Abstract
Ataxia-Telangiectasia (AT) is an immunodeficiency most often associated with T cell abnormalities. We describe a patient with a hyper-IgM phenotype and immune cell abnormalities that suggest a distinct clinical phenotype. Significant B cell abnormalities with increased unswitched memory B cells, decreased naive transitional B cells, and an elevated frequency of CD19+CD38loCD27−CD10−CD21−/low B cells expressing high levels of T-bet and Fas were demonstrated. The B cells were hyporesponsive to in vitro stimulation through the B cell receptor, Toll like receptors (TLR) 7 and 9, and CD40. T cell homeostasis was also disturbed with a significant increase in γδ T cells, circulating T follicular helper cells (Tfh), and decreased numbers of T regulatory cells. The ATM mutations in this patient are posited to have resulted in the perturbations in the frequencies and distributions of B and T cell subsets, resulting in the phenotype in this patient.
Keywords: Ataxia-telangiectasia, hyper-IgM, CD38loCD21-/loW B cells, T-bet, Fas, Tregs, Tfh, γδ T cells
Capsule summary:
A patient presented with ataxia-telangiectasia, cutaneous granulomas, and a hyper-IgM phenotype; a novel combination of mutations in the ATM gene was associated with abnormal distributions, frequencies, and function of T and B lymphocyte subsets.
Introduction
Ataxia-Telangiectasia (AT) is caused by mutations in the ATM gene located on chromosome 11q22-23. AT is characterized by ataxia, neurologic decline, oculocutaneous telangiectasia, and a wide range of immune function abnormalities (1-4). One subset of patients exhibits a hyper-IgM phenotype with an abnormal B-cell profile (5). Patients with the hyper-IgM phenotype and low serum levels of IgG and IgA have been associated with more severe disease and an increased risk of malignancy (1). The underlying relationships between AT and hypogammaglobulinemia and the hyper-IgM phenotype have not been well delineated. It is known that a significant percentage of AT patients, but not all, show diverse abnormalities in B and T cell homeostasis primarily marked by low circulating B and T cell numbers (6, 7). The presentation of oculocutaneous manifestations may appear later in childhood so that consideration of AT in the context of hyper-IgM may be delayed (5). Elevated serum alpha-fetoprotein levels, rearrangement of chromosomes 7 and/or 14, and radiosensitivity establish the diagnosis of AT (1). There have been a number of reports of patients with AT who develop severe and often progressive cutaneous granulomatous lesions, some with the hyper-IgM phenotype (8). The relationship between these granulomatous lesions and AT is unclear but has been thought to reflect the underlying immunodeficiency. Persistence of rubella vaccine virus in cutaneous lesions in AT patients as well as other primary immunodeficiency diseases is now well documented, perhaps serving as an antigenic trigger of the granulomas (5, 9, 10). To date, there have been no distinguishing genetic features that align AT with this unique hyper-IgM phenotype or presence of cutaneous granulomas. We present a patient with AT, a novel mutation in ATM, cutaneous granulomas, a hyper-IgM phenotype, and extensive B- and T-cell abnormalities that may define this subgroup of AT patients.
Case Presentation
A 5 year-old female presented for evaluation of concern for immunodeficiency because of frequent upper respiratory tract illnesses. None required hospitalization and were presumed to be viral. She was found to have an ataxic gait, some speech delay, and mild ocular and ear pinna telangiectasia. In addition, she had an ulcerative rash initially present on her left upper and right lower extremity. Initial blood work showed elevated α-fetoprotein levels (50 ng/ml), elevated serum IgM (719 mg/dL), and low IgG (<75 mg/dL) and IgA (0.9 mg/dL). Skin biopsies of the granulomatous lesions showed lymphoid hyperplasia with granuloma-like formation and lymphoplasmacytic infiltrate with macrophages. A repeat skin biopsy showed pseudoepithiomatous hyperplasia with underlying non-necrotizing granulomatous inflammation. Mycobacterial, fungal, and bacterial cultures were negative, and repeat cultures performed on follow-up visits were negative for mycobacterial infection as well. She had no improvement in her skin lesions despite initiation of Ig replacement, topical and systemic corticosteroids, clarithromycin, azithromycin, and topical anti-fungal and calcineurin inhibitor therapy. Vaccine-strain rubella virus was identified in the granulomatous tissue by immunofluorescence staining (10). Progression of the granulomatous lesions was arrested along with improvement following initiation of anti-TNFα therapy. Both parents were healthy. Over the course of her 2-year follow-up, repeated assessments by polymerase chain reactions for respiratory viral pathogens or Epstein-Barr, cytomegalovirus, Herpes simplex and parvovirus were negative.
Methods
Genetic analysis
Next generation sequencing was used to identify the mutations in the ATM gene. A panel of 345 genes was designed based on reports of the relationship of mutations in those genes to primary immune deficiencies (11, 12). Exon coordinates for all exons ±50 base pairs resulted in 4,360 targets with a region of interest (ROI) of ca. 2 Mb. These coordinates were submitted to Agilent (Santa Clara, CA) for synthesis of RNA capture nucleotides (Sure Select QXT custom library kit). Genomic DNA was isolated from whole blood and enriched for targets using the custom capture kit according to manufacturer’s directions. Sequencing was performed on a MiSeq (MiSeq, San Diego, CA). Initially, sequence data were analyzed for variants using NextGENe (softgenetics.com), and mutations confirmed by Sanger sequencing of targeted PCR amplicons.
Serum cytokine analysis
The patient’s serum was analyzed for various cytokines by ELISA on two separate occasions.
Flow cytometry
B cells were purified from the patient’s peripheral blood by positive selection using CD20 magnetic beads (Miltenyi Biotec, Cambridge, MA). CD4+ T cells were isolated from the peripheral blood of research subjects using the EasySep human CD4+ T cell enrichment kit (STEMCELL Technologies, Cambridge, MA). The following antibodies were used for flow cytometric staining: CD19 APC-Cy7, CD27 PerCP-Cy5.5, CD10 PE-Cy7, CD21 V450, CD69 PE, CD86 APC, FAS Alexa 647, CD4 APC-Cy7, CD127 PerCP-Cy5.5, CD45RO Alexa 700, CXCR5 PerCP-Cy5.5, PD-1 PE-Cy7, ICOS APC (all from Biolegend, San Diego, CA), CD3 eFluor 605NC (from eBioscience, San Diego, CA), and CD21 BD Horizon V450 (BD). Intracellular staining for FOXP3 Alexa 488 (eBioscience) and T-bet PE was performed using the FOXP3/Transcription Factor Staining Buffer Set (eBioscience) in accordance with the manufacturer’s directions.
Mitogen and antigen stimulated proliferation assays
Patient’s peripheral blood mononuclear cells (PBMC) were isolated following gradient centrifugation and cultured for 3 days with mitogen (phytohemagglutinin, Concanavalin A (Con A) and pokeweed mitogen) or for 5 days with antigen (tetanus toxoid, C. albicans) as described using age-matched controls (13).
B cell activation assay
B cells were purified from the peripheral blood by positive selection using CD20 magnetic beads (Miltenyi Biotec) and plated for 48 hours at 150,000-200,000 cells per well in a 96-well plate in RPMI containing 10% FBS alone or RPMI+10% FBS and either 2 μg/ml polyclonal F(ab')2 rabbit anti-human IgM (Jackson ImmunoResearch, West Grove, PA), multimeric, soluble, 0.05 μg/ml recombinant-human CD40L (Alexis Biochemicals, San Diego, CA), 2 μg/ml gardiquimod (TLR7 agonist; Invitrogen, Waltham, MA), or 0.5 μg/ml CpG (TLR9 agonist; Invitrogen).
In-vitro regulatory T cell (Treg) suppression assay
CD4+ T cells were enriched using the EasySep Human CD4+ T cell enrichment kit (STEMCELL Technologies). CD4+CD25hiCD127lo Tregs and CD4+CD25−CD127+ responder T cells (Tresp) were sorted by fluorescence activated cell sorting (FACS) and then labeled with 5 μM of CFSE using the CellTrace CFSE Cell Proliferation kit (Invitrogen). Treg and Tresp cells were co-cultured at a 1:1 ratio in the presence of beads loaded with anti-CD2, anti-CD3, and anti-CD28 (Human Treg Suppression Inspector; Miltenyi Biotec). On day 4.5, proliferation of the Tresp cells was analyzed by flow cytometry measuring CFSE dilution.
Results
Identification of ATM mutations
Next generation sequencing revealed two pathogenic mutations in the ATM gene, a novel mutation creating a premature stop codon [c.237delA,(p.Lys79Asnfs370)] (inherited from the mother) and a nonsense mutation [c.3372C>G, (p.Tyr124Ter)] (inherited from the father).
Immune evaluation
The results of initial peripheral blood lymphocyte evaluation are summarized in Table 1. Over a 2-year follow-up, these numbers remained relatively unchanged. Compared to laboratory controls, absolute numbers of CD3+ T cells were slightly low; CD8+ T cell numbers were low and γδ T cell numbers were slightly elevated as previously reported in AT (14). Proliferative responses of PBMC to mitogens (PHA, Con A, PWM) were reduced to roughly half of the age-matched control responses; the response to tetanus was normal whereas the response to C. albicans was absent. Serum cytokine analyses demonstrated elevations in levels of TNFα on two occasions (14.5 and 16.7 μg/mL) and IL-10 (7.0 and 7.9 μg/mL); levels of IFN-γ, IL-2, IL-5, IL-6, and IL-12 were below the limits of detection.
Table 1.
Lymphocyte Studies
| Patient | Reference Range | |
|---|---|---|
| WBC | 5,200 cells/μL | 4.0-12.0 |
| Absolute Lymph Count | 2122 | 1.0-4.4 |
| CD8+ | 119 cells/μL (5.6%)* | 490-1,300 |
| CD4+ | 963 cells/μL (45.4%) | 700-2,200 |
| CD3+ | 1,133 cells/μL (53.4%)* | 1,400-3,700 |
| CD19+ | 526 cells/μL (24.8%) | 390-1,400 |
| CD16+/56+ | 429 cells/μL (20.2%) | 130-720 |
| CD4+/CD45RA+ | 13.0% T cells | 9.5-41.9% |
| CD4+/CD45RO+ | 84.0% T cells | 16.5-42.2% |
| TCR/αβ | 93.2% T cells | >85% |
| TCR/γδ | 8.4% T cells* | 0.5-6% |
| IgD+/CD27+ | 54.0% CD20+ cells* | 4.9-14.2% |
| IgD−/CD27+ | 3.1% CD20+ cells | 2.9-9.2% |
| CD38hi/IgMhi | 0.2% CD20+ cells | 0.3-9.2% of B cells |
| CD38hi/IgMlo | 0.4% CD20+ cells | 0.1-4.7% of B cells |
Denotes abnormal value
An altered B cell compartment in the AT patient
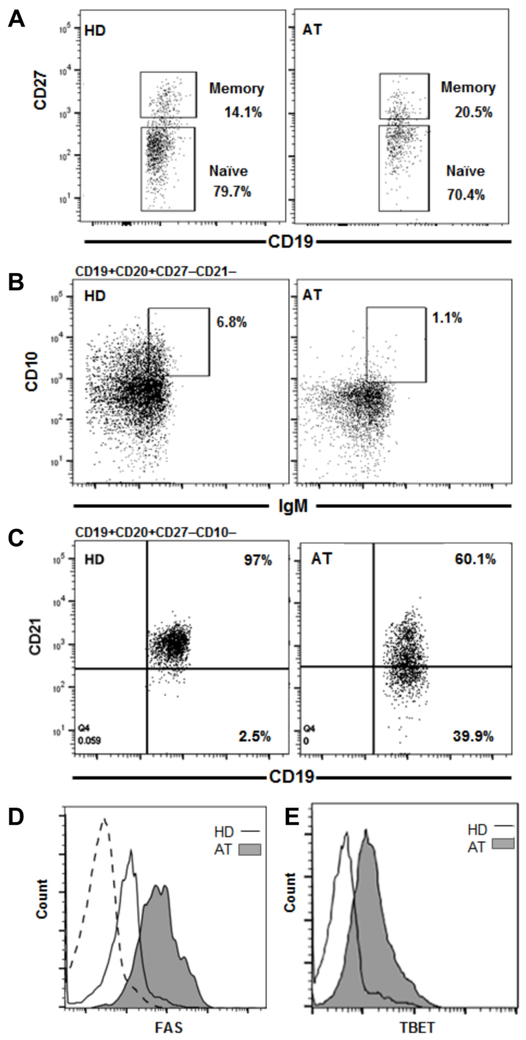
Though the number of total CD19+CD20+ B cells was within the normal range, we examined the frequencies of various subtypes of B cells as perturbations of the distribution of B cell subset frequencies has been reported in patients with other ATM mutations (6). The frequencies of total CD19+CD20+CD27− naive and CD19+CD20+CD27+ conventional memory B cells were similar to that of age-matched healthy controls (reference value 76.6±7.4% and 18.9±7.1% for naive and conventional memory B cells, respectively (15) (Fig. 1A). Within the memory B cell compartment however, there was a marked increased frequency of unswitched CD27+IgD+memory B cells (54%) compared to age-matched healthy controls (reference value: 8.3%, range 4.8-16.1% (16). The CD27+IgD− switched memory proportion (3.1%) was within the reference range (0.9-9.3%) (16) (Table 1). Among the naive B cells, the proportion of CD19+CD27−CD21−CD10+IgMhi transitional B cells that newly emigrated from the bone marrow (BM) was found to be diminished to 1.1% of the naive B cell compartment (age-matched reference value 5.8%, range 3.4-9.0%, (16) (Fig. 1B).
Figure 1.
ATM mutations result in altered new emigrant and mature naive B cell population frequencies. A) B cells isolated from PBMC by anti-CD20 magnetic bead separation from a healthy donor (HD) control sample processed in parallel with the AT patient sample show similar frequencies of the CD19+CD20+CD27+ memory and CD19+CD20+CD27− naive B cell compartments. B) Despite similar overall naive B cell compartment frequencies, the naive CD19+CD20+CD27−CD21−CD10+IgMhi new emigrant B cell population transitioning from the bone marrow is decreased in the AT patient. C) Conversely, the mature naive B cell compartment in the AT patient contains an elevated frequency of atypical CD19+CD20+CD27−CD10−CD21−/low B cells. D) This compartment in the AT patient exhibits elevated Fas expression and E) is enriched in TBET+ cells compared to lower Fas and TBET expression in the HD control (dashed histogram represents isotype control for both Fas and TBET).
While the occurrence of CD19+CD38loCD27−CD10−CD21−/low (referred henceforth as CD21−/low) B cells is rare compared to conventional CD19+CD27−CD10−CD21+ mature naive B cells, we found an elevated frequency of CD21−/low B cells (40%) in the AT patient (Fig. 1C). The frequency of this subset of B cells in the parents was similar to that in controls (<3.5%). This marked elevation in the patient is in agreement with the increased proportion of CD21−/low B cells previously described in some AT patients with other ATM mutations and also reported in Freiburg class 1A CVID patients (6, 17-19). In CVID patients and murine models where CD21−/low B cell frequency is elevated, gene microarray data revealed that these cells express high levels of TBET and FAS (17, 20). We similarly found elevated B cell expression of T-bet and Fas in this AT patient with a high CD21−/low B cell frequency compared to levels in healthy donor control B cells processed simultaneously (Figs. 1D and 1E).
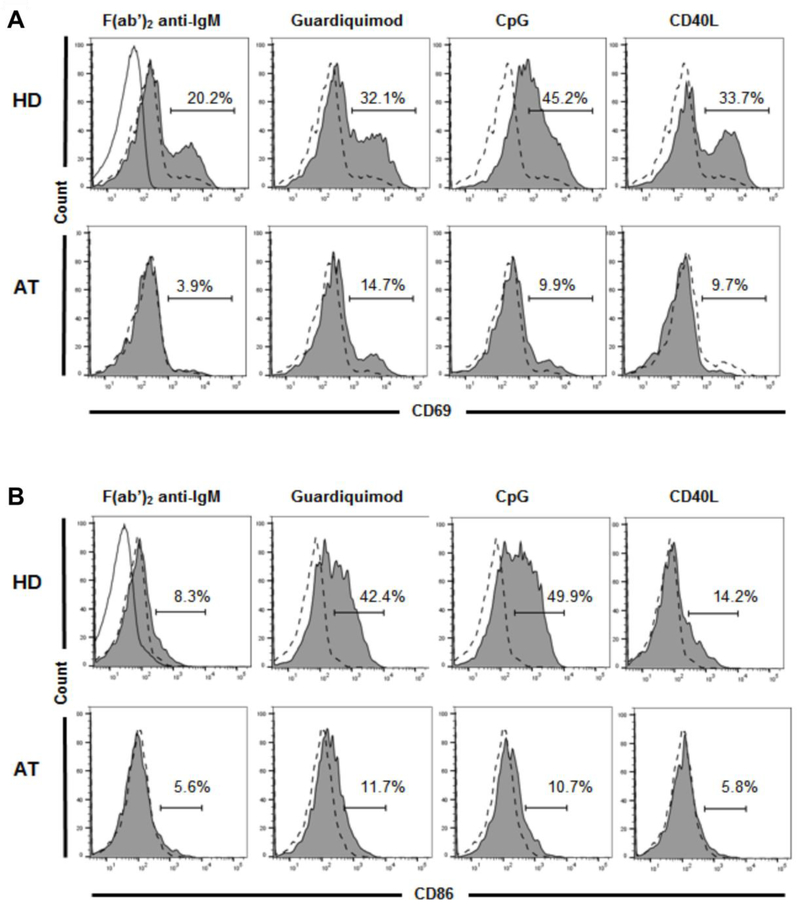
In addition to differences at the gene and protein expression levels in CD21−/low B cells compared to conventional CD21+ mature naive B cells, functional studies reported CD21−/low B cells to be hyporesponsive to stimulation in vitro (17, 21). We assessed the responses of these B cells after activation through the B cell receptor (BCR), TLR7 and TLR9, and CD40 as previously described (17, 21). We found decreased B cell activation in response to all of these stimuli as evidenced by a lower percentage of B cells expressing the activation markers CD69 and CD86 relative to the healthy control samples processed in parallel (Figs. 2A and 2B).
Figure 2.
ATM mutations result in defects in in vitro B cell activation. A) Purified CD19+CD20+CD27− naive B cells from a representative HD (top) and the AT patient (bottom) show decreased expression of the activated B cell marker CD69 in the AT patient after 48 hours of stimulation with the specified B cell activating agents (Solid line with unshaded area in F(ab’)2 anti-IgM panel represents isotype control for all panels; dotted line with unshaded area in all panels represents unstimulated HD or AT B cells; shaded area in all panels represents HD or AT B cells after stimulation). B) Similar B cell activation assay for expression of the activated B cell marker CD86 shows lower CD86 levels in the AT patient.
Dysregulated T cell populations in peripheral blood
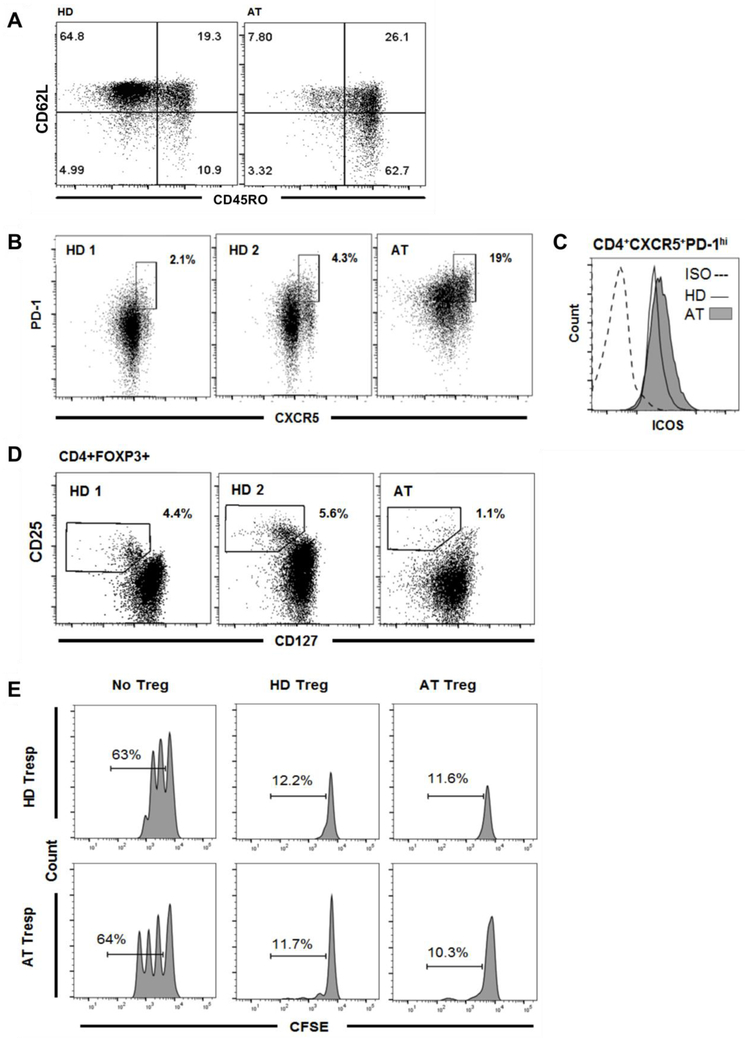
Along with perturbed B cell population frequencies and activation in the patient, we also found alterations in the T cell compartment. There was a decreased frequency of total CD3+ cells but there appeared to be a normal absolute number of CD3+CD4+ T cells (Table 1). There was also a decreased proportion of naive CD3+CD4+CD45RO−CD62L+ T cells (7.8%) (Fig. 3A) compared to age-matched healthy controls (reference median: 65.1%, range: 55.6-75.8% (16,22)). Conversely, there was a striking increase in the CD3+CD4+CD45RO+ memory T cell compartment frequency (89.8%) compared to the reference value in healthy donors (median (22): 29%, range: 22.1-36.6%) (Fig. 3A). This appeared to be largely attributed to the increased proportion of CD3+CD4+CD45RO+CD62L− effector memory T cells (62.7%) (Fig. 3A).
Figure 3.
Altered T cell compartments in the setting of the ATM mutations. A) There are fewer naive CD4+CD62L+CD45RO− T cells in the AT patient. Conversely, there is an elevated memory T cell compartment (CD45RO+) in the AT patient compared to the HD control. There is an increase in the central memory (CD62L+CD45RO+) compartment, and the increased memory T cell frequency is particularly evident in the effector memory ( CD62L−CD45RO+) T cell population. B) Purified CD4+ T cells from two representative HD and the AT patient show expansion of CD4+CXCR5+PD-1hi circulating Tfh (cTfh) cells in the peripheral blood of the AT patient. C) Despite an increase in the frequency of CD4+CXCR5+PD-1hi cTfh cells, the ATM mutations do not affect levels of ICOS, which is important for Tfh cell differentiation and function. (ISO = isotype control). D) Purified CD4+ T cells from two representative HD and the AT patient show a decreased frequency of CD4+FOXP3+CD25+CD127lo Tregs in the peripheral blood of the AT patient. E) Despite this, Treg suppressive capacity is preserved as shown in representative histograms of Treg-mediated suppression of autologous and heterologous CFSE-labeled Tresp cell proliferation on day 4.5 from the AT patient and a HD control. Percentages of Tresp cells undergoing division.
Given the presence of elevated serum IgM and decreased levels of other immunoglobulin isotypes, we examined T follicular helper (Tfh) cells as these play an important role in class-switch recombination (CSR) (23). While Tfh cells are typically found in secondary lymphoid organs, peripheral blood is the most readily accessible tissue for human immunology studies. A population of CXCR5+PD-1hiICOS+CD4+ T cells circulating in the peripheral blood shares similar functional characteristics to secondary lymphoid Tfh cells, including the ability to help naive B cells produce antibodies of different isotypes (24-25). This population was thus referred to as circulating Tfh (cTfh) cells. The average frequency of cTfh cells in a cohort of healthy controls has been reported at around 4% with the 90th percentile below 10% (21). We found that the cTfh cell frequency in the patient was 5-fold higher (19%) than the average for healthy donors (Fig. 3B). ICOS expression is important for Tfh cell development and function in the germinal centers (GCs) of secondary lymphoid organs (26). Despite differences in the frequency of cTfh cells, ICOS expression levels in the AT patient cTfh cells were similar to those in the healthy donor cTfh cells processed in parallel (Fig. 3C).
Decreased frequencies of Tregs have been reported in CD40L- and AID-deficient patients who display a hyper-IgM phenotype and suffer from impaired CSR (27). Given the hyper-IgM phenotype in the patient, we examined Treg numbers and function. Similar to CD40L- and AID-deficient patients, we found a decreased Treg frequency (1.1%) compared to healthy donor controls processed simultaneously and to the age-matched reference value (median: 5.4%, range: 2.3-7.7% (16)) (Fig. 3D). In addition to decreased numbers of Tregs, AID-deficient patients also have decreased Treg suppressive capacity (28). The AT patient described here exhibited strikingly elevated CD4+CD45RO+ memory T cell frequencies which is also seen in IPEX syndrome patients where there are mutations in the FOXP3 transcription factor important for Treg function (29). Observing a decreased frequency of Treg in this AT patient and in light of functional Treg impairment in other conditions with shared immunophenotypes, we evaluated Treg function and found that the suppressive capacity of Tregs was not impaired as Tregs from this patient were capable of suppressing both autologous and heterologous (from healthy donor controls) Tresp cells (Fig. 3E). However, despite intact functional capacity, decreased Treg numbers may have resulted in a similar outcome as decreased Treg function.
Discussion
AT is a complex disorder that not only encompasses oculocutaneous telangiectasias or the progressive neurodegeneration leading to cerebellar ataxia, but also immune dysregulation, which is present in about 2 out of every 3 AT patients (1). The disease is heterogeneous in the variability of the clinical manifestations and constellation of symptoms (1, 3, 4, 6). We report here the clinical phenotype and immunologic consequences of two mutations in ATM, the previously reported c.3372C>G, (p.Tyr124Ter) nonsense mutation and a novel c.237delA, (p.Lys79Asnfs370) mutation encoding a premature stop codon. ATM is important in recognizing, stabilizing, and mediating repair of double-strand breaks (DSB) in DNA. Thus, it is a key player in the V(D)J gene recombination process involved in generating a diverse repertoire of functional BCRs/immunoglobulins and T cell receptors (TCRs) (30-32). Furthermore, ATM plays a role in cell-cycle checkpoint regulation, and its dysfunction also results in continued DNA replication in the presence of unrepaired DSB leading to genomic instability and, as a result, increased cell death (30). As V(D)J recombination in developing B cells takes place in the BM, the decreased frequency of transitional B cells emigrating from the BM into the peripheral blood in the setting of these ATM mutations may be attributed to increased BM B cell apoptosis as a consequence of unrepaired DNA DSB during BCR gene recombination and resultant increased genomic instability. Similarly, the decreased naive T cell population frequency is likely also attributable to defects in DSB repair during TCR V(D)J recombination and consequent decreased viable T-cell output from the thymus (7). The observed decreased transitional B cell and naive T cell frequencies are likely directly related to these mutations (and not to secondary effects of the disease such as poor nutrition or increased infection) as these findings have been demonstrated in Atm− mouse models and also in AT patients with other ATM mutations, particularly those in the AT subgroup with hypogammaglobulinemia (6, 7, 32).
In the B cell compartment, the mutations in ATM described here were associated with abnormal development of CD21−/low B cells expressing high levels of T-bet an FAS. Of note, the parents, each carriers of one of the mutations, showed no increase in this population of B cells. Increased numbers of CD21−/low B cells have been reported in several conditions from CVID to infections such as hepatitis C, HIV-, and malaria and also in autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, and Sjogren syndrome (17, 18, 33, 34). In addition, animal models of autoimmunity display elevated frequencies of CD21−/low B cells (20). CD21−/low B cells have also been reported in AT patients with other ATM mutations where the highest proportions are noted among AT patients with hypogammaglobulinemia as in the current patient (6). The description of CD21−/low B cells is as heterogeneous as the diseases in which they have been found with some reports describing them as unresponsive to stimulation in vitro while others describe them as more similar to exhausted tissue memory B cells or innate-like B cells (18, 19, 35). In terms of similarity to other primary immunodeficiency states, the mutations in ATM described here were associated with the hyporesponsive, pro-apoptotic phenotype that has been described in CVID patients (17). Elevated levels of CD21−/low cells with high T-bet expression in the B cell compartment was unexpected and intriguing. T-bet expression in B cells has recently been associated with autoimmune diseases in animal models (37). Elevated frequency of T-bet-expressing B cells and the presence of IgG anti-chromatin antibodies were also reported in SLE patients (38). This presence of T-bet expressing B cells in ATM patients has not been reported. To date, with limited screening, we have not detected any autoantibodies in this patient, including IgG anti-chromatin or anti-nuclear autoantibodies. Given her age, we cannot rule out the potential auto-reactive nature of T-bet-expressing B cells at a later time.
It remains to be further investigated whether there is any connection between T-bet expression in B cells and the high Tfh counts in this patient. One possibility is that the elevated frequency of Tfh leads to higher levels of IL-21 production which in turn can influence T-bet expression in B cells as has been recently demonstrated (39). While the exact nature and function of this atypical subgroup of CD21−/lowT-bet+ B cells is the subject of ongoing research, it is apparent that they are a marker of dysregulated immunity from a variety of causes. Whether they are a driver or consequence of an abnormal immune system remains to be fully clarified, and further examination of ATM in the development of these cells may help elucidate their origin and role.
The hyper-IgM hypogammaglobulinemic phenotype of this patient may be a direct sequela of these novel mutations in ATM as CSR is impaired in ATM deficiency (40). CSR occurs in the light zone of GCs of secondary lymphoid organs where Tfh cells provide signals to B cells and mediate isotype switching, which requires DNA DSB and gene rearrangement (23). Given the role of Tfh cells in CSR, it is intriguing that an increased frequency of cTfh cells is observed in the presence of mutations in ATM. Interestingly, there is also increased frequency of cTfh cells in patients deficient in AID, a key enzyme induced in B cells by GC Tfh cells to mediate CSR (28). Of note, ATM deficiency also impairs AID activation and function in CSR (36). Thus, there appears to be an association between key enzymes involved in CSR and cTfh cell frequency, and ATM acts at multiple critical points in the CSR pathway. The exact mechanism for the increase in cTfh cells in the setting of ATM deficiency is unclear; however, it is thought to be from impaired B and T cell interactions and defects in multiple successful GC reactions (6).
Another clinical phenotype in this AT patient that is potentially related to the increase in cTfh cells is the presence of noninfectious, non-caseating cutaneous granulomas. Significantly higher percentages of cTfh cells were shown in CVID patients with granulomatous skin disease compared to CVID patients without granulomas despite similar frequencies of overall CD4+ T cells (41). In addition, as in the patient discussed here, CVID patients with elevated cTfh cell frequencies had a concomitantly decreased proportion of Tregs (21). This inverse relationship between cTfh cells and Tregs was also demonstrated in hyper-IgM, AID-deficient patients (28). In further support of a relationship between cTfh cells and Tregs, IPEX syndrome patients, LRBA-deficient, and CTLA-4-deficient patients lacking functional Tregs also exhibited an elevated cTfh cell percentage (29, 42). Thus, finding this inverse relationship in the setting of the ATM mutations described here supports observations in other primary immunodeficiencies that share similar immunologic phenotypes of elevated CD21−/low B cells and defects in CSR, and suggests a potential role for this imbalance in the pathogenesis of cutaneous granulomas. As in the patient presented here, descriptions of other AT patients with cutaneous granulomas report a common phenotype of hyper-IgM with hypogammaglobulinemia of other isotypes and decreased frequency of naive CD4+ T cells but increased T cells bearing γδ TCRs (1, 8, 39 43, 44). This immunophenotype is different from that seen in AT patients without cutaneous granulomas (8).
In terms of a trigger for granuloma formation in this patient, vaccine-strain rubella virus DNA has been reported to persist in the granulomas of patients with AT. It is suggested that the viral DNA serves as a continuous antigen trigger leading to macrophage and T cell activation; however, the exact causal mechanism by which this occurs needs further investigation (9, 10). Another postulated theory for the increased granulomatous skin disease in AT patients with a hyper-IgM, hypogammaglobulinemic phenotype is related to the elevated frequency of γδ T cells, which are a major source of IFN-γ in granulomatous disease (8). IFN-γ stimulates macrophage granuloma formation, and individuals with altered IFN-γ signaling cannot form granulomas in response to mycobacterial infections (45). In this patient, at least at the time point assessed, the IFN-γ level was within normal limits pointing to an alternative mechanism for cytokine stimulation of macrophage activation for granuloma formation. The decreased Treg frequency observed in this patient and others with primary immunodeficiency and cutaneous granulomas may account for increased T effector cell frequency and production of other pro-inflammatory cytokines important in granuloma formation and maintenance, such as TNFα. In addition, γδ T cells are also a known source of TNFα (46, 47). Furthermore, in addition to its elevation in diseases like sarcoidosis, TNFα may play a role in cutaneous granulomas in immunodeficiency as seen in granulomatous CVID (48) and anti-TNFα therapy has been used to treat granulomatous skin disease in both CVID and AT (49, 50). Given the elevated TNFα levels in this patient, anti-TNFα therapy was initiated and appeared helpful in reducing the size and progression of the cutaneous granulomas.
Conclusion
The mutations in ATM described here add to the growing understanding of the heterogeneity in degree and complex nature of the immunodeficiency seen in patients with AT. It is unclear why this variability exists among AT patients, but further examination and categorization of novel mutations in ATM may help clarify this issue. The mutations presented here resulted in perturbations in frequencies and distributions of normal and atypical B and T cell subsets, which can explain some immunologic aspects of the clinical phenotype of this patient. Our findings confirm observations in other AT patients with different ATM mutations and also in patients with other primary immunodeficiencies that share similar clinical and immunologic phenotypes. The clinical phenotype of cutaneous granulomas, hyper-IgM, and hypogammaglobulinemia helps to subcategorize the type of AT resulting from these identified mutations. This hyper-IgM subtype of AT is associated with worse disease outcome and higher lymphoid malignancy risk when combined with low IgA and IgG. Thus, further understanding of the mechanistic role of these novel mutations may have prognostic and therapeutic implications for those likely to have a more clinically severe disease course than AT patients with mutations linked to milder presentations. The information gained from examination of the immunologic effects of these novel mutations will be helpful for further understanding of the role of ATM in normal and pathologic immune system development and function.
Key messages: A novel mutation creating a premature stop codon and a nonsense mutation in the ATM gene are postulated to have resulted in the unique clinical picture characterized by abnormal B and T cell populations, lymphocyte subset dysfunction, granuloma formation, and a hyper-IgM phenotype.
Highlights.
A small subset of patients with AT present with hyper-IgM and a unique immunologic phenotype.
B cell abnormalities include increased CD38loCD21−/low B cells expressing T-bet and Fas.
B cells were hyporesponsive to stimulation through the B cell receptor, TLR 7 and 9, and CD40.
T cell homeostasis was disturbed with increases in γδ T cells and Tfh.
Acknowledgments
Funding Support: This manuscript was supported in part by Grifols, the Joanne Siegel Fund, the Dreizessen Fund (EWG), grants from NIAMS T32 AR007107-41 (KM), NIAID R01 AI071087 (EM), and NIH HL-36577 and AI-77609 (EWG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rothblum-Oviatt C, Wright J, Lefton-Greif MA, McGrath-Morrow SA, Crawford TO, Lederman HM. Ataxia telangiectasia: a review. Orphanet J Rare Dis 2016; 11:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perry TL, Kish SJ, Hinton S, Becker LE, Gelfand EW. Neurochemical abnormalities in a patient with ataxia-telangiectasia. Neurology 1984; 34:187–191. [DOI] [PubMed] [Google Scholar]
- 3.Jason JM, and Gelfand EW. Diagnostic considerations in ataxia telangiectasia. Arch Dis Child 1979; 54;682–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roifman CM and Gelfand EW. Heterogeneity of the immunological deficiency in ataxia-telangiectasia: absence of a clinical-pathological correlation. Kroc Found Ser. 1985; 19:1273–1285. [PubMed] [Google Scholar]
- 5.Noordzij JG1, Wulffraat NM, Haraldsson A, Meyts I, van't Veer LJ, Hogervorst FB, Warris A, Weemaes CM. Ataxia-telangiectasia patients presenting with hyper-IgM syndrome. Arch Dis Child 2009; 94:448–449. [DOI] [PubMed] [Google Scholar]
- 6.Driessen GJ, Ijspeert H, Weemaes CM, Haraldsson A, Trip M, Warris A, et al. Antibody deficiency in patients with ataxia telangiectasia is caused by disturbed B- and T-cell homeostasis and reduced immune repertoire diversity. J Allergy Clin Immunol 2013; 131:1367–1375 e9. [DOI] [PubMed] [Google Scholar]
- 7.Giovannetti A, Mazzetta F, Caprini E, Aiuti A, Marziali M, Pierdominici M, et al. Skewed T-cell receptor repertoire, decreased thymic output, and predominance of terminally differentiated T cells in ataxia telangiectasia. Blood 2002; 100:4082–4089. [DOI] [PubMed] [Google Scholar]
- 8.Chiam LY, Verhagen MM, Haraldsson A, Wulffraat N, Driessen GJ, Netea MG, et al. Cutaneous granulomas in ataxia telangiectasia and other primary immunodeficiencies: reflection of inappropriate immune regulation? Dermatology 2011; 223:13–19. [DOI] [PubMed] [Google Scholar]
- 9.Bodemer C, Sauvage V, Mahlaoui N, Cheval J, Couderc T, et al. Live rubella virus vaccine long-term persistence as an antigenic trigger of cutaneous granulomas in patients with primary immunodeficiency. Clin Microbiol Infect. 2014; 20:O656–O663. [DOI] [PubMed] [Google Scholar]
- 10.Perelygina L, Plotkin S, Russo P, Hautala T, Bonilla F, et al. Rubella persistence in epidermal keratinocytes and granuloma M2 macrophages in patients with primary immunodeficiencies. J Allergy Clin Immunol 2016; 138:1436–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picard C, Al-Herz W, Bousfiha A, Casanova JL, Chatila et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. J Clin Immunol 2015; 35:696–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Mousa H, Abouelhoda M, Monies DM, Al-Tassan N, Al-Ghonaium A, et al. Unbiased targeted next-generation sequencing molecular approach for primary immunodeficiency diseases. J Allergy Clin Immunol 2016; 137:1780–1787. [DOI] [PubMed] [Google Scholar]
- 13.Salt BH, Jain A, Pandey R, Hanson EP, Niemela JE, et al. IKBKG (NEMO) mutation can be associated with opportunistic infection without impairing TLR function. J. Allergy Clin. Immunol 2008; 121:976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbonari M, Cherchi M, Paganelli R, Giannini G, Galli E, Gaetano C, Papetti C, Fiorilli M. Relative increase of T cells expressing the gamma/delta rather than the alpha/beta receptor in ataxia-telangiectasia. N Engl J Med 1990; 322:73–76. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz A, Balint B, Korporal-Kuhnke M, Jarius S, von Engelhardt K, Furwentsches A, et al. B-cell populations discriminate between pediatric- and adult-onset multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2017; 4:e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Gent R, van Tilburg CM, Nibbelke EE, Otto SA, Gaiser JF, Janssens-Korpela PL, et al. Refined characterization and reference values of the pediatric T- and B-cell compartments. Clin Immunol 2009; 133:95–107. [DOI] [PubMed] [Google Scholar]
- 17.Isnardi I, Ng YS, Menard L, Meyers G, Saadoun D, Srdanovic I, et al. Complement receptor 2/CD21- human naive B cells contain mostly autoreactive unresponsive clones. Blood 2010; 115:5026–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorarinsdottir K, Camponeschi A, Gjertsson I, Martensson IL. CD21 −/low B cells: A Snapshot of a Unique B Cell Subset in Health and Disease. Scand J Immunol 2015; 82:254–261. [DOI] [PubMed] [Google Scholar]
- 19.Rakhmanov M, Keller B, Gutenberger S, Foerster C, Hoenig M, Driessen G, et al. Circulating CD21low B cells in common variable immunodeficiency resemble tissue homing, innate-like B cells. Proc Natl Acad Sci U S A 2009; 106:13451–13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubtsova AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood 2011; 118:1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romberg N, Chamberlain N, Saadoun D, Gentile M, Kinnunen T, Ng YS, et al. CVID-associated TACI mutations affect autoreactive B cell selection and activation. J Clin Invest 2013; 123:4283–4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tosato F, Bucciol G, Pantano G, Putti MC, Sanzari MC, Basso G, et al. Lymphocytes subsets reference values in childhood. Cytometry A 2015; 87:81–85. [DOI] [PubMed] [Google Scholar]
- 23.Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol 2012; 8:337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011; 34:108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma CS, Phan TG. Here, there and everywhere: T follicular helper cells on the move. Immunology 2017; 152:382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J Immunol 2006; 177:4927–4932. [DOI] [PubMed] [Google Scholar]
- 27.Meyers G, Ng YS, Bannock JM, Lavoie A, Walter JE, Notarangelo LD, et al. Activation-induced cytidine deaminase (AID) is required for B-cell tolerance in humans. Proc Natl Acad Sci U S A 2011; 108:11554–11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantaert T, Schickel JN, Bannock JM, Ng YS, Massad C, Delmotte FR, et al. Decreased somatic hypermutation induces an impaired peripheral B cell tolerance checkpoint. J Clin Invest 2016; 126:4289–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinnunen T, Chamberlain N, Morbach H, Choi J, Kim S, Craft J, et al. Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells. Blood 2013; 121:1595–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavin MF, Shiloh Y. The genetic defect in ataxia-telangiectasia. Annu Rev Immunol 1997; 15:177–202. [DOI] [PubMed] [Google Scholar]
- 31.Lavin MF. ATM and the Mre11 complex combine to recognize and signal DNA double-strand breaks. Oncogene 2007; 26:7749–7758. [DOI] [PubMed] [Google Scholar]
- 32.Bredemeyer AL, Sharma GG, Huang CY, Helmink BA, Walker LM, Khor KC, et al. ATM stabilizes DNA double-strand-break complexes during V(D)J recombination. Nature 2006; 442:466–470. [DOI] [PubMed] [Google Scholar]
- 33.Saadoun D, Terrier B, Bannock J, Vazquez T, Massad C, Kang I, et al. Expansion of autoreactive unresponsive CD21-/low B cells in Sjogren's syndrome-associated lymphoproliferation. Arthritis Rheum 2013; 65:1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quach TD, Manjarrez-Orduno N, Adlowitz DG, Silver L, Yang H, Wei C, et al. Anergic responses characterize a large fraction of human autoreactive naive B cells expressing low levels of surface IgM. J Immunol 2011; 186:4640–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thorarinsdottir K, Camponeschi A, Cavallini N, Grimsholm O, Jacobsson L, Gjertsson I, et al. CD21(−/low) B cells in human blood are memory cells. Clin Exp Immunol 2016; 185:252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khair L, Guikema JE, Linehan EK, Ucher AJ, Leus NG, Ogilvie C, et al. ATM increases activation-induced cytidine deaminase activity at downstream S regions during class-switch recombination. J Immunol 2014. 192:4887–4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubtsova K, Rubtsova AV, Thurman JM, Mennona JM, Kappler JW, et al. B cells expressing the transcription factor T-bet drive lupus-like autoimmunity. J Clin Invest 2017; 127:1392–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Zhou S, Qian J, Wang Y, Yu X et al. T-bet+CD11C+ B cells are critical for anti-chromatin immunoglobulin G production in the development of lupus. Arthritis Res Ther 2017; 19:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naradikian MS, Myles A, Beiting DP, Roberts KJ, Dawson L,. Cutting edge: IL-4, IL-21, and IFN-γ interact to govern T-bet and CD11c expression in TLR-activated B cells. J Immunol 2016;197:1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohammadinejad P, Abolhassani H, Aghamohammadi A, Pourhamdi S, Ghosh S, et al. Class switch recombination process in Ataxia-Telangiectasia patients with elevated serum levels of IgM. J Immunoassay Immunochem 2015; 36:16–26. [DOI] [PubMed] [Google Scholar]
- 41.Coraglia A, Galassi N, Fernandez Romero DS, Juri MC, Felippo M, Malbran A, et al. Common variable immunodeficiency and circulating TFH. J Immunol Res 2016; 2016:4951587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alroqi FJ, Charbonnier LM, Baris S, Kiykim A, Chou J, Platt CD, et al. Exaggerated follicular helper T-cell responses in patients with LRBA deficiency caused by failure of CTLA4-mediated regulation. J Allergy Clin Immunol 2018;141:1050–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Folgori L, Scarselli A, Angelino G, et al. Cutaneous granulomatosis and combined immuno-deficiency revealing Ataxia-Telangiectasia: a case report. Ital J Pediatr 2010; 36;29:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joshi RK, Asiri AL, Haleem A, et al. Cutaneous granuloma with ataxia-telangiectasia- a case report and review of literature. Clin Exp Dermatol 1993; 18:458–561. [DOI] [PubMed] [Google Scholar]
- 45.Lammas DA, De Heer E, Edgar JD, Novelli V, Ben-Smith A, Baretto R, et al. Heterogeneity in the granulomatous response to mycobacterial infection in patients with defined genetic mutations in the interleukin 12-dependent interferon-gamma production pathway. Int J Exp Pathol 2002; 83:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lafont V, Liautard J, Liautard JP, Favero J. Production of TNF-alpha by human V gamma 9V delta 2 T cells via engagement of Fc gamma RIIIA, the low affinity type 3 receptor for the Fc portion of IgG, expressed upon TCR activation by nonpeptidic antigen. J Immunol 2001; 166:7190–7199. [DOI] [PubMed] [Google Scholar]
- 47.Lawand M, Dechanet-Merville J, Dieu-Nosjean MC. Key features of gamma-delta T-cell subsets in human diseases and their immunotherapeutic implications. Front Immunol 2017; 8:761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mullighan CG, Fanning GC, Chapel HM, Welsh KI. TNF and lymphotoxin-alpha polymorphisms associated with common variable immunodeficiency: role in the pathogenesis of granulomatous disease. J Immunol 1997; 159:6236–6241. [PubMed] [Google Scholar]
- 49.Lin JH, Liebhaber M, Roberts RL, Dyer Z, Stiehm ER. Etanercept treatment of cutaneous granulomas in common variable immunodeficiency. J Allergy Clin Immunol 2006; 117:878–882. [DOI] [PubMed] [Google Scholar]
- 50.Mitra A, Gooi J, Darling J, Newton-Bishop JA. Infliximab in the treatment of a child with cutaneous granulomas associated with ataxia telangiectasia. J Am Acad Dermatol 2011; 65:676–677. [DOI] [PubMed] [Google Scholar]