Abstract
The purpose of this study was to evaluate the use of four fluorescent dyes (rhodamine B, uranine O, auramine O, and erythrosin B) and two nonfluorescent dyes (carmoisine and indigotine) incorporated into sugar baits as biomarkers for phlebotomine sand flies. Each dye could be detected in sand flies fed baits with dye for 24 h when examined using bright field microscopy, although there was considerable variability in the marking produced; all sand flies that had ingested rhodamine B-treated sucrose solution were marked clearly. Sand flies that had ingested sucrose solution containing rhodamine B or uranine O at concentrations as low as 10 mg/L were consistently detected under fluorescence microscopy. None of the treatments in this study reduced the longevity of sand flies. All sand flies fed sucrose solution containing rhodamine B or uranine O were marked for at least 14 d, whereas only 20% of sand flies were marked 3 d after feeding on a carmoisine-treated solution. When rhodamine B and uranine O were combined in a single sucrose solution or when the dyes were fed sequentially to sand flies, both dyes could be detected in sand flies using fluorescence microscopy. We propose that rhodamine B- or uranine O-treated sucrose baits could be used in ecological studies or to identify portions of the adult sand fly population that could be targeted with insecticide-treated sugar baits.
Keywords: Phlebotomus papatasi, biomarker, sugar bait, sand fly
Phlebotomine sand flies can be a significant biting nuisance to humans, and are the vectors of the protozoan parasites that cause leishmaniasis, as well as several viruses and the bacterium Bartonella bacilliformis. Worldwide, leishmaniasis remains an uncontrolled disease with an estimated two million new cases occurring annually (WHO 2009).
Male and female adult sand flies require sugars, which they obtain from sources including plant tissues (Schlein and Warburg 1986, Schlein and Muller 1995) and honeydew excreted by aphids and coccids (Cameron et al. 1995), for energy and reproduction. In arid locations with sparse vegetation, the sugar sources available to sand fly populations are limited to a few species of plants (Schlein and Warburg 1986, Schlein and Yuval 1987). As a demonstration of the shortage of natural sources in arid habitats, Schlein (1987) sprayed sugar solutions containing indigotine (a nonfluorescent food dye) on plants in Israel, and up to 50% of the sand flies subsequently collected in the area were marked by the dye. Marking sand flies with dye-treated sugar baits also has been used as a technique to determine the origin of sand flies collected in an evaluation of sand fly control using net barriers (Faiman et al. 2009).
Sugar baits containing dyes could be a useful tool for studying sand fly ecology, and could be used in conjunction with sand fly control studies. However, there have been no studies to evaluate the toxicity of indigotine (or other nonfluorescent food dyes) or their persistence in sand flies. Furthermore, if a portion of sand flies that feed on sugar solutions containing dyes cannot be detected by visual inspection, there would be limits to the usefulness of these markers in field studies. Certain fluorescent dyes have been shown to be potential biomarkers for blood-fed sand flies, and these fluorescent dyes, even at very low concentrations, could be detected objectively in sand flies feeding on sugar solutions using fluorescence microscopy (Mascari and Foil 2009).
The objective of this study was to evaluate two nonfluorescent dyes (indigotine and carmoisine) and four fluorescent dyes (rhodamine B, erythrosin B, auramine O, and uranine O) as potential biomarkers for sugar-fed sand flies. The dyes were evaluated in five experiments to determine their efficacy to mark sand flies, their effects on the survival of sand flies, their persistence in sand flies, their effectiveness when used in combination, and their minimum concentrations that could be used to mark sand flies.
Materials and Methods
Sand Flies.
A longstanding laboratory colony of sand flies (Phlebotomus papatasi originating from Turkey) was used in this study. The sand fly larvae in the colony were reared using a diet comprised of a composted and dried mixture of equal parts rabbit feces and alfalfa pellets. Adult sand flies were provided with 20% sucrose solution ad libitum, and female sand flies were blood fed using Syrian hamsters. The colony was maintained in environmental chambers in darkness at 28°C, 90% RH.
Experiment 1: Baseline Efficacy of Dyes as Biomarkers.
A sucrose bait solution was made by adding sucrose to deionized water at a rate of 200 g/L. Stock solutions of dyes also contained 1 g/L carmoisine (Stern, Netanya, Israel), indigotine (Stern, Netanya, Israel), rhodamine B (Sigma-Aldrich, St. Louis, MO), erythrosin B (Sigma-Aldrich, St. Louis, MO), uranine O (Sigma-Aldrich, St. Louis, MO), or auramine O (Sigma-Aldrich, St. Louis, MO); sucrose bait solution without dye was the control.
Bioassays were conducted in 250-ml glass jars that had been fitted with fine mesh lids. Ten unfed male and 10 unfed female sand flies (1–2 d old) were transferred into each jar using a mouth aspirator. Approximately 5 ml of sucrose bait solution was applied to a cotton ball, which then was placed on the mesh lid of a jar. The sand flies were allowed to feed on the solution ad libitum for 24 h. After 24 h, the sand flies were killed by freezing and were stored at −80°C. Four jars were prepared for each dye or for control.
Sand flies were placed in the well of a glass concavity slide and covered with a glass coverslip to prevent air currents in the laboratory from moving specimens during observation. The slide was placed on the stage of a fluorescence stereomicroscope (Zeiss SteREO Lumar.V12, Zeiss, Göttingen, Germany), and the sand flies were observed under bright field illumination. Digital images were captured utilizing Zeiss AxioVision (version 4.6) using a 200-ms exposure time. Using bright field microscopy, sand flies were considered positive for the presence of carmoisine, rhodamine B, or erythrosin B if they appeared red; uranine O if they appeared orange; auramine O if they appeared yellow; or indigotine if they appeared blue.
The sand flies also were observed under fluorescence microscopy using a rhodamine filter (excitation wavelength, 540 nm; emission wavelength, 625 nm) and green fluorescent protein (GFP) filter (excitation wavelength, 480 nm; emission wavelength, 535 nm). Preliminary analysis was conducted to determine autofluorescence of untreated sand flies at different exposure times using rhodamine or GFP filters. The maximum exposure time at which untreated sand flies were not fluorescent (appeared as a black field) was determined, and these values were used to set exposure times for examination of sand flies using fluorescence microscopy. Sand flies examined using a rhodamine filter were considered positive if they appeared red, and sand flies examined using the GFP filter were considered positive if they appeared green.
Images of sand flies were assigned random numbers and evaluated using a single-blind evaluation scheme to determine whether sand flies were marked or unmarked. The appearance of the sand flies and the distribution of the dyes in the sand flies were recorded.
Experiment 2: Effects of Dyes on Longevity.
Carmoisine, rhodamine B, uranine O, and control sucrose bait solutions were prepared, as described above; treated cotton balls and bioassay jars were prepared, as described above. Ten male and 10 female sand flies were introduced into each jar and were allowed to feed on the solutions ad libitum. Three jars of sand flies were prepared for each dye or for control.
Daily mortality of sand flies was recorded every 24 h. Mortality data were tested for normality using the Shapiro-Wilk test for normality (SAS Institute 2001). The mean longevity of sand flies fed different sucrose bait solutions was compared using analysis of variance performed with the generalized linear model (GLM) procedure (SAS Institute 2001). The Tukey multiple comparison procedure was used to separate significantly different means.
Experiment 3: Persistence of Dyes.
Carmoisine, rhodamine B, uranine O, and control sucrose bait solutions were prepared, as described above. Adult feeding bioassays were conducted in 3.8-liter polyethylene cages with a plaster surface on the floor and one wall. Treated cotton balls were prepared as above and placed in glass dishes inside the cages. Approximately 50 unfed male and 50 unfed female sand flies were transferred into each cage and were allowed to feed on the sucrose bait solution ad libitum. Three cages were prepared for each dye, and one for the control. After 24 h, sand flies that had been offered bait solution containing a dye, but were not marked, were removed from the cages and excluded from further experimentation. All marked sand flies then were provided with control sucrose bait solution ad libitum.
Five sand flies from each cage that had been fed sucrose bait solutions containing a dye were killed by freezing on 0, 1, 2, 3, 7, 10, and 14 d after the sand flies were withdrawn from dye-treated sucrose bait solution. Control sand flies also were killed on the same days. All sand flies that had been killed at each time period were examined using bright field and fluorescence microscopy, as described above. The percentage of sand flies that were marked on each day was recorded.
Experiment 4: Use of Dyes in Combination.
Rhodamine B, uranine O, and control sucrose bait solutions were prepared, as described above; a sucrose bait solution also was prepared that contained both 1 g/L rhodamine B and 1 g/L uranine O. Bioassays were conducted in four 3.8-liter cages (one cage for each of the three dye solutions and one control cage). A cotton ball was treated with one of the sucrose bait solutions and placed in a glass dish inside each bioassay cage. Thirty unfed male and 30 unfed female sand flies were transferred into each cage and were allowed to feed on the solutions ad libitum.
After 24 h, 15 male and 15 female sand flies that had ingested solutions containing rhodamine B (were visibly marked red) were transferred to a new cage and were offered a cotton ball that had been treated with uranine O; the remaining sand flies in the cage were allowed to continue feeding on rhodamine B-treated cotton balls. Similarly, 15 male and 15 female sand flies that had ingested uranine O (were visibly marked orange) were transferred to a new cage and fed a solution containing rhodamine B, and the remaining uranine O-treated sand flies were allowed to continue feeding on uranine O-treated cotton balls. Sand flies fed sucrose bait solution containing both rhodamine B and uranine O, or control sucrose bait solution were fed these solutions throughout the experiment. The experiment was repeated three times.
After 48 h, all sand flies were killed by freezing. The sand flies then were examined using bright field and fluorescence microscopy, as described above, and the percentage of sand flies that were marked with the dyes was recorded.
Experiment 5: Minimum Effective Concentrations.
Sucrose bait solutions that contained 10-fold concentrations of rhodamine B or uranine O ranging from 0.001 to 1000 mg/L were prepared; a control sucrose bait solution also was prepared. Cotton balls were treated with 5 ml of sucrose bait solution, and bioassays were conducted in 250-ml jars. Ten male and 10 female sand flies were introduced into each jar and were allowed to feed on the solutions ad libitum. Three jars of male and female sand flies were prepared for each dye concentration or control.
The sand flies were killed after being allowed to feed on the bait solutions for 24 h and were stored at −80°C. The sand flies then were examined using bright field and fluorescence microscopy, as described above. The lowest concentration of rhodamine B or uranine O that could be detected in bait-fed sand flies was determined using fluorescence microscopy, followed by single-blind analysis of images.
Results
Experiment 1: Baseline Efficacy of Dyes as Biomarkers.
For all of the dyes tested, sand flies that ingested dye-treated sucrose bait solution were visibly marked when examined using bright field microscopy. However, there was variability in the marking of sand flies that had ingested sucrose bait solution containing carmoisine, indigotine, uranine O, auramine O, or erythrosin B. Without bright and direct illumination, the dye was not apparent in some of the sand flies that had ingested a dye other than rhodamine B; rhodamine B apparently marked all sand flies that ingested dye-treated sucrose bait solution. For sand flies that had ingested sucrose bait solution containing carmoisine, indigotine, erythrosin B, or auramine O, dyes were only visible in the gut (Fig. 1). The dyes were observed throughout the thorax and abdomen of sand flies that had ingested sucrose solution containing rhodamine B or uranine O (Fig. 1).
Fig. 1.
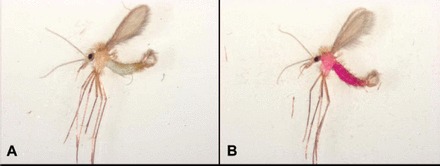
Images taken using bright field microscopy of male sand flies fed sucrose bait solutions containing 1 g/L indigotine (A) or rhodamine B (B). Indigotine was only visible in the gut (A), whereas rhodamine B was observed throughout the thorax and abdomen (B).
Autofluorescence of sand flies under fluorescence microscopy was observed when an exposure time >15 s was used with a rhodamine filter or 100 ms with a GFP filter. Therefore, a 15-s exposure time was used with a rhodamine filter, and 100-ms exposure times were used with a GFP filter when capturing images of sand flies.
Under fluorescence microscopy with a rhodamine filter, sand flies that had ingested sucrose bait solution containing carmoisine, indigotine, uranine O, auramine O, erythrosin B, or control sucrose bait solution were not marked (appeared as a black field); sand flies that had ingested sucrose bait solution containing rhodamine B were marked (appeared red). Sand flies that had ingested rhodamine B were marked throughout the body, except for the wings (Fig. 2).
Fig. 2.
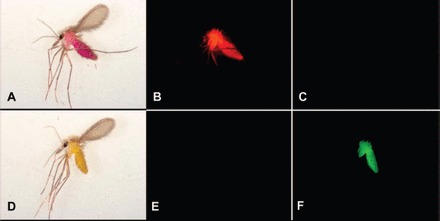
Images of two female sand flies taken using bright field microscopy (A and D), fluorescence microscopy with a rhodamine filter (B and E), and fluorescence microscopy with a GFP filter (C and F). The sand fly pictured in the first row (A–C) had fed on sucrose bait solution containing 1 g/L rhodamine B, and the sand fly pictured in the second row (D–F) had fed on sucrose bait solution containing 1 g/L uranine O.
Using a GFP filter, sand flies that had ingested sucrose solution containing carmoisine, indigotine, rhodamine B, erythrosin B, aurmaine O, or control sucrose solution were not marked (appeared as a black field); sand flies that had ingested sucrose solution containing uranine O were marked (appeared green). Sand flies that had ingested uranine O were marked throughout the body, except for the wings and the legs distal to the femur (Fig. 2).
Experiment 2: Effects of Dyes on Longevity.
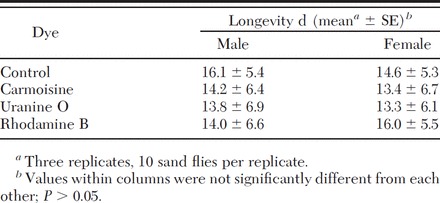
The mean longevity of sand flies that fed on sucrose bait solutions was 14.3 ± 6.0 d for female sand flies and 14.5 ± 6.3 d for male sand flies. The distribution of mortality data was significantly different from normal (W = 0.98627, df = 3, P = 0.3362 for females; W = 0.988302, df = 3, P = 0.4737 for males). There were no significant differences between the longevity of sand flies fed a sucrose bait solution containing any of the dyes tested in this experiment and controls for either sex (Table 1; F = 1.35, df = 3, P = 0.2622 for females; F = 0.86, df = 3, P = 0.4625 for males).
Table 1.
Mean longevity of male and female sand flies fed sucrose bait solution containing carmoisine, uranine O, or rhodamine B
Experiment 3: Persistence of Dyes.
None of the control sand flies were marked when examined using bright field microscopy or fluorescence microscopy with a GFP or rhodamine filter at any of the time periods (0–14 d posttreatment).
Using bright field microscopy, all sand flies that had been fed sucrose solution containing uranine O or rhodamine B were marked 0–14 d posttreatment. The mean percentage of male and female sand flies that were marked after feeding on sucrose bait solution containing carmoisine declined over time and after 3 d was significantly different from the percentage of sand flies marked with uranine O or rhodamine B (Fig. 3; F = 31.41, df = 27, P < 0.0001 for females; F = 41.52, df = 27, P < 0.0001 for males).
Fig. 3.
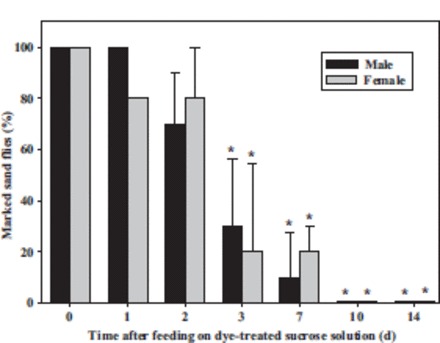
The mean percentage (±SE) of male and female sand flies that were marked when examined under bright field microscopy on 0, 1, 2, 3, 7, 10, and 14 d after having fed on sucrose bait solution containing 1 g/L carmoisine. Values that are significantly different (P < 0.05) from 100% (the mean percentage of sand flies marked by rhodamine B or uranine O) are indicated by an asterisk (*).
When examined under fluorescence microscopy with a rhodamine filter, none of the sand flies that had been fed sucrose solutions containing carmoisine or uranine O were marked at any time period; all sand flies that had been fed sucrose solution containing rhodamine B were marked 0–14 d posttreatment. Under fluorescence microscopy with a GFP filter, none of the sand flies that had been fed sucrose solution containing carmoisine or rhodamine B were marked at any time period; all sand flies that had been fed sucrose solution containing uranine O were marked 0–14 d posttreatment.
Experiment 4: Use of Dyes in Combination.
When examined under bright field microscopy, sand flies that had ingested sucrose bait solution containing uranine O alone appeared orange. Sand flies that ingested sucrose bait solution containing rhodamine B either alone or in combination with uranine O appeared red.
When examined under fluorescence microscopy with a rhodamine filter, sand flies that had ingested sucrose bait solution containing either rhodamine B followed by solution containing uranine O, uranine O followed by solution containing rhodamine B, both rhodamine B and uranine O, or rhodamine B alone appeared red. Sand flies fed sucrose bait solution containing uranine O alone or control sucrose bait solution were not marked when examined using a rhodamine filter.
Using a GFP filter, sand flies that had ingested sucrose bait solution containing either rhodamine B followed by a bait solution containing uranine O, uranine O followed by a bait solution containing rhodamine B, both rhodamine B and uranine O, or uranine O alone appeared green. Sand flies fed sucrose bait solution containing rhodamine B alone or control sucrose bait solution were not marked when examined using a GFP filter.
Experiment 5: Minimum Effective Concentrations.
When examined under fluorescence microscopy with a rhodamine filter, all sand flies that ingested sucrose bait solutions containing 10.0 mg/L rhodamine B or higher were marked. Fewer than half of sand flies (36.8% of males and 28.3% of females) fed sucrose bait solution containing 1.0 mg/L rhodamine B were marked, and none of the sand flies fed sucrose bait solution containing <1.0 mg/L rhodamine B were marked.
All sand flies that had ingested sucrose bait solutions with a concentration of 10.0 mg/L uranine or higher were marked when examined under fluorescence microscopy with a GFP filter. None of the sand flies fed sucrose bait solutions with a concentration of 1.0 mg/L uranine O or lower were marked.
Discussion
To be a useful biomarker for sugar-feeding sand flies, a dye incorporated into sucrose bait solution must consistently mark sand flies so that they can be distinguished from unmarked sand flies. In most instances, each of the dyes tested in this study could be detected in sand flies using bright field microscopy. However, with the exception of sand flies marked with rhodamine B, some dye-fed sand flies could not be differentiated from unmarked specimens using bright field microscopy.
Fluorescence microscopy made it possible to clearly identify all sand flies that had been marked with rhodamine B or uranine O. Using filters with appropriate excitation and emission wavelengths, unmarked sand flies could not be distinguished from the black field, whereas marked sand flies appeared green (uranine O) or red (rhodamine B). Although auramine O and erythrosin B also are fluorophores, they were ineffective as fluorescent biomarkers for sugar-feeding sand flies because the exposure times required to detect the dyes using fluorescence microscopy produced autofluorescence in the sand flies. As expected, the nonfluorescent food dyes carmoisine and indigotine were not detected with fluorescence microscopy.
Our results show that sand flies that had ingested a sucrose bait solution containing carmoisine at the concentrations used did not have reduced longevity compared with sand flies fed untreated sucrose bait solution. Previous studies have reported that indigotine was not toxic to sand flies, but the methods used to make this determination were not described (Schlein 1987). We show in this study that the longevity of sand flies that ingested either rhodamine B or uranine O also was not significantly different from that of control sand flies. Therefore, all three dyes should not interfere with the longevity of sand flies in mark-release-recapture studies.
Sand flies that had ingested a sucrose bait solution containing carmoisine were marked for a limited period of time; after 3 d, only 20% of the sand flies remained marked. In experiment 1, we found that carmoisine and indigotine remained in the gut of sand flies. The fact that carmoisine could no longer be detected in the sand flies after a few days suggests that it could have been voided by the sand flies as the sugar meal was digested. However, both rhodamine B and uranine O were detected throughout most the body of sand flies and remained there for at least 14 d. Being able to accurately detect marked sand flies for at least 2 wk postmarking should allow more comprehensive ecological studies on sand fly populations.
Our results indicate that uranine O and rhodamine B could be detected in sand flies after they have fed on a single sucrose bait solution containing both dyes and a sucrose bait solution containing one dye and then the other. Rather than being blended, the individual fluorescent properties (the specific excitation and emission wavelengths) of rhodamine B and uranine O were retained, and each dye could be detected using fluorescence microscopy. This finding suggests that these fluorescent dyes could be used in combination in sophisticated field studies; for example, multiple sucrose bait treatments could be evaluated in a single site to evaluate the frequency of sugar feeding by sand flies.
Both rhodamine B and uranine O are efficient fluorophores with high quantum yields, meaning they can be detected using fluorescence microscopy at very low concentrations (Magde et al. 1999). Using fluorescence microscopy, sand flies fed sucrose bait solutions containing 10 mg/L either rhodamine B or uranine O could be distinguished from control sand flies. While uranine O and rhodamine B are not known to persist in the environment, their effectiveness as biomarkers for sand flies at such low concentrations would require only very small amounts of the dyes to be applied in field studies (Wang et al. 2008).
The results of this study indicate that the fluorescent dyes rhodamine B and uranine O are very effective biomarkers when they are incorporated into sucrose bait solution and presented to sugar-feeding sand flies. Like the nonfluorescent food dyes that have been used previously to mark sand flies, sugar solutions containing rhodamine B and uranine O do not affect the survival of sand flies (Schlein 1987). However, using fluorescent dyes (and fluorescence microscopy) affords several advantages over nonfluorescent dyes, including the ability to consistently and objectively mark sand flies for at least 2 wk and the ability to detect more than one dye in a single sand fly specimen. Field testing of these methods should be conducted to ensure that these markers are not affected by the different physiological states in natural sand fly populations or by environmental conditions.
Acknowledgements
We thank Matthew Brown (Socolofsky Microscopy Center, Department of Biological Sciences, Louisiana State University) for his helpful support with the fluorescence microscope. This work was supported financially by a grant from the Deployed War-Fighter Protection Research Program, funded by the United States Department of Defense through the Armed Forces Pest Management Board. This is published with approval of the Director of Louisiana Agricultural Experiment Station.
References Cited
- Cameron M. M., Milligan P. J., Llanos-Cuentas A., Davies C. R. 1995. An association between phlebotomine sandflies and aphids in the Peruvian Andes. Med. Vet. Entomol. 9: 127–132. [DOI] [PubMed] [Google Scholar]
- Faiman R., Cuno R., Warburg A. 2009. Control of phlebotomine sand flies with vertical fine-mesh nets. J. Med. Entomol. 46: 820–831. [DOI] [PubMed] [Google Scholar]
- Magde D., Rojas G. E., Seybold P. 1999. Solvent dependence of the fluorescence lifetimes of xanthene dyes. Photochem. Photobiol. 70: 737–743. [Google Scholar]
- Mascari T. M., Foil L. D. 2009. Evaluation of rhodamine B as an orally delivered biomarker for rodents and a feed-through transtadial biomarker for phlebotomine sand flies (Diptera: Psychodidae). J. Med. Entomol. 46: 1131–1137. [DOI] [PubMed] [Google Scholar]
- SAS Institute 2001. SAS system for Windows, release 8.2. SAS Institute, Cary, NC. [Google Scholar]
- Schlein Y. 1987. Marking of Phlebotomus papatasi (Diptera: Psychodidae) by feeding on sprayed, colored sugar bait: a possible means for behavioral and control studies. Trans. R. Soc. Trop. Med. Hyg. 81: 599. [DOI] [PubMed] [Google Scholar]
- Schlein Y., Muller G. 1995. Assessment of plant tissue feeding by sand flies (Diptera: Psychodidae) and mosquitoes (Diptera: Culicidae). J. Med. Entomol. 32: 882–887. [DOI] [PubMed] [Google Scholar]
- Schlein Y., Warburg A. 1986. Phytophagy and the feeding cycle of Phlebotomus papatasi (Diptera: Psychodidae) under experimental conditions. J. Med. Entomol. 23: 11–15. [DOI] [PubMed] [Google Scholar]
- Schlein Y., Yuval B. 1987. Leishmaniasis in the Jordan Valley. IV. Attraction of Phlebotomus papatasi (Diptera: Psychodidae) to plants in the field. J. Med. Entomol. 24: 87–90. [DOI] [PubMed] [Google Scholar]
- Wang H., Wang W.X., Yang Y., Cai W.M. 2008. Visible light induced photodegradation and phototoxicity of phloxine B and uranine. Biomed. Environ. Sci. 21: 438–441. [DOI] [PubMed] [Google Scholar]
- [WHO] World Health Organization 2009. Leishmaniasis. (http://www.who.int/leishmaniasis/en/).


