Abstract
Feline herpesvirus type 1 (FHV-1) is a widespread cause of respiratory and ocular disease in domestic cats. A spectrum of disease severity is observed in host animals, but there has been limited prior investigation into viral genome factors which could be responsible. Stocks of FHV-1 were established from oropharyngeal swabs obtained from twenty-five cats with signs of infection housed in eight animal shelters around the USA. A standardized numerical host clinical disease severity scoring scheme was used for each cat from which an isolate was obtained. Illumina MiSeq was used to sequence the genome of each isolate. Genomic homogeneity among isolates was relatively high. A general linear model for fixed effects determined that only two synonymous single nucleotide polymorphisms across two genes (UL37/39) in the same isolate (from one host animal with a low disease severity score) were significantly associated (p ≤ 0.05) with assigned host respiratory and total disease severity score. No variants in any isolate were found to be significantly associated with assigned host ocular disease severity score. A concurrent analysis of missense mutations among the viral isolates identified three genes as being primarily involved in the observed genomic variation, but none were significantly associated with host disease severity scores. An ancestral state likelihood reconstruction was performed and determined that there was no evidence of a connection between host disease severity score and viral evolutionary state. We conclude from our results that the spectrum of host disease severity observed with FHV-1 is unlikely to be primarily related to viral genomic variations, and is instead due to host response and/or other factors.
Keywords: Feline herpesvirus, Virulence, Herpes, Host response, Disease severity, Cat
Introduction
Feline herpesvirus type 1 (order Herpesvirales, family Herpesviridae, genus Varicellovirus, species Felid alphaherpesvirus 1, FHV-1) is a widespread and common cause of upper respiratory and ocular disease in cats [1]. Serological studies indicate that up to 97% of cats have been exposed to the virus [2], 80% will become persistently infected following exposure and 45% will continue to intermittently shed the pathogen [3]. The initial clinical signs of FHV-1 in feline hosts are conjunctivitis, keratitis and upper respiratory disease. This phase is often self-limiting but can result in permanent sequelae such as corneal scarring, symblepharon formation and blindness [4]. There are several vaccines currently available for FHV-1, but no currently available vaccine can prevent infection or the chronic, carrier state [1] and FHV-1-associated clinical disease remains highly prevalent in both privately owned pets and shelter cats. Although recently there have been advances in treatment of FHV-1 with antivirals (notably oral famciclovir) [5, 6], clinical disease remains a significant issue in feline populations.
Ocular and respiratory disease in cats affected with FHV-1 can vary markedly in severity [4, 7]. Some animals will be affected by mild ocular discharge and blepharospasm and/or occasional sneezing while others will demonstrate severe ocular lesions such as corneal ulceration and blindness with concurrent marked nasal discharge, lethargy and anorexia [1]. Limited prior studies have found conflicting evidence that different viral isolates of FHV-1 induce very similar [8] or quite different [9] disease severities in host animals. In both cases, a genome-based assessment of the experimental FHV-1 isolates used was not performed. A recent genomic and phylogenetic analysis of FHV-1 isolates from the USA (most of which are included in the present study) and Australia demonstrated that there is low world-wide intra-species genomic variation for this virus [10]. In a recent genomic assessment of the available sequences of the varicelloviruses, FHV-1 was found to have one of the lowest degrees of intra-species variation for this genus [11]. For related viruses such as Herpes simplex type 1 and 2 (order Herpesvirales, family Herpesviridae, genus simplexvirus, species Human alphaherpesvirus 1 and 2, HSV-1/2), it has been established that different viral isolates can cause different disease severities in various hosts [12, 13], and that there is a much higher degree of genomic variation in these viruses relative to FHV-1 [10, 14, 15].
The primary goal of this study was to establish if genomic variation in FHV-1 isolates may be associated with disease severity in feline hosts. Our hypothesis was that genomic variants would be detected in the sequences of the isolates which were significantly associated with host disease severity scores.
Materials and methods
Sample acquisition and clinical disease severity scoring
All samples were obtained in accordance with an approved University of Wisconsin-Madison Institutional Animal Care and Use Committee protocol (identification code V005353). Cats in animal shelters with signs of respiratory disease with or without ocular involvement were identified by shelter veterinarians in eight geographically distinct areas of the USA (Table 1). The veterinarians were instructed to include any animals which were showing clinical signs of respiratory disease of any severity with or without ocular involvement. Each animal was assigned a clinical disease severity score according to the scheme in Table 2. This scoring scheme was designed by a panel of experienced veterinary ophthalmologists, shelter veterinarians and virologists. The number of samples submitted by each shelter was limited by the number of animals showing clinical signs at the time of sampling. A single oropharyngeal swab was taken from each cat by brushing the oropharyngeal area firmly for around 10 s. The swabs were then placed into a transport medium (Universal Viral Transport, Becton, Dickinson and Company, Sparks, MD 21152 USA), labelled and double bagged to prevent cross-contamination. Gloves were changed between animals. The swabs were shipped overnight from the animal shelters for viral isolation.
Table 1.
Viral isolate sample identification, host age, host sex, host geographic location, sampling date, Genbank sequence identification, host ocular score, host respiratory score, host general signs score and host overall score for each individual isolate included in the present study
| Sample ID | Host approximate age (months) | Host sex | Host geographic origin | Sampling date | Genbank ID | Host ocular score | Host respiratory score | Host general signs score | Host total score |
|---|---|---|---|---|---|---|---|---|---|
| CALI11 | 24 | F | Oakland, CA | 11/9/16 | MH070326 | 6 | 2 | 0 | 8 |
| CALI14 | 4 | F | Oakland, CA | 11/9/16 | MH070339 | 3 | 0 | 0 | 3 |
| FLOR04 | 3 | M | Orlando, FL | 11/29/16 | MH070341 | 0 | 3 | 0 | 3 |
| FLOR05 | 3 | M | Orlando, FL | 11/29/16 | MH070327 | 0 | 2 | 0 | 2 |
| KANS02 | 10 | M | Kansas City, MO | 8/25/16 | MH070348 | 6 | 4 | 2 | 12 |
| KANS04 | 24 | F | Kansas City, MO | 8/25/16 | MH070337 | 3 | 2 | 0 | 5 |
| KANS08 | 54 | M | Kansas City, MO | 12/1/16 | MH070325 | 3 | 3 | 3 | 9 |
| KANS09 | 72 | M | Kansas City, MO | 12/1/16 | MH070329 | 3 | 4 | 3 | 10 |
| KANS10 | 54 | M | Kansas City, MO | 12/1/16 | MH070331 | 3 | 3 | 3 | 9 |
| MILW02 | 18 | F | Milwaukee, WI | 8/24/16 | MH070342 | 0 | 2 | 1 | 3 |
| MILW03 | 36 | M | Milwaukee, WI | 8/24/16 | MH070335 | 3 | 4 | 2 | 9 |
| MILW09 | 60 | F | Milwaukee, WI | 10/5/16 | MH070346 | 0 | 2 | 3 | 5 |
| MILW10 | 3 | M | Milwaukee, WI | 10/5/16 | MH070332 | 7 | 2 | 0 | 9 |
| MILW11 | 24 | M | Milwaukee, WI | 10/5/16 | MH070347 | 2 | 3 | 1 | 6 |
| MILW12 | 12 | F | Milwaukee, WI | 10/5/16 | MH070338 | 1 | 2 | 1 | 4 |
| NEWY01 | 24 | F | Long Island, NY | 11/7/16 | MH070324 | 5 | 3 | 1 | 9 |
| NEWY03 | 4 | M | Long Island, NY | 11/7/16 | MH070343 | 2 | 2 | 0 | 4 |
| Peebles-1 | 60 | F | Milwaukee, WI | 3/22/16 | MH070340 | 3 | 2 | 0 | 5 |
| PHIL01 | 12 | F | Philadelphia, PA | 8/18/16 | MH070336 | 1 | 2 | 1 | 4 |
| PHIL03 | 72 | M | Philadelphia, PA | 8/18/16 | MH070334 | 3 | 3 | 1 | 7 |
| PHIL04 | 72 | F | Philadelphia, PA | 8/18/16 | MH070333 | 2 | 4 | 0 | 6 |
| PHIL10 | 120 | M | Philadelphia, PA | 11/9/16 | MH070344 | 1 | 2 | 0 | 3 |
| SANJ01 | 3 | F | San Jose, CA | 11/15/16 | MH070330 | 3 | 3 | 0 | 6 |
| WASH01 | 48 | M | Auburn, WA | 11/8/16 | MH070328 | 3 | 2 | 0 | 5 |
| WASH03 | 48 | M | Auburn, WA | 11/29/16 | MH070345 | 2 | 3 | 2 | 7 |
All samples were obtained from animals housed in animal shelters located in the USA. A total disease score of 1–4 was assigned a ‘low’ severity category, a total disease score of 5–8 was assigned a ‘medium’ severity category and a total disease score of 9–12 was assigned a ‘high’ disease score severity category
Table 2.
Clinical scoring scheme used by veterinarians to assign scores to host animals from which isolates were obtained
| Clinical parameter assessed | Assigned score | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| Conjunctival hyperemia | None present | Mild redness of the conjunctiva; conjunctival vessels are obvious and detectable with the naked eye | Severe redness of the conjunctiva; vessels are more intensely injected and it may be difficult to distinguish individual vessels or conjunctival vessels from episcleral vessels |
| Conjunctival chemosis | None present | Mild swelling of the conjunctiva; detectable only with eversion of the lids during examination | Severe swelling of the conjunctiva; present with no eversion of the lids, swelling obvious when cat is at rest |
| Ocular discharge | None present | Mild, clear discharge or black crusts | Severe, yellow/green discharge |
| Other ocular disorders | None present | Corneal Ulceration, Neovascularization, Symblepharon, Blindness, Sequestrum, Phthisis Bulbi (1 point assigned per problem if present in one or both eyes) | N/A |
| Nasal discharge | None present | Serous (watery), nasal discharge present either/both nostrils | Mucopurulent nasal discharge present either/both nostrils |
| Other respiratory disorders | None present | Sneezing | N/A |
| Coughing | |||
| Dyspnea | |||
| Open mouth breathing | |||
| Increased respiratory rate (1 point assigned per problem if present) | |||
| Other general disorders | None present | Lethargy | N/A |
| Anorexia | |||
| Pyrexia | |||
| Oral Ulceration (1 point assigned per problem if present) | |||
The maximum possible ocular disease score is 12, the maximum possible respiratory disease score is 7, the maximum possible general signs disease score is 4. The total score is the sum of these 3 scores with a maximum total score of 23
Virus isolation, DNA extraction and Illumina sequencing
After shipping, the samples were immediately unpacked and refrigerated prior to viral isolation, with viral DNA being subsequently extracted using an existing protocol [16] as previously described [10]. DNA was submitted to the University of Wisconsin-Madison Biotechnology Center for sequencing.
Processing and mapping of reads
All processing and mapping procedures were performed using the BBTools Suite v.38.37. Raw paired-end reads were merged using BBMerg, and duplicates were removed using Dedupe. Reads were trimmed using BBDuk, at a quality threshold Phred Score (Q) of 20, trimming low quality reads from both ends (indicating a 99% chance of reads being correct) and a kmer length of 27. Reads were also filtered to keep only reads with a % G + C content between 25 and 75%. Finally, any reads shorter than 30 base pairs were discarded to avoid erroneously aligning short reads to the genome in random locations. Reads from each sample were then individually mapped to the reference FHV-1 genome (FJ478159 [17]) using BBMap with highest sensitivity, a kmer length of 13, and mapping to the first best match when multiple matches were discovered.
Virulence association
Mapped reads were analyzed using FreeBayes v1.1.0 to perform haplotype-based variant calling [18]. Because all variant callers bias variant calls in some way and we were treating this study as an uninformed approach to finding viral genome variants associated with host disease severities, it was determined that it was important that the variants be more accurately defined in the viral samples than the reference sequence. FreeBayes is currently the only variant caller that shifts the bias in calls towards ignoring variants in the reference genome over the samples [19]. Additionally, FreeBayes performs well regardless of genome alignment software chosen, as it calls variants based on the literal sequences of the reads aligned to the target, regardless of their precise alignment [20, 21].
To test for association mapping of virulence to haplotype variants, a generalized linear model (GLM) was constructed using R version 3.5.3 and the rTASSEL interface package version 5.0 [22]. We tested for association between variants and all disease severity scores (ocular, respiratory, general and total) for each host animal with 1000 permutations. Additionally, a main effect only model was built using all variables in the input data (each disease severity score). Finally, a separate model was built and solved for each variant and disease severity score. All significance values reported are for the permutation-based experiment-wide tests of marker effect. We chose this as the value to report as it controls for the probability of any false positives, by handling dependency between our hypotheses and non-normality in the data.
Ancestral state analysis
In order to estimate the ancestral disease state of each sample, a likelihood reconstruction of the ancestral states using a likelihood framework was conducted in Mesquite v3.6 [23]. This analysis functions by attempting to maximize the probability that the states observed evolved under a stochastic model of evolution. For each node, the state assignment that maximized the probability of arriving at the observed states in the terminal taxa, given the model of evolution, and allowing the states at all other nodes to vary, is estimated. As discrete character states are necessary for this analysis, host total disease scores were reclassified and assigned as ‘low’ (1–4), ‘medium’ (5–8) and ‘high’ (9–12). The Mk1 model (Markov k-state 1 parameter model) with the single parameter being the rate of change was used [24]. Any particular change (from low severity to medium severity or high severity to medium severity, for example) was set as equally probable. The tree topology used a majority-rule consensus tree generated from the posterior distribution of a Bayesian estimation generated in MrBayes v3.2.6 with 4 independent runs and 4 Metropolis-coupled chains per run and 5 million generations. All substitution model parameters, with the exceptions of topology and branch lengths, were unlinked across data subsets. Each gene-by-gene dataset was aligned with MAFFT v7 using the E-INS-i algorithm for the highest level of accuracy. The best-fit substitution model was estimated for each of the 248 individual partitions. The best fitting model of evolution was selected using Bayesian Information Criterion, BIC as implemented in JModelTest2 v2.1.9. The tree was rooted on Canid herpesvirus 1 (NC_030117, [25]) to provide directionality.
Results
Disease severity scoring
Viable isolates of FHV-1 were obtained from 25 cats housed in 8 animal shelters across the USA (Table 1). The estimated age of the host animals ranged from 3 to 120 months (mean ± SD, 34.6 ± 30.4 months). Eleven of the animals were female and the remainder male. All animals had clinical disease consistent with a diagnosis of FHV-1, determined by a veterinarian. All assigned disease scores varied markedly between hosts. The assigned ocular disease scores ranged from 0 to 7 (mean ± SD, 2.6 ± 1.8). The assigned respiratory disease scores ranged from 0 to 4 (mean ± SD, 2.6 ± 0.9). The assigned general disease scores ranged from 0 to 3 (mean ± SD, 1.0 ± 1.1). Finally, the assigned total disease scores ranged from 2 to 12 (mean ± SD, 6.1 ± 2.7). Details of scores for individual host animals can be found in Table 1. Based on the scoring criteria, 8 animals were designated as having ‘low’ total disease severity, 10 animals were designated as having ‘medium’ total disease severity and 7 animals designated as having ‘high’ total disease severity.
Processing and mapping of reads
The mean raw sequence reads were 1,273,829 per sample. Quality trimming and duplicate removal left a mean of 1,115,729 reads per sample. Prior to trimming reads, the minimum and maximum read length was 301 base pairs. Post trimming, minimum read length, maximum read length, and mean read length were 30, 301, and 248.1 base pairs respectively. Following trimming and filtering, quality scores were good for the reads of all viral isolates and were consistently higher than Phred quality score of 30 throughout the genomes, as was previously reported [10]. Coverage was also good for all samples, with more than 150× for all samples (Fig. 1, Supplementary Material). Overall, the quality and coverage of reads obtained from the viral isolates was considered to be excellent.
Virulence association
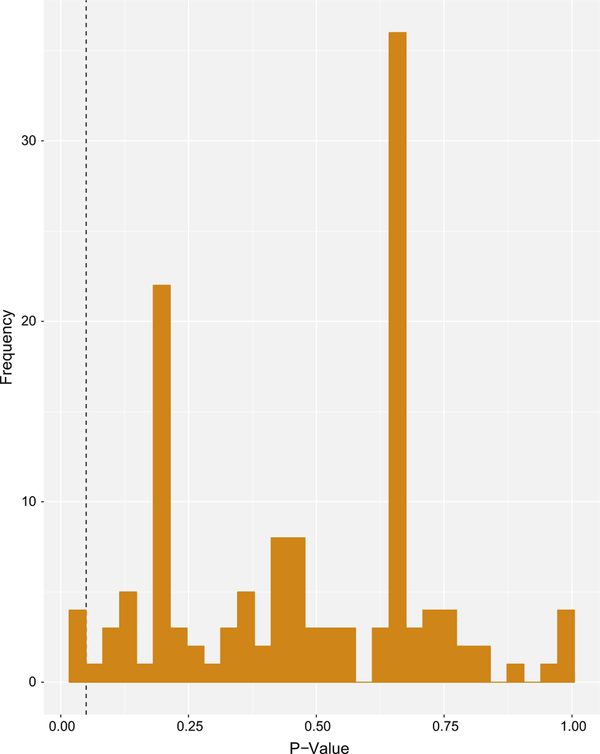
A total of 129 variants were found across all viral genomes when compared to the reference genome. Variants were filtered to only those which were present in all viral samples in comparison to the prototypic referenced genome. Only 8 possible variants (6.2%) were found to be present in all isolates, all of which were variants compared to the reference genome. The GLM recovered a significant association (p < 0.05) for only two synonymous single nucleotide polymorphisms (SNPs) with respiratory disease severity scores and total disease severity scores. The distribution of p values from the GLM is shown in Fig. 1. Both of the SNPs significantly associated with disease scores occurred in the same viral isolate, FLOR05, which was obtained from a host animal housed in Orlando, Florida. This animal had the lowest overall total disease severity score of any of the host animals assessed. The SNPs in FLOR05 which were found to be associated with a specific disease severity score were located in UL37 (position 26995) and UL39 (position 25840). No significant associations were found between variants in the viral genomes and ocular disease severity scores or general disease severity scores. Numerous SNPs causing missense mutations were detected within the isolate genomes, as have been previously reported [10]. While none of these were significantly associated with assigned host disease scores, these mutations were found to almost exclusively occur in 3 genes (UL36, UL52 and ICP4).
Fig. 1.
Distribution of p values from GLM Model. A fixed effect linear model was constructed to test for association between segregating sites (variants) and disease severity scores. A main effect only model was built using all variables in the input data (ocular disease severity score, respiratory disease severity score, general signs disease severity score and total disease severity score). A separate model was built and solved for each trait and variant. Significant associations shown to the left of the dashed line (n = 4)
Ancestral state analysis
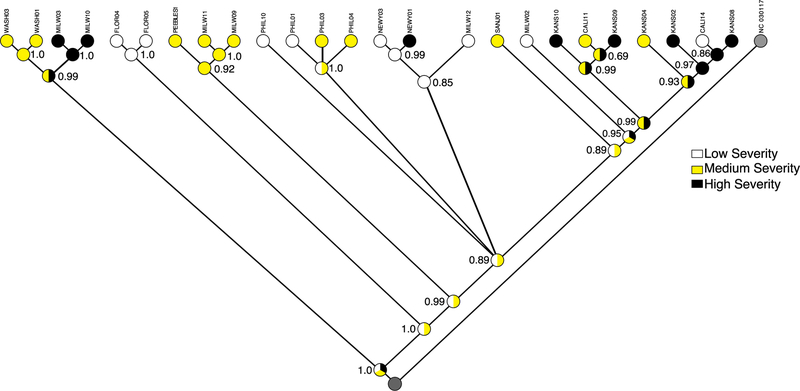
The ancestral state likelihood reconstruction determined that there was no clear evidence of a connection between host total disease severity scores and predicted viral evolutionary state (in terms of host disease severity) (Fig. 2). Each of the deepest estimated ancestral nodes were equally likely to be from all three of the disease severity groups. The topology of the tree recovered was similar to previous work [10]. Many recovered clades in the tree clustered on geographic sampling region. For example, the two isolates recovered from host animals housed in New York (NEWY01, NEWY03) clustered together. However, there were some exceptions to this pattern. For example, the samples from Kansas and California formed two interspersed clades. Other individual samples clustered in geographically distant clades such as the WASH01 isolate clustering with the MILW group. Despite some isolates from the same geographic location clustering together, host disease severity for these groups was variable. For example, although the isolates from New York formed a cluster, the host disease severity was very different (NEWY01 = high, NEWY03 = low).
Fig. 2.
Likelihood reconstruction of the ancestral states that maximize the probability the observed states evolved under a stochastic model of evolution. This model uses the Markov k-state 1 parameter model with the single parameter being the rate of change. Any particular change is equally probable. The tree topology is a majority-rule consensus tree generated from the posterior distribution of a Bayesian estimation generated in MrBayes 3.2.6 with 4 independent runs and 4 Metropolis-coupled chains per run and run for 5 million generations. All substitution model parameters, with the exceptions of topology and branch lengths, were unlinked across data subsets. Each gene-by-gene dataset was aligned with MAFFT version 7 using the E-INS-i algorithm for the highest level of accuracy. The best-fit substitution model was estimated for each of the 248 gene partitions. The tree is rooted on (NC_030117, Canid herpesvirus 1 strain 0194, isolated in 1985) to provide directionality
Discussion
It is known that variation in host disease severity scores for related viruses such as HSV-1 is due to a complicated interaction of viral genomic factors, innate immunity and host immune response [12, 26–29]. HSV-1 causes a similar spectrum of disease to FHV-1 in its respective host, and it is reasonable to assume that the disease severity seen with FHV-1 is influenced by a similarly wide variety of factors. Our findings that host disease severity was minimally affected by FHV-1 genomic variants are supported by evidence from prior studies [8, 9, 30]. Using full genome sequencing we have previously determined that FHV-1 isolates obtained from both Australia and the USA contain very few genomic variants relative to other varicelloviruses [10, 11]. This would suggest that the spectrum of clinical disease seen in feline hosts is unlikely to be primarily related to genomic variants in FHV-1. Previous work has established that there was very little difference in clinical disease severity for host animals infected with 5 different FHV-1 isolates when environment and host were homogenized in the experimental setting [8]. Another study using 2 unmodified FHV-1 isolates demonstrated similar host disease severities for each isolate [30]. However, another study which utilized the experimental infection of feline hosts (n = 6) with 3 different FHV-1 isolates found that while all 3 caused disease, one isolate caused subjectively less severe disease than the others [9]. Overall, there is minimal prior evidence of significant differences in the severity of host disease which can be solely attributed to the use of different isolates of FHV-1.
There have been a number of prior studies utilizing experimental feline subjects to assess various treatments or preventative measures for FHV-1 [31–35]. All of these studies utilized one isolate of FHV-1 to induce infection (with different isolates used in individual studies), mostly in specific pathogen free cats. The finding that host disease severity is unlikely to be strongly influenced by viral isolate genomic sequence is important for the interpretation of these studies and suggests that the choice of unmodified FHV-1 isolate for use in such studies is of limited importance.
Our GLM detected significant associations between two SNPs and host disease severity scores. One SNP was in UL37, a highly conserved tegument protein which is involved in many essential viral functions [36]. The other was in UL39, ribonucleotide reductase subunit 1, mutations in which can result in attenuation of viral activity [37]. Both SNPs were associated with the lowest respiratory disease severity score found in the study, which could indicate that the isolate in which these variants were found (FLOR05) was attenuated in some way compared to the other wild-type isolates examined. However, neither of the SNPs would be predicted to result in amino acid alterations and it is unlikely that these variations would have a significant impact on the products of these coding sequences. It is therefore considered unlikely that these SNPs would have a clinically meaningful impact on disease severity scores.
Our additional ancestral character state reconstruction supported the GLM results, showing that disease severity did not have a strong correlation with evolutionary history. For example, the two isolates recovered from host animals housed in New York (NEWY01, NEWY03) clustered together and yet the hosts had markedly different total disease severities (NEWY01 = high, NEWY03 = low), suggesting that virus genome factors are less likely to be important than host response in determination of host disease severity. Instead, geographic location was the strongest predictor of evolutionary history suggesting that strong genetic isolation occurs between viruses in these geographic regions. As mentioned earlier, the few exceptions to this pattern include single samples landing in geographically distant clades, likely a signal of convergence in the viral genomes (or movement of host animals). A more powerful result is found in the Kansas and California based samples. These isolates are found interspersed on two clades which suggests that at some point, these viral isolates shared a common ancestor or were founded by the same viral genome.
Certain regions of the FHV-1 genomes which were assessed accounted for the majority of gaps and missense mutations which were identified. These included the Inverted Repeat Short (IRS) and Tandem Repeat Short (TRS) regions. These repeat regions in herpesviruses can invert and give rise to up to four different isomers of a genome [38], with these isomers usually being present in equimolar proportions. These repeat regions in certain isomers of herpesviruses are known to be extremely unstable during even short periods of viral replication [39]. Although none of the missense variants in UL36 (large tegument protein), UL52 (helicase-primase primase subunit) or ICP4 (transcriptional regulator) were significantly associated with host disease scores, almost all of the missense variants which were detected were located in these three regions of the viral genomes. Possible explanations for this finding are the relative size of these regions of the genome (UL36 is one of the largest genes in FHV-1) or lower sequencing coverage in certain regions of the genome.
The present study utilized samples obtained from animals in the real-world setting of an animal shelter, allowing for a reasonable number of animals to be sampled without the limitations of obtaining and housing experimental animals. When using samples of this nature it is not possible to adequately control for factors such as age and sex of the host or for environmental variables such as population density, temperature or humidity, all of which could conceivably impact host disease severity for FHV-1. Further studies to collect more samples from a larger population (to control for age and sex) in each location (to control for environment) are warranted. In addition, a simultaneous collection of host DNA could shed light on the interplay between host response, viral isolate genome and environment.
There are several limitations of the current study. For example, due to the use of clinical cases rather than experimentally naïve animals, we cannot know if the observed disease traits are due to initial infection or reactivation of latent virus. In addition, because the scoring was performed at only one time point for each animal, it is possible that the host disease severity score had not yet reached maximum intensity. Our finding that there were limited variants associated with clinical disease severity scores could have been secondary to sample size. It is possible that by sampling further animals and repeating the analysis that additional associations would be made between viral genomic variants and disease severity scores. However, the results from the present study represent the most comprehensive attempt to date to detect associations between FHV-1 viral genome and feline host clinical disease severity scores.
Due to minimal evidence of FHV-1 viral genome variants impacting host disease severity scores, it was concluded that the wide spectrum of host disease severities reported was most likely due to other factors such as host response. Because this work presents compelling evidence that FHV-1 disease severity is not primarily associated with specific viral genomic variants, further efforts are warranted to directly assess the impact of variations in host response in FHV-1 infection on feline host disease severity outcomes.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Curtis Brandt, Aaron Kolb, Dr. Kristen Bernard, Dr. Sandra Newbury and the University of Wisconsin-Madison Biotechnology Center for their assistance and advice in obtaining samples, viral isolation and sequencing. The authors would also like to thank Kristen Cooley CVT, Dr. McLean Gunderson, Jenni Rose McKay, Dr. Aleisha Swartz, Dr. Sharon Oster-mann, Dr. Sarah Frei, Dr. Christine Solis, Dr. Julie Andersen, Dr. Emily Purvis, Dr. Margaret Wixson, Dr. Lauren Park, Dr. Cooper Brookshire, Dr. Mark Verdino, Dr. Hillary Herendeen, Dr. Jen Dalmasso, Dr. Libby Gutting and Dr. Kimberly Woodruff for their assistance in acquiring the samples from the cats in this study. Finally the authors would like to thank the LSU Center for Computation and Technology and High-Performance Computing Clusters for computational resources and support.
Funding This project was partially funded by a Grant from the University of Wisconsin-Madison Veterinary Teaching Hospital Companion Animal Fund; NIH Vision Research Core Grant, P30EY016665, and NIH National Center for Advancing Translational Sciences (NCATS), Grant UL1TR000427. This work was also supported by an unrestricted Grant from Research to Prevent Blindness, Inc. to the Department of Ophthalmology and Visual Sciences, University of Wisconsin-Madison. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest None of the authors have any conflict of interest to report in regards to this manuscript.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11262-019-01718-3) contains supplementary material, which is available to authorized users.
References
- 1.Gaskell R, Dawson S, Radford A, Thiry E (2007) Feline herpesvirus. Vet Res 38(2):337–354. 10.1051/vetres:2006063 [DOI] [PubMed] [Google Scholar]
- 2.Maggs DJ, Clarke HE (2005) Relative sensitivity of polymerase chain reaction assays used for detection of feline herpesvirus type 1 DNA in clinical samples and commercial vaccines. Am J Vet Res 66(9):1550–1555 [DOI] [PubMed] [Google Scholar]
- 3.Gaskell RM, Povey RC (1977) Experimental induction of feline viral rhinotracheitis virus re-excretion in FVR-recovered cats. Vet Rec 100(7):128–133 [DOI] [PubMed] [Google Scholar]
- 4.Gould D (2011) Feline herpesvirus-1: ocular manifestations, diagnosis and treatment options. J Feline Med Surg 13(5):333–346. 10.1016/j.jfms.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sebbag L, Thomasy SM, Woodward AP, Knych HK, Maggs DJ (2016) Pharmacokinetic modeling of penciclovir and BRL42359 in the plasma and tears of healthy cats to optimize dosage recommendations for oral administration of famciclovir. Am J Vet Res 77(8):833–845. 10.2460/ajvr.77.8.833 [DOI] [PubMed] [Google Scholar]
- 6.Thomasy SM, Shull O, Outerbridge CA, Lim CC, Freeman KS, Strom AR, Kass PH, Maggs DJ (2016) Oral administration of famciclovir for treatment of spontaneous ocular, respiratory, or dermatologic disease attributed to feline herpesvirus type 1: 59 cases (2006–2013). J Am Vet Med Assoc 249(5):526–538. 10.2460/javma.249.5.526 [DOI] [PubMed] [Google Scholar]
- 7.Stiles J (2003) Feline herpesvirus. Clin Tech Small Anim Pract 18(3):178–185. 10.1053/svms.2003.ysvms28 [DOI] [PubMed] [Google Scholar]
- 8.Nasisse MP, Guy JS, Davidson MG, Sussman WA, Fairley NM (1989) Experimental ocular herpesvirus-infection in the cat—sites of virus-replication, clinical-features and effects of corticosteroid administration. Investig Ophthalmol Vis Sci 30(8):1758–1768 [PubMed] [Google Scholar]
- 9.Hamano M, Maeda K, Mizukoshi F, Une Y, Mochizuki M, Tohya Y, Akashi H, Kai K (2003) Experimental infection of recent field isolates of feline herpesvirus type 1. J Vet Med Sci 65(8):939–943 [DOI] [PubMed] [Google Scholar]
- 10.Lewin AC, Kolb AW, McLellan GJ, Bentley E, Bernard KA, Newbury SP, Brandt CR (2018) Genomic, recombinational and phylogenetic characterization of global feline herpesvirus 1 isolates. Virology 518:385–397. 10.1016/j.virol.2018.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolb AW, Lewin AC, Moeller Trane R, McLellan GJ, Brandt CR (2017) Phylogenetic and recombination analysis of the herpesvirus genus varicellovirus. BMC Genom 18(1):887 10.1186/s12864-017-4283-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandt CR (2005) The role of viral and host genes in corneal infection with herpes simplex virus type 1. Exp Eye Res 80(5):607–621. 10.1016/j.exer.2004.09.007 [DOI] [PubMed] [Google Scholar]
- 13.Lee K, Kolb AW, Larsen I, Craven M, Brandt CR (2016) Mapping murine corneal neovascularization and weight loss virulence determinants in the herpes simplex virus 1 genome and the detection of an epistatic interaction between the UL and IRS/US regions. J Virol 90(18):8115–8131. 10.1128/Jvi.00821-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolb AW, Adams M, Cabot EL, Craven M, Brandt CR (2011) Multiplex sequencing of seven ocular herpes simplex virus type-1 genomes: phylogeny, sequence variability, and SNP distribution. Investig Ophthalmol Vis Sci 52(12):9061–9073. https://do.org/10.1167/iovs.11-7812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolb AW, Larsen IV, Cuellar JA, Brandt CR (2015) Genomic, phylogenetic, and recombinational characterization of herpes simplex virus 2 strains. J Virol 89(12):6427–6434. 10.1128/JVI.00416-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kintner RL, Brandt CR (1994) Rapid small-scale isolation of herpes-simplex virus-DNA. J Virol Methods 48(2–3):189–196. 10.1016/0166-0934(94)90118-X [DOI] [PubMed] [Google Scholar]
- 17.Tai SHS, Niikura M, Cheng HH, Kruger JM, Wise AG, Maes RK (2010) Complete genomic sequence and an infectious BAC clone of feline herpesvirus-1 (FHV-1). Virology 401(2):215–227. 10.1016/j.virol.2010.02.021 [DOI] [PubMed] [Google Scholar]
- 18.Garrison E, Marth G (2012) Haplotype-based variant detection from short-read sequencing
- 19.Liu XT, Han SZ, Wang ZH, Gelernter J, Yang BZ (2013) Variant callers for next-generation sequencing data: a comparison study. PLoS ONE 8(9):e75619 10.1371/journal.pone.0075619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ni GY, Strom TM, Pausch H, Reimer C, Preisinger R, Simianer H, Erbe M (2015) Comparison among three variant callers and assessment of the accuracy of imputation from SNP array data to whole-genome sequence level in chicken. BMC Genom 16:824 10.1186/s12864-015-2059-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang S, Kim E, Lee I, Marcotte EM (2015) Systematic comparison of variant calling pipelines using gold standard personal exome variants. Sci Rep 5:17875 10.1038/srep17875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23(19):2633–2635. 10.1093/bioinformatics/btm308 [DOI] [PubMed] [Google Scholar]
- 23.Maddison WPJE (2008) Mesquite: a modular system for evolutionary analysis. Evolution 62:1103–111818298648 [Google Scholar]
- 24.Lewis PO (2001) A likelihood approach to estimating phylogeny from discrete morphological character data. Syst Biol 50(6):913–925. 10.1080/106351501753462876 [DOI] [PubMed] [Google Scholar]
- 25.Papageorgiou KV, Suarez NM, Wilkie GS, McDonald M, Graham EM, Davison AJ (2016) Genome Sequence of Canine Herpesvirus. PLoS ONE 11(5):e0156015 10.1371/journal.pone.0156015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doymaz MZ, Rouse BT (1992) Immunopathology of herpes-simplex virus-infections. Curr Top Microbiol 179:121–136 [DOI] [PubMed] [Google Scholar]
- 27.Kastrukoff LF, Lau AS, Puterman ML (1986) Genetics of natural-resistance to herpes-simplex virus type-1 latent infection of the peripheral nervous-system in mice. J Gen Virol 67:613–621. 10.1099/0022-1317-67-4-613 [DOI] [PubMed] [Google Scholar]
- 28.Pollara G, Katz DR, Chain BM (2004) The host response to herpes simplex virus infection. Curr Opin Infect Dis 17(3):199–203. 10.1097/01.qco.0000129616.14121.07 [DOI] [PubMed] [Google Scholar]
- 29.Thompson RL, Williams RW, Kotb M, Sawtell NM (2014) A forward phenotypically driven unbiased genetic analysis of host genes that moderate herpes simplex virus virulence and stromal keratitis in mice. PLoS ONE 9(3):e92342 10.1371/journal.pone.0092342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruger JM, Sussman MD, Maes RK (1996) Glycoproteins gI and gE of feline herpesvirus-1 are virulence genes: safety and efficacy of a gI-gE(−) deletion mutant in the natural host. Virology 220(2):299–308. 10.1006/viro.1996.0318 [DOI] [PubMed] [Google Scholar]
- 31.Thomasy SM, Lim CC, Reilly CM, Kass PH, Lappin MR, Maggs DJ (2011) Evaluation of orally administered famciclovir in cats experimentally infected with feline herpesvirus type-1. Am J Vet Res 72(1):85–95. 10.2460/ajvr.72.1.85 [DOI] [PubMed] [Google Scholar]
- 32.Spertus CB, Pennington MR, Van de Walle GR, Badanes ZI, Judd BE, Mohammed HO, Ledbetter EC (2019) Effects of orally administered raltegravir in cats with experimentally induced ocular and respiratory feline herpesvirus-1 infection. Am J Vet Res 80(5):490–497 [DOI] [PubMed] [Google Scholar]
- 33.Fontenelle JP, Powell CC, Veir JK, Radecki SV, Lappin MR (2008) Effect of topical ophthalmic application of cidofovir on experimentally induced primary ocular feline herpesvirus-1 infection in cats. Am J Vet Res 69(2):289–293. 10.2460/ajvr.69.2.289 [DOI] [PubMed] [Google Scholar]
- 34.Hoover EA, Griesemer RA (1971) Experimental feline herpesvirus infection in pregnant cat. Am J Pathol 65(1):173. [PMC free article] [PubMed] [Google Scholar]
- 35.Gaskell RM, Povey RC (1979) Dose-response of cats to experimental-infection with feline viral rhinotracheitis virus. J Comp Pathol 89(2):179–191. 10.1016/0021-9975(79)90057-4 [DOI] [PubMed] [Google Scholar]
- 36.Koenigsberg AL, Heldwein EE (2018) The dynamic nature of the conserved tegument protein UL37 of herpesviruses. J Biol Chem 293(41):15827–15839. 10.1074/jbc.RA118.004481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mostafa HH, Thompson TW, Konen AJ, Haenchen SD, Hilliard JG, Macdonald SJ, Morrison LA, Davido DJ (2018) Herpes simplex virus 1 mutant with point mutations in UL39 is impaired for acute viral replication in mice, establishment of latency, and explant-induced reactivation. J Virol 92(7):e01654–17. 10.1128/jvi.01654-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roizman B (1979) Structure and isomerization of herpes-simplex virus genomes. Cell 16(3):481–494. 10.1016/0092-8674(79)90023-0 [DOI] [PubMed] [Google Scholar]
- 39.Maertzdorf J, Remeijer L, Van der Lelij A, Buitenwerf J, Niesters HGM, Osterhaus ADME, Verjans GMGM (1999) Amplification of reiterated sequences of herpes simplex virus type 1 (HSV-1) genome to discriminate between clinical HSV-1 isolates. J Clin Microbiol 37(11):3518–3523 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.