another studying showing that MSC CCM attenuated renal fibrosis in a rat model of CKD caused by unilateral ureteral obstruction (UUO)90. In this experiment, animals underwent unilateral ureteral ligation or sham injury, followed by intravenous injection of MSC or MSC CCM. This study is limited by the absence of renal functional assessment. However, the authors did demonstrate that 14 days after treatment, both MSC and CCM decreased expression of inflammatory cytokines in the kidney, collagen formation, fibrosis, and apoptosis.
Together, these studies indicate that the MSC secretome is not only able to prevent acute kidney injury but also reverse chronic kidney damage. The specific mechanisms responsible for this reno-protective effect have yet to be elucidated but are most likely multi-factorial. From in vitro studies, we can speculate that cytokines present in the secretome such as TGF-β1 or PGE2 47,91 may contribute to suppressing the acute inflammatory phase of renal injury. For the progression to CKD, MSC cytokines responsible for modulating fibrosis and apoptosis have been implicated63,77. It is unclear whether renal protection stems from the direct action of secreted factors or from their activation of regenerative pathways in the injured native tissue. The latter hypothesis is more compelling and seems to be supported by the CKD studies in which reno-protection was observed 2–6 weeks after initial treatment. Even though the specific mechanisms remain unclear, clinical trials are currently underway to investigate the use of MSCs for the treatment of acute and chronic kidney disease92–94.
4c. Bladder Dysfunction
Bladder dysfunction is a term encompassing a wide range of pathologies affecting micturition. It can include urinary retention, overactive bladder (OAB), neurogenic bladder, and interstitial cystitis, amongst many other diseases. While many pharmacologic and surgical treatments exist, none offer the regenerative and reparative potential possessed by MSCs and their secretome. While no studies have examined the secretome directly, stem cell studies can give us insight into their mechanism (Table 1). Zhang et. al studied the use of ADSCs in a rat model of diabetic bladder dysfunction (DBD) produced by a high-fat diet and streptozocin95. Experimental animals received intra-detrusor or intravenous injections of labeled ADSCs. One month after treatment, all rats receiving PBS had voiding dysfunction as measured by conscious cystometry, while only 60% and 40% of rats receiving tail vein and bladder stem cell injections, respectively, showed bladder dysfunction. However, only a small fraction of injected ADSCs were seen in the bladder mucosa after 1 month, suggesting that the functional effect of the stem cells were primarily paracrine in nature. The pathogenesis of diabetic bladder dysfunction is likely related to detrusor decompensation and neuronal damage96. Therefore, the benefit of stem cells for DBD could result from upregulation of smooth muscle and neuronal growth factors. This study would have benefited from an analysis of gene expression in the ADSC-injected bladder compared to controls.
In another study, OAB was induced with urethral ligation of female rats12. Four weeks after injury, either labeled human MSCs or phosphate-buffered saline (PBS) were injected into the detrusor muscle of the bladder. These groups were compared to a control group receiving the anti-muscarinic, solifenacin, intravenously. Two and 4 weeks after treatment, both MSC and solfinacin groups had significant decreases in their detrusor contraction frequencies compared to those of the control groups, but at 4 weeks, the therapeutic effect was superior in the stem cell-treated group. In MSC-treated bladders, there was upregulation of native pluripotent stem cell markers, such as Oct4, Sox2, and Stella, without any engraftment of transplanted human MSCs. Lastly, consistent with other previously reviewed studies, the authors demonstrate secretion of trophic cytokines such as SDF-1, hepatocyte-derived growth factor (HGF), PDGF, and VEGF. This study validates findings from other organ systems, demonstrating that without engraftment, transplanted MSCs release soluble factors which may treat overactive bladder. Moreover, the mechanism of action may be through mobilization of the bladder’s endogenous stem cells. It is worth noting that stem cell treatment was more effective than anti-cholinergic therapy after 4 weeks. While effective for the management of OAB in humans, anti-cholinergics are a symptomatic treatment and not without side effects. MSCs and their secretome may offer a safe, effective treatment for bladder dysfunction which targets the pathophysiology rather than symptoms.
4d. Erectile Dysfunction
Another area of urology where the stem cell secretome has shown benefit is male erectile dysfunction (Table 1). Albersen et al. developed a bilateral cavernosal nerve injury model in rats designed to simulate erectile dysfunction after radical prostatectomy97. Immediately after injury, experimental animals received intracavernosal ADSC or ADSC cell lysate. Four weeks later, erectile function was assessed by measuring intracavernous pressure (ICP) after electrostimulation of the distal cavernosal nerve. Both ASDC and lysate-treated animals had significant increases in ICP/mean arterial pressure (MAP) ratio as compared to injured animals treated with controls. Animals treated with ADSCs or lysate had significantly higher nitric oxide synthase positive nerve fibers, more preservation of smooth muscle content, and less fibrosis compared to controls. In the stem cell group, only a small fraction of labeled stem cells were observed in the cavernosal tissue after 28 days, suggesting the benefit of the stem cells do not result from incorporation or transdifferentiation into the host tissue. The authors conclude that the mechanism of therapeutic effect of the ADSCs in this case may be from soluble neurotrophins released by the cells.
Sun et al. studied the effect of MSC and its secretome for erectile dysfunction in diabetic rats98. Animals received intracavernosal injections of MSC or MSC CCM. Four weeks later, partial restoration of erectile function (as measured by ICP/MAP ratio) occurred in both stem cell and CCM groups, although the effect was smaller in the CCM group. This was accompanied by increased staining of neuronal nitric oxide synthase (nNOS) and neurofilament (NF) positive nerve fibers in the cavernosal tissue. These effects were likely due to neurotrophins that the authors found to be highly expressed in the MSC CCM such as brain-derived neurotrophic factor (BDNF) and neuronal growth factor (NGF). Studies in the neurologic literature have established that MSCs, through secretion of neurotrophins such as BDNF, glial cell-derived neurotrophic factor (GDNF), and neurotrophin-3 (NT3), improve central nervous system recovery in animal models of neurodegenerative disease 99–101. Similar to the neuroprotective effects of MSC CCM after pudendal nerve injury in a model of SUI81, the recovery of erectile function after stem cell injection may in part be due to the neuro-regenerative effects of the MSC secretome. In the future, treatment with MSC CCM could promote recovery of potency after prostatectomy or bladder function after spinal cord injury.
5a. Characterizing the Secretome
The emerging paradigm that stem cells work through secretion of active molecules has led the stem cell research community to begin to characterize these molecules. Through a variety of proteomic methods, researchers have begun to characterize the MSC secretome, but much work is left to be done. The study of proteomics can utilize targeted detection using antibodies, or shotgun-based, antibody-free methods. Detection using antibodies, such as with enzyme-linked immunosorbent assay (ELISA), is sensitive, reliable, and reproducible. However, its main limitation is the need for pre-selection of the antibody102, so, the investigator must know what he or she is looking for. For example, using an antibody array of 120 common cytokines and chemokines, Park and colleagues assessed the secretion profile of human bone MSCs103. They demonstrated that IL-6, TIMP-2, MCP-1, VEGF, and OPG were constitutively secreted by the cells in culture, independent of donor characteristics. In another study Western blot analysis, another antibody based detection method, demonstrated that MSC CCM contained high levels of the angiogenic cytokines VEGF and angiopoietin-1104. This approach is also limited by the availability and ability of antibodies to detect certain proteins. Moreover, targeted protein detection fails to detect active molecules that have not yet been defined.
Using untargeted proteomics techniques, investigators have been able to study the secretome more broadly. A commonly-used approach is tandem liquid chromatography and mass spectrometry (LC-MS)102,105. This is a powerful analytical tool in which a sample can be separated into components which can subsequently be analyzed in detail using the detection of charged ions106. Researchers have been able to use this technique to discover novel proteins involved in the MSC secretome. Sarjoni and colleagues used LC-MS to detect pigment epithelium-derived factor (PEDF), a major chemoattractant of fibroblasts, and cysteine-rich protein 61 (Cyr61), a pro-angiogenic cytokine, in the MSC CCM107,108. Untargeted proteomics approaches enable definition of novel active molecules important for stem cell therapy, but they are limited by their ability to detect small quantities of secreted cytokines. With this in mind, Sze et al. used a combination of LC-MS and antibody arrays to identify 201 unique gene products in embryonic stem cell-derived MSCs109. The investigators used computational analysis to confirm that these gene products were involved in important biological pathways such as metabolism, immune response, and differentiation.
5b. Extracellular Vesicles
Recently, it has been proposed that the therapeutic benefit of MSCs stems not from individual cytokines working in conjunction, but through cytokines packaged in groups of extracellular vesicles (EVs). A group of researchers from the Netherlands first demonstrated that MSC CCM reduced infarct size and improved ventricular function in a porcine model of myocardial infarction110. Size fractionation of the CCM demonstrated that the cardioprotection was only provided by the fraction of the CCM containing products >1000 kDa and a size between 100220nm110. Hypothesizing that this fraction consisted of extracellular vesicles called exosomes, they went on to purify the fraction even further using high-performance liquid chromatography (HPLC). They found that a population of phospholipid-bound structures, with a radius of 55–65nm that stained positive for the exosome-associated proteins CD81, CD9, and Alix, were cardioprotective in their porcine myocardial infarction model111.
Exosomes, a class of extracellular vesicles, are membrane-bound nanovesicles ranging in size from 30–100nm which are released by MSCs and other cells through exocytosis112. Once thought to be the recycling center for cellular debris, they have recently been shown to contain a variety of proteins, lipids, and even genetic material such as micro RNA (miRNA) responsible for intracellular signaling113. The contents of exosomes reflect those of the parent cell and can be transported to distant targets via ligand/receptor interactions114. Microvesicles (MVs) are a larger type of extracellular vesicle (between 100–1000nm) with similar contents and functions as exosomes115,116, and are often used interchangeably in the literature117,118. Both types of EVs are released constitutively, and in response to stimuli, by MSCs and other types of stem cells. They may influence the behavior of the target cell by transference of cell surface receptors119, delivery of proteins115, or horizontal transmission of mRNA or miRNA120. A growing body of evidence demonstrates that they are partially responsible for the beneficial effects of stem cells in a number of different pathologies111,121.
In a glyercol-induced model of AKI in mice, MSC-derived MVs incorporated into tubular cells reduced apoptosis and protected against acute renal dysfunction14. Interestingly, this effect was abolished when the MVs were exposed to RNase, suggesting that tubular cell regeneration may be dependent on RNA transfer by extracellular vesicles. The same group tested MSC MVs in a rat model of renal ischemia. MV treatment protected rats from developing AKI and also prevented chronic renal dysfunction15. Zhou and colleagues treated cisplatin-induced AKI in rats with intraparenchymal injections of MSC exosomes. Exosome-treated rats demonstrated improved renal function and cell morphology after 5 days16. A similar result was observed by Reis et al. in a gentamicin-induced AKI model in rats treated with intravenous injections of MSC exosomes118. MSC extracellular vesicles have been shown to diminish the growth of bladder tumors17 and promote erectile function in diabetic rats122. While the study of MSC EVs for regenerative urology is still in its infancy, their great potential should motivate researchers to explore cell-free stem cell therapies.
6. Conclusion
Regenerative medicine gives hope to patients with diseases that lack effective treatments. In urology, MSC-based therapy has demonstrated efficacy for the treatment of SUI in several clinical trials. In pre-clinical studies, cell therapy has shown a benefit in animal models of acute and chronic renal disease, bladder dysfunction, and erectile dysfunction. We have yet to examine their potential for anti-fibrosis in urethral strictures, angiogenesis in hypospadias repair, or anti-apoptosis in many urologic malignancies. Most now believe that the therapeutic effects of MSCs stem from active factors present in their secretions, as cell-free treatments have demonstrated benefit as well. These factors may be individual cytokines, or more likely, extracellular vesicles acting as messengers for the parent stem cell. In fact, there is a movement in the stem cell research community to change the name of MSCs to “medicinal signaling cells” to more accurately reflect this fact123.
Many questions regarding the mechanism of action of MSC secretome require further investigation. For example, how do its effects persist days or even weeks after treatment, given the short half-life of cytokines? This finding, replicated in several studies, implies that secreted factors activate innate regenerative pathways in the host tissue that achieve a durable effect. Moreover the MSC secretome appears to protect against different mechanisms of injury (i.e., ischemic versus cytotoxic kidney injury), suggesting that the local microenvironment somehow influences the regenerative pathway. Finally, long-term data regarding the effect of MSC secretome administration is still required to translate this therapy into effective clinical treatment.
Figure 1. Putative mechanisms of action of MSCs. MSCs either differentiate or secrete soluble factors, some of which are contained in extracellular vesicles. They exert trophic, angiogenic, immunosupressive, anti-fibrotic, and anti-apoptotic effects which promote regeneration of injured tissue.
Figure 2.
A summary of the effects of the MSC secretome on urological pathology as demonstrated in pre-clinical studies
Key Points.
Stem cells possess anti-inflammatory, angiogenic, and anti-apoptotic properties which may benefit urologic diseases for which conventional therapies are lacking.
The acellular secretome of mesenchymal stem cells has been found to exert similar therapeutic benefits as the cells themselves.
The mesenchymal stem cell secretome avoids problems associated with traditional stem cell therapy including oncogenic transformation, immune-reactivity, and cost.
The mesenchymal stem cell secretome has been shown in pre-clinical studies to benefit models of stress urinary incontinence, acute and chronic renal disease, bladder dysfunction, and erectile dysfunction
The specific mechanisms by which the stem cell secretome exerts its benefits requires further investigation, but it is likely due to multiple bioactive cytokines working synergistically.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Thomson JA Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 282, 1145–1147 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Odorico JS, Kaufman DS & Thomson JA Multilineage Differentiation from Human Embryonic Stem Cell Lines. Stem cells (Dayton, Ohio) 19, 193–204 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Cananzi M, Atala A & De Coppi P Stem cells derived from amniotic fluid: new potentials in regenerative medicine. Reproductive biomedicine online 18 Suppl 1, 17–27 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Hipp J & Atala A Sources of stem cells for regenerative medicine. Stem cell reviews 4, 3–11 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Wezel F & Southgate J Reprogramming Stromal Cells from the Urinary Tract and Prostate: A Trip to Pluripotency and Back? European Urology 64, 762–764 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Wagers AJ & Weissman IL Plasticity of Adult Stem Cells. Cell 116, 639–648 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Gunsilius E, Gastl G & Petzer AL Hematopoietic stem cells. Biomedicine & Pharmacotherapy 55, 186–194 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Copelan EA Hematopoietic Stem-Cell Transplantation http://dx.doi.org.ccmain.ohionet.org/10.1056/NEJMra052638 354, 1813–1826 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Sadeghi Z et al. Mesenchymal stem cell therapy in a rat model of birthtrauma injury: functional improvements and biodistribution. International urogynecology journal 27, 291–300 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dissaranan C et al. Rat mesenchymal stem cell secretome promotes elastogenesis and facilitates recovery from simulated childbirth injury. Cell Transplantation 23, 1395–1406 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song M et al. The paracrine effects of mesenchymal stem cells stimulate the regeneration capacity of endogenous stem cells in the repair of a bladder-outlet-obstruction-induced overactive bladder. Stem cells and development 23, 654–663 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Shabbir A, Zisa D, Suzuki G & Lee T Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. American Journal of Physiology - Heart and Circulatory Physiology 296, H1888–H1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruno S et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. Journal of the American Society of Nephrology: JASN 20, 1053–1067 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gatti S et al. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrology Dialysis Transplantation 26, 1474–1483 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Research & Therapy 4, 34 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu S, Ju G-Q, Du T, Zhu Y-J & Liu G-H Microvesicles derived from human umbilical cord Wharton’s jelly mesenchymal stem cells attenuate bladder tumor cell growth in vitro and in vivo. PloS one 8, e61366 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF & Keiliss-Borok IV STROMAL CELLS RESPONSIBLE FOR TRANSFERRING THE MICROENVIRONMENT OF THE HEMOPOIETIC TISSUES: Cloning In Vitro and Retransplantation In Vivo. Transplantation KW - 17, (1974). [DOI] [PubMed] [Google Scholar]
- 19.Ding D-C, Shyu W-C & Lin S-Z Mesenchymal stem cells. Cell Transplantation 20, 5–14 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Caplan AI Mesenchymal stem cells. Journal of orthopaedic research: official publication of the Orthopaedic Research Society 9, 641–650 (1991). [DOI] [PubMed] [Google Scholar]
- 21.Jackson WM, Nesti LJ & Tuan RS Potential therapeutic applications of muscle-derived mesenchymal stem and progenitor cells. Expert opinion on biological therapy 10, 505–517 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caplan AI All MSCs Are Pericytes? Cell Stem Cell 3, 229–230 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Lv F-J, Tuan RS, Cheung KMC & Leung VYL Concise Review: The Surface Markers and Identity of Human Mesenchymal Stem Cells. STEM CELLS 32, 1408–1419 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Covas DT et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Experimental Hematology 36, 642–654 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Carr LK et al. 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. International urogynecology journal 19, 881–883 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Stangel-Wojcikiewicz K, Piwowar M, Jach R, Majka M & Basta A Quality of life assessment in female patients 2 and 4 years after muscle-derived cell transplants for stress urinary incontinence treatment. Ginekologia polska 87, 183–189 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Sèbe P et al. Intrasphincteric injections of autologous muscular cells in women with refractory stress urinary incontinence: a prospective study. International urogynecology journal 22, 183–189 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Usas A & Huard J Muscle-derived stem cells for tissue engineering and regenerative therapy. Biomaterials 28, 5401–5406 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou T et al. Generation of induced pluripotent stem cells from urine. Journal of the American Society of Nephrology: JASN 22, 1221–1228 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karp JM & Leng Teo GS Mesenchymal Stem Cell Homing: The Devil Is in the Details. Cell Stem Cell 4, 206–216 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Zhuang Y, Chen X, Xu M, Zhang L-Y & Xiang F Chemokine stromal cell-derived factor 1/CXCL12 increases homing of mesenchymal stem cells to injured myocardium and neovascularization following myocardial infarction. Chinese medical journal 122, 183–187 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Zhang D et al. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. Journal of molecular and cellular cardiology 44, 281–292 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin G et al. Treatment of Stress Urinary Incontinence with Adipose TissueDerived Stem Cells. Cytotherapy 12, 88–95 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honczarenko M et al. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem cells (Dayton, Ohio) 24, 1030–1041 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Baek SJ, Kang SK & Ra JC In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Experimental & molecular medicine 43, 596–603 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sohni A & Verfaillie CM Mesenchymal Stem Cells Migration Homing and Tracking. Stem Cells International 2013, 130763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chermansky CJ et al. Intraurethral muscle-derived cell injections increase leak point pressure in a rat model of intrinsic sphincter deficiency. Urology 63, 780–785 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Huard J et al. Muscle-derived cell-mediated ex vivo gene therapy for urological dysfunction. Gene Therapy 9, 1617 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Iso Y et al. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochemical and biophysical research communications 354, 700–706 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y et al. Human marrow stromal cell therapy for stroke in rat: Neurotrophins and functional recovery. Neurology 59, 514–523 (2002). [DOI] [PubMed] [Google Scholar]
- 41.Ranganath SH, Levy O, Inamdar MS & Karp JM Harnessing the Mesenchymal Stem Cell Secretome for the Treatment of Cardiovascular Disease. Cell Stem Cell 10, 244–258 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Breitbach M et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood 110, 1362–1369 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Kunter U et al. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. Journal of the American Society of Nephrology: JASN 18, 1754–1764 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Foudah D et al. Monitoring the genomic stability of in vitro cultured rat bone-marrow-derived mesenchymal stem cells. Chromosome research: an international journal on the molecular, supramolecular and evolutionary aspects of chromosome biology 17, 1025–1039 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeong J-O et al. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circulation Research 108, 1340–1347 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi H, Lee RH, Bazhanov N, Oh JY & Prockop DJ Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood 118, 330–338 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maggini J et al. Mouse Bone Marrow-Derived Mesenchymal Stromal Cells Turn Activated Macrophages into a Regulatory-Like Profile. PloS one 5, e9252 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spaggiari GM et al. Mesenchymal stem cells inhibit natural killer–cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 111, 1327–1333 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Chiesa S et al. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proceedings of the National Academy of Sciences 108, 17384–17389 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bai L et al. Human Bone Marrow-derived Mesenchymal Stem Cells Induce Th2-Polarized Immune Response and Promote Endogenous Repair in Animal Models of Multiple Sclerosis. Glia 57, 1192–1203 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duffy MM et al. Mesenchymal stem cell inhibition of T-helper 17 cell- differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur J Immunol 41, 2840–2851 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Luz-Crawford P et al. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Research & Therapy 4, 65 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.English K et al. Cell contact, prostaglandin E2 and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25Highforkhead box P3+ regulatory T cells. Clinical & Experimental Immunology 156, 149–160 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ren G et al. Concise Review: Mesenchymal Stem Cells and Translational Medicine: Emerging Issues. STEM CELLS Translational Medicine 1, 51–58 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R & Fibbe WE Mesenchymal stem cells inhibit generation and function of both CD34+derived and monocyte-derived dendritic cells. The Journal of Immunology 177, 2080–2087 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Jiang Y-H, Peng C-H, Liu H-T & Kuo H-C Increased Pro-Inflammatory Cytokines, C-Reactive Protein and Nerve Growth Factor Expressions in Serum of Patients with Interstitial Cystitis/Bladder Pain Syndrome. PloS one 8, e76779 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.KHADRA A, FLETCHER P, LUZZI G, SHATTOCK R & HAY P Interleukin8 levels in seminal plasma in chronic prostatitis/chronic pelvic pain syndrome and nonspecific urethritis. BJU International 97, 1043–1046 (2006). [DOI] [PubMed] [Google Scholar]
- 58.Gurtner GC, Callaghan MJ & Longaker MT Progress and Potential for Regenerative Medicine. http://dx.doi.org.ccmain.ohionet.org/10.1146/annurev.med.58.082405.0953 29 58, 299–312 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Colwell AS, Longaker MT & Lorenz HP Fetal wound healing. Front Biosci 8, s1240–8 (2003). [DOI] [PubMed] [Google Scholar]
- 60.Thorgeirsson SS Hepatic stem cells in liver regeneration. The FASEB Journal 10, 1249–1256 (1996). [PubMed] [Google Scholar]
- 61.Levy V, Lindon C, Harfe BD & Morgan BA Distinct Stem Cell Populations Regenerate the Follicle and Interfollicular Epidermis. Developmental Cell 9, 855–861 (2005). [DOI] [PubMed] [Google Scholar]
- 62.Chen L, Tredget EE, Wu PY & Wu Y Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PloS one 3, e1886 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alfarano C et al. Intraparenchymal injection of bone marrow mesenchymal stem cells reduces kidney fibrosis after ischemiareperfusion in cyclosporine-immunosuppressed rats. 21, 2009–2019 (2012). [DOI] [PubMed] [Google Scholar]
- 64.Semedo P et al. Mesenchymal Stem Cells Attenuate Renal Fibrosis Through Immune Modulation and Remodeling Properties in a Rat Remnant Kidney Model. Stem cells (Dayton, Ohio) 27, 3063–3073 (2009). [DOI] [PubMed] [Google Scholar]
- 65.van Buul GM et al. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthritis and Cartilage 20, 1186–1196 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Roddy GW et al. Action at a Distance: Systemically Administered Adult Stem/Progenitor Cells (MSCs) Reduce Inflammatory Damage to the Cornea Without Engraftment and Primarily by Secretion of TNF-α Stimulated Gene/Protein 6. Stem cells (Dayton, Ohio) 29, 1572–1579 (2011). [DOI] [PubMed] [Google Scholar]
- 67.Yagi H et al. Reactive Bone Marrow Stromal Cells Attenuate Systemic Inflammation via sTNFR1. Molecular Therapy 18, 1857–1864 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leung DW, Cachianes G, Kuang W-J, Goeddel DV & Ferrara N Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246, 1306–1309 (1989). [DOI] [PubMed] [Google Scholar]
- 69.Kinnaird T et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 109, 1543–1549 (2004). [DOI] [PubMed] [Google Scholar]
- 70.Kinnaird T et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circulation Research 94, 678–685 (2004). [DOI] [PubMed] [Google Scholar]
- 71.Togel F et al. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. American journal of physiology. Renal physiology 292, F1626–F1635 (2007). [DOI] [PubMed] [Google Scholar]
- 72.Zhang T et al. Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Research & Therapy 4, 70 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takahashi M et al. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. American Journal of Physiology - Heart and Circulatory Physiology 291, H886–H893 (2006). [DOI] [PubMed] [Google Scholar]
- 74.Li B et al. Bone marrow mesenchymal stem cells protect alveolar macrophages from lipopolysaccharide-induced apoptosis partially by inhibiting the Wnt/β-catenin pathway. Cell Biology International 39, 192–200 (2015). [DOI] [PubMed] [Google Scholar]
- 75.Fall PA et al. Apoptosis and Effects of Intracavernous Bone Marrow Cell Injection in a Rat Model of Postprostatectomy Erectile Dysfunction. European Urology 56, 716–726 (2009). [DOI] [PubMed] [Google Scholar]
- 76.Bonegio R & Lieberthal W Role of apoptosis in the pathogenesis of acute renal failure. Current opinion in nephrology and hypertension 11, 301–308 (2002). [DOI] [PubMed] [Google Scholar]
- 77.Imberti B et al. Insulin-like growth factor-1 sustains stem cell mediated renal repair. Journal of the American Society of Nephrology: JASN 18, 2921–2928 (2007). [DOI] [PubMed] [Google Scholar]
- 78.Morigi M et al. Human Bone Marrow Mesenchymal Stem Cells Accelerate Recovery of Acute Renal Injury and Prolong Survival in Mice. Stem cells (Dayton, Ohio) 26, 2075–2082 (2008). [DOI] [PubMed] [Google Scholar]
- 79.Chong EC, Khan AA & Anger JT The financial burden of stress urinary incontinence among women in the United States. Current urology reports 12, 358–362 (2011). [DOI] [PubMed] [Google Scholar]
- 80.Brubaker L et al. Adverse events over two years after retropubic or transobturator midurethral sling surgery: findings from the Trial of Midurethral Slings (TOMUS) study. American Journal of Obstetrics and Gynecology 205, 498.e1–498.e6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deng K et al. Mesenchymal stem cells and their secretome partially restore nerve and urethral function in a dual muscle and nerve injury stress urinary incontinence model. American journal of physiology. Renal physiology 308, F92–F100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.SMITH ARB, HOSKER GL & WARRELL DW The role of pudendal nerve damage in the aetiology of genuine stress incontinence in women. BJOG:An international journal of O&G 96, 29–32 (1989). [DOI] [PubMed] [Google Scholar]
- 83.Saran R et al. US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. American Journal of Kidney Diseases 69, A7–A8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Collins AJ, Foley RN, Gilbertson DT & Chen S-C United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney international supplements 5, 2–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gnecchi M, Zhang Z, Ni A & Dzau VJ Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy. Circulation Research 103, 1204–1219 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Togel F et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. American journal of physiology. Renal physiology 289, F31–F42 (2005). [DOI] [PubMed] [Google Scholar]
- 87.Bi B, Schmitt R, Israilova M, Nishio H & Cantley LG Stromal cells protect against acute tubular injury via an endocrine effect. Journal of the American Society of Nephrology 18, 2486–2496 (2007). [DOI] [PubMed] [Google Scholar]
- 88.Venkatachalam MA et al. Acute kidney injury: a springboard for progression in chronic kidney disease. American journal of physiology. Renal physiology (2010). doi: 10.1152/ajprenal.00017.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Koppen A et al. Human embryonic mesenchymal stem cell-derived conditioned medium rescues kidney function in rats with established chronic kidney disease. PloS one 7, e38746 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Da Silva AF, Silva K, Reis LA, Teixeira VP & Schor N Bone marrow-derived mesenchymal stem cells and their conditioned medium attenuate fibrosis in an irreversible model of unilateral ureteral obstruction. Cell Transplantation 24, 2657–2666 (2015). [DOI] [PubMed] [Google Scholar]
- 91.Ren G et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2, 141–150 (2008). [DOI] [PubMed] [Google Scholar]
- 92.Tögel FE & Westenfelder C Kidney Protection and Regeneration Following Acute Injury: Progress Through Stem Cell Therapy. American Journal of Kidney Diseases 60, 1012–1022 (2012). [DOI] [PubMed] [Google Scholar]
- 93.Makhlough A et al. Bone marrow-mesenchymal stromal cell infusion in patients with chronic kidney disease: A safety study with 18 months of follow-up. Cytotherapy 20, 660–669 (2018). [DOI] [PubMed] [Google Scholar]
- 94.Gourabi H, Aghdami N, Makhloogh A & Moghadasali R Autologous Bone Marrow Derived Mesenchymal Stromal Cells (BM-MSCs) in Patients With Chronic Kidney Disease (CKD). Clinicaltrials.gov Identifier: (2014).
- 95.Zhang H et al. Adipose Tissue-Derived Stem Cells Ameliorate Diabetic Bladder Dysfunction in a Type II Diabetic Rat Model. (2011). doi: 10.1089/scd.2011.0244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Golbidi S & Laher I Bladder Dysfunction in Diabetes Mellitus. Front. Pharmacol. 1, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Albersen M et al. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. The Journal of Sexual Medicine 7, 3331–3340 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun C et al. Neurotrophic effect of bone marrow mesenchymal stem cells for erectile dysfunction in diabetic rats. International Journal of Andrology 35, 601–607 (2012). [DOI] [PubMed] [Google Scholar]
- 99.Wagih A, Elhawary S, Ellessy RM, of, B. E. A. J. 2015. Stem Cells for Neuro-regeneration: State of the Art. researchgate.net [Google Scholar]
- 100.Hu S-L et al. Functional recovery in acute traumatic spinal cord injury after transplantation of human umbilical cord mesenchymal stem cells. Critical Care Medicine 38, 2181–2189 (2010). [DOI] [PubMed] [Google Scholar]
- 101.Drago D et al. The stem cell secretome and its role in brain repair. Biochimie 95, 2271–2285 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tran C & Damaser MS Stem cells as drug delivery methods: application of stem cell secretome for regeneration. Advanced drug delivery reviews 82, 1–11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park CW et al. Cytokine Secretion Profiling of Human Mesenchymal Stem Cells by Antibody Array. International Journal of Stem Cells 2, 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu Y, Chen L, Scott PG & Tredget EE Mesenchymal Stem Cells Enhance Wound Healing Through Differentiation and Angiogenesis. Stem cells (Dayton, Ohio) 25, 2648–2659 (2007). [DOI] [PubMed] [Google Scholar]
- 105.Choi Y-A et al. Secretome Analysis of Human BMSCs and Identification of SMOC1 as an Important ECM Protein in Osteoblast Differentiation. J. Proteome Res. 9, 2946–2956 (2010). [DOI] [PubMed] [Google Scholar]
- 106.Lee MS & Kerns EH LC/MS applications in drug development. Mass Spectrometry Reviews 18, 187–279 (1999). [DOI] [PubMed] [Google Scholar]
- 107.SAROJINI H et al. PEDF from mouse mesenchymal stem cell secretome attracts fibroblasts. Journal of Cellular Biochemistry 104, 1793–1802 (2008). [DOI] [PubMed] [Google Scholar]
- 108.ESTRADA R et al. Secretome From Mesenchymal Stem Cells Induces Angiogenesis Via Cyr61. Journal of cellular physiology 219, 563–571 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sze SK et al. Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells. Molecular & cellular proteomics: MCP 6, 1680–1689 (2007). [DOI] [PubMed] [Google Scholar]
- 110.Timmers L et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Research 1, 129–137 (2008). [DOI] [PubMed] [Google Scholar]
- 111.Lai RC et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Research 4, 214–222 (2010). [DOI] [PubMed] [Google Scholar]
- 112.Lai RC, Chen TS & Lim SK Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regenerative medicine 6, 481–492 (2011). [DOI] [PubMed] [Google Scholar]
- 113.Valadi H et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature 9, 654 (2007). [DOI] [PubMed] [Google Scholar]
- 114.Mathivanan S, Ji H & Simpson RJ Exosomes: Extracellular organelles important in intercellular communication. Journal of Proteomics 73, 1907–1920 (2010). [DOI] [PubMed] [Google Scholar]
- 115.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A & Ratajczak MZ Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20, 1487 (2006). [DOI] [PubMed] [Google Scholar]
- 116.Baglio SR, Pegtel DM & Baldini N Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Frontiers in Physiology 3, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Camussi G, Deregibus MC, Bruno S, Cantaluppi V & Biancone L Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney international 78, 838–848 (2010). [DOI] [PubMed] [Google Scholar]
- 118.Reis LA et al. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PloS one 7, e44092 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tomasoni S et al. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem cells and development 22, 772–780 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Collino F et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PloS one 5, e11803 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li T et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem cells and development 22, 845–854 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu LL et al. Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia 50, e12871 (2018). [DOI] [PubMed] [Google Scholar]
- 123.Caplan AI Mesenchymal Stem Cells: Time to Change the Name! STEM CELLS Translational Medicine 6, 1445–1451 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sarojini W et al. PEDF from mouse mesenchymal stem cell secretome attracts fibroblasts. J. Cell. Biochem. 104, 1793–1802 (2008). [DOI] [PubMed] [Google Scholar]
- 125.Estrada R et al. Secretome from mesenchymal stemcells induces angiogenesis via Cyr61. J. Cell. Physiol. 219, 563–571 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sze SK et al. Elucidating the secretion proteome of human embryonic stem cell- derived mesenchymal stem cells. Mol. Cell. Proteomics 6, 1680–1689 (2007). [DOI] [PubMed] [Google Scholar]
- 127.Lai RC et al. Exosome secreted by MSC reduces myocardial ischemia / reperfusion injury. Stem Cell Res. 4, 214–222 (2010). [DOI] [PubMed] [Google Scholar]
- 128.Valadi H et al. Exosome- mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature 9, 654 (2007). [DOI] [PubMed] [Google Scholar]
- 129.Mathivanan S, Ji H & Simpson RJ Exosomes: extracellular organelles important in intercellular communication. J. Proteomics 73, 1907–1920 (2010). [DOI] [PubMed] [Google Scholar]
- 130.Ratajczak J, Wysoczynski M, Hayek F, Janowska- Wieczorek A & Ratajczak MZ Membranederived microvesicles: important and underappreciated mediators of cell- to-cell communication. Leukemia 20, 1487 (2006). [DOI] [PubMed] [Google Scholar]
- 131.Baglio SR, Pegtel DM & Baldini N Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell- free therapy. Front. Physiol 3, 359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Camussi G, Deregibus MC, Bruno S, Cantaluppi V & Biancone L Exosomes/microvesicles as a mechanism of cell- to-cell communication. Kidney Int. 78, 838–848 (2010). [DOI] [PubMed] [Google Scholar]
- 133.Reis LA et al. Bone marrow- derived mesenchymal stem cells repaired but did not prevent gentamicininduced acute kidney injury through paracrine effects in rats. PLOS ONE 7, e44092 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tomasoni S et al. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 22, 772–780 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Collino F et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLOS ONE 5, e11803 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li T et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 22, 845–854 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Timmers L et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 1, 129–137 (2008). [DOI] [PubMed] [Google Scholar]
- 138.Zhu LL et al. Transplantation of adipose tissuederived stem cell- derived exosomes ameliorates erectile function in diabetic rats. Andrologia 50, e12871 (2018). [DOI] [PubMed] [Google Scholar]
- 139.Nassar W et al. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 20, 21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Carr LK et al. Autologous muscle derived cell therapy for stress urinary incontinence: a prospective, dose ranging study. J. Urol. 189, 595–601 (2013). [DOI] [PubMed] [Google Scholar]
- 141.Caplan AI Mesenchymal stem cells: time to change the name! Stem Cells Transl. Med 6, 1445–1451 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang Y et al. Urine derived cells are a potential source for urological tissue reconstruction. J. Urol. 180, 2226–2233 (2008). [DOI] [PubMed] [Google Scholar]
- 143.Bharadwaj S et al. Multipotential differentiation of human urine- derived stem cells: potential for therapeutic applications in urology. Stem Cells 31, 1840–1856 (2013). [DOI] [PubMed] [Google Scholar]
- 144.Bharadwaj S et al. Characterization of urine- derived stem cells obtained from upper urinary tract for use in cell- based urological tissue engineering. Tissue Eng. A 17, 2123–2132 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lang R et al. Self- renewal and differentiation capacity of urine- derived stem cells after urine preservation for 24 hours. PLOS ONE 8, e53980 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ouyang B et al. Human urine- derived stem cells alone or genetically- modified with FGF2 improve type 2 diabetic erectile dysfunction in a rat model. PLOS ONE 9, e92825 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jiang Z-Z et al. Exosomes secreted by human urinederived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res. Ther 7, 24 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]