Abstract
Background:
To assess clinical and epidemiological trends of severe sepsis.
Methods:
Ecological study of patients presenting to the emergency department with severe sepsis or septic shock between 2005 and 2013. Patients were identified using the statewide hospital administrative database. Key outcomes included incidence rates (IR) and mortality rates (per 1,000 population) by age and medically underserved areas (MUA), sepsis case fatality rate (deaths per 100 sepsis cases), and proportions of transfer and co-morbidities.
Results:
There were 154,019 sepsis cases identified. In 2005, 85+ yo in non-MUAs had a 44% increase in IR compared to those in MUAs, and this difference rose to 74% by 2013. Mortality rates were 1.6 [95%CI: 1.3, 1.8] times greater among 85+yo in non-MUAs. Mortality rates increased by 1.8% annually, while the sepsis case fatality rate decreased by 7.7%. The proportion of transfer among sepsis cases decreased by 2.1% per year (3.8% in non-MUA, 0.7% in MUAs).
Conclusions:
Sepsis incidence varies geographically, and access to health care is one proposed mechanism that may explain heterogeneity. Over time, we may be capturing higher acuity sepsis cases with better recognition and management, as well as observing differential diagnostic coding documentation by location.
Keywords: Epidemiology of aging, geography, inequalities, mortality, infection
INTRODUCTION
One in four severe sepsis cases results in death, and each year approximately 300 per 100,000 persons are diagnosed with severe sepsis.1 Across several studies, three key trends have been observed: 1) the incidence of severe sepsis has been increasing, 2) population rates of mortality due to sepsis have been steadily increasing, and 3) sepsis case fatality rates have been decreasing.1–4 Older individuals (>65 years) and those with medical co-morbidities have the highest incidence of sepsis, and geography has been proposed to impact incidence and severity. One marker of local health system capacity is medically underserved area (MUA) status. MUAs often have fewer healthcare providers per capita, a higher population below the federal poverty level, more of the population over 65 years, the higher infant mortality rates.5 A recent study showed that those living in MUAs experienced higher sepsis incidence and mortality, but this study was a one-year analysis, serving as a point prevalence of the impact of living in medically underserved areas on sepsis epidemiology.6
The purpose of this study was 1) to measure trends in sepsis incidence and mortality, and 2) to evaluate whether disparities in these outcomes varied over time by demographic and geographical factors between 2005 and 2013.
MATERIAL AND METHODS
Study design, data sources, and patient identification
This study was a retrospective analysis of patients treated for severe sepsis or septic shock in Iowa emergency departments (ED) between January 1, 2005 and December 31, 2013. Patients were identified from the Iowa Hospital Association inpatient and outpatient administrative data sets using a definition for severe sepsis commonly used in the literature using International Classification of Disease, 9th Edition, Clinical Modification (ICD-9-CM) diagnosis criteria for infection and organ failure. Records were restricted to patients who resided in the state. To match cases across inter-hospital transfer, ED records for patients with a discharge diagnosis consistent with transfer were matched to inpatient records using a probabilistic linkage algorithm that incorporated date of birth, sex, patient zip code, county of residence and date of visit. A one-day window for linkage of visits was allowed to account for patients whose transfers may have spanned overnight. Patients who were identified as transferred but for whom no linkage was identified were not assessed for clinical outcomes (i.e. mortality measures). The study population and identification of transfers has been previously described.7 This study was approved by the local Institutional Review Board under waiver of informed consent (Protocol ID# 201409761), and the manuscript is reported in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.8
We used the patient’s county of residence to determine MUA status, defined by the Health Resources & Services Administration Data Center.9 MUAs are evaluated by the Index of Medically Underserved (IMU) Score and can range from 0 to 100, where zero represents the completely underserved. MUAs are designated when a township has an Index of Medically Underserved score equal to or below 62. For this study, any county that contains a MUA designation was defined as an “MUA County.” We categorized age as <40 years, 40–64 years, 65–84 years, and 85+ years to be consistent with aggregating census data to determine rates. Comorbidities were identified using the Elixhauser method, a widely used technique for measuring patient comorbidity through administrative datasets ICD-9-CM codes.10 Data on population classified by age and sex by county were obtained from U.S. Census Bureau Estimates.11
Outcomes
The primary outcomes for this study were measures of sepsis incidence and mortality. Specifically, the annual sepsis incidence rate (IR) was measured as the number of sepsis cases per 1,000 population. We defined sepsis mortality as an overall annual hospital mortality rate in patients who died in hospitals indexed at the population level (deaths per 1,000 population), and as sepsis case fatality rate (proportion of diagnosed sepsis cases resulting in death). As a secondary outcome, we evaluated the proportions of transferred patients and select morbidities per year.
Data analysis
Sepsis IRs were measured for each age group and compared across MUA status and time. Rates were estimated using generalized estimating equations with log link and Poisson distribution, clustered on age group within a county. Least square mean estimates were used to calculate incidence, mortality rates, percent mortality, transfer, and co-morbidities. We compared estimates across age groups and MUA status through incident rate ratios. Temporal trends were assessed as annual changes for all rates and proportions, deaths and transfers among sepsis cases. All analyses were conducted using SAS software (Version 9.4, SAS Institute, Cary, NC).
RESULTS
Over the study period, 154,019 records were identified with severe sepsis through the statewide hospital administrative database. Less than three percent of the records were excluded from the study as they were transfers with no appropriate linkage to an inpatient stay. Approximately half of records identified (46.8%) were among 65–84 year olds (yo), followed by 26.6% among 40–64 yo, 20.8% among ≥85 yo, and 5.9% among <40 yo.
Incidence Rates
Statewide IR over the nine-year study period was 7.07 [95%CI: 5.34–9.26] per 1,000 population, ranged from 0.66 [95%CI: 0.60, 0.71] in those <40 years to 48.96 [95%CI: 43.07, 55.65] among 85+ yo [Table 1]. The annual IR of sepsis increased by 9% [95% CI: 1.08, 1.10] each year, primarily among 85+ yo [Figure 1]. While the rate of change per year did not vary by MUA at the population level [Figure 2], non-MUA rates were greater than MUA rates across most age groups. For example, there was a 9% increase per year in sepsis incidence in both regions, but the IR in non-MUAs was 31% [95%CI: 1.11, 1.55] greater among those 40–64 yo, 48% [95%CI: 1.24, 1.76] greater among those 65–84 yo, and 81% [95%CI: 1.48, 2.22] greater among 85+ yo compared to MUAs.
Table 1.
Sepsis Incidence Rates, Mortality Rates, and Case Fatality Rates in Iowa, 2005–2013
| Characteristic | Incidence Rate (1,000 population) | Mortality Rate (per 1,000 population) | Case Fatality Rate (deaths per 100 sepsis cases) | |||
|---|---|---|---|---|---|---|
| IR | 95% CI | IR | 95% CI | IR | 95% CI | |
| Overall | 7.07 | 5.34, 9.26 | 0.56 | 0.56, 0.56 | 9.96 | 9.95, 9.98 |
| Age Category | ||||||
| < 40 years | 0.66 | 0.60, 0.71 | 0.03 | 0.03, 0.04 | 5.37 | 4.87, 5.92 |
| 40–64 years | 4.73 | 4.25, 5.27 | 0.39 | 0.35, 0.44 | 8.65 | 8.16, 9.17 |
| 65–84 years | 21.99 | 19.69, 24.56 | 2.20 | 1.97, 2.46 | 10.40 | 9.78, 11.06 |
| 85+ years | 48.96 | 43.07, 55.65 | 5.27 | 4.70, 5.91 | 10.95 | 10.31, 11.64 |
| Medically Underserved | ||||||
| Yes | 5.83 | 4.85, 7.01 | 0.55 | 0.45, 0.66 | 10.00 | 9.61, 10.41 |
| No | 7.93 | 5.21, 12.08 | 0.57 | 0.36, 0.89 | 9.15 | 8.53, 9.82 |
| Year | ||||||
| 2005 | 3.82 | 2.85, 5.14 | 0.48 | 0.37, 0.64 | 14.96 | 14.03, 14.95 |
| 2006 | 4.40 | 3.27, 5.90 | 0.50 | 0.38, 0.67 | 13.35 | 12.58, 14.17 |
| 2007 | 5.14 | 3.85, 6.86 | 0.55 | 0.41, 0.74 | 12.36 | 11.58, 13.19 |
| 2008 | 5.98 | 4.50, 7.96 | 0.57 | 0.43, 0.75 | 10.86 | 10.26, 11.49 |
| 2009 | 6.56 | 4.96, 8.68 | 0.57 | 0.44, 0.74 | 9.82 | 9.24, 10.43 |
| 2010 | 7.03 | 5.28, 9.36 | 0.54 | 0.40, 0.72 | 8.69 | 8.18, 9.23 |
| 2011 | 7.50 | 5.63, 9.99 | 0.56 | 0.42, 0.74 | 8.41 | 7.92, 8.92 |
| 2012 | 7.94 | 5.98, 10.54 | 0.56 | 0.42, 0.75 | 7.95 | 7.42, 8.53 |
| 2013 | 8.02 | 6.07, 10.59 | 0.59 | 0.44, 0.78 | 8.27 | 7.69, 8.91 |
Figure 1. Sepsis Incidence Rates, Mortality Rates, and Case Fatality Rates in Iowa, 2005–2013.
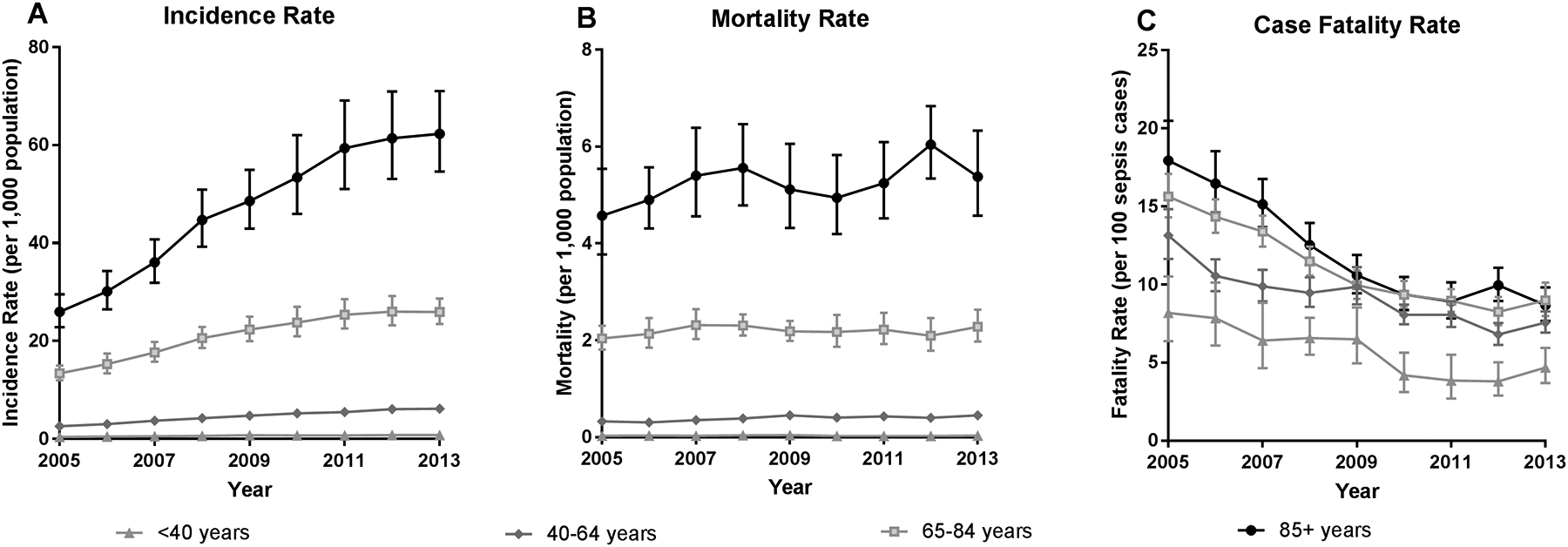
A) Presents the incidence rate per 1,000 population for each age group. B) Presents the mortality rate per 1,000 population for each age group. C) Presents sepsis case fatality rates (i.e. deaths per 100 sepsis cases).
Figure 2. Mortality Rates of Sepsis (per 1,000 Population) by Medically Underserved Area Status, 2005–2013.
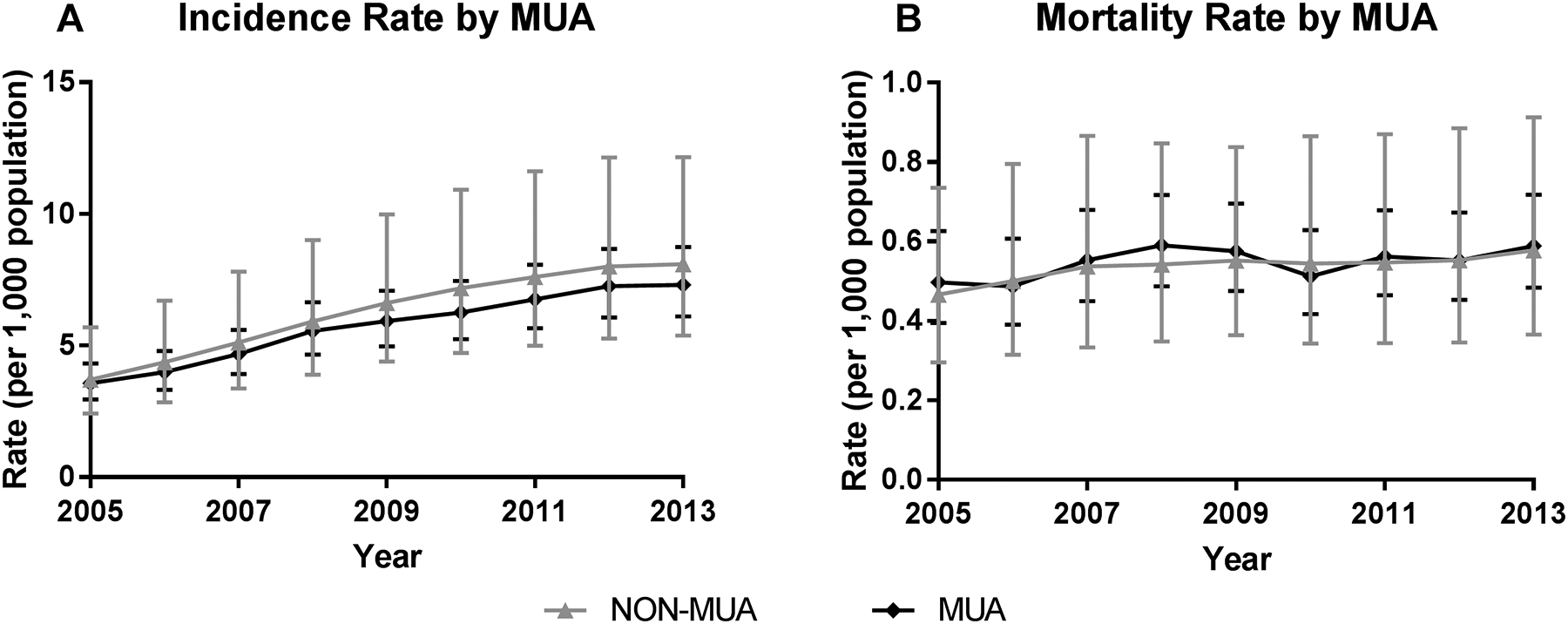
A) Incidence rate (sepsis cases per 1,000 population) and B) Mortality rate (deaths per 1,000 population) by county type. MUA = medically underserved area; Non-MUA = non-medically underserved area.
Mortality Rates
Mortality rates by age, MUA status, and year are presented in Table 1. Overall, mortality rates increased by 1.8% [95%CI: 1.01, 1.03] per year, though this change was driven by an increase among 40–64 yo. There was no change over time in mortality rate for other age groups or by MUA, as rates remained stable at the population level [Figure 2]. Mortality rates varied by MUA status for older age groups; the mortality rate was 1.26 [95%CI: 1.05, 1.51] times greater in 40–64 yo, 1.39 [95%CI: 1.17, 1.64] times greater among 65–84 yo, and 1.57 [95%CI: 1.35, 1.83] times greater among 85+ yo in non-MUA counties compared to MUA counties [Figure 1].
Sepsis Case Fatality Rates
When considering case fatality rates, there was a 7.7% [95%CI: 0.91, 0.93] decrease in the proportion per year, with up to a 9.2% [95%CI: 0.88, 0.94] decrease among those <40 yo. Compared to 2005, there was a 51% decreased in the case fatality rate among 85+ yo and a 43% decrease in the other age groups by 2013. Case fatality rates did not vary by MUA status within any age group.
Transfer and Co-morbidities
Transfer among sepsis cases decreased by 2.1% [95%CI: 0.97, 0.99] per year, with a 3.8% [95%CI: 0.93, 0.99] and 0.7% [95%CI: 0.98, 1.00] decrease per year in non-MUAs and MUAs, respectively (data not shown). Overall, 35.0% of those in MUAs were transferred compared to 7.8% in non-MUAs. Of the co-morbidities investigated, the most frequently identified comorbidities included all deficiency anemias (n=43,853; 28.5%), congestive heart failure (n=30,758; 20.0%), chronic pulmonary disease (n=41,027; 26.6%), diabetes with chronic complications (n=65,576; 42.6%), hypertension (n=76,830; 49.9%), and renal failure (n=38,082; 24.7%). The proportions of these comorbidities documented among sepsis cases increased over time [Figure 3], most notably in hypertension, which increased by 41%. With the exception of depression, there was no difference in the proportion of sepsis cases with each co-morbidity by MUA status.
Figure 3. Proportion of Co-morbidities among Patients with Sepsis.

Number of cases with each co-morbidity per 100 sepsis cases. CHF = coronary heart failure.
DISCUSSION
In this study, we identified several important epidemiological trends in sepsis care and management over a nine-year study period. First, we noted the rise in sepsis incidence at the population level over time. Specifically, the age-adjusted rate went from 3.8 to 8.0 per 1,000 population between 2005 and 2013. Second, we observed that the sepsis case fatality rates decreased during this study period, from 15.0% in 2005 to 8.3% in 2013. Third, sepsis mortality rates at the population level remained stable over the nine years with no significant difference.
There may be several public health and clinical explanations for these trends. In addressing the first primary finding, we found that the increasing IR over time varied by demographic and geographical factors. For example, we observed that the increase was primarily driven by 85+ yo in non-MUAs, and that the gap between MUA and non-MUA grew over the years. These findings are different from a previous study by Goodwin, et al., where those in MUAs experienced a higher incidence rate of admission with severe sepsis and higher in-hospital mortality.6 However, the findings between the studies may not be comparable due to the vast differences in the population demographics between the two states examined.
Other public health-related explanations include potential change in migration patterns with aging. The findings from this study conflict with several others that have identified that the burden of mental and physical illness is greater in rural or medically underserved areas compared to more urban or urban-adjacent areas.12–16 It is plausible that the sicker, aging population is moving towards the cities where more advanced medical care is available.17 If this is true, this may indicate that health care needs are not adequately provided in more rural areas or MUAs, thereby driving people to move toward locations where appropriate care exists. A review of state county-level census data for the 85+ yo population provides some evidence of this between 2005 and 2013; overall, 42% of MUAs experienced a decline in the 85+ yo population, while 91% of non-MUA counties experienced an increase. Furthermore, 27% of non-MUA counties experienced over a 20% increase in the 85+ yo population in the study period.
Several changes in clinical practice may also be influencing better outcomes. There have been significant improvements in sepsis recognition and management, leading to earlier administration of therapeutics and resulting in better patient outcomes.18 The Surviving Sepsis Campaign first published sepsis treatment guidelines in 2004, and initiatives by private foundations, professional organizations, and government entities increased hospital-based screening programs and increased use of treatment guidelines since.19–20 The National Quality Forum published the first sepsis quality metric in 2008, which was adopted as a composite measure by the Centers for Medicare and Medicaid Services for the Hospital Inpatient Quality Reporting System in 2015. Each initiative was designed to improve early sepsis recognition and timely administration of antibiotic therapy, hemodynamic resuscitation, and appropriate early monitoring.
There are certain limitations to this study. It is limited to one state that may not be representative of other states in terms of demographics and overall health behaviors and wellness. Despite this, data from this state captures a variety of settings (urban, suburban, and rural regions), as well as hospital types and facilities throughout the state. Furthermore, as we are relying on a statewide administrative database and diagnostic coding, we can anticipate some under-reporting, under-coding of severity, or misclassification of sepsis cases and co-morbidities that were documented.21,22 It was not possible to determine whether medical management and therapies are related to the decline in mortality given sepsis in this study, as these data were not available. Similarly, socioeconomic status (SES) is associated with MUA status and affects outcomes, and may confound this relationship and/or serve as an effect modifier (whereby outcomes vary by MUA status differentially across SES levels); however, at the patient-level we did not have access to SES levels, and therefore could not adjust for this factor in our analysis.
These limitations aside, there are some key questions or considerations this study highlights in shifting patterns of severe sepsis epidemiology and management. Despite improvements in management, more timely recognition of sepsis, and more investment of financial resources in sepsis care, we have to ask why there are no differences in population-level mortality? There may be several explanations for the observed pattern. First, this could be driven by an increase in illness severity and improved case fatality rates proportions over time. Perhaps the population was getting sicker as indicated with the rising incidence rate and proportion of co-morbidities documented. The earlier recognition, improved treatments, and financial resources that are allocated for severe sepsis management may be effective at offsetting this increasing illness burden in our society. As a result, this would drive the case fatality rate down while keeping population-level mortality stable over time.
Second, the concurrent rising incidence rates with stable population level mortality may be due to a level of ascertainment bias that varies differentially by MUA status; case identification may be different between hospitals in larger metropolitan areas compared to rural or critical access care hospitals. For example, prospective payment system hospitals are reimbursed through a diagnostic-related group based system,23,24 whereby identifying comorbidities and severe diagnoses will significantly impact reimbursement. This explanation is further supported by the proportion of several co-morbidities that also rose over time. In contrast, smaller rural hospitals or critical access hospitals that are often in MUAs may have fewer financial incentives with reimbursements to document more severe diagnoses in patients.
Third, as it further relates to ascertainment bias, larger facilities that are incentivized to increase accurate coding for reimbursement may also have chart-based auditing of hospital billing data. As a result, coding practices may differ by location and availability of billing auditors and will be captured in the hospital administrative databases. If this is accurate, then even small changes in real population-adjusted sepsis mortality could be exaggerated strictly through increased recognition and coding more severe illness in administrative datasets. This is also reflected in our data, as we can see the higher rate of sepsis diagnoses in non-MUA counties as well as the rapid change in the elderly populations within these counties compared to MUA counties.
As it relates to administrative data, this presents a unique challenge in sepsis research, as sepsis diagnosis is captured and defined by the type of administrative codes. We may ultimately only know the burden of sepsis at the population level by the quality of data used for hospital billing and reimbursement. This finding has important ramifications for using administrative data for tracking the burden of disease on society, because financial pressures may actually be contributing to case-finding, and those pressures may be magnified or suppressed based on rurality and health system structure. In the future, studies should also focus on delineating the extent to which severe sepsis incidence and outcomes are impacted by coding practices due to hospital-level characteristics as well as the contributions of improved clinical management.
CONCLUSIONS
Overall, this study demonstrates the shifting patterns of sepsis epidemiology through a statewide administrative healthcare database. Although the sepsis case mortality rates are declining, population mortality rate is largely unchanged over time. These may be driven by public health and demographic factors, as well as changes in practice associated with clinical documentation and management of severe sepsis patients that vary by geographical measures such as MUAs.
Thumbnail sketch.
What is already known on this subject?
One in four severe sepsis cases results in death, and each year approximately 300 per 100,000 persons are diagnosed with severe sepsis
What does this study add?
Sepsis incidence increased, sepsis case fatality rates and transfer proportions decreased, and population-level mortality rates remained fairly stable.
Sepsis incidence rates increased most among adults 85+ years in non-medically underserved areas.
While earlier recognition, improved treatments, and financial resources that are allocated for severe sepsis management may be effective at offsetting sepsis case fatality, there is potential ascertainment bias in more urban areas.
ACKNOWLEDMENTS
This work was supported by the Emergency Medicine Foundation, the University of Iowa Department of Emergency Medicine, and the University of Iowa Institute for Clinical and Translational Science.
Abbreviations:
- MUA
medically underserved area
- IR
incidence rate
- ED
emergency department
Footnotes
Competing Interest: None declared.
Conflicts: The authors have no conflicts to report.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29(7):1303–10. [DOI] [PubMed] [Google Scholar]
- 2.Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013;41(5):1167–74. doi: 10.1097/CCM.0b013e31827c09f8 [DOI] [PubMed] [Google Scholar]
- 3.Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med 2007;35(5):1244–50. doi: 10.1097/01.CCM.0000261890.41311.E9 [DOI] [PubMed] [Google Scholar]
- 4.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003;348(16):1546–54. doi: 10.1056/NEJMoa022139 [DOI] [PubMed] [Google Scholar]
- 5.Health Resources & Services Administration. Medically Underserved Areas and Populations (MUA/Ps) 2016. [Available from: https://bhw.hrsa.gov/shortage-designation/muap accessed March 21, 2017.
- 6.Goodwin AJ, Nadig NR, McElligott JT, et al. Where You Live Matters: The Impact of Place of Residence on Severe Sepsis Incidence and Mortality. Chest 2016;150(4):829–36. doi: 10.1016/j.chest.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohr NM, Harland KK, Shane DM, et al. Inter-hospital transfer is associated with increased mortality and costs in severe sepsis and septic shock: An instrumental variables approach. J Crit Care 2016;36:187–94. doi: 10.1016/j.jcrc.2016.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370(9596):1453–57. doi: Doi 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 9.Health Resources & Services Administration. MUA Find 2017. [Available from: https://datawarehouse.hrsa.gov/tools/analyzers/muafind.aspx accessed July 15, 2016.
- 10.Elixhauser AME. Clinical Classifications Software (CCS) for ICD-9-CM [Available from: https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp accessed June 15, 2015.
- 11.Population Division U.S. Census Bureau. Population and Housing Unit Estimates. [updated June 23, 2016. Available from: https://www.census.gov/programs-surveys/popest.html accessed 1/17/2017.
- 12.Singh GK, Azuine RE, Siahpush M, et al. All-cause and cause-specific mortality among US youth: socioeconomic and rural-urban disparities and international patterns. J Urban Health 2013;90(3):388–405. doi: 10.1007/s11524-012-9744-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh GK, Siahpush M. Widening rural-urban disparities in life expectancy, U.S., 1969–2009. Am J Prev Med 2014;46(2):e19–29. doi: 10.1016/j.amepre.2013.10.017 [DOI] [PubMed] [Google Scholar]
- 14.Baernholdt M, Yan G, Hinton I, et al. Quality of life in rural and urban adults 65 years and older: findings from the National Health and Nutrition Examination survey. J Rural Health 2012;28(4):339–47. doi: 10.1111/j.1748-0361.2011.00403.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eberhardt MS, Pamuk ER. The importance of place of residence: examining health in rural and nonrural areas. Am J Public Health 2004;94(10):1682–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartley D. Rural health disparities, population health, and rural culture. Am J Public Health 2004;94(10):1675–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hillier SM B G. Aging, the Individual, and Society. 10th Edition ed. Stamford: Cengage Learning; 2014. [Google Scholar]
- 18.Ferrer R, Artigas A, Suarez D, et al. Effectiveness of treatments for severe sepsis: a prospective, multicenter, observational study. Am J Respir Crit Care Med 2009;180(9):861–6. doi: 10.1164/rccm.200812-1912OC [DOI] [PubMed] [Google Scholar]
- 19.Levy MM, Rhodes A, Phillips GS, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med 2015;43(1):3–12. doi: 10.1097/ccm.0000000000000723 [published Online First: 2014/10/03] [DOI] [PubMed] [Google Scholar]
- 20.van Zanten AR, Brinkman S, Arbous MS, et al. Guideline bundles adherence and mortality in severe sepsis and septic shock. Crit Care Med 2014;42(8):1890–8. doi: 10.1097/ccm.0000000000000297 [published Online First: 2014/03/29] [DOI] [PubMed] [Google Scholar]
- 21.Whittaker SA, Mikkelsen ME, Gaieski DF, et al. Severe sepsis cohorts derived from claims-based strategies appear to be biased toward a more severely ill patient population. Crit Care Med 2013;41(4):945–53. doi: 10.1097/CCM.0b013e31827466f1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jolley RJ, Sawka KJ, Yergens DW, et al. Validity of administrative data in recording sepsis: a systematic review. Crit Care 2015;19:139. doi: 10.1186/s13054-015-0847-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Department of Health and Human Services. Acute Care Hospital Inpatient Prospective Payment System 2016. [Available from: https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/AcutePaymtSysfctsht.pdf accessed 9/21/2017 2017.
- 24.Centers for Medicare & Medicaid Services. Acute Inpatient PPS 2017. [Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/index.html accessed 9/21/2017.


