Abstract
Autism spectrum disorder is a common neurodevelopmental disorder with an unknown etiology. The correlation between neonatal jaundice and the risk of developing autism spectrum disorder was investigated previously. Some studies showed significant associations, whereas others demonstrated no association. In this meta-analysis, we pooled the results of observational studies to examine the association between neonatal jaundice and the risk of autism spectrum disorder among children. We identified all studies published through April 2018 by conducting a literature search using Web of Science, PubMed, and Scopus databases as well as the reference lists of the retrieved studies. The pooled odds ratios (ORs), rate ratio (RR), and their 95% confidence intervals (CIs) were calculated as random effect estimates of association among studies. We conducted a subgroup analysis to explore any potential sources of intergroup heterogeneity. The pooled estimates of OR and RR showed a considerable correlation between neonatal jaundice and ASD among children (OR, 1.35; 95% CI, 1.02–1.68) and (RR, 1.39; 95% CI, 1.05–1.74). A larger effect size was shown in the pooled estimated crude OR than in the adjusted OR (1.75 [0.96–2.54] vs. 1.19 [1.07–1.30]). This study showed that neonatal jaundice may be associated with ASD and may increase the risk of ASD among children.
Keywords: Autism spectrum disorder, Autism, Pre-eclampsia, Bilirubin, Neonatal Jaundice
Introduction
Autism spectrum disorders (ASDs), a group of neurodevelopmental disorders among children, are manifesting a dramatically increased prevalence in most societies [1]. Deficits in verbal and non-verbal communication, restricted and repetitive patterns of behavior, and impaired social preferences are observed among children with ASD [2]. While the etiology of ASD is unknown, evidence that genetic factors, environmental characteristics, and pregnancy complications such as preeclampsia and antenatal hemorrhage are involved in its pathogenesis [3-6].
Unconjugated hyperbilirubinemia (jaundice) is common in the first days after birth. Heme catabolism produces the bilirubin, a low level of which is beneficial and acts as an antioxidant agent; however, in higher concentrations, can be neurotoxic to neuronal cells in neonates [7]. The literature shows that the prevalence of jaundice is 50% and 80% in term and preterm neonates, respectively [8].
The risk of ASD in relation with neonatal jaundice has been investigated previously. Some studies showed a significant association [9-11], while some others demonstrated no association [12-14]. To date, only one meta-analysis examined the association between neonatal jaundice and the risk of ASD [15]. The authors reorted a significant correlation between neonatal jaundice and the risk of ASD among children (odds ratio [OR], 1.43; 95% confidence interval [CI], 1.22–1.67). However, that meta-analysis was limited to the PubMed and MEDLINE databases and included only studies published through 2009. Therefore, the current meta-analysis pooled all case-control and cohort studies within 3 databases to determine the risk of ASD in children in relation with neonatal jaundice.
Methods
1. Data sources
The present meta-analysis assessed the association between neonatal jaundice and the risk of ASD among children. We systematically searched for relevant studies using the published PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement checklist. We identified all studies published through April 2018 in the Web of Science, PubMed, and Scopus databases and manually checked the reference lists of the published manuscripts.
The protocol of this study with the code of 9703081248 was approved by Hamadan University of Medical Sciences.
2. Search strategy
The index terms used in the present meta-analysis included: (autism or ASDs) and (jaundice or hyperbilirubinemia or icterus) and (infants or neonates or neonatal). We searched studies regardless of language of publication.
3. Inclusion and exclusion criteria
In this systematic search, studies that evaluated the association between neonatal jaundice measured by total serum bilirubin (TSB) and the risk of ASD among children were included. Case reports, case series studies, reviews, letters to the editor, and multiple publications on the same material from the same author were excluded.
4. Data extraction
The titles were screened first, followed by the abstracts and full texts of the included studies by 2 authors (SK and EJ). The predesigned extraction form included publication year, first author, country, study design, ASD diagnostic criteria, number of participants, OR, rate ratio (RR) and the associated 95% CI, adjustment for confounders, child age (range), and study quality. The confounding variables related to the correlation between neonatal jaundice and the risk of ASD among children in the included studies were sex, maternal age, child’s birth year, birth season, prematurity, multiple gestation, birth order, and level of urbanization.
5. Quality assessment
We used the improved Newcastle-Ottawa Scale (NOS) to assess the quality of the included studies [16]: comparability using 2 items, election using 4 items, and exposure or outcomes using 3 items with scores of 0–9. The quality assessment of included studies was performed independently by 2 authors (SK and EJ). Finally, According to NOS scale, the studies were categorized as low quality (<7 points) and high quality (≥7 points).
6. Sensitivity analysis
We used a sequential algorithm to obtain the minimum desired I2 threshold (50%) [17]. Hence, we conducted 21 new meta-analyses in which one study for each was excluded from the estimations. The study was accountable for the greatest reduction in I2, and a new set of 1–21 studies was made. If by excluding 2 or more studies created the same decrease in the I2, the study with the greatest decrease in Q was excluded. The procedure was continued until the I2 diminished to the below of desired preset threshold. Finally, we revealed that a minimum I2 in one omitted study could result in the I2 falling below the desired threshold.
7. Statistical analysis
We calculated pooled OR, RR, and their 95% CI from eligible studies used as random effect estimates of association among studies. The subgroup analyses were performed according to crude and adjusted reporting of studies and preterm labor to identify potential sources of heterogeneity. To assess the degree of intergroup heterogeneity, the I2 statistic was used. According to the I2 statistic, low and high heterogeneity were considered at values of 25% and 75%, respectively.
The Begg and Egger’s regression model was used to examine publication bias [18]. The statistical analyses were performed using Stata 13 (Stata Corp., College Station, TX, USA), and values of P<0.05 were considered significant.
Results
1. Description of studies
A total of 1,008 studies were included in the initial search by searching the mentioned major international databases for studies published until April 2018 and manually reviewing the reference lists. A total of 287 duplicates were identified and eliminated; the title and abstract review identified and eliminated another 681 irrelevant studies. Accordingly, 40 studies were subjected to further screening. Of them, 19 studies did not meet our eligibility criteria or were not original articles. Ultimately, 21 articles were included in the meta-analysis. The selection process of the included studies is presented in Fig. 1. The meta-analysis included 5 cohort studies [19-23] and 16 case-control studies [9-14,24-33] with a total of 826,330 participants. All included studies were published in English.
Fig. 1.
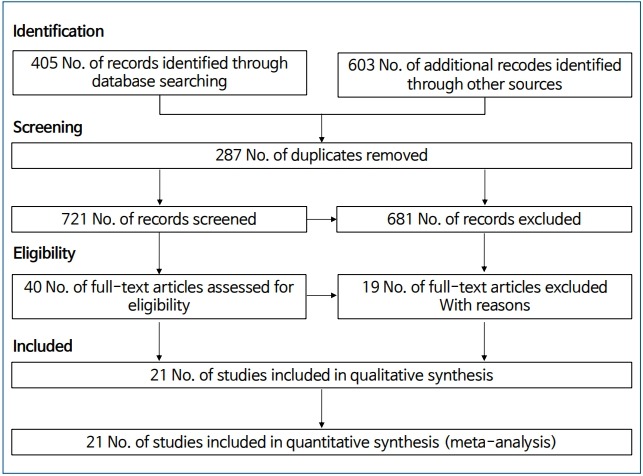
Diagram of studies through the different phases of the systematic review.
1) Main analysis
The correlation between jaundice in neonates and the risk of ASD among children is shown in a forest plot in Fig. 2. The pooled estimates of OR and RR showed a significant association between neonatal jaundice and the risk of ASD among children (OR, 1.35; 95% CI, 1.02–1.68; and RR, 1.39; 95% CI, 1.05–1.74), respectively. There was moderate heterogeneity among the studies reporting the risk of ASD among children based on OR (I2=52.5%, P=0.006). However, there was no heterogeneity in the studies reporting the risk of ASD among children based on RR (I2=0.0%, P=0.457).
Fig. 2.
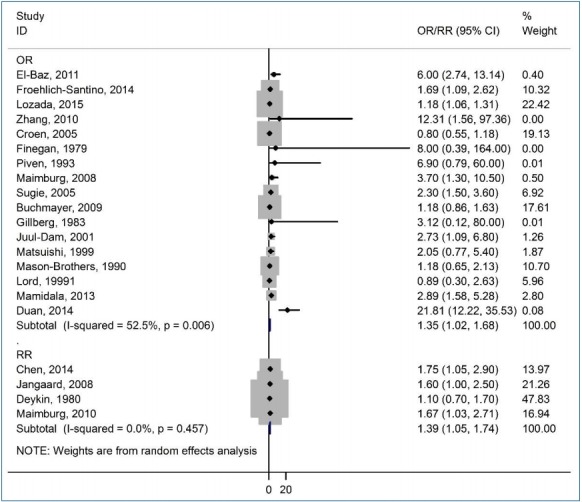
Forest plot of the association between jaundice and autism spectrum disorder among children. OR, odds ratio; RR, rate ratio; CI, confidence interval.
Fig. 3 shows asymmetry based on funnel plot shape. This asymmetry indicated evidence of no publication bias. The P values for Begg and Egger's regression were 0.090 and 0.056, respectively.
Fig. 3.
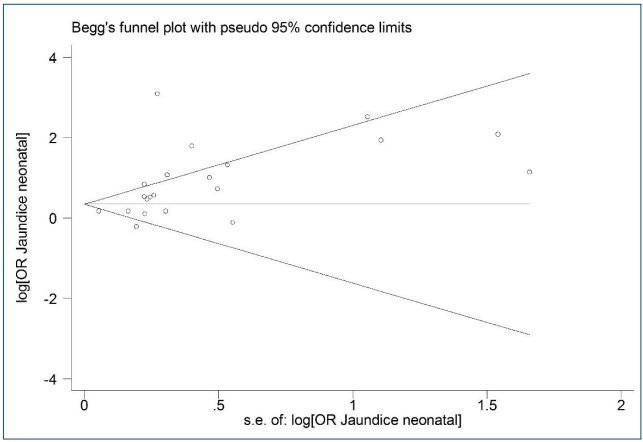
Funnel plot of the association between jaundice and autism spectrum disorder among children. s.e., standard error; OR, odds ratio.
2) Subgroup analysis
The subgroup analyses were conducted based on the adjusted form of studies and preterm labor. The OR of the crude and adjusted studies were 1.75 (95% CI, 0.96–2.54) and 1.19 (95% CI, 1.07–1.30), respectively. A significant association was found in the adjusted studies, and no heterogeneity was identified among them (Table 1). There was no significant association between neonatal jaundice and ASD in preterm neonates (OR, 0.89; 95% CI, 0.65–1.13). Most of the enrolled studies had determined both preterm (<37 weeks gestational age) and term neonates with jaundice, but only 4 studies [11,19,24,26] separately assessed the association between neonatal jaundice and ASD in preterm and term neonates (Table 2).
Table 1.
Results of subgroup analysis of neonatal jaundice and autism spectrum disorders
| Subgroup | Studies |
||
|---|---|---|---|
| No. of studies | OR (95% CI) | I2 | |
| Adjusted form (studies based on OR) | |||
| Crude analysis | 11 | 1.75 (0.96–2.54) | 64.9% |
| Adjusted analysis | 6 | 1.19 (1.07–1.30) | 0% |
| Gestational age | |||
| Preterm delivery | 4 | 0.89 (0.65–1.13) | 0% |
| Term delivery | 4 | 1.20 (1.08–1.32) | 0% |
OR, odds ratio; CI, confidence interval.
Table 2.
Summary results of the included studies
| Study | Country | Design | Sample size | Estimate | Adjustment | Degree of jaundice | Autism criteria | Gestational age (wk) | Child age range (yr) | Study quality |
|---|---|---|---|---|---|---|---|---|---|---|
| El-Baz, [9] 2011 | Egypt | Case-control | 300 | Odds ratio | Crude | No reported | DSM-4 | <37 | 2–13 | High |
| Froehlich-Santino, [21] 2014 | USA | Pros. cohort | 388 | Odds ratio | Crude | No reported | ADI-R; ADOS | No reported | 4–18 | High |
| Lozada, [11] 2015 | USA | Case-control | 11,668 | Odds ratio | Adjusted | No reported | ICD-9 | <37 | 3.8–7.2 | High |
| Zhang, [30] 2010 | China | Case-control | 19 | Odds ratio | Crude | No reported | ICD-10; CARS | 3–21 | 38–40 | High |
| Croen, [12] 2005 | USA | Case-control | 2,028 | Odds ratio | Crude | TSB > 15 | ICD | >35 | 4–7 | High |
| Chen, [19] 2014 | Taiwan | Pros. cohort | 10,080 | Hazard ratio | Adjusted | No reported | ICD-9 | 40 | 4–7 | High |
| Finegan, [32] 1979 | USA | Case-control | 46 | Odds ratio | Crude | TSB > 16 | Clinical | No reported | No reported | Low |
| Piven, [31] 1993 | USA | Case-control | 78 | Odds ratio | Adjusted | TSB > 20 | ADI; ADOS | 36–41 | 5–25 | High |
| Maimburg, [26] 2008 | Denmark | Case-control | 946 | Odds ratio | Adjusted | No reported | ICD | No reported | <10 | High |
| Jangaard, [22] 2008 | Canada | Pros. cohort | 55,671 | Rate ratio | Adjusted | TSB > 13.5 | Data linkage of the study cohort | >35 | <5 | High |
| Sugie, [29] 2005 | Japan | Case-control | 1789 | Odds ratio | Crude | Based on need for phototherapy | DSM | 38–40 | <3 | High |
| Buchmayer [36], 2009 | Sweden | Case-control | 6,776 | Odds ratio | Adjusted | ICD | No reported | <10 | High | |
| Deykin, [20] 1980 | USA | Cohort | 364 | Rate ratio | Adjusted | No reported | Clinical | No reported | 6 | High |
| Gillberg, [33] 1983 | Sweden | Case-control | 50 | Odds ratio | Crude | TSB>20 | Clinical | 36–41 | >7 | Low |
| Juul-Dam, [10] 2001 | USA | Case-control | 128 | Odds ratio | Crude | TSB>10 | ADI; ADOS | <37 | 2.5–4 | High |
| Matsuishi, [14] 1999 | Japan | Case-control | 232 | Odds ratio | Crude | No reported | DSM | No reported | 5–8 | High |
| Mason-Brothers, [28] 1990 | USA | Case-control | 280 | Odds ratio | Crude | No reported | DSM | No reported | 0–5 | High |
| Lord, [13] 1991 | USA | Case-control | 100 | Odds ratio | Crude | No reported | DSM | No reported | 6–10 | High |
| Mamidala, [27] 2013 | India | Case-control | 942 | Odds ratio | Adjusted | No reported | ICD-10; CARS; DSM | No reported | 2–10 | High |
| Maimburg, [23] 2010 | Denmark | Cohort | 733,826 | Hazard ratio | Adjusted | No reported | ICD-10 | 33–42 | No reported | High |
| Duan, [25] 2014 | China | Case-control | 572 | Odds ratio | Adjusted | No reported | CARS; DSM | >37 | 3–6 | High |
Adjusted to eliminate or reduce the effect of confounding variables on the association between neonatal jaundice and autism spectrum disorder among children.
DSM, Diagnostic and Statistical Manual of Mental Disorders; ADI-R, Autism Diagnostic Interview, Revised; ADOS, Autism Diagnostic Observation Schedule; ICD, International Classification of Diseases; CARS, Childhood Autism Rating Scales; ADOS, Autism Diagnostic Observation Schedule; Pros. Cohort, prospective cohort; Ret cohort, retrospective cohort.
3) Study quality
According to the NOS, only 2 of the included studies were categorized as low quality and others had high quality (Table 2).
2. Sensitivity analysis
We observed evidence for considerable intergroup heterogeneity. Thus, the sensitivity analysis was performed according to the sequential algorithm to gain interstudy homogeneity. We achieved the least desired I2 threshold (50%) by ignoring 2 studies [12,25] from the meta-analysis and the risk of ASD (OR, 1.31; 95% CI, 1.09–1.53; I2=10.9%).
Discussion
In the present meta-analysis, the existing evidence derived from epidemiological studies related to assessing the association between the risk of ASD and neonatal jaundice was summarized. In this meta-analysis, we included all case-control and cohort studies to assess the correlation between neonatal jaundice and the risk of ASD among children in 21 individual studies. Our results indicated a significant association between neonatal jaundice and the risk of ASD among children. The pooled estimate of the included studies reported that the OR and RR of ASD among children with neonatal jaundice were 1.35 and 1.39, respectively. There was no heterogeneity among the studies that estimated the risk of ASD among children based on RR, while there was moderate heterogeneity among the studies that estimated the risk of ASD based on OR.
Only one meta-analysis was conducted on the risk of ASD in relation to neonatal jaundice. In 2011, Amin et al. [15] showed that neonatal jaundice was a risk factor for ASD among children (OR, 1.43; 95% CI, 1.22–1.67). This meta-analysis was restricted to 13 studies published until 2009 retrieved from the PubMed and MEDLINE databases, whereas the present study included searches of the PubMed, Scopus, Web of Science databases up to April 2018 and included 21 studies.
Maimburg et al. [26] and Sugie et al. [29] reported a dose-response association between neonatal jaundice and the risk of ASD among children. Other studies reported that the TSB concentration consists of free or bound unconjugated and conjugated bilirubin. The free unconjugated bilirubin can be bound to protein, while freely unconjugated or unbound bilirubin is neurotoxic and can cross the intact blood brain barrier [34].
In 2014, Bhutani et al. [7] showed that bilirubin-induced neurologic dysfunction may manifest as muscle tone abnormalities, neuromotor abnormalities, neurobehavior manifestations, hyperexcitable reflexes in neonates, abnormalities in speech and language, and dysfunctions in central processing.
In the present meta-analysis, the correlation between neonatal jaundice and ASD in preterm neonates was not significant. In preterm neonates, the binding affinity may change because of several endogenous and exogenous competitors of bilirubin for albumin binding, while the protein concentration may be lower. Therefore, despite the low concentration of TSB, the concentration of free unconjugated bilirubin can be considerably higher. Thus, it may be necessary to measure free unconjugated bilirubin in preterm neonates rather than relying on TSB values alone. In contrast, for term neonates, the correlation between TSB and free unconjugated bilirubin concentrations is reportedly a sensitive marker for bilirubin-induced neurotoxicity. Therefore, jaundice in term neonates may be a risk factor for ASD [35].
The present study has some limitations: (1) in some studies, no adjustment was made for confounding factors; (2) data that were presented by included studies were insufficient for a subgroup analysis; (3) different diagnostic criteria for ASD were used and some studies did not use standardized diagnostic instruments; and (4) not all of the included studies mentioned a correlation between blood bilirubin concentration and ASD. Also, jaundice levels were not unified.
The above limitations might enhance the probability of selection bias. However, this meta-analysis evaluated the association between neonatal jaundice and ASD among 826,330 participants.
Neonatal jaundice may be associated with ASD in children. Despite existing controversy, our findings indicated that neonatal jaundice may increase the risk of ASD among children.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Getahun D, Fassett MJ, Peltier MR, Wing DA, Xiang AH, Chiu V, et al. Association of perinatal risk factors with autism spectrum disorder. Am J Perinatol. 2017;34:295–304. doi: 10.1055/s-0036-1597624. [DOI] [PubMed] [Google Scholar]
- 2.Bailey A, Phillips W, Rutter M. Autism: towards an integration of clinical, genetic, neuropsychological, and neurobiological perspectives. J Child Psychol Psychiatry. 1996;37:89–126. doi: 10.1111/j.1469-7610.1996.tb01381.x. [DOI] [PubMed] [Google Scholar]
- 3.Buchmayer S, Johansson S, Johansson A, Hultman CM, Sparén P, Cnattingius S. Can association between preterm birth and autism be explained by maternal or neonatal morbidity? Pediatrics. 2009;124:e817–25. doi: 10.1542/peds.2008-3582. [DOI] [PubMed] [Google Scholar]
- 4.Jenabi E, Karami M, Khazaei S, Bashirian S. The association between preeclampsia and autism spectrum disorders among children: a metaanalysis. Korean J Pediatr. 2019;62:126–30. doi: 10.3345/kjp.2018.07010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenabi E, Ataei S, Bashirian S. Evaluation of drug interventions for the treatment of sleep disorders in children with autism spectrum disorders: a systematic review. Korean J Pediatr. 2019;62:405–9. doi: 10.3345/kjp.2019.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenabi E, Bashirian S, Khazaei S. Is breech presentation associated with autism spectrum disorders among children: a meta-analysis. Adv Hum Biol. 2019;9:12–5. [Google Scholar]
- 7.Bhutani VK, Johnson-Hamerman L. The clinical syndrome of bilirubininduced neurologic dysfunction. Semin Fetal Neonatal Med. 2015;20:6–13. doi: 10.1016/j.siny.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Maisels MJ, McDonagh AF. Phototherapy for neonatal jaundice. N Engl J Med. 2008;358:920–8. doi: 10.1056/NEJMct0708376. [DOI] [PubMed] [Google Scholar]
- 9.El-Baz F, Ismael NA, El-Din SMN. Risk factors for autism: an Egyptian study. Egypt J Med Hum Genet. 2011;12:31–8. [Google Scholar]
- 10.Juul-Dam N, Townsend J, Courchesne E. Prenatal, perinatal, and neonatal factors in autism, pervasive developmental disorder-not otherwise specified, and the general population. Pediatrics. 2001;107:E63. doi: 10.1542/peds.107.4.e63. [DOI] [PubMed] [Google Scholar]
- 11.Lozada LE, Nylund CM, Gorman GH, Hisle-Gorman E, Erdie-Lalena CR, Kuehn D. Association of autism spectrum disorders with neonatal hyperbilirubinemia. Glob Pediatr Health. 2015;2:2333794. doi: 10.1177/2333794X15596518. X15596518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Croen LA, Yoshida CK, Odouli R, Newman TB. Neonatal hyperbilirubinemia and risk of autism spectrum disorders. Pediatrics. 2005;115:e135–8. doi: 10.1542/peds.2004-1870. [DOI] [PubMed] [Google Scholar]
- 13.Lord C, Mulloy C, Wendelboe M, Schopler E. Pre- and perinatal factors in high-functioning females and males with autism. J Autism Dev Disord. 1991;21:197–209. doi: 10.1007/BF02284760. [DOI] [PubMed] [Google Scholar]
- 14.Matsuishi T, Yamashita Y, Ohtani Y, Ornitz E, Kuriya N, Murakami Y, et al. Brief report: incidence of and risk factors for autistic disorder in neonatal intensive care unit survivors. J Autism Dev Disord. 1999;29:161–6. doi: 10.1023/a:1023048812202. [DOI] [PubMed] [Google Scholar]
- 15.Amin SB, Smith T, Wang H. Is neonatal jaundice associated with Autism Spectrum Disorders: a systematic review. J Autism Dev Disord. 2011;41:1455–63. doi: 10.1007/s10803-010-1169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. Ottawa (ON): Ottawa Hospital Research Institute; 2009. Sep 15, The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet] [cited 2019 Jul 1]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 17.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of betweenstudy heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37:1148–57. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen MH, Su TP, Chen YS, Hsu JW, Huang KL, Chang WH, et al. Is neonatal jaundice associated with autism spectrum disorder, attention deficit hyperactivity disorder, and other psychological development? A nationwide prospective study. Res Autism Spectr Disord. 2014;8:625–32. [Google Scholar]
- 20.Deykin EY, MacMahon B. Pregnancy, delivery, and neonatal complications among autistic children. Am J Dis Child. 1980;134:860–4. doi: 10.1001/archpedi.1980.02130210044012. [DOI] [PubMed] [Google Scholar]
- 21.Froehlich-Santino W, Londono Tobon A, Cleveland S, Torres A, Phillips J, Cohen B, et al. Prenatal and perinatal risk factors in a twin study of autism spectrum disorders. J Psychiatr Res. 2014;54:100–8. doi: 10.1016/j.jpsychires.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jangaard KA, Fell DB, Dodds L, Allen AC. Outcomes in a population of healthy term and near-term infants with serum bilirubin levels of >or=325 micromol/L (>or=19 mg/dL) who were born in Nova Scotia, Canada, between 1994 and 2000. Pediatrics. 2008;122:119–24. doi: 10.1542/peds.2007-0967. [DOI] [PubMed] [Google Scholar]
- 23.Maimburg RD, Bech BH, Vaeth M, Møller-Madsen B, Olsen J. Neonatal jaundice, autism, and other disorders of psychological development. Pediatrics. 2010;126:872–8. doi: 10.1542/peds.2010-0052. [DOI] [PubMed] [Google Scholar]
- 24.Buchmayer S, Johansson S, Johansson A, Hultman CM, Sparén P, Cnattingius S. Can association between preterm birth and autism be explained by maternal or neonatal morbidity? Pediatrics. 2009;124:e817–25. doi: 10.1542/peds.2008-3582. [DOI] [PubMed] [Google Scholar]
- 25.Duan G, Yao M, Ma Y, Zhang W. Perinatal and background risk factors for childhood autism in central China. Psychiatry Res. 2014;220:410–7. doi: 10.1016/j.psychres.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 26.Maimburg RD, Vaeth M, Schendel DE, Bech BH, Olsen J, Thorsen P. Neonatal jaundice: a risk factor for infantile autism? Paediatr Perinat Epidemiol. 2008;22:562–8. doi: 10.1111/j.1365-3016.2008.00973.x. [DOI] [PubMed] [Google Scholar]
- 27.Mamidala MP, Polinedi A, P T V PK, Rajesh N, Vallamkonda OR, Udani V, et al. Prenatal, perinatal and neonatal risk factors of Autism Spectrum Disorder: a comprehensive epidemiological assessment from India. Res Dev Disabil. 2013;34:3004–13. doi: 10.1016/j.ridd.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 28.Mason-Brothers A, Ritvo ER, Pingree C, Petersen PB, Jenson WR, McMahon WM, et al. The UCLA-University of Utah epidemiologic survey of autism: prenatal, perinatal, and postnatal factors. Pediatrics. 1990;86:514–9. [PubMed] [Google Scholar]
- 29.Sugie Y, Sugie H, Fukuda T, Ito M. Neonatal factors in infants with Autistic Disorder and typically developing infants. Autism. 2005;9:487–94. doi: 10.1177/1362361305057877. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Lv CC, Tian J, Miao RJ, Xi W, Hertz-Picciotto I, et al. Prenatal and perinatal risk factors for autism in China. J Autism Dev Disord. 2010;40:1311–21. doi: 10.1007/s10803-010-0992-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piven J, Simon J, Chase GA, Wzorek M, Landa R, Gayle J, et al. The etiology of autism: pre-, peri- and neonatal factors. J Am Acad Child Adolesc Psychiatry. 1993;32:1256–63. doi: 10.1097/00004583-199311000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Finegan JA, Quarrington B. Pre-, peri-, and neonatal factors and infantile autism. J Child Psychol Psychiatry. 1979;20:119–28. doi: 10.1111/j.1469-7610.1979.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 33.Gillberg C, Gillberg IC. Infantile autism: a total population study of reduced optimality in the pre-, peri-, and neonatal period. J Autism Dev Disord. 1983;13:153–66. doi: 10.1007/BF01531816. [DOI] [PubMed] [Google Scholar]
- 34.Amin SB, Ahlfors C, Orlando MS, Dalzell LE, Merle KS, Guillet R. Bilirubin and serial auditory brainstem responses in premature infants. Pediatrics. 2001;107:664–70. doi: 10.1542/peds.107.4.664. [DOI] [PubMed] [Google Scholar]
- 35.Amin SB. Clinical assessment of bilirubin-induced neurotoxicity in premature infants. Semin Perinatol. 2004;28:340–7. doi: 10.1053/j.semperi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Buchmayer S, Johansson S, Johansson A, Hultman CM, Sparén P, Cnattingius S. Can association between preterm birth and autism be explained by maternal or neonatal morbidity? Pediatrics. 2009;124(5):e817–25. doi: 10.1542/peds.2008-3582. [DOI] [PubMed] [Google Scholar]


