Consistent use of continuous glucose monitoring (CGM) has been associated with lower HbA1c levels in adolescents and adults with Type 1 diabetes [1]; however, frequent CGM use in young childen did not result in HbA1c-lowering in earlier studies by the DirecNet Group [2,3], raising the possibility that parents and other caregivers of the children wearing the devices may have been using CGM to avoid hypoglycaemia rather than to lower HbA1c values.
During the past 5–6 years there have been several changes in care for young children with Type 1 diabetes. The American Diabetes Association (ADA) lowered glycaemic targets to <58 mmol/mol (<7.5%) [4], the International Society for Paediatric and Adolescent Diabetes (ISPAD) lowered targets to <53 mmol/mol (<7.0%) [5], improved CGM systems with remote monitoring were introduced, and levels of CGM and insulin pump use in young children rose [6,7]. Nevertheless, whether parents of very young children are using additional glucose information and advanced diabetes technologies to attempt to achieve lower glycaemic targets has not been established. As part of a qualitative evaluation of parents’ attitudes and perspectives related to the burdens of managing their child’s Type 1 diabetes, we asked parents to report the glycaemic target ranges they used (including lower and upper limits) and ranges that were recommended to them by their diabetes clinicians. We aimed to explore the hypothesis that parents using diabetes technology to manage their young children would have lower glycaemic targets than those not using technology. Further, target ranges in which parents felt comfortable would be higher than parental perceptions of the glycaemic target ranges recommended by their clinicians.
Seventy-nine parents of children aged < 8 years diagnosed with Type 1 diabetes for ≥6 months, HbA1c levels of <91 mmol/mol (<10.5%), and no profound developmental delays were recruited from major diabetes centres in the USA (Texas Children’s Hospital, Indiana University, Joslin Diabetes Centre and Yale School of Medicine) to participate in semistructured interviews exploring parental experiences with diabetes management. Interviews were transcribed and reviewed. Cross-sectional data are presented from 64 interviews in which parents provided numeric responses regarding glycaemic ranges with which they felt comfortable and the target ranges that their clinicians recommended. The most recent HbA1c value was obtained from the medical record. Glucose data are presented as frequencies and means with 95% CIs. The Wilcoxon signed rank test was used to compare parental targets and parental-reported clinician targets. The Mann–Whitney test was used to report the primary outcome, comparison of the parental target ranges for diabetes technology users and non-users. HbA1c values of CGM users and non-users were reported as means and 95% CIs, and compared using an unpaired t-test. P values <0.05 were taken to indicate statistical significance.
The mean age of the children with Type 1 diabetes was 5.0±1.5 years, duration of diabetes 2.2±1.1 years, and HbA1c level 64±10 mmol/mol (8.0±0.9%); 67% used pumps and 58% used CGM. Interviewees were mostly mothers (86%) and of non-Hispanic-white race/ethnicity (80%), while 6% were African-American, 6% Hispanic/Latino, and 8% mixed race/ethnicity. Sixty-one percent of the parents had earned a bachelor or graduate degree, and annual income for 55% of families was ≥$75,000, while for 32% of families it was <$50,000.
Although parental-reported glucose target ranges were higher compared with parental-reported clinician target ranges, the differences were not statistically significant. This was true both at the lower limit of target range, with values of 5.1 mmol/l (95% CI 4.7, 5.4) vs 4.8 mmol/l (95% CI 4.5, 5.1) or 92 mg/dl (95% CI 85, 98) vs 87 mg/dl (95% CI 81,92), respectively (P=0.09) and at the upper limit of target range, with values of 9.9 mmol/l (95% CI 9.2, 10.5) vs 9.2 mmol/l (95% CI 8.7, 9.7) or 178 mg/dl (95% CI 166, 189) vs 166 mg/dl (95% CI 156,175), respectively (P=0.2) (Table 1).
Table 1.
Upper and lower limits of parental target glucose ranges by use of diabetes technology
| Pump users | No pump | CGM users | No CGM | |
|---|---|---|---|---|
| Upper limit, mean (95% CI) | ||||
| mmol/l | 9.6 (9, 10.1) | 10.5 (8.7, 12.4) | 9.8 (8.9, 10.8) | 9.9 (9.2, 10.6) |
| mg/dl | 172 (163, 182) | 190 (157, 223) | 177 (159, 195) | 178 (165, 192) |
| Lower limit, mean (95% CI) | ||||
| mmol/l | 5.1 (4.6, 5.6) | 5.1 (4.7, 5.5) | 4.6 (4.3, 4.8)* | 5.8 (5, 6.5)* |
| mg/dl | 91 (82, 100) | 92 (84, 99) | 82 (78, 87) | 104 (91, 117) |
CGM, continuous glucose monitoring.
P=0.0017 for CGM vs no CGM.
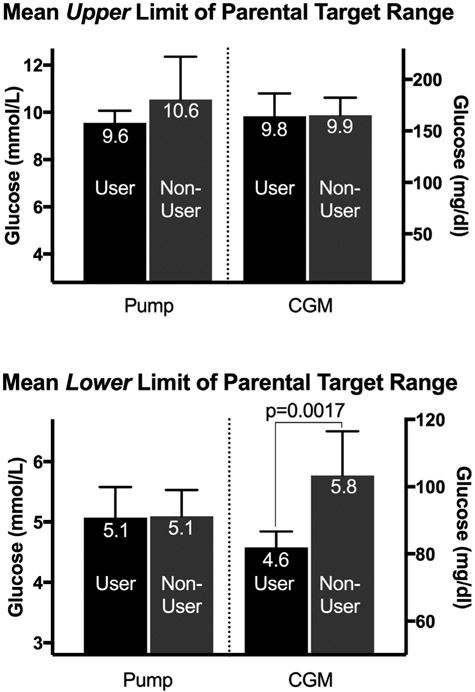
When participants were divided by technology use (Fig. 1), the upper limits of target ranges were not different between users and non-users of CGM or pumps; however, parents of children on CGM reported using a lower glucose target for the lower range than non-users (Fig. 1; P=0.0017), and their children had lower HbA1c values [CGM user 61 mmol/mol (95% CI 57, 64) or 7.7% (95% CI 7.4, 8.0) vs non-user 67 mmol/mol (95% CI 64, 72) or 8.3% (95% 8.0, 8.7); P=0.005].
FIGURE 1.
Upper and Lower limits of parental target ranges by use of diabetes technology (glucose values above bars are in mmol/L, error bars reflect 95% confidence interval).
This is the first study to report the glycaemic target ranges used by parents in the diabetes management of very young children. While the upper and lower bounds of the target glucose range tended to be higher than ranges parents recalled as recommended by their clinicians, the differences were not statistically significant in this small sample. Importantly, parents of very young children with Type 1 diabetes who used CGM reported being comfortable with significantly lower minimum glucose values than parents who relied solely on intermittent blood glucose measurements. These lower minimum glucose values fall within the ranges in the ADA and ISPAD guidelines, but above the recommended absolute threshold for a low value [5].
It is particularly noteworthy that the young children who were using CGM in our sample also had lower HbA1c levels, which was not reported in prior randomized CGM studies in children aged < 10 years [2]. We are unable to determine whether this was related to differences in parental education/ socio-economic status, CGM data/alerts affording parents more comfort with lower glucose values as they expect CGM to alert them of an impending hypoglycaemic event, or another reason. Furthermore, causal attribution cannot be made as this was a cross-sectional study. Additionally, the results should be interpreted with caution given that both the HbA1c and glycaemic targets were self-reported.
Further research regarding the impact of CGM use on glycaemic targets, especially with remote monitoring and predicted low-glucose-suspend functionalities, can inform novel strategies for parents and clinicians to work together to improve time-in-range in these young children.
Acknowledgments
Funding sources
This study was supported by the Leona M. and Harry B. Helmsley Charitable Trust.
Footnotes
Competing interests
None declared.
References
- 1.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Beck RW, Buckingham B et al. Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes Diabetes Care 2009;32:1947–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsalikian E, Fox L, Weinzimer S, Buckingham B, White NH, Beck R et al. Feasibility of prolonged continuous glucose monitoring in toddlers with type 1 diabetes. Pediatr Diabetes 2012; 13: 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mauras N, Beck R, Xing D, Ruedy K, Buckingham B, Tansey M et al. A randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to <10 years. Diabetes Care 2012; 35: 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiang JL, Kirkman MS, Laffel LM, Peters AL. Type 1 Diabetes Sourcebook Authors. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 2014; 37: 2034–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiMeglio LA, Acerini CL, Codner E, Craig ME, Hofer SE, Pillay K et al. Glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes 2018; 19(Suppl. 27): 105–114. [DOI] [PubMed] [Google Scholar]
- 6.DeSalvo DJ, Miller KM, Hermann JM, Maahs DM, Hofer SE, Clements MA et al. Continuous glucose monitoring and glycemic control among youth with type 1 diabetes: International comparison from the T1D Exchange and DPV Initiative. Pediatr Diabetes 2018; 19: 1271–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015; 38: 971–978. [DOI] [PubMed] [Google Scholar]