Abstract
Abnormality in the number and function of CD4+CD25+FOXP3+ regulatory T cells (Tregs) in peripheral blood has been linked to the initiation and progression of rheumatoid arthritis (RA). Effect of chemokine CCL22 on the number of Tregs in CD4+ T cells and the underlying mechanism were investigated. Downregulation of peripheral Tregs were observed while upregulation of serum chemokine CCL22 in RA patients. Tregs count and the expression of FOXP3 (Tregs function-related maker) and phosphorylated-signal transducer and activator of transcription 5 (p-STAT5) in CD4+ T cells from RA patients were increased while C-C chemokine receptor 4 (CCR4) was decreased by anti-CCL22 antibody, however, recombinant CCL22 resulted in the opposite effects in CD4+ T cells from the healthy control. STAT5 inhibitor significantly reversed the effects of anti-CCL22 antibody. Similarly, sinomenine, an anti-arthritis drug, which decreased CCL22 and CCR4, showed the same trends as the above events, and was reversed by recombinant CCL22 or STAT5 inhibitor. Collectively, anti-CCL22 induced the number of Tregs via STAT5 pathway, leading to expansion of Tregs and subsequently to control of the autoimmune reaction in RA patients. Our study provides s novel strategy for RA treatment.
Keywords: rheumatoid arthritis, CCL22, Tregs, STAT5 pathway
Introduction
Rheumatoid arthritis (RA), a chronic inflammatory autoimmune disease, arises from a breakdown in self-tolerance which is characterized by the infiltration of massive inflammatory cells into both synovial tissues and synovial fluid, leading to chronic synovitis, progressive erosions and cartilage destruction (synovium, cartilage, and bone of multiple joints (1). CD4+CD25+ regulatory T cells (Tregs), a lymphocyte subpopulation, are engaged in peripheral tolerance and anti-autoimmunity by directly or indirectly suppressing self-reactive T cells and B cells. The decreased number of Tregs in circulation, as well as deficiency of their suppressive and anti-inflammatory activity in the joints have been regarded as an indispensable factor of RA (2,3). Although Tregs are unable to undergo mitosis, they do proliferate in response to exogenous stimulation (4). Induction or amplification of Tregs may serve as helpful and innovative therapies for RA (5).
In humans, Tregs have been identified in the peripheral circulation and in the thymus (4). Tregs express certain chemokines and chemokine receptors (such as CCR4, −5, and −6), rendering it migrate into rheumatic joint to retard the activation of auto-aggressive cells that have escaped central tolerance mechanisms (6). The macrophage-derived chemokine (MDC)/CCL22 belongs to the CC family, and selectively interactes with the C-C chemokine receptor 4 (CCR4). Evidence suggests that CCL22 is significantly enhanced in both synovial fluid and plasma in patients with RA (7). However, whether and how CCL22 functioned in RA remain largely unknown.
Signal transducer and activator of transcription 5 (STAT5) is implicated in maintaining immune function, particularly in Tregs development and function (8,9). Moreover, high expression of FOXP3 and the in vitro suppressive activity of Tregs was maintained in the presence of STAT5-activating cytokines, such as IL-2 and IL-15 (10). Evidence suggests that the expression of chemokine CCL1 in primary human dermal fibroblasts is depended on the activation of STAT5 (11).
To study the effect of chemokine CCL22 on Tregs, CD4+ T cells obtained from RA patients and healthy control were treated with anti-CCL22 antibody and recombinant CCL22 protein, respectively. STAT5 inhibitor (CAS 285986-31-4) was used to block the STAT5 pathway (12). Our data suggested that CCL22 decreased the number of Tregs and FOXP3 expression via STAT5 pathway in human RA. Further, we found that Sinomenine, which has been used in RA therapy for several decades (13), regulated Tregs by CCL22/STAT5 pathway.
Patients and methods
Patients
This study was approved by the Ethics Committee of Shuguang Hospital Affiliated to Shanghai University of TCM (Shanghai, China), and written informed consent from the participants was obtained. Blood samples were collected from 30 pairs of RA patients and age-matched healthy controls for isolation of serum.
Enzyme-linked immunosorbent assay (ELISA)
Human Macrophage-Derived Chemokine, MDC ELISA Kit 96T/48T (XY-E10131, X-Y Biotechnology) was used to determine serum concentration of CCL22 (pg/ml) in accordance with the manufacturer's procedure.
Isolation and stimulation of CD4+ T cells
Peripheral blood mononuclear cells (PBMCs) were isolated from blood of RA patients (n=15) and healthy controls (n=10) by using density gradient centrifugation method. CD4+ T cells were isolated from PBMCs, using MagCellect Human CD4+ T cell Isolation Kit (MAGH102; R&D Systems), and then cultured in a medium of RPMI media (HyClone) with 10% fetal calf serum (Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml penicillin (Solarbio).
For cytokine responses, CD4+ T cells from RA patients and healthy controls were exposed to anti-CCL22 antibody (ab9847; Abcam) at a dose of 0, 0.1, 0.2, 0.5, 1.0 and 2.0 µg/ml, and recombinant CCL22 (ab243277; Abcam) at a dose of 0, 2, 10 and 50 ng/ml, respectively. To study the involvement of STAT5 pathway in the promoted effect of anti-CCL22 antibody on Tregs population, CD4+ T cells from RA patients were treated with 50 ng/ml of anti-CCL22 antibody (0.5 µg/ml) plus 1 µM of STAT5 inhibitor (Millipore) or vehicle (DMSO). To study the effect of sinomenine (SIN), CD4+ T cells from RA patients were treated with 0, 1.0, 2.5, 5.0, 10 and 20 µg/l of SIN (SS8560; Solarbio).
Flow cytometric analysis of Tregs
CD4+ T cells were stained with Anti-Human FOXP3 Staining Kit (560133; BD Biosciences), and the percentage of CD4+CD25+FOXP3+ cells in CD4+ T cell population was analyzed on Accuri C6 flow cytometer (BD Biosciences) with FlowJo 7.6.1 software (Tree Star Inc.).
Real-time (RT)-PCR
Total RNA from CD4+ T cells was extracted by TRIzol reagent (Sigma-Aldrich; Merck KGaA) and reverse transcribed using cDNA synthesis kit (Takara). The primers for CCL22 (NCBI NM_002990.5) were: 5′-CCTGCTTAAACCCTTCCATGAC-3′ and 5′-TTGGAGAACAGGGAGCTAGAAC-3′; primers for CCR4 (NCBI NM_005508.4) were: 5′-CCTTCCTGGCTTTCTGTTC-3′ and 5′-CATCTTCACCGCCTTGTTC-3′, primers for FOXP3 (NCBI NM_001114377.1) were: 5′-AGGAGGATGGACGAACAG-3′ and 5′-GGCAAGACAGTGGAAACC-3′; and primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (NCBI NM_001256799.2) were: 5′-AATCCCATCACCATCTTC-3′ and 5′-AGGCTGTTGTCATACTTC-3′. SYBR-Green PCR Kit (Thermo Fisher Scientific, Inc.) with an ABI 7300 system (Applied Biosystems) was used for analysis. mRNA levels of CCL22, CCR4 and FOXP3 were normalized by GAPDH.
Western blot analysis
After fully lysed of CD4+ T cells, total protein was quantified using BCA protein assay kit (Thermo Fisher Scientific, Inc.), and 25 µg of which was separated using 15% SDS-PAGE. After transferring onto PVDF membranes (Millipore), the membrane were incubated with antibody against CCL22 (Ab9847), CCR4 (Ab832F50), FOXP3 (Ab20034), STAT5 (Ab230670), antibody against phosphorylated (p)-STAT5 (Ab32364) (all from Abcam) and antibody against GAPDH (#5174; Cell Signaling Technology) at 4°C overnight followed by secondary antibodies (A0208 and A0216; Beyotime Institute of Biotechnology) for 1 h at 25°C. ECL system (GE Healthcare/Amersham Biosciences) was used for analysis.
Statistical analysis
Data were calculated using GraphPad Prism 7.0 software (GraphPad Software, Inc.) and described as mean ± standard error of the mean (SEM). Comparison between RA and HC group, as well as CD4+ T cells with various stimulations and the corresponding Control group were conducted by one-way ANOVA analysis with post-hoc Tukey's test, and P-value <0.05 was considered significant.
Results
Serum CCL22 is upregulated while Tregs are downregulated in RA
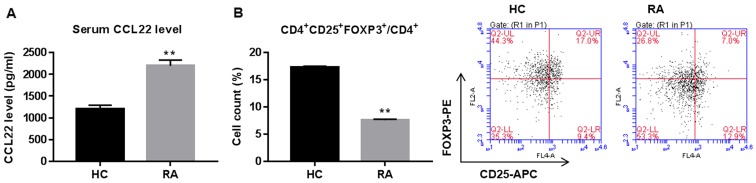
Serum concentration of CCL22 and Tregs proportion in CD4+ T cells are shown in Fig. 1. CCL22 was significantly increased while peripheral Tregs were reduced in RA patients when compared with healthy controls (HC).
Figure 1.
Serum CCL22 concentration and percentage of Tregs in CD4+ T cells of rheumatoid arthritis (RA) patients and healthy controls (HC). (A) ELISA assay showing CCL22 concentration in serum of RA (n=30) and HC (n=30). (B) Flow cytometry analysis showing CD4+CD25+FOXP3+ cells (Tregs) in RA (n=15) and HC (n=10), as a % of total CD4+ cells. **P<0.01 vs. HC. Tregs, regulatory T cells.
Roles of CCL22 in regulating the number of Tregs in CD4+ T cells
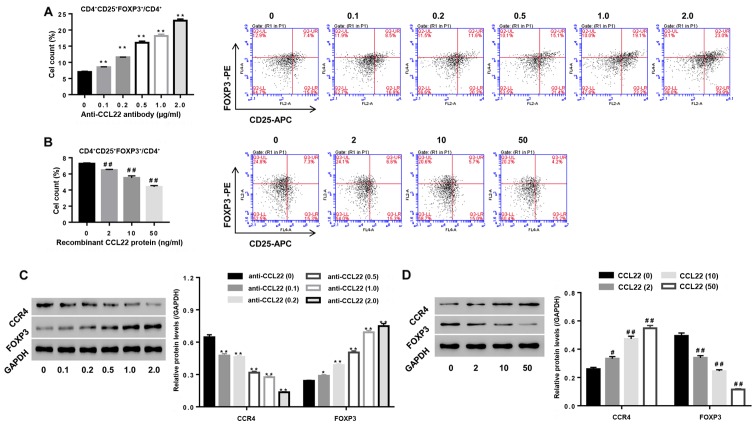
To study the roles of CCL22 in regulating Tregs expansion, CD4+ T cells isolated from RA were stimulated with anti-CCL22 antibody (0, 0.1, 0.2, 0.5, 1.0 and 2.0 µg/ml) while CD4+ T cells, isolated from HC, were stimulated with exogenous CCL22 protein (0, 2, 10 and 50 ng/ml), then the percentage of CD4+CD25+FOXP3+ regulatory T cells, and the expression of CCL22, CCR4 and FOXP3 within CD4+ T cells were determined. Our data showed that, in comparison to their corresponding control, the percentage of CD4+CD25+FOXP3+ regulatory T cells in CD4+ T cell population from RA patients was significantly enhanced 1, 65, 129, 157 and 228% under a stimulation dose of anti-CCL22 antibody at 0.1, 0.2, 0.5, 1.0 and 2.0 µg/ml, respectively (all P<0.01) (Fig. 2A). On the contrary, the percentage of CD4+CD25+FOXP3+ regulatory T cells in CD4+ T cell population from HC was significantly reduced to 11, 23 and 40% under a stimulation dose of recombinant CCL22 protein at 2, 10 and 50 ng/ml, respectively (all P<0.01) (Fig. 2B). FOXP3, the Tregs function associated maker, was dose-dependently upregulated by anti-CCL22 antibody, however, downregulated by recombinant CCL22 protein (Fig. 2C and D) further confirming the roles of CCL22 on Tregs function at a molecular level.
Figure 2.
Roles of CCL22 in regulating the number and function associated makers of Tregs. (A and C) Stimulation of CD4+ T cells of RA patients with anti-CCL22 antibody (0.1, 0.2, 0.5, 1.0 and 2.0 µg/ml) caused apromoted effect on the number of Tregs, as well as a decrease of CCR4 while an increase in FOXP3 (C). (B and D) Stimulation of CD4+ T cells of HC with CCL22 protein (2, 10 and 50 ng/ml) caused inhibition of the number of Tregs (B), as well as increase in CCR4 while decreased FOXP3 (D). *P<0.05, **P<0.01 vs. control CD4+ T cells from RA; #P<0.05, ##P<0.01 vs. control CD4+ T cells from HC. Tregs, regulatory T cells; CCR4, C-C chemokine receptor 4.
In addition, Fig. 2C and D indicated that anti-CCL22 antibody reduced the expression of CCR4 in a dose-dependent manner, however, recombinant CCL22 protein resulted in the opposite effects (all P<0.01), confirming inhibition of CCR4 expression by anti-CCL22 antibody, while stimulation of CCR4 expression by exogenous CCL22 protein.
Anti-CCL22 antibody enhances Tregs content by increasing p-STAT5
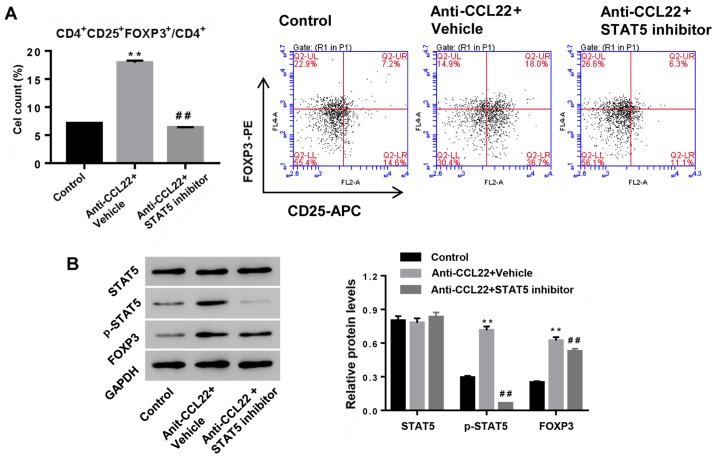
To study the possible mechanism, CD4+ T cells, isolated from RA patients, were exposed to anti-CCL22 antibody (0.5 µg/ml) in the presence of STAT5 inhibitor (1 µM) or vehicle, and then Tregs content and expression of p-STAT5 and FOXP3 were determined. Fig. 3 shows that additional STAT5 inhibitor significantly reversed the effects of anti-CCL22 antibody on Tregs content and the expression of p-STAT5 and FOXP3, suggesting that activation of STAT5 was the mechanism, by which anti-CCL22 exerted promoting effect on the number of Tregs in CD4+ T cells.
Figure 3.
Anti-CCL22 antibody enhances the number of Tregs via the activation of STAT5 pathway. CD4+ T cells, isolated from RA, were treated with anti-CCL22 antibody (0.5 µg/ml) in the presence of STAT5 inhibitor (1 µM) or vehicle (DMSO) at 48 h, and then the number of Tregs (A) and protein levels of FOXP3, STAT5 and p-STAT5 (B) were assessed. GAPDH was used a loading control. **P<0.01 vs. control; ##P<0.01 vs. anti-CCL22. Tregs, regulatory T cells; STAT5, signal transducer and activator of transcription 5.
Sinomenine promotes the number of Tregs and enhances CCL22 expression in CD4+ T cells
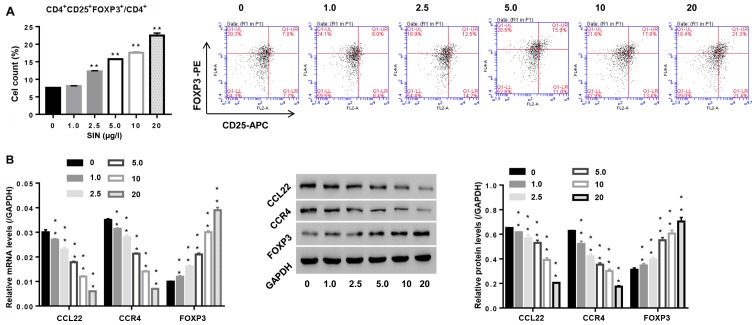
Sinomenine has been used in RA therapy for several decades (13). To study the roles of SIN in Tregs and its effect on CCL22 expression in CD4+ T cells, isolated CD4+ T cells from RA patients were stimulated with SIN (1, 2.5, 5, 10 and 20 µg/l), and then the number of Tregs was assessed, as well as expression of CCL22, CCR4 and FOXP3 in CD4+ T cells. Fig. 4A indicates that SIN promoted Treg contents, and was associated with the downregulation of CCL22 and CCR4, and the upregulation of FOXP3, substantiating that SIN enhanced the number of Tregs and Tregs function at the molecular level, and the involvement of CCL22 and CCR4 in this process.
Figure 4.
Effect of sinomenine (SIN) on the number of Tregs and function-associated makers. CD4+ T cells, isolated from peripheral blood of RA patients, were exposed to SIN (0, 1.0, 2.5, 5.0, 10 and 20 µg/l). (A) After 24 h, the number of Tregs in CD4+ T cells was measured by flow cytometric analysis. (B) 48 h later, mRNA and protein levels of FOXP3, CCL22 and CCR4 were assessed by real-time PCR and western blotting, respectively. **P<0.01 vs. 0 (CD4+ T cells stimulated with vehicle PBS). Tregs, regulatory T cells; CCR4, C-C chemokine receptor 4.
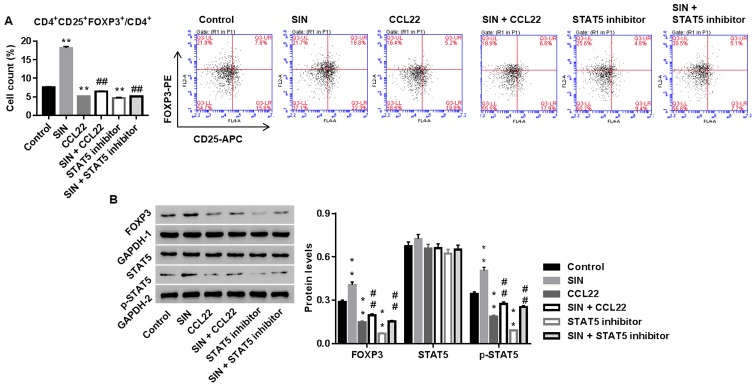
Sinomenine promotes the number of Tregs via CCL22/STAT5 pathway
We studied whether CCL22 and STAT5 was the underlying mechanism, by which SIN regulated the number of Tregs in CD4+ T cells. Tregs were treated with SIN, CCL22, STAT5 inhibitor, SIN plus CCL22, or SIN plus STAT5 inhibitor. As shown in Fig. 5, SIN significantly enhanced the number of Tregs and protein levels of FOXP3 and p-STAT5, and the effects were weakened by additional CCL22 or STAT5 inhibitor treatment. Thus, our data indicated that SIN increased the number of Tregs probably via CCL22/STAT5 pathway.
Figure 5.
Sinomenine (SIN) promotes the number of Tregs via CCL22/STAT5 pathway. CD4+ T cells, isolated from RA, were treated with vehicle, CCL22 (50 ng/ml) + vehicle, CCL22 + SIN (5 µg/l), STAT5 inhibitor (1 µM), or SIN + STAT5 inhibitor. Twenty-four hours later, the number of Tregs (A) and protein levels of FOXP3, STAT5 and p-STAT5 (B) were assessed. GAPDH, loading control. **P<0.01 vs. control; ##P<0.01 vs. CCL22. Tregs, regulatory T cells; STAT5, signal transducer and activator of transcription 5.
Discussion
A hallmark of RA is the reduction of Tregs function in peripheral blood, and Tregs expansion and transfer have been involved in therapeutic implications in RA (2,4). FOXP3, a specific maker for Tregs function, is responsible for Tregs specific detection and enumeration. In the present study, we confirmed that peripheral Tregs were decreased while serum CCL22 was increased in RA which was in line with a reported study (Fig. 1) (7). Evidence suggests that injection of CCL22 induces accumulation of CCR4 expressing T cells in vivo (14). Given the roles of CCL22 in regulating Tregs proliferation in tumor immunity (15), we studied whether CCL22 function in modulating the number of Tregs in RA. Our data elucidated that CCL22 increased CCR4 expression, and decreased the number of Tregs and expression of FOXP3 in CD4+ T cells of healthy controls, and anti-CCL22 showed the reverse effect in CD4+ T cells from RA patients (Fig. 2). Our data indicated that the elevated CCL22/CCR4 may involve in the pathogenesis of RA by decreasing the number of Tregs.
Activation of STAT5 is sufficient to increase the number of Tregs and favors Tregs homeostasis and self-tolerance (16). Tregs with the high levels of p-STAT5 is frequently associated with the high suppressive activity in vitro (17). Expression levels of p-STAT5 is down regulated in Tregs in RA patients (17). In this study, we suggest that the activation of STAT5 was accelerated by anti-CCL22 antibody (Fig. 3). The STAT5 inhibitor abolished anti-CCL22 and increased the number of Tregs and FOXP3 expression. Thus, we deduced that CCL22 inactivated STAT5 signals, which lead to the reduction of the number of Tregs in CD4+ T cells of RA patients.
Alkaloid SIN, known as an anti-arthritis drug, has been used in RA therapy for several decades. Li et al (13) reported that treatment with SIN reduced the proportion of Th17 (CD4+IL-17+) and elevated the proportion of Tregs in PBMC of RA patients. Tong et al (18) suggested that SIN treatment suppressed collagen-induced arthritis by regulating Th17/Treg cells in intestinal lymph nodes. This study substantiated the promotion effect of SIN on the number of Tregs and FOXP3 expression in CD4+ T cells of RA patients in vitro simultaneously with the decreased CCL22 and CCR4 (Fig. 4). Further experiments showed that recombinant CCL22 and STAT5 inhibitor blocked the effect of SIN (Fig. 5), suggesting that CCL22/CCR4/STAT5 axis mediated the function of SIN on Tregs. Thus, compounds which can modulate CCL22/CCR4/STAT5 axis may be applied for the treatment of RA.
In conclusion, CCL22 plays a role in regulating the number of Tregs and the function, and blocking STAT5 activation is the underlying mechanism. Drugs targeting CCL22/CCR4/STAT5 axis might represent the immunomodulatory effect in the long-term treatment of RA. Our study provides a novel strategy for RA treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
LW (first in the author list) conceived the study and drafted the manuscript. PH and QC acquired the data; ZZ and LW (second in the author list) analyzed the data and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Shuguang Hospital Affiliated to Shanghai University of TCM (Shanghai, China). Patients who participated in this research had complete clinical data. Signed informed consents were obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kurkó J, Besenyei T, Laki J, Glant TT, Mikecz K, Szekanecz Z. Szekanecz, immunology, genetics of rheumatoid arthritis - A comprehensive review. Clin Rev Allergy Immunol. 2013;45:170–179. doi: 10.1007/s12016-012-8346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong Q, Song XY, Huang JY, Guo Y. CD4+CD25+Foxp3+ regulatory T cell content in peripheral blood and synovial fluid of patients with rheumatoid arthritis and osteoarthritis. Zhongguo Shengwuzhipinxue Zazhi. 2011;24:169–172. (In Chinese) [Google Scholar]
- 3.Bayry J, Sibéril S, Triebel F, Tough DF, Kaveri SV. Rescuing CD4+CD25+ regulatory T-cell functions in rheumatoid arthritis by cytokine-targeted monoclonal antibody therapy. Drug Discov Today. 2007;12:548–552. doi: 10.1016/j.drudis.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Leipe J, Skapenko A, Lipsky PE, Schulze-Koops H. Regulatory T cells in rheumatoid arthritis. Arthritis Res Ther. 2005;7:93. doi: 10.1186/ar1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu Q, Xu M, Yu F, Jin Y. CD4(+)CD25(+) regulatory T cells as a therapeutic target in rheumatoid arthritis. Cent Eur J Immunol. 2014;39:100–103. doi: 10.5114/ceji.2014.42133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi H, Zhao Y. Chemokines, chemokine receptors and CD4+CD25+ regulatory T cells. Expert Rev Clin Immunol. 2007;3:343–349. doi: 10.1586/1744666X.3.3.343. [DOI] [PubMed] [Google Scholar]
- 7.Flytlie HA, Hvid M, Lindgreen E, Kofod-Olsen E, Petersen EL, Jørgensen A, Deleuran M, Vestergaard C, Deleuran B. Expression of MDC/CCL22 and its receptor CCR4 in rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Cytokine. 2010;49:24–29. doi: 10.1016/j.cyto.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 9.Mahmud SA, Manlove LS, Farrar MA. Interleukin-2 and STAT5 in regulatory T cell development and function. JAK-STAT. 2013;2:e23154. doi: 10.4161/jkst.23154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Passerini L, Allan SE, Battaglia M, Di Nunzio S, Alstad AN, Levings MK, Roncarolo MG, Bacchetta R. STAT5-signaling cytokines regulate the expression of FOXP3 in CD4+CD25+ regulatory T cells and CD4+CD25− effector T cells. Int Immunol. 2008;20:421–431. doi: 10.1093/intimm/dxn002. [DOI] [PubMed] [Google Scholar]
- 11.Hintzen C, Haan C, Tuckermann JP, Heinrich PC, Hermanns HM. Oncostatin M-induced and constitutive activation of the JAK2/STAT5/CIS pathway suppresses CCL1, but not CCL7 and CCL8, chemokine expression. J Immunol. 2008;181:7341–7349. doi: 10.4049/jimmunol.181.10.7341. [DOI] [PubMed] [Google Scholar]
- 12.Betts BC, Veerapathran A, Pidala J, Yu XZ, Anasetti C. STAT5 polarization promotes iTregs and suppresses human T-cell alloresponses while preserving CTL capacity. J Leukoc Biol. 2014;95:205–213. doi: 10.1189/jlb.0313154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li N, Deng Y, Zhou JR, Song GZ, Wang JY. Effect of sinomenine on the proportion of T helpers 17 and regulatory T cells in puerperal blood mononuclear cells from patients with rheumatoid arthritis. Shanghai Med J. 2013;36:254–258. [Google Scholar]
- 14.Fahy O, Porte H, Sénéchal S, Vorng H, McEuen AR, Buckley MG, Walls AF, Wallaert B, Tonnel AB, Tsicopoulos A. Chemokine-induced cutaneous inflammatory cell infiltration in a model of Hu-PBMC-SCID mice grafted with human skin. Am J Pathol. 2001;158:1053–1063. doi: 10.1016/S0002-9440(10)64052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 16.Antov A, Yang L, Vig M, Baltimore D, Van Parijs L. Essential role for STAT5 signaling in CD25+CD4+ regulatory T cell homeostasis and the maintenance of self-tolerance. J Immunol. 2003;171:3435–3441. doi: 10.4049/jimmunol.171.7.3435. [DOI] [PubMed] [Google Scholar]
- 17.Huang Z, Chen L, Yang B, Chen J, Wang L. Decreased phosphostat5 contributed to impairment of CD4+CD25+Foxp3+ Tregs in RA patients. Ann Rheum Dis. 2016;75(Suppl 2):987. doi: 10.1136/annrheumdis-2016-eular.4760. [DOI] [Google Scholar]
- 18.Tong B, Yu J, Wang T, Dou Y, Wu X, Kong L, Dai Y, Xia Y. Sinomenine suppresses collagen-induced arthritis by reciprocal modulation of regulatory T cells and Th17 cells in gut-associated lymphoid tissues. Mol Immunol. 2015;65:94–103. doi: 10.1016/j.molimm.2015.01.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.