Abstract
Background
The stability of orthodontic treatment is thought to be significantly affected by the compression and retraction of gingival tissues, but the underlying molecular mechanism is not fully elucidated. The objectives of our study were to explore the effects of mechanical force on the ECM-integrin-cytoskeleton linkage response in human gingival fibroblasts (HGFs) cultured on 3-dimension (3D) lactide-co-glycolide (PLGA) biological scaffold and to further study the mechanotransduction pathways that could be involved.
Material/Methods
A compressive force of 25 g/m2 was applied to the HGFs-PLGA 3D co-cultured model. Rhodamine-phalloidin staining was used to evaluate the filamentous actin (F-actin) cytoskeleton. The expression level of type I collagen (COL-1) and the activation of the integrin α5β1/focal adhesion kinase (FAK) signaling pathway were determined by using real-time PCR and Western blotting analysis. The impacts of the applied force on the expression levels of FAK, phosphorylated focal adhesion kinase (p-FAK), and COL-1 were also measured in cells treated with integrin α5β1 inhibitor (Ac-PHSCN-NH 2, ATN-161).
Results
Mechanical force increased the expression of integrin α5β1, FAK (p-FAK), and COL-1 in HGFs, and induced the formation of stress fibers. Blocking integrin α5β1 reduced the expression of FAK (p-FAK), while the expression of COL-1 was not fully inhibited.
Conclusions
The integrin α5β1/FAK signaling pathway and actin cytoskeleton appear to be involved in the mechanotransduction of HGFs. There could be other mechanisms involved in the promotion effect of mechanical force on collagen synthesis in addition to the integrin α5β1 pathway.
MeSH Keywords: Actin Cytoskeleton; Fibroblasts; Focal Adhesion Kinase 1; Gingival Overgrowth; Integrin alpha5beta1; Mechanotransduction, Cellular
Background
Mechanical force is applied to an orthodontic attachment and transmitted to the periodontal tissue (e.g., periodontal ligament, alveolar bone, gingival) to move certain teeth during orthodontic treatment [1,2]. Unlike periodontal ligament and bone, which can always be absorbed, gingival tissue usually accumulates after rotation, labial tooth movement, and closure of an extraction site. Retraction and compression are the causes of gingival tissue accumulation. Such gingival tissue, like compressed rubber, tends to develop toward the pre-treatment position after treament, which can result in the extraction site re-opening or the tooth returning to its original position [3]. It was reported that orthodontic force can upset the balance between collagen degradation and synthesis of gingival tissue, which is of great importance in maintaining homeostatic balance, and also increases the proliferation of human gingival fibroblasts (HGFs) and the synthesis of type I collagen (COL-1), which are the major structural components in the extracellular matrix (ECM) of the gingiva [4,5]. Such excessive ECM accumulation in gingival tissues is a primary driver of relapse after orthodontic treatment [6]. However, the mechanisms by which HGFs detect and react to orthodontic force, thus inducing ECM accumulation, are largely unknown.
The conversion of mechanical cues into chemical signals is known as mechanotransduction [7]. ECM-integrin-cytoskeleton linkage is considered to be a critical component in cell mechanotransduction and signaling [8]. Integrins are cellular transmembrane receptors that mediate cell–ECM two-way signal interactions by binding with a variety of ECM proteins, such as fibronectin (FN) and laminin [9]. Integrins are composed of a and b subunits, including α2β1, α6β1, α3β1, and α5β1. Among all these subunits, α5β1 is the most highly expressed receptor for FN in fibroblasts [10].
When mechanical loading is applied to cells, integrins bind to small amino acid sequences of FN ligands in the ECM, and then rapid cluster on the cell membrane [11]. The clustered integrins further recruit and activate various signaling molecules and kinases, such as Src family kinases (SFKs) and focal adhesion kinase (FAK), to form focal adhesions (FA) [12], which are considered as the adhesion organelles connecting the ECM and the reorganized cytoskeleton to favor the outside-in signal transmission during mechanical stimuli [13]. The cytoskeleton is a complex network structure in which filaments can disassemble and reassemble due to different mechanical stimuli and chemical signals. Filamentous actin (F-actin), as the most important component of the cytoskeleton, is an active sensor of mechanical tension [14]. A large body of evidence indicates that the integrin-mediated cell adhesion in the ECM regulates the formation of mechanosensitive structures, which is believed to be of great significance in mechanotransduction. Christopher et al. found that the expression levels of integrin subunits could be improved by pressure loading on cardiac myocytes such as α1, α5, α7, and β1 [15]. FAK plays an important role in organization of the cytoskeleton, which also has been shown to mediate integrin signaling in cardiac myocytes and fibroblasts during pressure overload [16,17].
Although previous findings demonstrated integrin complex activation by the mechanism of mechanotransduction, fibroblasts from different tissues exhibit performance variation in the biochemical reactions and mechanotransduction properties when loaded with different mechanical forces [18]. Little is known about the effects of compressive force stimulation on the integrin α5β1/FAK pathway and actin cytoskeleton in HGFs. Additionally, compared with two-dimensional (2D) culture, three-dimensional (3D) culture can better reflect the actual cell morphology in tissues. For instance, HGFs cultured on 3D nanofibrous scaffolds exhibit spindle shape rather than forming a clustered plane on 2D matrices [19]. The poly lactide-co-glycolide (PLGA) biological scaffold, which can simultaneously simulate the effects of hypoxia and mechanical force environment, can provide a 3D HGFs culture model that is more similar to the tissue-like environment of natural ECM [4].
In this study, we established a 3D co-culture model of HGFs and PLGA scaffold and elicited a compressive stress analogous to produce an orthodontical force on cells to investigate the role of integrin α5β1, FAK, p-FAK, as well as F-actin cytoskeleton in HGFs mechanotransduction, and also to explore the role of integrin α5β1 in HGFs ECM accumulation. Our study provides additional insights into the mechanisms of gingival remodeling during orthodontic therapy.
Material and Methods
3D culture of HGFs
The gingival tissue samples (n=15) were obtained from healthy premolar teeth from patients ages 10–14 years during orthodontic extraction. Informed consent was obtained from each patient. All the study procedures were approved by the Ethics Review Board (approval no. 20150304-22) of Guangxi Medical University (Nanning, China). The gingival tissues attached to the root surface were scraped off and cut into small pieces. These tissues were cultivated in Eagle’s minimum essential medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), with an addition of 2 mM L-glutamine and antibiotics of 100 U/ml (Gibco; Thermo Fisher Scientific, Inc.). The cells were cultured at 37°C and 5% CO2. As described previously [20], the PLGA scaffold (size, 1×1 cm×300 μm; average pore size, 80–120 μm; porosity, 85%) was synthesized by the solvent casting/particulate leaching technique. The HGFs between 4 and 6 passages (1×105) were inoculated on a single sheet of PLGA scaffold to established a co-culture model of HGFs and PLGA scaffold.
Application of mechanical force
The above PLGA-HGF co-culture model was completely placed into a well for 24 h and then moved to another well. After 6-h incubation, the cells on the PLGA scaffold were subjected to a compressive stress of 25 g/cm2 for 3, 6, 12, 24, and 48 h by placing glass bottles containing lead granules on the top. The stress value was determined based on previous research demonstrating the peak HGF on the PLGA scaffold proliferation activity under the applied force of 25 g/cm2 [4]. In addition, the stress value was also consistent with the level reported by Schwarz [21], who proposed that the optimal force to move the tooth should be within the range of capillary pressure, which is 7–26 g/cm2. Control group cells were cultured under the same conditions without the application of compressive stress. In another experiment, the 3D-cultured cells were treated with 25 g/cm2 compressive force in the presence of 1 μM Ac-PHSCN-NH 2 (ATN-161), an integrin α5β1 inhibitor (MedChemExpress Monmouth Junction, NJ, USA), for 3, 6, 12, 24, and 48 h. To assess the cytotoxicity of ATN-161, a dose-response assay using the Cell Counting Kit-8 (CCK8; Dojindo, Japan) was performed. RT-qPCR was performed to evaluate the effect of the concentration of ATN-161 on integrin α5β1 in HGFs.
Immunofluorescence microscopy
The HGFs-PLGA complex that was treated with compressive force was washed with phosphate-buffered saline (PBS) 3 times, 5 min each time. Then, 4% paraformaldehyde was applied for cell fixation for 10 min, followed by permeabilization with 0.05% Triton X-100 for 5 min. The visualization of F-actin and nuclei was performed in accordance with the manufacturer’s instructions. Briefly, the complex was treated with rhodamine-phalloidin (100 nM) and DAPI (10 μg/ml) (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) for 30 min and 30 s at room temperature, respectively. Finally, the immunofluorescence micrographs were obtained by using a confocal laser scanning microscope (C1si; NIKON Corporation, Tokyo, Japan).
Quantitative real-time PCR (RT-qPCR)
TRIzol® reagent (Aidlab Biotechnologies., Ltd., Beijing, China) was used for extraction of total RNA from the HGFs-PLGA complex. The obtained RNAs were reversely-transcribed into cDNAs using an ExScript RT reagent kit (Takara Bio, Inc., Otsu, Japan). The RT-qPCR was performed based on the manufacturer’s instructions. Specifically, an ABI Prism 7500 system with the corresponding primers and an SYBR® Green and fluorescein qPCR Master Mix (Bio-Rad Laboratories, Inc., Hercules, CA, USA) were used for the quantification. The mRNA expression of integrin α5β1, FAK, and COL-1 were normalized to GAPDH. The primer sequences were:
ITGA5, Forward: 5′-CAAAGCCCTGAAGA TGCCCTA-3′;
ITGA5, Reverse: 5′-ATCCACAGTGGGACGCCATA-3′;
ITGB1, Forward: 5′-GGTTTCACTTTGCTGGAGATGG-3′;
ITGB1, Reverse: 5′-CAGTTTCTGGACAAGGTGAGCAATA-3′;
FTK, Forward: 5′-GCCTGTGGGTAAACCAGGTAA-3′;
FTK, Reverse: 5′-ACACCCTCGTTGTAGCTGTCA-3′;
COL-1, Forward: 5′-GAGGGCAACAGCAGGTTCACTTA-3′;
COL-1, Reverse: 5′-TCAGCACCACCGA TGTCCA-3′;
GAPDH, Forward: 5-GCACCGTCAAGGCTGAGAAC-3′;
GAPDH, Reverse: 5′-TGGTGAAGACGCCAGTGGA-3′;
All reactions were performed in triplicate and the 2−ΔΔCT method was used to determine the quantitative values of the relative expression levels.
Western blot analysis for protein expression
Western blot analysis was performed following standardized procedures to evaluate the expression levels of the proteins related to integrin α5β1, FAK, p-FAK, and COL-1 [22]. HGFs from the 3D models were dissociated from the PLGA scaffold by using 0.25% trypsin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and were lysed with ice-cold RIPA buffer (Beyotime, Shanghai, China) to isolate proteins. Then, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed and the protein specimens were thereby transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA), which were blocked with 5% non-fat dried milk in TBST for 1 h at 37°C. Subsequently, the primary antibodies were applied for overnight incubation at 4°C, including anti-integrin α5 (ab150361, Abcam), anti-integrin β1 (ab179471, Abcam), anti-FAK (ab40794, Abcam), anti-COL-1(ab138492, Abcam), anti-p-FAK (141-9, Invitrogen), and anti-Alpha Tubulin (Cell Signaling Technology), after which the membranes were incubated with the horseradish peroxidase (HRP)-conjugated secondary antibodies (Cell Signaling Technology) for 1 h at room temperature. Protein samples were normalized according to GAPDH (Sigma-Aldrich, T9026, clone DM1A). The relative density of protein expression was analyzed quantitatively with Image Lab v3.0 software (Bio-Rad, USA).
Statistical analysis
The experiments were conducted in triplicate, and results are presented as mean±standard deviation (SD). After testing for normality, one-way ANOVA was performed to evaluate the group differences. P value of less than 0.05 was considered as the criteria for statistical significance. All analyses were performed in SPSS v22.0 (IBM, Chicago, IL, USA).
Results
Induction effects of mechanical force on F-actin remodeling and stress fiber formation in HGFs
As presented in Figure 1, the application of mechanical force improved the regularity of the HGFs arrangement compared with the control group. Furthermore, mechanical stimulation also resulted in reorganization of actin cytoskeleton and stress fibers formed by the F-actin fiber polymerization. However, in the 48-h mechanical loading group, the F-actin cytoskeleton was depolymerized and reconstructed towards lack of any mechanical levels.
Figure 1.
Mechanical force induced the formation of stress fibers in HGFs. Rhodamine-phalloidin staining was used to evaluate the F-actin cytoskeleton in HGFs cultured on 3D PLGA scaffolds after applying a compressive force of 25 g/m2 at different time points (red, actin; blue, nuclei).
Improvement in the expression levels of integrin α5β1, FAK, and p- FAK in HGFs
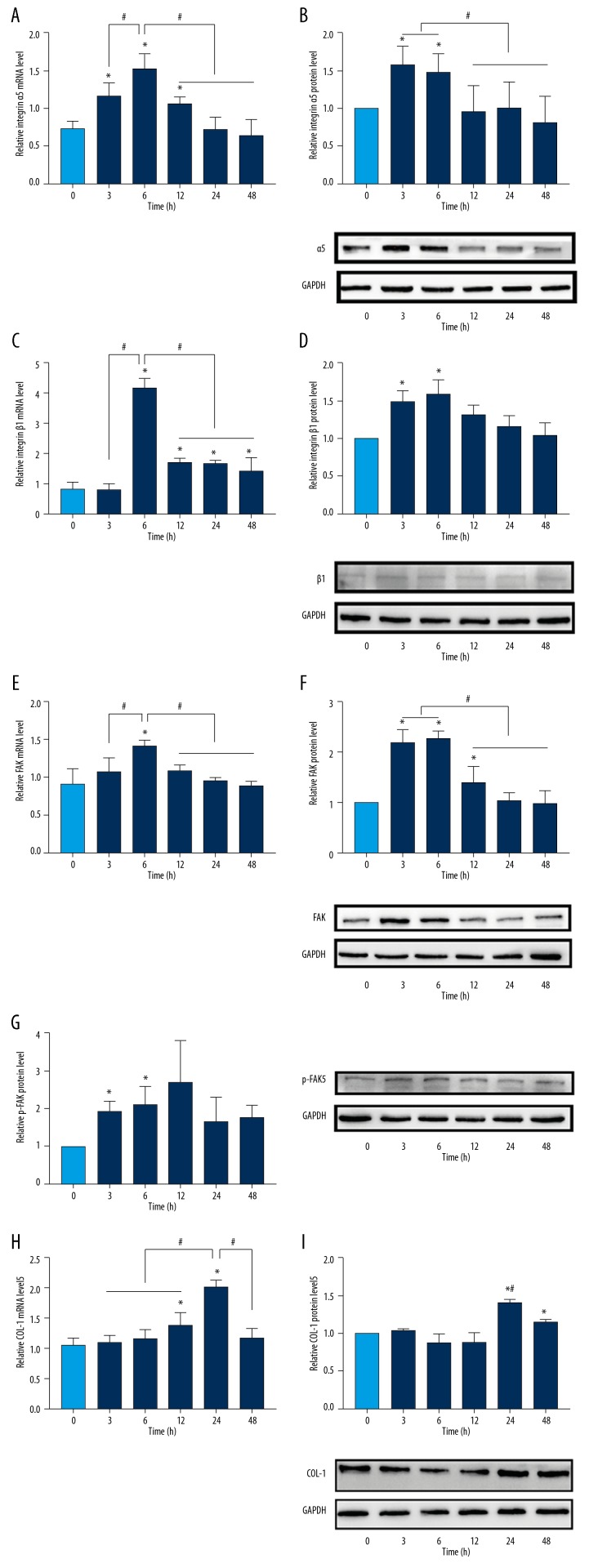
As shown in Figure 2A, 2C, and 2E, RT-qPCR showed a significant increase in the levels of integrin α5, integrin β1, and FAK under the mechanical stimulation compared with the control group, with the peak mRNA expression levels in the 6-h group. Integrin β1 and FAK showed the highest protein expression at 6 h (Figure 2D, 2F), while the highest protein expression of integrin α5 was detected at 3 h (Figure 2B). With respect to the levels of FAK phosphorylation, the protein expression of p-FAK was upregulated within the first 12 h and then decreased over time (Figure 2G). These findings suggest that the mechanical force was a sufficient stimulus to promote integrin α5β1 and FAK activation in HGFs.
Figure 2.
Mechanical force increased integrin α5β1, FAK, p-FAK, and COL-1 expression in HGFs. mRNA expression: (A) integrin α5, (C) integrin β1, (E) FAK, (H) COL-1 and protein expression: (B) integrin α5, (D) integrin β1, (F) FAK, (G) p-FAK, (I) COL-1, were evaluated after applying a compressive force of 25 g/m2 on HGFs at different time points. Data are presented as means±standard deviations, and were obtained from 3 independent experiments. * p<0.05 vs. 0 h control. # p<0.05 vs. mechanical stress group.
Improvement in the expression levels of COL-1 in HGFs
We also examined COL-1 expression of HGFs in response to compressive force. Compared with the control group, the expression level of COL-1 was upregulated, and the highest expression level was detected at 24 h (Figure 2H, 2I). Thus, the mechanical force stimulus led to higher collagen expression in gingival tissue.
Reversal of the mechanical force effects by blocking integrin α5β1
Regarding the effective concentration of ATN-161 in HGFs, a low dose of ATN-161 (10 nM, 100 nM, 1 μM, 10 μM) showed no significant effect on the proliferation of HGFs, but a high dose of ATN-161 (100 μM, 1 mM) significantly inhibited the proliferation of HGFs on the PLGA scaffold (Figure 3A, 3B). Additionally, as described by Doñate [23], ATN-161 exhibited a U-shaped dose-response curve in several pre-clinical models. Our results also suggested that 1 μM ATN-161 can strongly inhibit integrin a5b1 expression, while 10 μM did not exert similar effects on the expression in HGFs (Figure 3C, 3D).
Figure 3.
1 μM ATN-161 strongly inhibited integrin α5β1. (A, B) The cytotoxicity of ATN-161 was evaluated using Cell Counting Kit-8. The mRNA expression of (C) integrin α5 and (D) integrin β1 were evaluated using quantitative real-time polymerase chain reaction (RT-qPCR). Data are presented in the form of means±standard deviations, and were obtained from 3 independent experiments. * p<0.05 vs. 0 h control. # p<0.05 vs. mechanical stress group.
According to our analysis, the significantly increased levels of FAK and p-FAK in HGF under mechanical force were largely reversed by the integrin α5β1 inhibitor (Figure 4A, 4C, 4D). It is noteworthy that integrin α5β1 blockade decreased the mRNA level of COL-1 in HGF, which was most significant after 6 h (Figure 4B). Although the protein expression of COL-1 decreased after 24 h, a temporary increase in the protein expression of COL-1 was observed within 3–6 h (Figure 4E). These data suggest that integrin α5β1 is an effective mediator in increasing the levels of FAK and phosphorylated FAK, and there could be other biomarkers that are involved in the production of COL-1 in HGFs under mechanical force.
Figure 4.
The mRNA expression: (A) FAK, (B) COL-1 and protein expression: (C) FAK, (D) p-FAK, (E) COL-1, were evaluated after treated with compressive force of 25 g/m2 and integrin α5β1 inhibitor on HGFs at different time points. Data are presented as means±standard deviations, and were obtained from 3 independent experiments. * p<0.05 vs. 0 hour control. # p<0.05 vs. mechanical stress group.
Discussion
Our study simulated the real condition by using the HGF-PLGA 3D co-cultured model and showed the role of a typical ECM-integrin-cytoskeleton pathway in the mechanotransduction of HGFs. In this process, compressive forces can allosterically modulate the function of integrin a5b1 in the adhesion structures and thus cause biochemical signaling cascades, which not only trigger rapid changes in signal transduction by affecting the dynamics of actin cytoskeleton, but also induce long-term changes in collagen synthesis by regulating gene expression [7].
In our study, the expression levels of FAK and p-FAK were upregulated under the application of mechanical stress, which was assumed to be related to the activation of integrin through binding with the FAK FERM domain. This elevated activity of FAK might be associated with the cytoskeletal dynamics and focal contacts, and facilitates a direct signal pathway between the focal contacts and the nucleus [24]. The most characterized FAK phosphorylation event is auto-phosphorylation at Tyr397. The upregulated p-FAK promotes Src binding, which activates the FAK-Src signaling complex. This FAK-Src-mediated complex can promote maximal FAK catalytic activation and lead to the activation of Ras-extracellular signal-regulated kinase-2 (ERK2) and Rac–Jun N-terminal kinase (JNK) signaling cascades, and further promote the formation of stress fibers [25]. Stress fibers are large bundles of F-actin that passes through the cell and are anchored at both ends by focal adhesions [26]. They are considered to be the orbit of extracellular-intracellular signal transduction, which also enables HGFs to resist and buffer external pressure, thus maintaining cell morphology. Live-cell microscopy has revealed that polymerized actin can drive the actin network to “flow” from the outer edge of the synapse inward, and provide power for the activation of downstream signaling pathways and regulation of gene transcription [27]. In our study, 48-h continuous compressive force did not make the F-actin polymerize further, but rather it began to depolymerize, which may be related to the downregulation of integrin α5β1 and FAK, which weakened its effect on actin polymerization.
Mechanical force can promote collagen synthesis in HGFs, which is consistent with the results from our previous study and other research [4]. It was reported that fibroblasts are sensitive to the mechanics of their microenvironment, and aberrant tissue mechanics drive fibrotic responses [28]. In recent years, numerous studies have reported the key role of the integrin-mediated signaling pathway in ECM accumulation in the process of tissues fibrosis, such as in renal fibrosis and cardiac fibroblasts [29–31]. In these fibrotic tissues, ECM synthesis was significantly suppressed when an integrin inhibitor was administrated. In the present study, the mRNA expression of COL-1 was downregulated after 6 h of mechanical force stimulation in the presence of integrin α5β1 inhibitor, suggesting that gingival collagen synthesis under mechanical force can be mediated, in part, by integrin α5β1 engagement. However, a temporary increase was also observed in the production of COL-1 protein in the above HGFs within 3–6 h. These findings could be of great significance to clinical practice because the post-transcriptional gene regulation was independent of their upregulation or downregulation. The downstream process of transcription has a strong regulatory effect on protein abundance [32]. The production of collagen is also involved in the feedback regulation of translation and secretion [33]. Additionally, Wenjing et al. demonstrated that although corneal fibrosis can be inhibited by integrin α5β6 knockout, certain alternate mechanisms can initiate fibrosis 7–14 days later [34]. In conclusion, we speculate that different signals are amplified to the mRNA transcription of COL-1 through certain regulatory factors via a post-transcriptional mechanism in the process of mechanical stimulation of HGFs, thus improving the COL-1 protein abundance in the early period. HGFs use a multitude of mechanisms to respond to mechanical forces and initiate collagen synthesis. However, as this study only used ATN-161 to inhibit integrin α5β1, and additional rigorous methods, such as small interfering RNAs and gene knockout, are required to further investigate this hypothesis.
In addition to integrins, the formation of stress fibers is also considered to cause fibrosis [35]. Stress fibers are only produced when cells are subjected to mechanical stress and are considered to be tissue-specific structures [36]. Parrish et al. explored the role of the cytoskeleton in the process of renal fibrosis, and found that the polymerization of actin cytoskeleton resulted in tissue fibrosis. Destruction of stress fibers can reduce the expression of fibrogenic factors such as TGF-β1 in human glomerular endothelial cells, thus inhibiting the renal fibrosis pathway [37]. Sandeep et al. also found that continuous activation of the epithelial-interstitial transformation process in response to chronic inflammation can lead to fibrosis. In this process, the formation of stress fibers increases cell contractility and affects cell mechanics, and also promotes the process of tissue and organ fibrosis, which results in tissue and organ texture hardening [38]. Thus, we speculate that the orthodontic force changed the ECM of HGFs, which increased the synthesis of collagen fibers and the formation of stress fibers. The abnormal production of collagen and stress fibers can lead to accumulation of gingival tissue, which ultimately affects the efficiency and stability of orthodontic treatment.
Conclusions
This study demonstrated activation of the integrin α5β1/FAK signaling pathway, reorganization of cytoskeleton, and increased COL-1 synthesis in HGFs cultured on 3D PLGA scaffolds under mechanical force. Specifically, blocking integrin α5β1 can partially but not completely inhibit collagen synthesis. These findings provide additional insights into the mechanisms of mechanotransduction and ECM synthesis in HGFs, and may contribute to the development of targeted therapies by promoting gingival tissue remodeling.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (No. 81560188) and the Scientific Research Project Foundation of Health Department of Guangxi (No. Z2013155)
Conflicts of interest
None.
References
- 1.Dutra EH, Ahmida A, Lima A. The effects of alveolar decortications on orthodontic tooth movement and bone remodelling in rats. Eur J Orthod. 2018;40:423–29. doi: 10.1093/ejo/cjx080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang N, Guo W, Chen M, et al. Periodontal ligament and alveolar bone in health and adaptation: Tooth movement. Front Oral Biol. 2016;18:1–8. doi: 10.1159/000351894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redlich M, Shoshan S, Palmon A. Gingival response to orthodontic force. Am J Orthod Dentofacial Orthop. 1999;116:152–58. doi: 10.1016/s0889-5406(99)70212-x. [DOI] [PubMed] [Google Scholar]
- 4.Nan L, Zheng Y, Liao N, et al. Mechanical force promotes the proliferation and extracellular matrix synthesis of human gingival fibroblasts cultured on 3D PLGA scaffolds via TGFbeta expression. Mol Med Rep. 2019;19:2107–14. doi: 10.3892/mmr.2019.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong S, Aoki A, Iwasaki K. Biological effects of laser irradiation on the proliferation of primary human gingival fibroblasts. J Biophotonics. 2018;11:e201700157. doi: 10.1002/jbio.201700157. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan V, Ambili R, Davidovitch Z. Gingiva and orthodontic treatment. Seminars in Orthodontics. 2007;13:257–71. [Google Scholar]
- 7.Sun Z, Costell M, Fassler R. Integrin activation by talin, kindlin and mechanical forces. Nat Cell Biol. 2019;21:25–31. doi: 10.1038/s41556-018-0234-9. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal A, Lingappa J, Leppla SH, et al. Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin. Nature. 2003;424:329–34. doi: 10.1038/nature01794. [DOI] [PubMed] [Google Scholar]
- 9.Georgiadou M, Lilja J, Jacquemet G, et al. AMPK negatively regulates tensin-dependent integrin activity. J Cell Biol. 2017;216:1107–21. doi: 10.1083/jcb.201609066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bharadwaj M, Strohmeyer N, Colo GP, et al. alphaV-class integrins exert dual roles on alpha5beta1 integrins to strengthen adhesion to fibronectin. Nat Commun. 2017;8:14348. doi: 10.1038/ncomms14348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strohmeyer N, Bharadwaj M, Costell M, et al. Fibronectin-bound α5β1 integrins sense load and signal to reinforce adhesion in less than a second. Nat Mater. 2017;16:1262. doi: 10.1038/nmat5023. [DOI] [PubMed] [Google Scholar]
- 12.Schnittert J, Bansal R, Storm G. Integrins in wound healing, fibrosis and tumor stroma: High potential targets for therapeutics and drug delivery. Adv Drug Deliv Rev. 2018;129:37–53. doi: 10.1016/j.addr.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Shams H, Hoffman BD, Mofrad MRK. The “stressful” life of cell adhesion molecules: on the mechanosensitivity of integrin adhesome. J Biomech Eng. 2018;140(2) doi: 10.1115/1.4038812. [DOI] [PubMed] [Google Scholar]
- 14.Ghatak S, Misra S, Moreno-Rodrigue RA, et al. Periostin/β1integrin interaction regulates p21-activated kinases in valvular interstitial cell survival and in actin cytoskeleton reorganization. Biochim Biophys Acta Gen Subj. 2019;1863(5):813–29. doi: 10.1016/j.bbagen.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Babbitt CJ, Shai SY, Harpf AE, et al. Modulation of integrins and integrin signaling molecules in the pressure-loaded murine ventricle. Histochem Cell Biol. 2002;118:431–39. doi: 10.1007/s00418-002-0476-1. [DOI] [PubMed] [Google Scholar]
- 16.Saucerman JJ, Tan PM, Buchholz KS, et al. Mechanical regulation of gene expression in cardiac myocytes and fibroblasts. Nat Rev Cardiol. 2019;16:361–78. doi: 10.1038/s41569-019-0155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu M, Dai J, Lin Y, et al. Effect of the cyclic stretch on the expression of osteogenesis genes in human periodontal ligament cells. Gene. 2012;491:187–93. doi: 10.1016/j.gene.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 18.Xu QC, Kuang RC, Wei SQ, et al. Analysis of mechanical behavior of dermal fibroblasts obtained from various anatomical sites in human to explore the optimum stretch magnitude. Ann Plast Surg. 2017;79:438–43. doi: 10.1097/SAP.0000000000001121. [DOI] [PubMed] [Google Scholar]
- 19.Sachar A, Strom TA, San Miguel S, et al. Cell-matrix and cell-cell interactions of human gingival fibroblasts on three-dimensional nanofibrous gelatin scaffolds. Tissue Eng Regen Med. 2012;8:862–73. doi: 10.1002/term.1588. [DOI] [PubMed] [Google Scholar]
- 20.Thadavirul N, Pavasant P, Supaphol P. Improvement of dual-leached polycaprolactone porous scaffolds by incorporating with hydroxyapatite for bone tissue regeneration. J Biomater Sci. 2014;25:1986–2008. doi: 10.1080/09205063.2014.966800. [DOI] [PubMed] [Google Scholar]
- 21.Schwarz AM. Tissue changes incidental to orthodontic tooth movement. Int J Orthod. 1932;18:331–52. [Google Scholar]
- 22.Qin Q, Cui L, Zhou Z, et al. Inhibition of microRNA-141-3p reduces hypoxia-induced apoptosis in H9c2 rat cardiomyocytes by activating the RP105-dependent PI3K/AKT signaling pathway. Med Sci Monit. 2019;25:7016–25. doi: 10.12659/MSM.916361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doñate F, Parry GC, Shaked Y, et al. Pharmacology of the novel antiangiogenic peptide ATN-161 (Ac-PHSCN-NH2): Observation of a U-shaped dose-response curve in several preclinical models of angiogenesis and tumor growth. Clin Cancer Res. 2008;14:2137–44. doi: 10.1158/1078-0432.CCR-07-4530. [DOI] [PubMed] [Google Scholar]
- 24.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 25.Elizabeth MB, Erika S, Wittchena CM, et al. A Rnd3/p190RhoGAP pathway regulates RhoA activity in idiopathic pulmonary fibrosis fibroblasts. Mol Biol Cell. 2018;29:2165–75. doi: 10.1091/mbc.E17-11-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burridge K, Guilluy C. Focal adhesions, stress fibers and mechanical tension. Exp Cell Res. 2016;343:14–20. doi: 10.1016/j.yexcr.2015.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy NH, Burkhardt JK. The actin cytoskeleton: A mechanical intermediate for signal integration at the immunological synapse. Front Cell Dev Biol. 2018;6:116. doi: 10.3389/fcell.2018.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiore VF, Wong SS, Tran C, et al. alphavbeta3 Integrin drives fibroblast contraction and strain stiffening of soft provisional matrix during progressive fibrosis. JCI Insight. 2018;3(20) doi: 10.1172/jci.insight.97597. pii: 97597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun M, Krauszman A, Breitling S, et al. Experimental right ventricular hypertension induces regional β1-integrin – mediated transduction of hypertrophic and profibrotic right and left ventricular signaling. J Am Heart Assoc. 2018;7:e007928. doi: 10.1161/JAHA.117.007928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrucci GL, Barbagallo VA, Corliano M, et al. Integrin alphanubeta5 in vitro inhibition limits pro-fibrotic response in cardiac fibroblasts of spontaneously hypertensive rats. J Transl Med. 2018;16:352. doi: 10.1186/s12967-018-1730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bon H, Hales P, Lumb S, et al. Spontaneous extracellular matrix accumulation in a human in vitro model of renal fibrosis is mediated by alphaV integrins. Nephron. 2019;142:328–50. doi: 10.1159/000499506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christine V, Marcott EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2013;13:227–34. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwarz RI. Collagen I and the fibroblast: high protein expression requires a new paradigm of post-transcriptional, feedback regulation. Biochem Biophys Rep. 2015;3:38–44. doi: 10.1016/j.bbrep.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu W, Hutcheon AEK, Sriram S, et al. Initiation of fibrosis in the integrin αvβ6 knockout mice. Exp Eye Res. 2019;180:23–28. doi: 10.1016/j.exer.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsoyi K, Chu SG, Patino-Jaramillo NG, et al. Syndecan-2 attenuates radiation-induced pulmonary fibrosis and inhibits fibroblast activation by regulating PI3K/Akt/ROCK pathway via CD148. Am J Respir Cell Mol Biol. 2018;58:208–15. doi: 10.1165/rcmb.2017-0088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Theodosiou M, Widmaier M, Böttcher RT, et al. Kindlin-2 cooperates with talin to activate integrins and induces cell spreading by directly binding paxillin. Elife. 2016;52:10130. doi: 10.7554/eLife.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parrish A. The cytoskeleton as a novel target for treatment of renal fibrosis. Pharmacol Ther. 2016;166:1–8. doi: 10.1016/j.pharmthera.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Nalluri SM, O’Connor JW, Gomez EW. Cytoskeletal signaling in TGFβ-induced epithelial – mesenchymal transition. Cytoskeleton. 2015;72:557–69. doi: 10.1002/cm.21263. [DOI] [PubMed] [Google Scholar]