Abstract
Acute myocardial infarction (AMI) evokes a temporally coordinated immune response, in which monocytes are critically involved in the clearance of cell debris; however, excessive inflammation induced by the classical sub-population of monocytes frequently limits the endogenous reparative process. In the present study, the potential of the anti-inflammatory adipokine complement C1q tumor necrosis factor (TNF)-related protein-3 (CTRP3) to induce intermediate switch of monocytes to an anti-inflammatory phenotype was explored. Circulating monocytes were isolated from patients with AMI at various time-points (3–5 h, 3 days and 7 days) and categorized by flow cytometry/immunostaining into three sub-divisions based on the expression of CD14 and CD16 epitopes: Classical (CD14++/CD16−), non-classical (CD14+/CD16++) and intermediate populations (CD14++/CD16+). The phagocytic activity was evaluated by the ingestion of FITC-Zymosan and 19F-nanoemulsion and the migratory activity using Thin Cert™ Transwell assay. Monocytes were cultured using autologous serum in the presence of CTRP3 (1 µg/ml) for 24 h and the expression of interleukin 6 (IL-6) and TNF-α was quantified by reverse-transcription quantitative PCR. In addition, SB203580, a p38 mitogen-activated protein kinase (MAPK)/ERK inhibitor, was used to examine the downstream pathways of CTRP3. AMI evoked a transient increase in monocyte counts of the classical subset after onset of the ischemic insult, while the non-classical and intermediate subsets persistently expanded (P<0.01). The monocytes from patients at 3 days after AMI displayed enhanced phagocytic and migratory activities in comparison with those from healthy volunteers (P<0.01). Of note, addition of CTRP3 induced an intermediate switch of monocyte subsets and antagonized the enhanced expression of cytokines, particularly IL-6, in monocytes stressed by lipopolysaccharides, likely by blunting the ERK1/2 and P38 MAPK signaling pathway. In conclusion, the present study demonstrated a dynamic fluctuation of monocyte subsets and enhanced phagocytic and migratory activities in patients with AMI. Furthermore, the ‘proof-of-concept’ evidence pinpoints CTRP3 as an alternative candidate to modulate the ‘uncontrolled’ inflammatory response and thus to augment cardiac reparative processes in patients with AMI.
Keywords: complement C1q tumor necrosis factor-related protein-3, CD14++CD16+ monocytes, acute myocardial infarction, phagocytosis, migration, interleukin-6
Introduction
Acute myocardial infarction (AMI), commonly known as heart attack, results in ischemic damage of cardiomyocytes, which, by activation of a damage-associated molecular pattern, leads to initiation of the innate immune response (1,2). Inflammatory cells infiltrate to the injured heart, ensuring the clearance of harmful cell debris and repair of the damaged area via formation of a fibrotic scar (3) and activation of the endogenous stem cell pool (4). While the default immune response to AMI appears to ensure a quick fix of the heart, the scarring leads to pathological remodeling of the heart and compromised cardiac function over time. Therefore, timely balanced transformation from pro-inflammatory in the acute stage to an anti-inflammatory state is rather critical to ensure a reparative course after AMI (5).
Monocytes are cornerstones of local inflammatory response that are causatively involved in numerous inflammatory diseases and tissue homeostasis (6). Monocytes are recruited to the site of necrosis where they differentiate into distinct subsets of tissue macrophages (M1/M2) and function synergistically, but at times counteractively, with other cellular components in a reparative process, orchestrated mainly by the local milieu and cytokine profile (7,8). Although circulating monocytes develop from a single, common precursor, heterogeneity of the monocyte population has been widely acknowledged based on the differential expression of the lipopolysaccharide (LPS) receptor (CD14) and the FccII I receptor (CD16), with cell subsets being the classical (CD14++/CD16−), non-classical (CD14+/CD16++) and intermediate (CD14++/CD16+) populations (9). Each subset has different immune functions, phagocytic activity, cytokine profile and reparative capacity (10). Classical monocytes express high levels of chemokine receptor 2 (CCR2) and are highly responsive to LPS stimulation; they produce a broad range of cytokines and chemokines with high myeloperoxidase and phagocytic activity, and are thus associated with the severity of inflammatory response and worse clinical outcome in patients with AMI (11). By contrast, non-classical subsets are distinguished by high levels of complement C1q tumor necrosis factor (TNF)-related protein-3 (CTRP3), have numerous patrolling properties and are therefore thought to be involved in the innate surveillance of tissues (12). They may be derived from classical monocytes and have a lifespan of several days in humans. Non-classical monocytes contribute to the formation of granulation tissue and exert an array of similarities to tissue macrophages; they are thus considered to be more mature than classical monocytes and frequently exert anti-inflammatory and pro-homeostatic effects (13).
The intermediate subpopulation, although it has the smallest proportion and appears to only display an intermediate expression of surface markers, it is a clearly distinguishable subset and has a distinct gene expression profile and functionality (14). Intermediate monocytes selectively express C-C chemokine receptor type 5 (CCR5), which, upon activation, secretes numerous inflammatory cytokines, including interleukins (ILs) and TNF-α (5). The proportion of the inflammatory monocytes in peripheral blood was determined to be elevated in numerous pathogeneses of cardiovascular diseases and diabetes (15,16), and has been used as a predictor of prognosis for patients with cardiovascular disease (CVD) (17); however, the mechanistic association of the intermediate monocyte subset with the severity of CVD remains elusive (18).
Given the growing body of evidence on the functional complexity of different monocyte subsets, modulation of the inflammatory response by targeting the monocyte sub-population has conceptually emerged as a novel intervention that supports endogenous tissue homeostasis in patients with AMI (19). Kinetic studies have provided circumstantial evidence for this monocyte conversion during the course of an inflammatory response, which occurred in the peripheral blood and locally at the site of injury (20). This theory is further supported by the fact that the level of transcription of genes associated with maturation progressively increases from classical monocytes via intermediate to non-classical monocytes (13). Phenotypic switch of monocyte subsets is known to be regulated by numerous factors. For, instance, in vitro, CD34+ hematopoietic stem cells first differentiate into CD14++CD16− monocytes, which, following subsequent culture, express CD16, suggesting that a phenotypic transition of the monocyte sub-population may occur under specific conditions (21).
In the present study, the potential of an adipokine to limit the excessive inflammatory response was explored by inducing an intermediate switch of monocytes. The CTRP superfamily is a cluster of adipokines and 15 isotypes of proteins in humans have been recently identified (22). CTRP3 is ubiquitously expressed in chondrocytes and adipose tissue and is able to improve insulin sensitivity as well as promote glucose and lipid metabolism (23). CTRP3 also serves as a potent anti-inflammatory adipokine; it may be beneficial in the prevention of cardiovascular diseases and provide a promising therapeutic strategy to attenuate vascular remodeling (24). In this context, the present study demonstrated that CTRP3 induced an intermediate switch of monocyte subsets and antagonized pro-inflammatory cytokine expression in monocytes, providing a ‘proof-of-concept’ evidence of using CTRP3 to limit inflammatory response in patients following AMI.
Materials and methods
Sample collection
Blood samples (10 ml) were collected from the patients during primary hospitalization (3–5 h following AMI) and follow-up (3 and 7 days) at the Department of Cardiology of the People's Hospital of Danyang (Danyang, China) between November 2018 and February 2019, as well as from young staff members (age range, 26–53 years) within the department who volunteered for this study. All patients were angiographically proven to have ST-elevated AMI and all physical parameters are summarized in Table I. A total of 12 patients with AMI and 8 healthy volunteers were included in the present study. Diagnosed inflammatory diseases within the last three months were the only exclusion criterion for all volunteers. The study was approved by the Ethics Committee of People's Hospital of Danyang (Danyang, China) and was performed in accordance with the Declaration of Helsinki. All participants or accompanying relatives provided written consent prior to the obtainment of peripheral blood samples, and they all agreed to participate in the present experimental study regarding the isolation of monocytes for medical research (according to the Declaration of Helsinki).
Table I.
Clinicopathological parameters of subjects included in the present study.
| Parameter | AMI (n=12) | Volunteers (n=8) | Reference range | P-value |
|---|---|---|---|---|
| Age (years) | 67.31±24.1 | 31.35±14.2 | – | 0.007 |
| Sex (M/F) | 7/5 | 5/3 | – | – |
| Diastolic blood pressure (mmHg) | 91.28±8.1 | 81.31±4.8 | 80–89 | 0.006 |
| Systolic blood pressure (mmHg) | 147.26±34.6 | 121.31±8.2 | 120–139 | 0.053 |
| WBC count (cells/µl) | 11,917±2212 | 10,191.31±1812 | 4,000-11,000 | 0.084 |
| PBMC count (cells/µl) | 767±212 | 512±117 | 500-3,000 | 0.006 |
| Sub-category of circulating monocytes (% of total monocytes)a | ||||
| Classical | 91.31±4.12 | 94.66±3.44 | 86–95 | 0.07 |
| Non-classical | 2.58±0.31 | 2.85±0.38 | 2–4 | 0.098 |
| Intermediate | 6.10±1.21 | 2.49±0.13 | 3–10 | 0.0001 |
Classical, CD14++/CD16−; non-classical, CD14+/CD16++; intermediate, CD14++/CD16+. AMI, acute myocardial infarction; WBC, white blood cells; PBMC, peripheral blood mononuclear cells; M, male; F, female.
Isolation, cultivation and analysis of monocyte subsets
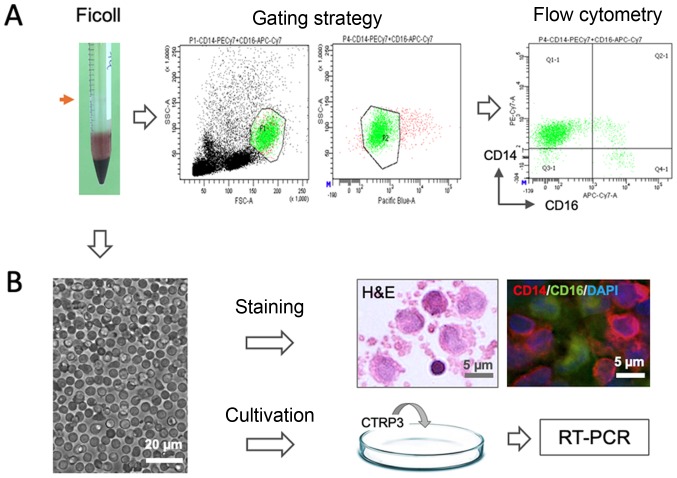
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient centrifugation (1,000 × g for 30 min) at 4°C. The mononuclear fraction (indicated by the red arrow in Fig. 1) was washed with saline and RPMI-1640 medium (Sigma-Aldrich; Merck KGaA), suspended in autologous serum (AS) and counted. The samples were sequentially subjected to either flow cytometric analysis or cultivation as schematically illustrated in Fig. 1. For subset analysis, the cell fraction was resuspended in MACS buffer [2% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) in PBS with 1 mM EDTA] and stained with CD14 and CD16 monoclonal antibodies using the methodology described in detail below. For pharmacological experiments, the isolated cells were inoculated into a 10-cm petri dish containing Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich; Merck KGaA) culture medium supplemented with 30% AS, penicillin (100 U/ml), streptomycin (0.1 mg/ml) and glutamine (2 mM) and incubated at 37°C with 5% CO2 for treated with CTRP3 for 24 h prior to extraction of total RNA for reverse transcription-quantitative (RT-q)PCR.
Figure 1.
Schematic illustration of experimental design. Mononuclear cells (red arrow) were isolated by Ficoll gradient centrifugation from peripheral blood sample and were (A) stained with CD14 and CD16 antibodies for flow cytometric analysis and (B) cultivated for pharmacological testing of CTRP3, histological H&E staining and immunostaining for CD14 and CD16 (scale bar, 5 or 20 µm). PE, phocoerythrin; APC, allophycocyanine; Cy, cyanine; FSC, forward scatter; SSC, side scatter; Q, quadrant; RT-PCR, reverse transcription PCR; CTRP3, complement C1q tumor necrosis factor-related protein-3.
Flow cytometric analysis
For immunostaining, cells were diluted at a concentration of 2×105 in 100 µl MACS buffer (2% FBS in PBS with 1 mM EDTA) and stained with the following fluorescence-conjugated antibodies: Phycoerythrin-cyanine (Cy)7-conjugated anti-human CD14 (clone 63D3; cat. no. 367112; 1:200 dilution; Biolegend) and allophycocyanine-Cy7-conjugated anti-human CD16 (clone B73.1; cat. no. 360710; 1:200 dilution; Biolegend) for 10 min at 4°C in the dark. Thereafter, cell suspensions were washed twice by using MACS buffer and stained with DAPI (1:250 dilution) for 30 sec. In the gating strategy, subpopulations of leukocytes (monocytes and lymphocytes) were identified by distinguishing their locations on the forward and side scatter scale, and the duplicates and dead cells were excluded in the channel of pacific blue based on DAPI staining. Monocyte subsets were discriminated based on the intensity/presence of expression of CD14 and CD16, namely, classical (CD14++/CD16−), non-classical (CD14+/CD16++) and intermediate populations (CD14++/CD16+). All samples were analyzed using a flow cytometer (FACSCanto II; BD Biosciences) and the data were stored and analyzed using Flowjo 7.6 software (Tree Star, Inc.).
Assessment of phagocytic and migratory activity
To compare the biological nature of monocytes from two cohorts of subjects (healthy, n=8 vs. AMI on day 3, n=12), two complementary approaches were used to assess the phagocytic activity. The cells isolated by Ficoll gradient centrifugation were seeded into 6-well plates [1×105 in 2 ml DMEM supplemented with 10% FBS, penicillin (100 U/ml), streptomycin (0.1 mg/ml) and glutamine (2 mM)] and incubated at 37°C for 30 min. In the first part, FITC-labeled Zymosan (Thermo Fisher Scientific, Inc.) was used as a reference tracer to mimic infectious conditions. Zymosan was added into the culture medium at the final concentration of 200 µg/ml and plates were again placed at 37°C under gentle shaking (60 rpm) for an additional 1 h. Thereafter, cells were collected and washed 3 times with PBS and stained again with antibodies against CD14 and CD16, as described above. For flow cytometric analysis of particle uptake, the mean fluorescence intensity (MFI) in the FITC channel (wavelength, 495 nm) was measured as an indicator for the amount of ingested Zymosan particles. In the second part, 19F-perfluorocarbon (PFC) nanoparticles were used as a sterile inflammatory tracer as previously described (25). In brief, 100 µl (10% in PBS) was added to each well of a 6-well plate and incubation was maintained at 37°C under gentle shaking (60 rpm) for 60 min, following which cells were collected. After several washing steps, the cell pellet was fixed with 4% paraformaldehyde and the 19F content was measured by using an NMR spectrometer (9.4 Tesla; Bruker) at frequencies of 400.13 MHz for 1H and 376.46 MHz for 19F detection. For superimposing the images of the same nuclei, the ‘hot iron’ color lookup table (ParaVision; Bruker) was applied to 19F images and the intensity of 19F was considered as a phagocytic tracer in the comparison of the activity in different subpopulations from individual subjects.
Migratory analysis was performed using a commercial migration assay in a 12-well plate with a pore size of 3 µm (Thin Cert™; Austria). In brief, 500,000 monocytes were transferred to the upper layer of a Thin Cert™ set in 10 ml culture medium and the lower compartment contained 2 ml DMEM with 10% FBS supplemented with monocyte chemoattractant protein 1 (MCP-1) at a concentration of 20 ng/ml to facilitate the migratory process of monocytes. The migratory unit was placed in an incubator at 37°C for 3 h and subsequently, the entire medium of the lower compartment was collected. Migrated cells were quantitatively counted via flow cytometry (FACSCanto II; BD Biosciences) using CountBright Absolute counting beads (Thermo Fisher Scientific, Inc.).
Pharmacological interventions
In the present series of experiments, to minimize potential skewing of epitopes and spontaneous differentiation into a macrophage phenotype of cultured monocytes, FBS was replaced by AS of individual subjects in the culture protocol. In doing so, monocytes from patients 3 days after AMI (3D-AMI) were isolated and cultivated under similar conditions as above, except that 10% FBS was changed to 30% AS, and under these conditions, the proportions of the three phenotypic subsets of monocytes, as well as the expression profile of cytokines (IL-6 and TNF-α), were unaffected after 24 h in culture. To test the induction effects of CTRP3 (Aviscera Bioscience) on phenotypic switch of monocytes that display anti-inflammatory properties, recombinant CTRP3 was added into the culture medium at a final concentration of 1 µg/ml and the subsets of monocytes were analyzed by flow cytometry, as specified above. To explore potential downstream cascades mediating this biological effect, the cells were first challenged with LPS (100 ng/ml; Sigma-Aldrich; Merck KGaA) and a p38 mitogen-activated protein kinase (MAPK)/ERK inhibitor, SB203580 (1 µM; MedChemExpress), was optionally added prior to treatment with CTRP3. All chemicals were dissolved in DMSO and added into the medium at a ratio of 1:1,000. The wells supplemented with 0.1% of DMSO were applied as DMSO control. In all interventions, cells were cultivated at 37°C with 5% CO2 for a period of 24 h prior to the extraction of total RNA was for RT-qPCR.
RT-qPCR
RT-qPCR was performed to determine the mRNA expression levels of IL-6 and TNF-α following the pharmacological treatments. Total RNA of monocytes was isolated using the RNeasy Micro Kit (Qiagen) and complementary DNA was synthesized using the QuantiTect Reverse Transcription Kit (Qiagen) according to the manufacturer's protocol and as previously described (8). The thermocycling conditions were as follows: Initial denaturation for 2 min at 50°C, and 40 cycles of 10 min at 95°C and 1.5 min at 60°C. Amplification efficiencies were calculated from the linear part of the quantification curve. Gene expression was normalized to GAPDH and calculated using the 2−∆∆Cq method (8). Pre-designed Taqman commercial primers (IL-6: Hs00174131_m1; TNF-α, Hs02621508_s1; GAPDH: Hs02786624_g1) were purchased from Thermo Fisher Scientific, Inc. and their efficiency was confirmed in all amplification plots. Quantification of gene expression was performed using a StepOnePlus Real-time PCR system (Thermo Fisher Scientific, Inc) following the manufacturer's protocol and all PCR assays were performed in duplicate.
Statistical analysis
Values are expressed as the mean ± standard deviation. Differences in sub-populations of monocytes over time, migratory activity and the phagocytic activity of FITC-Zymosan within different monocyte subsets were analyzed using two-way repeated-measures analysis of variance (ANOVA). The expression levels of IL-6 and TNF-α were analyzed using one-way ANOVA. Tukey's multiple-comparisons test was used as a post hoc analysis to reveal significant differences between the groups. An unpaired Student's t-test was performed to analyze the phagocytic activity of 19F-nanoparticles in the comparison of healthy vs. 3D-AMI monocytes. Statistical analysis was performed using GraphPad Prism© (version 7; GraphPad Software Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Subjects
In total, 12 AMI patients (age, 67.31±24.1 years; male/female, 7/5) and 8 volunteers (age, 31.35±14.2 years; male/female, 5/3) were included in the present study. Physical data of all subjects included in the present study are summarized in Table I.
Dynamic changes of circulating monocyte subsets after AMI
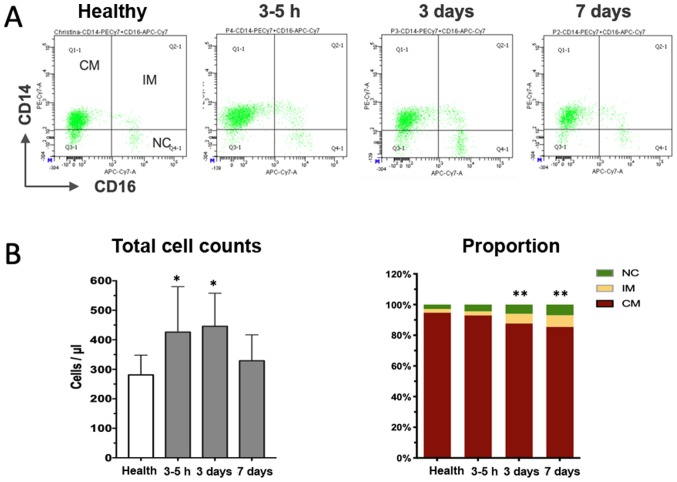
Peripheral whole blood samples were obtained directly after the angiographic exam (usually 3–5 h after hospitalization), as well as 3 and 7 days after the onset of AMI and subjected to flow cytometric analysis. A total of three distinct monocyte subpopulations were defined according to CD14 and CD16 expression in the cytometric plots (Fig. 2A), with the classical subset accounting for the majority of cells in healthy volunteers and patients with AMI (Table I). Although the relative percentage of the subsets in patients with AMI at the early stage was similar to that in the healthy controls, the absolute cell counts were already significantly increased, suggesting that recruitment of monocytes occurred as early as a few hours after the onset of MI. Thereafter, the total monocytes and classical subset further increased, peaking on day 3 after the onset of MI, and declined to a level close to that in the healthy controls on day 7 (Fig. 2B). The non-classical and intermediate subset increased more vigorously than the classical population and remained significantly increased at the end of the observation period of 7 days (Fig. 2B). Taken together, the time-course study indicates dynamical recruitment of monocyte subsets that is required to orchestrate the reparative process of the damaged heart tissue.
Figure 2.
Dynamical changes of monocyte subsets after myocardial infarction. (A) Representative illustration of three monocyte subsets according to CD14 and CD16 staining at different time-points. (B) Quantitative analysis indicated that the total cell counts initially increased (3–5 h) and then declined almost to the level of healthy controls (left panel in B). The right panel in B displays the proportional changes of three subsets of monocytes over time, revealing the progressive increase of the percentage of the IM subpopulation. *P<0.05, total cell counts vs. healthy; **P<0.01, proportion of IM vs. healthy. CM, classical monocytes; NC, non-classical subsets; IM, intermediate subpopulation; PE, phycoerythrin; APC, allophycocyanine; Cy, cyanine; FSC, forward scatter; SSC, side scatter; Q, quadrant.
Enhanced phagocytic and migratory activity of monocytes in patients with AMI
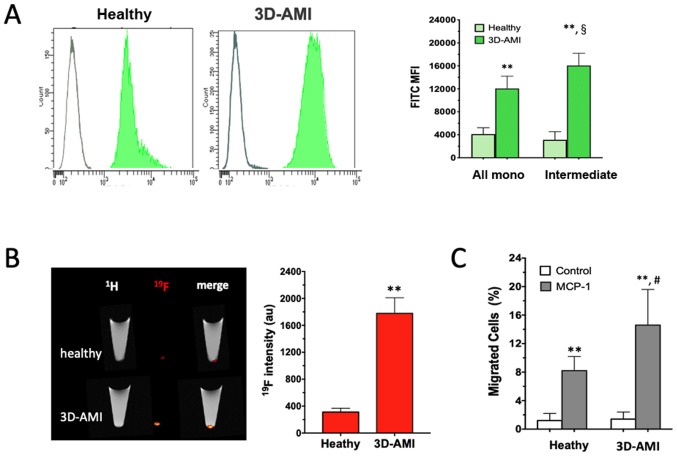
The functional enhancement of the circulating monocytes in patients with AMI was further analyzed. In this attempt, two complementary approaches were used. When FITC-Zymosan was added to the culture medium of the isolated monocytes, they exhibited strong phagocytic activity, as shown by the right-shifted MFI in Fig. 3A. In comparison to the healthy control, the monocytes derived from patients in the 3D-AMI group exhibited more vigorous phagocytic activity in response to Zymosan in all monocyte subsets, which was even more pronounced in the intermediate sub-population (P<0.01). In addition, 19F-containing nanoparticles were used as an alternative phagocytic tracer to compare monocytes from either healthy volunteers or patients with AMI. After 60 min of co-cultivation, the 19F content in the cell pellet, which represents the ingested nanoparticles, was analyzed by 19F-MRI detection, which consistently indicated a ~5-fold increase in 19F intensity in the AMI-derived monocytes (P<0.01; Fig. 3B). Therefore, the circulating monocytes in patients with AMI, likely triggered by the inflammatory milieu after AMI, reached a state with enhanced phagocytic activity.
Figure 3.
Enhanced phagocytic and migratory activity of monocytes in patients following AMI. (A) MFI determined by flow cytometry and (B) 19F-MRI were significantly increased in total monocytes and even more pronounced in the intermediate subpopulation derived from patients in the 3D-AMI group compared with that in the healthy control. (C) When primed by MCP-1 present in the lower chamber, the monocytes from the 3D-AMI group exhibited enhanced mobility in a trans-membrane assay. **P<0.01 compared to healthy; §P=0.0193 MFI between all mono and intermediate subset; #P<0.05 compared to unstimulated condition without MCP-1. MFI, mean fluorescence intensity; AMI, acute myocardial infarction; 3D, at 3 days; 19F-MRI, 19F intensity in MRI; all mono, total monocytes; MCP-1, monocyte chemoattractant protein 1.
Next, cell migration was examined as an additional function of monocytes by using a commercial Transwell assay (Thim Cert™). When primed with chemotactic MCP-1 in the lower chamber, monocytes mobilizing from the upper layer into the lower chamber were observed in the healthy as well as the 3D-AMI group. Of note, the monocytes from the 3D-AMI patients migrated more vigorously, with the proportion of monocytes moving across the membrane reaching 15±5.0% in comparison to only 8±2.0% in the healthy control group (P<0.01; Fig. 3C). Taken together, circulating monocytes in patients with AMI not only increased in quantity but were also induced to acquire an increased function, as evidenced by their elevated phagocytic and migratory activity.
CTRP3 induced a phenotypic switch of monocytes to acquire anti-inflammatory properties
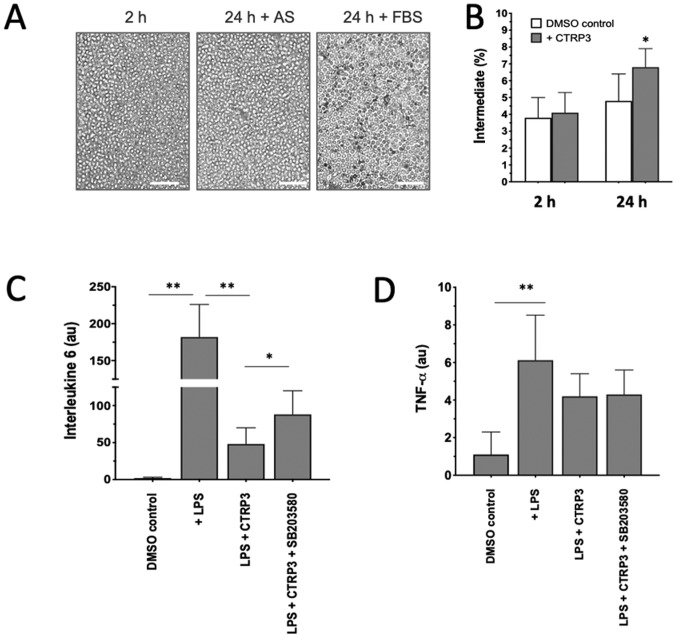
Given that an ‘uncontrolled’ excessive inflammatory response may cause secondary cardiac damage in patients with AMI and that dynamic changes of monocyte subsets may provide a novel therapeutic target in the treatment of AMI, the anti-inflammatory properties of a newly discovered adipokine, CTRP3, on monocyte functionality were then tested in vitro. When monocytes from patients in the 3D-AMI group were isolated and cultivated with 10% FBS, a significant spontaneous differentiation into macrophages was noticed over 24 h (24 h dark grey in Fig. 4A), but the phenomenon was substantially minimized and no phenotypic changes were observed (DMSO control in Fig. 4B) when FBS was replaced by AS, confirming the stability of in vitro monocyte cultivation for pharmacological interventions (Fig. 4A). When CTRP3 was added to the medium, the proportion of the classical (CD14++CD16−), non-classical (CD14+CD16++) and intermediate (CD14++CD16+) subset was analyzed by either immunostaining or flow cytometry. The results indicated that all three subsets did not change in the first 2 h, but that the percentage of the intermediate population was elevated to 6.8±1.1% after 24 h of culture in comparison to 4.1±1.2% in the DMSO control (P<0.05; Fig. 4B), suggesting that CTRP3 is able to induce a phenotypic switch among monocyte subpopulations.
Figure 4.
Anti-inflammatory effect of CTRP3 on phenotypic switch and cytokines. (A) When isolated monocytes were cultivated ex vivo with 30% AS for 24 h, they were morphologically similar to cells after 2 h cultivation with 10% FBS. Incubation with 10% FBS for 24 h appeared to have induced spontaneous macrophage differentiation, as characterized by the attached, elongated morphology. Scale bars, 50 µm. (B) Addition of CTRP3 induced a significant enrichment of the intermediate subset of cultivated monocytes. (C and D) LPS-induced stress stimulated expression of (C) interleukin 6 and (D) TNF-α, which was blunted in the presence of CTRP3, highlighting the anti-inflammatory effects of CTRP3 on monocytes. Of note, the inhibitory effect on IL-6 but not TNF-α was partially counteracted when SB203580, a p38 mitogen-activated protein kinase/ERK inhibitor, was added. *P<0.05 and **P<0.01. CTRP3, complement C1q TNF-related protein-3; AS, autologous serum; TNF, tumor necrosis factor; FBS, fetal bovine serum; LPS, lipopolysaccharides.
To demonstrate the effects of CTRP3 on the secretory profiles of monocytes, two representative cytokines, IL-6 and TNF-α were examined as major pro-inflammatory cytokines derived from activated monocytes (26). When monocytes were stressed by LPS, transcription of IL-6 increased by >100-fold in comparison with that in the DMSO control (P<0.01; Fig. 4C), while TNF-α was only moderately induced (P<0.05; Fig. 4D), indicating that individual cytokines may react diversely in response to LPS stimulation. Of note, the LPS-induced IL-6 activation was largely antagonized in the presence of CTRP3 and partially restored when the activity of the p38 MAPK/ERK cascade was blocked by the addition of SB203580 to the culture medium (Fig. 4C). SB203580 is a pyridinyl imidazole inhibitor widely used to investigate the roles of p38 MAPK. The present results highlight the inhibitory activity of CTRP3 on the response to LPS challenge in monocytes populations upon activation of the p38 MAPK/ERK cascade. On the other hand, CTRP3 exhibited a less pronounced effect on LPS-induced TNF-α activation in the present experimental setting (Fig. 4D).
Discussion
The present study revealed a sequential mobilization and phenotypical interchange of monocyte subpopulations from the onset of AMI to the sub-acute phase (7 days) with enhanced phagocytic and migratory activities, reflecting the salvaging requirement and severity of the systemic inflammatory response to tissue damage. The dynamic heterogeneity of circulating monocytes and their differential cell fates following tissue infiltration pinpoints monocytes as a novel therapeutic target, which, by pharmacological manipulation, may augment the cardiac reparative process in patients following AMI. In this attempt, it was further demonstrated that CTRP3, a member of the CTRP family, induced an intermediate switch to the CD14++CD16+ monocyte subpopulation and antagonized the LPS-induced expression of pro-inflammatory cytokines, suggesting that CTRP3 may represent an alternative candidate in preventing damage arising from excessive inflammatory responses by targeting circulating monocytes.
Cardiac ischemic insult evokes a complex, temporally and spatially well-coordinated inflammatory response at the damaged parts of the tissue where immune cells are sequentially recruited and functionally orchestrated (27). Among all infiltrating immune cells, monocytes as a part of the innate immune system are mobilized and appear concomitantly at different stages of the inflammatory response to pursue distinct functions in specific ways (5). In mice after myocardial infarction, Ly-6Chi monocytes, corresponding to CD14++CD16− monocytes in humans, accumulate via CCR2, predominate at the site of injury during the first 3 days and scavenge necrotic debris by a combination of expression of inflammatory mediators, proteolysis and phagocytosis, while Ly-6Clo monocytes, corresponding to CD14+CD16++ monocytes in humans, infiltrate preferentially via a CX3C chemokine receptor 1-mediated pathway to initiate a reparative process (5,27). This paradigm was demonstrated in the present study, as a few hours after infarction, the total monocyte counts (mainly classical subsets) in AMI patients expanded and were elevated as early as 3–5 h and retained for up to 3 days before starting to decline, whilst a persistent increase in intermediate and non-classical subsets at 3 and 7 days was observed, suggesting that the mobilization and recruitment of monocytes is a sequentially fine-tuned process upon the salvaging requirement for the maintenance of tissue homeostasis.
Of note, the peak level of the cell counts of classical monocytes (CD14++CD16−) has been proved to be linked to the impairment of myocardial salvage in the acute phase after AMI and adverse left ventricular remodeling, suggesting excessive mobilization of circulating monocytes is likely to be harmful to the initiation of the reparative course (11,28). The undue response is frequently associated with the enhanced innate immunity as evidenced by the phagocytic and migratory activities of the activated monocytes, mainly occurring in the intermediate subset (29). In the present study, two types of particle sizes and tracers were employed: FITC-Zymosan has a relatively big size (1 µm in average dimension) and represents infectious particles (produced from yeast particles known to be phagocytosed by various types of cell, including monocytes/macrophages) (30) and 19F-containing PFC is a small-particle size (100 nm) nanoemulsion that has been used as an experimental tracer of monocytes homing to the site of inflammation (25). The two independent approaches suggested that monocytes from patients with AMI exhibited an enhanced phagocytic activity, indicating that the monocytes were systemically tuned to a highly active state in response to infarction. Of note, the patient-derived monocytes more efficiently ingested the small-size particles (100 nm) than the big-size particles (1 µm), although the biological relevance of this phenomenon requires to be further investigated.
While phagocytic activity and production of a myriad of cytokines are necessary to ensure the clearance of cell debris in the damaged tissue, a timely switch of immune response and reduction of excessive inflammation are important to maintain tissue homeostasis (6). The nature of fluctuations observed in the transient increase of cell counts of monocyte subsets and persistent elevation of non-classical and intermediate cells points at a time-dependent change of monocyte counts in patients after AMI, which opens a possibility of manipulating CD14++CD16− monocytes as a novel therapeutic target for salvaging ischemic damage (19,11). In this attempt, the potential of CTRP3 as an immune modulator to induce phenotypic switch of monocyte subsets and to balance the immune response was tested. First, a culture system with individual AS was established, in which monocytes maintained their phenotypic stability for as long as 24 h in in vitro culture. It was also demonstrated that CTRP3 treatment caused a skew of cultured monocytes towards the intermediate subset (CD14++CD16+), which mimicked the scenario in patients, where classical CD14++CD16− monocytes convert to CD14+CD16++ non-classical monocytes through a CD14++CD16+ monocyte intermediate (14), although it remains elusive how CTRP3 brought about the phenotypic changes. Intermediate monocytes are particularly viewed as pro-atherogenic, as they selectively express CCR5, which has been associated with atherosclerosis in experimental and large epidemiological studies (31,32), but mainly with angiogenic (33) and reparative properties and reduced cytokine production (13). This paradigm was observed in the present study, which demonstrated that CTRP3 antagonized the LPS-induced upregulation of IL-6 expression but had a less pronounced influence on TNF-α expression. The limited ability of CTRP3 to inhibit the LPS-induced upregulation of TNF-α expression is likely associated with the minor response of monocytes to LPS-induced stress in the present experimental setting in comparison to that in a previous study (34), or CTRP3 is preferentially effective in the inhibition of IL-6 production. Furthermore, the inhibitory effect is likely exerted via blunting the activity of the p38 MAPK/ERK cascade, particularly in elderly patients (26).
Of note, the present study has certain limitations: i) The physical data and associated parameters (age, sex and hemodynamics) in the present study were not exactly matched between AMI patients and volunteer controls, which may yield pre-exiting bias on subject selection. Nevertheless, the comparison of monocyte subsets remain valid as the blood cell counts of all subjects were comparable. ii) The present study mainly focused the intermediate subset of monocytes as an interventional target. In the biology of blood monocytes, the intermediate subpopulation, although its proportion was small, is the most critical and informative to demonstrate the functional transition from pro-inflammatory to anti-inflammatory status in patients with AMI. The other two subsets (classical and non-classical) may be equally important and should be addressed in a future study. iii) In the ex vivo experiments, the cultivation time was restricted to 24 h, which may have somewhat limited the phenotypic changes, particularly after CTRP3 treatment. However, when the culture time was extended up to 36 or 48 h, most of the cultivated monocytes transformed into a macrophage phenotype on the dish (see supplementary Fig. S1), indicating phenotypic transition of monocytes and spontaneous differentiation of monocytes into macrophages even under culture conditions with AS. This inevitable phenomenon does not permit any prolongation of culture time in the present setting and requires technical circumvention in future studies. iv) There is limited evidence of p38 MAPK/ERK activation after CTRP3 intervention. As the major focus of the present study was to reveal the dynamic fluctuation of monocyte subsets and only provided ‘proof-of-concept’ evidence by using CTRP3 to modulate the immune response after AMI, further experiments to gain insight at the molecular level are currently being performed by our team and the results will be reported in a separate study in the near future.
In conclusion, the present study indicated a dynamical fluctuation of monocyte counts and the proportions of three subsets, which underscores the notion that the clinical use of monocytes as a predictive marker of cardiac risk should be performed with caution due to the time-dependent variation of circulating monocytes in patients with AMI. The increased monocyte counts and their elevated phagocytic and migratory activities pinpoint an enhanced immune response to tissue damage. Furthermore, CTRP3, a member of the CTRP family, may represent an alternative candidate to modulate the ‘uncontrolled’ inflammatory response and to augment the cardiac reparative process in patients following AMI.
Supplementary Material
Acknowledgements
The authors thank Ms Jiangfang Zhang, Department of Cardiology, People's Hospital of Danyang, The Affiliated Hospital of Nantong University (Danyang, China) for her technical support in the flow cytometric analysis and Prof. Dr. Linlin Qiu from the Department of Cardiology, People's Hospital of Danyang, The Affiliated Hospital of Nantong University (Danyang, China) for his constructive discussions that conceptually formed the major body of this study.
Glossary
Abbreviations
- AMI
acute myocardial infarction
- CTRP3
complement C1q tumor necrosis factor-related protein-3
- AS
autologous serum
- LPS
lipopolysaccharides
Funding
This study was supported by the Department of Zhenjiang S&T (grant no. SHW2015020) and the Social Development Foundation of Zhenjiang, China (grant no. FZ2017010).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HZ and WW conceived the study, designed the experiments and interpreted the data. YD participated in performing the experiments of monocyte isolation and characterization by flow cytometry. YZ, XD and JZ performed ex vivo experiments and RT-qPCR analysis of cytokine expression. WO, JG and YZ acquired all blood samples from patients and performed statistical analysis of all experimental data. XL provided conceptual advice, interpreted the results and wrote the manuscript. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the People's Hospital of Danyang (Danyang, China) and was performed in accordance with the Declaration of Helsinki. All participants or companying relatives provided written informed consent for the use of their monocytes for scientific research in accordance with the Declaration of Helsinki directly after AMI.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Godwin JW, Pinto AR, Rosenthal NA. Chasing the recipe for a pro-regenerative immune system. Semin Cell Dev Biol. 2017;61:71–79. doi: 10.1016/j.semcdb.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016;529:307–315. doi: 10.1038/nature17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen B, Frangogiannis NG. Immune cells in repair of the infarcted myocardium. Microcirculation. 2017;24 doi: 10.1111/micc.12305. [DOI] [PubMed] [Google Scholar]
- 4.Tang J, Wang X, Tan K, Zhu H, Zhang Y, Ouyang W, Liu X, Ding Z. Injury-induced fetal reprogramming imparts multipotency and reparative properties to pericardial adipose stem cells. Stem Cell Res Ther. 2018;9:218. doi: 10.1186/s13287-018-0959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arfvidsson J, Ahlin F, Vargas KG, Thaler B, Wojta J, Huber K. Monocyte subsets in myocardial infarction: A review. Int J Cardiol. 2017;231:47–53. doi: 10.1016/j.ijcard.2016.12.182. [DOI] [PubMed] [Google Scholar]
- 6.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: Protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437–2445. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barberà–Cremades M, Baroja-Mazo A, Pelegrín P. Purinergic signaling during macrophage differentiation results in M2 alternative activated macrophages. J Leukoc Biol. 2016;99:289–299. doi: 10.1189/jlb.1A0514-267RR. [DOI] [PubMed] [Google Scholar]
- 8.Tan K, Zhu H, Zhang J, Ouyang W, Tang J, Zhang Y, Qiu L, Liu X, Ding Z, Deng X. CD73 expression on mesenchymal stem cells dictates the reparative properties via its anti-inflammatory activity. Stem Cells Int. 2019;2019:8717694. doi: 10.1155/2019/8717694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 10.Ziegler-Heitbrock L. Blood monocytes and their subsets: Established features and open questions. Front Immunol. 2015;6:423. doi: 10.3389/fimmu.2015.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg KE, Ljungcrantz I, Andersson L, Bryngelsson C, Hedblad B, Fredrikson GN, Nilsson J, Björkbacka H. Elevated CD14++CD16- monocytes predict cardiovascular events. Circ Cardiovasc Genet. 2012;5:122–131. doi: 10.1161/CIRCGENETICS.111.960385. [DOI] [PubMed] [Google Scholar]
- 12.Buscher K, Marcovecchio P, Hedrick CC, Ley K. Patrolling mechanics of non-classical monocytes in vascular inflammation. Front Cardiovasc Med. 2017;4:80. doi: 10.3389/fcvm.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ancuta P, Liu KY, Misra V, Wacleche VS, Gosselin A, Zhou X, Gabuzda D. Transcriptional profiling reveals developmental relationship and distinct biological functions of CD16+ and CD16- monocyte subsets. BMC Genomics. 2009;10:403. doi: 10.1186/1471-2164-10-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zawada AM, Rogacev KS, Rotter B, Winter P, Marell RR, Fliser D, Heine GH. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 2011;118:e50–e61. doi: 10.1182/blood-2011-01-326827. [DOI] [PubMed] [Google Scholar]
- 15.Heine GH, Ulrich C, Seibert E, Seiler S, Marell J, Reichart B, Krause M, Schlitt A, Köhler H, Girndt M. CD14(++)CD16+ monocytes but not total monocyte numbers predict cardiovascular events in dialysis patients. Kidney Int. 2008;73:622–629. doi: 10.1038/sj.ki.5002744. [DOI] [PubMed] [Google Scholar]
- 16.Neuser J, Galuppo P, Fraccarollo D, Willig J, Kempf T, Berliner D, Bauersachs J, Widder JD. Intermediate CD14++CD16+ monocytes decline after transcatheter aortic valve replacement and correlate with functional capacity and left ventricular systolic function. PLoS One. 2017;12:e0183670. doi: 10.1371/journal.pone.0183670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wildgruber M, Aschenbrenner T, Wendorff H, Czubba M, Glinzer A, Haller B, Schiemann M, Zimmermann A, Berger H, Eckstein HH, et al. The ‘Intermediate CD14++CD16+ monocyte subset increases in severe peripheral artery disease in humans. Sci Rep. 2016;6:39483. doi: 10.1038/srep39483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stansfield BK, Ingram DA. Clinical significance of monocyte heterogeneity. Clin Transl Med. 2015;4:5. doi: 10.1186/s40169-014-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsujioka H, Imanishi T, Ikejima H, Kuroi A, Takarada S, Tanimoto T, Kitabata H, Okochi K, Arita Y, Ishibashi K, et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. 2009;54:130–138. doi: 10.1016/j.jacc.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Zhu L, Yin Y, Zhou R, Lin J, Li J, Ye J. Changes of monocyte subsets in patients with acute coronary syndrome and correlation with myocardial injury markers. Int J Clin Exp Pathol. 2015;8:7266–7271. [PMC free article] [PubMed] [Google Scholar]
- 21.Cappellari R, D'Anna M, Bonora BM, Rigato M, Cignarella A, Avogaro A, Fadini GP. Shift of monocyte subsets along their continuum predicts cardiovascular outcomes. Atherosclerosis. 2017;266:95–102. doi: 10.1016/j.atherosclerosis.2017.09.032. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Wright GL, Peterson JM. C1q/TNF-related protein 3 (CTRP3) function and regulation. Compr Physiol. 2017;7:863–878. doi: 10.1002/cphy.c160044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson JM, Wei Z, Wong GW. C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output. J Biol Chem. 2010;285:39691–39701. doi: 10.1074/jbc.M110.180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu D, Lei H, Wang JY, Zhang CL, Feng H, Fu FY, Li L, Wu LL. CTRP3 attenuates post-infarct cardiac fibrosis by targeting Smad3 activation and inhibiting myofibroblast differentiation. J Mol Med (Berl) 2015;93:1311–1325. doi: 10.1007/s00109-015-1309-8. [DOI] [PubMed] [Google Scholar]
- 25.Flögel U, Ding Z, Hardung H, Jander S, Reichmann G, Jacoby C, Schubert R, Schrader J. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation. 2008;118:140–148. doi: 10.1161/CIRCULATIONAHA.107.737890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyugen J, Agrawal S, Gollapudi S, Gupta S. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J Clin Immunol. 2010;30:806–813. doi: 10.1007/s10875-010-9448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol. 2015;15:117–129. doi: 10.1038/nri3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Laan AM, ter Horst EN, Delewi R, Begieneman MP, Krijnen PA, Hirsch A, Lavaei M, Nahrendorf M, Horrevoets AJ, Niessen HW, Piek JJ. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur Heart J. 2014;35:376–385. doi: 10.1093/eurheartj/eht331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shantsila E, Lip GY. Monocyte diversity in myocardial infarction. J Am Coll Cardiol. 2009;54:139–142. doi: 10.1016/j.jacc.2009.03.047. [DOI] [PubMed] [Google Scholar]
- 30.Nienhaus F, Colley D, Jahn A, Pfeiler S, Flocke V, Temme S, Kelm M, Gerdes N, Flögel U, Bönner F. Phagocytosis of a PFOB-nanoemulsion for 19F magnetic resonance imaging: First results in monocytes of patients with stable coronary artery disease and ST-elevation myocardial infarction. Molecules. 2019;24(pii):E2058. doi: 10.3390/molecules24112058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fingerle G, Pforte A, Passlick B, Blumenstein M, Ströbel M, Ziegler-Heitbrock HW. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82:3170–3176. doi: 10.1182/blood.V82.10.3170.3170. [DOI] [PubMed] [Google Scholar]
- 32.Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, Grosse-Dunker G, Heisel I, Hornof F, Jeken J, et al. CD14++CD16+ monocytes independently predict cardiovascular events. J Am Coll Cardiol. 2012;60:1512–1520. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 33.Jaipersad AS, Lip GY, Silverman S, Shantsila E. The role of monocytes in angiogenesis and atherosclerosis. J Am Coll Cardiol. 2014;63:1–11. doi: 10.1016/j.jacc.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Weigert J, Neumeier M, Schäffler A, Fleck M, Schölmerich J, Schütz C, Buechler C. The adiponectin paralog CORS-26 has anti-inflammatory properties and is produced by human monocytic cells. FEBS Lett. 2005;579:5565–5570. doi: 10.1016/j.febslet.2005.09.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.