Abstract
A biosecurity response was triggered by the detection of Aedes albopictus (Skuse) (Diptera: Culicidae) at the Port of Auckland, New Zealand. Ae. albopictus does not occur in New Zealand and is the most significant mosquito threat to this country. The possibility that a founding population had established, resulted in a large-scale biosecurity surveillance and control program. The response was initiated in early March 2007 and completed by mid-May 2007. No further exotic mosquitoes were detected. The response surveillance program consisted of larval habitat surveys and high density ovi- and light trapping. It was coordinated with a habitat modification and S-methoprene treatment control program. The response policies were guided by analysis of surveillance and quality assurance data, population modeling, and trace-back activities. Mosquito habitat and activity close to port were both more abundant than expected, particularly in storm water drain sumps. Sumps are difficult to treat, and during the response some modification was required to the surveillance program and the control regime. We were assured of the absence or eradication of any Ae. albopictus population, as a result of nil detection from surveillance, backed up by four overlapping rounds of insecticide treatment of habitat. This work highlights the importance of port surveillance and may serve as a guide for responses for future urban mosquito incursions.
Keywords: Aedes albopictus, biosecurity response, surveillance trapping, modeling, control
The Asian tiger mosquito, Aedes (Stegomyia) albopictus (Skuse) (Diptera: Culicidae), does not occur in New Zealand, and is considered as the most significant mosquito threat to this country. A single adult male Ae. albopictus was collected from a CO2-baited sentinel surveillance light trap at the Port of Auckland (POA) on 2 March 2007. The specimen was identified on 5 March 2007, and the identification was validated on 6 March 2007.
There was potential that detection of Ae. albopictus represented an established population, rather than a recent incursion from nearby containers. Evidence supporting the possibility of an established population was as follows: 1) the specimen was not associated with a specific risk good or a particular vessel; 2) the dispersive adult life stage detected indicated that local breeding may have occurred; and 3) the POA surveillance program was determined to have poor sensitivity with many of the mosquito traps inadequately maintained. Hence, there was the possibility that a small population at the port may not have been detected. Furthermore, immediate treatment of habitat with larvicides after the detection would have reduced the population, and subsequent zero catches from adult and larval surveys may not have reflected the population status.
Ae. albopictus is considered as one of the most invasive mosquito species in the world (Lowe et al. 2000). The aggressive colonizing capacity of Ae. albopictus (Hawley 1988) has been demonstrated by its global spread since the 1980s (Savage et al. 1992, Knudsen 1995, Eritja et al. 2005, Ritchie et al. 2006).
There were 12 confirmed interceptions of Ae. albopictus in New Zealand between 1998 and January 2007. In New Zealand, most exotic mosquito interceptions have been on ships, and generally associated with used tires and used machinery. The POA was the most frequent site of interception (Derraik 2004). These facts, coupled with habitat and climate suitability (Laird et al. 1994, Weinstein et al. 1995, de Wet et al. 2001), have led to the POA being forecast as the most likely point of establishment of Ae. albopictus in New Zealand (Derraik 2006).
New Zealand has only 16 mosquito species. Low species diversity along with low endemic mosquito population densities mean that mosquito larval habitats in New Zealand are underutilized (Laird 1990). Niche availability of larval habitats, together with an increase in the international mobility of people and their products (Plotkin and Kimball 1997), makes New Zealand vulnerable to further introductions of exotic mosquito species.
At the start of the response, we considered that the Auckland Port and the central business district (CBD) represent an environment suitable for Ae. albopictus colonization, as has been the case in several large cities overseas, including Kuala Lumpur and Tokyo (Hawley 1988, e.g., Honorio et al. 2003). The surveys carried out during the response confirmed the abundance of mosquito larval and adult habitats in the POA and Auckland CBD, as predicted by Derraik (2006).
Ae. albopictus is classified as an unwanted organism under the New Zealand Biosecurity Act 1993. If it became established, it is likely to have negative pest and ecological effects, as well as potential medical and veterinary impacts as a vector for a number of animal and human diseases (Gratz 2004).
The aim of the Ministry of Agriculture and Forestry Biosecurity New Zealand response was to determine whether a population of Ae. albopictus was present at the port. This work describes the actions taken to detect Ae. albopictus and mitigate risk, if present.
Materials and Methods
The area for the surveillance and control efforts was set by considering the distribution potential of Ae. albopictus in the context of the POA climate and our initial perception of larval and adult habitat availability. The initial assessment of the environment was that temperatures and rainfall were suitable, but the POA and immediately adjacent CBD areas presented limited sites for adult refuge, oviposition, and larval and adult development. This assessment was supported by the low mosquito numbers represented in the sentinel surveillance trap data. We therefore assumed that the dispersal pressure would be high (e.g., Richards et al. 2006), and that colonization could have potentially occurred over an area of up to 1,000 m. Therefore, the initial surveillance and control zones were set for 1,000 m from the epicenter. This was later modified to a zone A (600 m plus all contiguous highly suitable habitats) and zone B (600–1,000 m).
The surveillance program consisted of continuous light, tire, and ovitrapping, along with sticky emergence traps and a series of delimiting surveys. The delimiting surveys were rain event-triggered search, evaluations, and mapping of all potential habitats for Ae. albopictus in the 1,000-m radius zone from the POA epicenter, combined with larval sampling. Three delimiting surveys were carried out during 19–23 March, 25 April, and 2–4 May 2007. Final surveillance and delimiting activities were carried out during 14–18 May 2007 (reduced to 600 m). The delimiting surveys followed 5–7 d after a rainfall event that was 25 mm or greater. Suitable habitat for Ae. albopictus included areas of vegetation and artificial containers, discarded rubbish holding water, pot plant holders and trays, water features, etc. Samples and habitat were recorded using Global Positioning System (GPS) locations (where GPS capable), and coded using the habitat categories of Laird (1995).
Priority was placed on identifying, mapping, sampling, and treating larval habitats in zone A, followed by increased surveillance in zone B. Enhanced surveillance ovitraps and sentinel tire traps and larvitraps were deployed starting from the focal point.
Visual examination of the habitat was conducted before sampling. Pipettes were used to collect larvae from tree holes and similar habitats. Small white cotton nets (mesh size 200–300 μm) or ladle-type dippers were used in other small water bodies. Water from tires and ovitraps were emptied into a white tray to locate and collect mosquito larvae. Sumps and other deep drainage pits were sampled using a net (mesh size 200–300 μm) with long handles by a vortex technique, as explained by Montgomery et al. (2004), and samples were collected using a sample concentrator adapted from plankton collection methodology.
The survey teams recorded 338 storm water sumps within 600 m of the epicenter and >1,100 sumps in the 600- to 1,000-m zone. There were too many sumps for the response team to sample all sumps, and a sampling plan was instituted. The number of sumps to be sampled was determined using the following logic.
We calculated that if a sampled sump is infested, there is a 100% probability of detecting at least one Ae. albopictus larvae in that sump sample, based on the following. 1) The relationship between population rate of increase (r, day-1) and temperature (T, °C) reported for Ae. albopictus by Alto and Juliano (2001) was extrapolated to the lower temperatures experienced in Auckland by linear regression (r = 0.0147(T - 15.3), R2 = 0.849). This relationship was later validated by the results of Delatte et al. (2009). Daily rates of increase in Auckland were estimated using temperatures recorded at the nearby Kyber Pass weather station, suggesting that Ae. albopictus populations would have increased ≈11-fold over the period 2 March (time of detection) to 4 May (time of sump survey) (Fig. 1). Therefore, assuming only one female adult mosquito had been introduced, with a fertility output of 76 larvae (the mean number of eggs matured by a single female at 17°C [Briegel and Timmermann 2001]), then we would expect the population at the sample time to have been in the order of 809 larvae (Fig. 1). 2) Assuming that these are distributed across 5% (probability of infestation, Ipr) of the 338 sumps, suggests 47 larvae per infested sump. 3) Assuming that the probability of correctly identifying Ae. albopictus larva in a sample is 90%, we estimated the probability of not detecting the presence of Ae. albopictus larvae in an infested sump to be in the range (0.1)5 to (0.1)14, i.e., effectively zero.
Fig. 1.
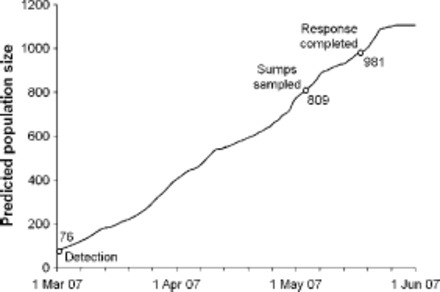
Predicted size of a hypothesized population at the final delimiting survey and response completion, estimated by a population growth model based on the daily rate of increase of Ae. albopictus extrapolated from the data of Alto and Juliano (2001) and Delatte et al. (2009).
To calculate the sample size required for the survey, we assumed that the true proportion of sumps infested with Ae. albopictus was 0.05 (denoted as Ipr). We set the desired overall detection probability for the sump survey to be 0.95 (power of the survey, probability of detection, Dpr) and assumed a binomial distribution for a sump being infested (yes/no). Then with a sample size of n randomly selected sumps tested, the probability of finding Ae. albopictus larvae in at least one sump (Dpr) is 1 - (1 - Ipr)n, because (1 - Ipr) is the probability of not finding any larvae in a randomly selected survey sump. Hence, a sample size of 59 sumps was required.
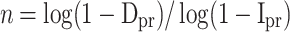 |
The sampling effort was stratified into six 100-m semicircles, with a plan to collect 10 samples from within each 100-m annuli. Therefore, the sampling intensity diminished moving outward, the closest 100 m being 6.4 n/ha and in the 500–600 ring 0.6 n/ha. However, because of field constraints, only two sumps were available to be sampled within the closest 100 m, and so additional samples were collected in subsequent annuli. Seventy sumps were sampled in total.
Both ovitraps and tire traps were used for sampling of eggs and larvae during the delimiting surveys. Ovitraps were prepared out of black glass/plastic containers of ≈500–700 ml and filled with aged water (tap water, supplemented by rabbit pellets) to a depth of 10 cm or near to the top. A wooden paddle (16 × 3 cm) placed in the container provided the surface for oviposition. The traps were placed 0–1 m above the ground. Each component of the ovitraps was numbered to avoid mixing up of samples. The exposed ovitraps were examined for egg or larval stages, the wooden paddles were removed, and all larvae were collected and transported securely to the approved containment facility for identification and rearing, if required. Used automobile tires with a drainage hole cut on the side wall and placed upright or at 60° angles, with 1 liter of aged water, were used as tire traps. The traps were serviced on a weekly basis in which the water was completely emptied into a white tray and the life stages were collected and transported to the laboratory.
Light traps using CO2, supplemented by octenol, and operated away from competing light sources, were used to trap adult mosquitoes within the surveillance area.
The sites for various traps were stationed in localities that were considered attractive for the Ae. albopictus species, being shaded, sheltered areas away from competing water sources, and close to vegetation, where possible. All areas were close to human population. All trap location sites were geo-referenced, and photographs, GPS way points, and street address descriptors (when available) were used to identify trap location. This information allowed efficient revisit of traps for servicing and maintenance.
At the POA, six light traps, 45 ovitraps, and four tire traps were deployed at the beginning of the first delimiting survey. In the CBD, 95 ovitraps, 20 tire traps, and eight adult traps were deployed after the first delimiting survey. After the second delimiting survey, all the ovitraps and tire traps from 600 to 1,000 m were relocated to within 600 m. The eight light traps in the 600- to 1,000-m area remained in their original locations. The adult traps were checked three times per week, and the ovitraps were serviced weekly. On 14 May, 56 sticky emergence adult traps were installed over the drain sumps, as described by Montgomery et al. (2004), to capture and identify the emerging adults from sumps within 600 m. The sticky adult traps served as an additional quality assurance on sump treatment efficacy. The sticky traps were recovered after 2 d.
The control program was directed at the larval habitat and sought to treat or remove all identified habitats to mitigate the possibility of Ae. albopictus establishment, limit the dispersal potential, and eradicate the mosquito if a local population was detected. Larval habitat unable to be mitigated or removed was treated with S-methoprene pellets at a rate that would have provided effective lethal dose up to 30 d after application. Prolink S-methoprene insect growth regulator pellets (Pacific BioLogics, Kippa Ring, Queensland, Australia) were applied at a rate of 4 kg/ha for Aedes and Culex spp when water is deep (>30 cm), vegetation and/or pollution are present, and/or mosquito larvae numbers are high (>10 per dip). This equated to approximately three pellets per square meter. All sumps in the surveillance area were also treated with S-methoprene. Treatment application in sump drains was calculated as per follows: sump dimensions were L700 mm, W450 mm, D500 mm (water depth), with a surface area: 0.315 m2; 4 kg/ha = 3 pellets per m2, one pellet per drain has an effective treatment rate of 4.23 kg/ha, but we conservatively applied a treatment rate of three or more pellets per sump (=3/0.315 = 9.52 kg/ha or more). The S-methoprene pellets had a batch certificate of analysis. Furthermore, quality control for this response was linked to lab aquaria, and field quality checking bioassays were conducted using Aedes antipodeus as a surrogate species to trial pellet efficacy. All sumps treated were marked with dazzle paint. The control program was reviewed and refined in early-mid April.
A trace-back exercise was conducted in an attempt to identify the source of the Ae. albopictus detected. Between 17 February 2007 and 2 March 2007, there were 16 ships from potential source countries unloaded within 600 m from where the Ae. albopictus specimen was found. In addition, goods from a number of ships were fumigated in close proximity to this location. Fumigated goods were not necessarily from ships berthed within the 600-m zone. Those ships identified could have provided a potential pathway for the specimen found, assuming that an adult was able to survive for up to 10 d. Stable isotope analysis was considered as a means of determining whether the Ae. albopictus specimen originated from Auckland or outside of New Zealand. However, neither the analytical method nor reference data are currently available (R. Frew, personal communication, May 2007). mtDNA haplotyping was also assessed as a response tool. Although this method could not resolve the point of origin query, if a population were to be detected, haplotyping could be used to determine whether the strain was a tropical or temperate/cold hardy, and also help identify risk pathways or risk locations.
Results and Discussion
The habitat delimiting survey/larval sampling detected five habitat types (Table 1). The vast majority of these (over 1,100) were street side storm water sumps. Other habitat categories found were gutters, tires, and other artificial containers, along with a moderate amount of vegetation. The distribution of these is shown in Fig. 2. The survey results detected a greater number of suitable habitats than expected. Within the 600-m zone, there were 338 storm water sumps, several other larval habitats, plus vegetation, and many hosts (rodents, humans, birds). These were considered ample to provide Ae. albopictus larval and adult habitat.
Table 1.
Total mosquito larvae counts by habitat category from the habitat delimit surveys
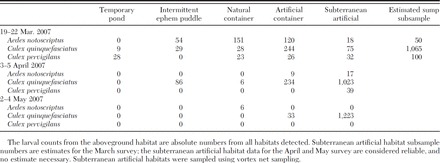
Fig. 2.
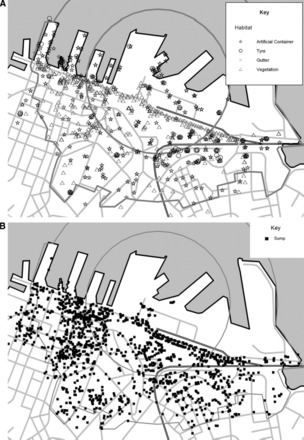
A and B, Maps of Auckland port and CBD showing 600- and 1,000-m response zones and mosquito habitat identified during the delimiting surveys. A, Gives the distribution of mosquito habitats identified; B, gives the distribution of sumps.
The mosquito activity detected within the POA and CBD in the first 4 wk of the response confirmed that the habitat present was indeed able to support mosquito populations, and was relatively rich (larval collections in the 100s per week) (see Figs. 3 and 4 [trapping] and Table 1 [first two delimiting surveys]). This mosquito activity and unexpected abundance of habitat within 600 m, along with the niche availability with only modest competition, led to the conclusion that the probability of Ae. albopictus distribution in a single generation beyond 600 m was less likely than initially thought. Culex quinquefasciatus was the most abundant species, often occurring at 10–100 times the numbers of Aedes notoscriptus, with the native Culex pervigilans least commonly detected. The occurrence of Ae. notoscriptus and Cx. quinquefasciatus, mosquito species that complete development in flooded artificial containers under similar ecological parameters to Ae. albopictus (S. Ritchie, personal communication, April 2007), within the POA area and CBD 600-m zone confirmed the suitability of those areas to support Ae. albopictus population. (An incidental observation of the surveillance data is the relative number of larvae collected was often 10 times the adult numbers. This could be interpreted as an indication of mosquito population structure, and/or indicates that adult trapping is less sensitive than larval monitoring as a surveillance method.).
Fig. 3.
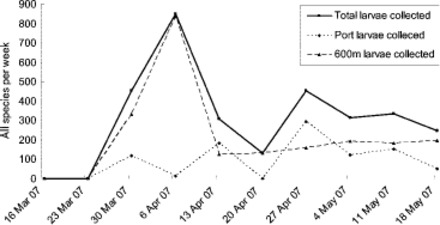
Total mosquito larvae and egg counts (all species) from tire and ovitrapping surveillance over the entire response period. No Ae. albopictus larvae were detected.
Fig. 4.
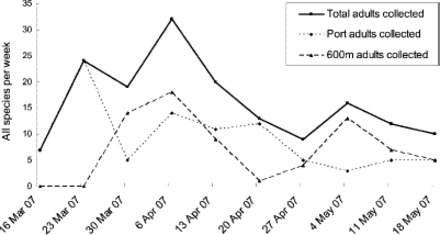
Total adult mosquito counts (all species) from adult surveillance over the entire response period. No Ae. albopictus adults were detected.
Trap and delimit survey data demonstrate that Aedes spp. populations tapered off to zero in the response area (Figs. 5 and 6; Table 1). This indicates that if Ae. albopictus had established, the control actions would have contributed to local eradication. However, Culex spp. activity and abundance decreased slowly over the duration of the response, particularly in the storm water sumps (Figs. 5 and 6; Table 1). This persistence may be because of their abundance as well as recruitment from outside the response area. Culex spp. are less susceptible to methoprene than Aedes spp., particularly in habitats with high organic load (e.g., Ritchie et al. 1997). As the habitat mitigation and control program either eliminated or minimized mosquito activity in all but artificial subterranean habitats (Table 1), the surveillance data showed that the storm water sumps were the most important habitat in the response area, and the mosquito populations persisted in the sumps the longest (Table 1).
Fig. 5.
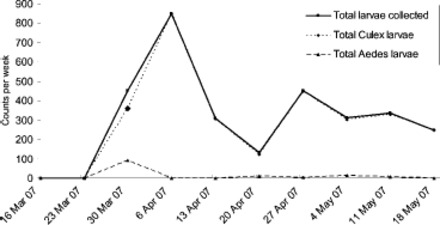
Comparison of Aedes spp. and Culex spp. larvae and egg counts from tire and ovitrapping surveillance over the entire response period, showing Aedes spp. trending to zero. No Ae. albopictus larvae were detected.
Fig. 6.
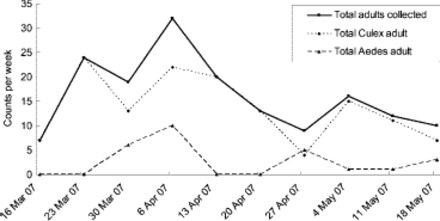
Comparison of Aedes spp. and Culex spp. adult counts from adult surveillance over the entire response period. No Ae. albopictus adults were detected.
Our data corroborate the observations of other authors that sumps provide good mosquito habitat, with consistent water and organic matter for larval development and humid shelter habitat for adult refuge (Figs. 7 and 8; Kay et al. 2000, Russell et al. 2002, Su et al. 2003, Hribar et al. 2004, and others). Areawide control targeting Culex spp. in urban areas is difficult, and future Culex responses need to be prepared for a prolonged campaign, with Culex-specific treatment rates, and especially targeting storm water sumps.
Fig. 7.

Example of a street side storm water sump in the Auckland CBD.
Fig. 8.

Leaves and water in a street side storm water sump. Sumps provide good urban mosquito habitat, with semipermanent bodies of water, high organic matter content, and adult refuge.
After the first delimit survey, we observed that the sump mosquito population was substantially greater than initially expected. The number of mosquitoes from the sumps was more than from all other habitats combined, and increasingly so as other habitats were eliminated during the response. A wide variation was noticed between the estimated sump population and the actual surveillance data up to the beginning of April. Therefore, it was assumed that initial subterranean habitat sampling probably would not have detected an Ae. albopictus population in the storm water sumps, and so we developed and conducted the specific sump sample plan for the later delimits.
It is known that Ae. albopictus uses drain sumps, including catch basins in urban Tokyo (30% utilization) (Hata and Kurihara 1982) and the underground storm drain systems in several southeastern states in the United States (Madon et al. 2002) and in Italy (Blackmore 1995), where Celli et al. (1994) found between 12.5 and 22.75% of manholes surveyed in the Brescia Province of northern Italy were infested (in June-September). Preliminary work confirms that New Zealand sumps are also good urban mosquito habitat, and suggests similar rates of utilization (Laird 1990, 1995).
A quality assessment detected possible treatment failures, including emergence of some adults from late instar larvae collected from sumps and ovitraps in early April (original water was used). However, rearing on in an environment separate to that in which the treatment has occurred may give false positive results (S. Richie, personal communication, April 2007). Therefore, although we were not certain treatment failures had occurred, we were concerned that the treatment rate was possibly too low, or some larval habitats were inadvertently untreated. Consequently, the control program was reviewed.
Three factors necessitated improvements in the surveillance and control programs, as follows: 1) The high density of mosquito habitat and activity close to the initial detection strongly suggested that dispersal pressure over 600 m would be unlikely. 2) As a result of limited resources available, it was possible that we may not have detected an Ae. albopictus population in the subterranean habitat sampling. 3) Some possible treatment failures were detected during the quality control assessment.
The response activity was redesigned to concentrate resources in the 600-m zone, with the intentions of both improving the probability of detection, and ensuring treatment actions were effective, by: 1) focusing the third delimit survey on the 600-m zone and only higher risk areas of the 600- to 1,000-m zone; 2) redeploying all tire and ovitraps to within the 600-m zone, and increasing the number of ovitraps to a density of ≈1 per 50 m2; 3) increasing the confidence level of sampling in the storm water sumps to a 95%; 4) reviewing the treatment regime (product and rate); and 5) placing sticky emergence traps on series of sumps to both give quality assurance of effective treatment, and to contribute to the surveillance effort if treatment failures occurred.
The more intensive 600-m zone delimiting survey and tire and ovitrapping followed the models recommended in Cowley et al. (1998) and Honorio et al. (2003). The 600-m intensive surveillance was complemented by ongoing enhanced surveillance consisted of continued light trapping at the already established sites out to 1,000 m. All traps continued to be inspected two to three times per week.
Survey design and specimen collection methods specifically designed for sumps provided efficient use of field worker time, and gave statistical confidence that the third delimit should have detected an Ae. albopictus population, if it was present.
Although the S-methoprene dose that has been applied followed label instructions, the supplier of the product (Pacific BioLogics) recommended that considering the high organic load, we increase the dose for the sumps to 6–10 pellets per sump per month, with reapplications after any rain event that will cause flushing. This rate was applied on the treatment rounds of 2–4 May and 14–18 May, and each of the treated habitats marked for quality assurance purposes. The sticky emergence adult traps detected no adult emergence from the sumps, indicating successful control had been achieved.
As the final delimit and sump surveys were considered adequate, and the quality checks gave assurance of the efficacy of the control program, the response was declared completed on 18 May 2007. The biosecurity agencies of New Zealand had a high degree of confidence in the absence or eradication of Ae. albopictus from Auckland.
Conclusion
There were several salient points and areas for improvement identified by this incursion and response. The most important observations of the response initiation phase are as follows: 1) Rapid response (action immediately after detection) control actions are wholly appropriate in mitigating nearby adult and larval populations. In addition, for future incursions, the initial actions taken need to be coordinated with biosecurity response and longer term biosecurity objectives. 2) The Port surveillance needs to be delivered to a standard that can give assurance of local area freedom and to provide an early warning of incursant mosquito populations (Ritchie and Russell 2002). 3) The period between notification to the beginning of areawide control actions should ideally be shorter than the 2 wk required in this response. Honorio et al. (2003) reported the distribution potential of the Ae. albopictus in 6 d is 800 m or more. The methods documented in this work will serve as a contingency plan and will enable a more rapid rollout of response actions for the next mosquito incursion.
The most significant biological findings and biosecurity response outcomes are as follows: 1) Storm water drain sumps and other subterranean artificial larval habitat have been confirmed as important urban mosquito habitat in New Zealand. 2) Sumps are difficult and time consuming to sample. Future responses need to be prepared to sample sumps effectively. 3) Sumps are a difficult environment to treat, and mosquito populations, Culex spp. in particular, can persist in drains under a control regime. 4) The population modeling suggests that recently incursant populations will be very small under temperate climates: potentially <2,500 individuals for the first 2 mo. Newly established populations will possibly be below detectable levels, or only marginally detectable. Therefore, we need to be highly confident of the effectiveness of the control program. 5) For this response, we are confident of the absence or eradication of any Ae. albopictus population. This confidence is based on the surveillance program detecting no further exotic specimens, backed up by four overlapping rounds of quality-assured insecticide treatment.
Acknowledgements
This response was a collaborative effort. Scott Ritchie and Professor Richard Russell contributed expert advice. Rachel Cane and Amy Snell compiled a review of the literature that was used in this work for habitat-type range. Graham Mackareth gave intelligence interpretation. The speed and efficiency of the New Zealand BioSecure field teams were greatly appreciated.
Footnotes
Biosecurity New Zealand is a division of the Ministry of Agriculture and Forestry, and is the lead agency in New Zealand’s biosecurity system.
References Cited
- Alto B. W., Juliano S. A. 2001. Precipitation and temperature effects on the populations of Aedes albopictus (Diptera: Culicidae): implications for range expansion. J. Med. Entomol. 38: 646–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore M. S. 1995. Aedes albopictus in Italy. Am. Mosq. Contr. Asso. Newsl. 21: 9. [Google Scholar]
- Briegel H., Timmermann S. E. 2001. Aedes albopictus (Diptera: Culicidae): physiological aspects of development and reproduction. J. Med. Entomol. 38: 566–571. [DOI] [PubMed] [Google Scholar]
- Celli G. H., Bellini R., Carrieri M. 1994. Survey on Aedes albopictus (Skuse) (Diptera: Culicidae) infestation in Desenzano del Garda (Italy). Boll. Inst. Entomol. Univ. Bologna 48: 211–217. [Google Scholar]
- Cowley J. M., Bullians M. S., Herrera V. E., Holder P. W., Whyte C. F., Kay B. H. 1998. National pest management strategy for exotic mosquitoes of significance to New Zealand, pp. 113–125. InProceedings of the Third National Conference of the Mosquito Control Association of Australia Conference, 23–25 September 1998, Gold Coast, Australia. [Google Scholar]
- Delatte H., Gimonneau G., Triboire A., Fontenille D. 2009. Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J. Med. Entomol. 46: 33–41. [DOI] [PubMed] [Google Scholar]
- Derraik J.G.B. 2004. Exotic mosquitoes in New Zealand: a review of species intercepted, their pathways and ports of entry. Aust. NZ. J. Public Health 28: 433–444. [DOI] [PubMed] [Google Scholar]
- Derraik J.G.B. 2006. A scenario for invasion and dispersal of Aedes albopictus (Diptera: Culicidae) in New Zealand. J. Med. Entomol. 43: 1–8. [DOI] [PubMed] [Google Scholar]
- de Wet N., Wei Y., Hales S., Warrick R., Woodward A., Weinstein P. 2001. Use of computer model to identify potential hotspots for dengue fever inNewZealand. N. Z. Med. J. 114: 420–422. [PubMed] [Google Scholar]
- Eritja R., Escosa R., Lucientes J., Marquès E., Molina R., Roiz D., Ruiz S. 2005. Worldwide invasion of vector mosquitoes: present European distribution and challenges for Spain. Biol. Invasions 7: 87–97. [Google Scholar]
- Gratz N. G. 2004. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 18: 215–227. [DOI] [PubMed] [Google Scholar]
- Hata K., Kurihara T. 1982. Mosquito larvae found in catch-basins in an urban area. Jpn. J. Sanitary Zool. 33: 247–248. [Google Scholar]
- Hawley W. A. 1988. The biology of Aedes albopictus. J. Am. Mosq. Control Assoc. 4(Suppl. 1): 1–40. [PubMed] [Google Scholar]
- Honorio N. A., Silva W. C., Leite P. J., Goncalves J. M., Lounibos L. P., Lourenco-de-Oliveira R. 2003. Dispersal of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in an urban endemic dengue area in the state of Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz 98: 191–198. [DOI] [PubMed] [Google Scholar]
- Hribar L. J., Vlach J. J., DeMay D. J., James S. S., Fahey J. S., Fussell E. M. 2004. Mosquito larvae (Culicidae) and other Diptera associated with containers, storm drains, and sewage treatment plants in the Florida Keys, Monroe County, Florida. Fla. Entomol. 87: 199–203. [Google Scholar]
- Kay B. H., Ryan P. A., Russell B. M., Hold J. S., Lyons S. A., Foley P. N. 2000. The importance of subterranean mosquito habitat to arbovirus vector control strategies in North Queensland, Australia. J. Med. Entomol. 37: 846–853. [DOI] [PubMed] [Google Scholar]
- Knudsen B. 1995. Global distribution and continuing spread of Aedes albopictus. Parassitologia 37: 91–97. [PubMed] [Google Scholar]
- Laird M. 1990. New Zealand’s northern mosquito survey 1988–89. J. Am. Mosq. Control Assoc. 6: 287–299. [PubMed] [Google Scholar]
- Laird M. 1995. Background and findings of the 1993–94 New Zealand mosquito survey. N.Z. Entomol. 18: 77–90. [Google Scholar]
- Laird M., Calder L., Thornton R., Syme R., Holder P., Mogi M. 1994. Japanese Aedes albopictus among four mosquito species reaching New Zealand in used tires. J. Am. Mosq. Control Assoc. 10: 14–23. [PubMed] [Google Scholar]
- Lowe S., Browne M., Boudjelas S., De Poorter M. 2000. 100 of the world’s worst invasive alien species: a selection from the global invasive species database: Aliens 12. (updated November 2004 http://www.issg.org/booklet.pdf).
- Madon M. B., Mulla M. S., Shaw M. W., Kluh S., Hazelrigg J. E. 2002. Introduction of Aedes albopictus (Skuse) in Southern California and potential for its establishment. J. Vector Ecol. 27: 149–154. [PubMed] [Google Scholar]
- Montgomery B. L., Ritchie S. A., Hart A. J., Long S. A., Walsh I. D. 2004. Subsoil drain sumps are a key container for Aedes aegypti in Cairns, Australia. J. Am. Mosq. Control Assoc. 20: 365–369. [PubMed] [Google Scholar]
- Plotkin B. J., Kimball A. M. 1997. Designing an international policy and legal framework for the control of emerging infectious diseases first steps. Emerg. Infect. Dis. 3: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S. L., Apperson C. S., Ghosh S. K., Cheshire H. M., Zeichner B. C. 2006. Spatial analysis of Aedes albopictus (Diptera: Culicidae) oviposition in suburban neighborhoods of a piedmont community in North Carolina. J. Med. Entomol. 43: 976–989. [DOI] [PubMed] [Google Scholar]
- Ritchie S. A., Russell R. C. 2002. A Review of the New Zealand Mosquito Surveillance Program. New Zealand Ministry of Health, Wellington, New Zealand. [Google Scholar]
- Ritchie S. A., Asnicar M., Kay B. H. 1997. Acute and sublethal effects of (S)-methoprene on some Australian mosquitoes. J. Am. Mosq. Control Assoc. 13: 153–155. [PubMed] [Google Scholar]
- Ritchie S. A., Moore P., Carruthers M., Williams C., Montgomery B., Foley P., Ahboo S., van den Hurk A. F., Lindsay M. D., Cooper B., Beebe N., Russell R. C. 2006. Discovery of a widespread infestation of Aedes albopictus in the Torres Strait, Australia. Med. J. Malaysia 61: 264–269. [DOI] [PubMed] [Google Scholar]
- Russell B. M., McBride W.J.H., Mullner H., Kay B. H. 2002. Epidemiological significance of subterranean Aedes aegypti (Diptera: Culicidae) breeding sites to dengue virus infection in Charters Towers, 1993. J. Med. Entomol. 39: 143–145. [DOI] [PubMed] [Google Scholar]
- Savage H. M., Ezike V. I., Nwankwo A.C.N., Spiegel R., Miller B. R. 1992. First record of breeding populations of Aedes albopictus in continental Africa: implications for arboviral transmission. J. Am. Mosq. Control Assoc. 8: 101–103. [PubMed] [Google Scholar]
- Su T., Webb J. P., Meyer R. P., Mulla M. S. 2003. Spatial and temporal distribution of mosquitoes in undergroung storm drain systems in Orange County, California. J. Vect. Ecol. 28(1): 79–89. [PubMed] [Google Scholar]
- Weinstein P., Laird M., Calder L. 1995. Australian arboviruses: at what risk New Zealand? Aust. NZ. J. Med. 25: 666–669. [DOI] [PubMed] [Google Scholar]


