Abstract
Background
This study aimed to investigate the effects of dexmedetomidine in a rat model of sepsis-induced lung injury and the role of the adenosine monophosphate-activated protein kinase (AMPK) gene and silent information regulator 1 (SIRT1) gene signaling pathway.
Material/Methods
Sixty 28-week-old healthy male Sprague-Dawley rats were randomly divided into three groups, the sham group, the model group, and the dexmedetomidine-treated group. The rat model of sepsis-induced lung injury was developed by surgical cecal ligation and puncture. Lung tissues examined histologically in the three study groups. Cell apoptosis was measured using the TUNEL assay, and the expression of inflammatory cytokines, tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-10 were measured in rat lung tissue by enzyme-linked immunosorbent assay (ELISA). Apoptosis-associated proteins and AMPK/SIRT1 pathway-associated protein expression levels were detected using Western blot.
Results
Dexmedetomidine significantly increased the survival rate and reduced the body temperature of rats in the model group with sepsis-induced lung injury, reduced lung injury, significantly reduced apoptosis in lung tissues, and reduced the expression levels of TNF-α, and IL-1β, and increased the levels of IL-10. Dexmedetomidine significantly reduced the expression of caspase-3 in the rat lung tissue (P<0.01), and significantly increased the expression of Bcl-2/Bax and the phosphorylation levels of AMPK, SIRT1, nuclear factor-κB (NF-κB), and forkhead box class O 3a (FOXO3a).
Conclusions
In a rat model of sepsis-induced lung injury, dexmedetomidine reduced lung damage by activating the AMPK/SIRT1 signaling pathway and reduced the expression of inflammatory cytokines and cell apoptosis.
MeSH Keywords: Inflammation, Lung Injury, Sepsis
Background
Sepsis is a potentially fatal complication of critical illness, such as trauma, shock, and major surgery, and results from systemic infection that involves all major organ systems, resulting in multiple organ failure [1]. The lung is a susceptible organ of sepsis, and acute lung injury is an important cause of death in patients with sepsis [2]. Sepsis-induced lung injury increases the permeability of pulmonary capillaries, leading to edema, alveolar atelectasis, and the formation of hyaline membranes, followed by pulmonary interstitial fibrosis [3]. Shen et al. [4] showed that in sepsis-induced lung injury, increased inflammation is an important cause of acute lung injury, which is associated with the expression of inflammatory cytokines and platelet activation, resulting in thrombotic vascular occlusion and further ischemic damage to the lung. Previously published studies have shown that reducing the sepsis-induced inflammatory response and reducing the levels of inflammatory cytokines significantly reduced tissue damage [5].
Dexmedetomidine is an adrenergic receptor agonist that is commonly used in anesthesia and analgesia during surgery. Ge et al. [6] found that the analgesic effect of dexmedetomidine was not significant under normal conditions, while it can exert a potent analgesic effect by inhibiting the sensory conduction pathway from the dorsal horn and of the spinal cord and following the inflammatory response in vivo. The findings from a study in a rat model of sepsis reported by Feng et al. [7] showed that dexmedetomidine could effectively increase the vascular reactivity of exogenous catecholamines.
Adenosine monophosphate-activated protein kinase (AMPK) is a crucial energy metabolism receptor in vivo, which is found in eukaryotic cells. After activation, AMPK can significantly reduce the inflammatory effect of tumor necrosis factor-α (TNF-α) and inhibit the release of inflammatory cytokines [8]. Silent information regulator 1 (SIRT1) is a classical histone deacetylase that is involved in the inflammatory response. Chen et al. [9] found that AMPK phosphorylates SIRT1 to activate downstream proteins and inhibit the release of inflammatory cytokines, resulting in an anti-inflammatory effect. Currently, there have been few studies on the effect of dexmedetomidine on the AMPK/SIRT1 signaling pathway in organ damage due to sepsis. Therefore, this study aimed to investigate the effects of dexmedetomidine in a rat model of sepsis-induced lung injury and the role of the AMPK and SIRT1 signaling pathway.
Material and Methods
Materials and equipment
Rat tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-10 enzyme-linked immunosorbent assay (ELISA) kits were obtained from Wuhan Boster Biological Technology Co., Ltd. (Wuhan, China). Rabbit antibodies to caspase-3, B-cell lymphoma-2 (Bcl-2), Bcl-2 associated X protein (Bax), AMPK, SIRT1, nuclear factor-κB (NF-κB), FoxO3a, p-AMPK, p-SIRT1, p-NF-κB, p-FoxO3a, and GAPDH were obtained from Cell Signaling Technology (Danvers, MA, USA), The terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) apoptosis kit was purchased from Beyotime (Shanghai, China). A TRIzol kit (Invitrogen, Carlsbad, CA, USA), pipettes(Eppendorf, Hamburg, Germany), electronic balance (Sartorious, Ilshofen, Germany), refrigerated centrifuge (Thermo Fisher Scientific, Waltham, MA, USA), freezing microtome (Leica, Wetzlar, Germany), and fluorescence microscope (Nikon, Tokyo, Japan) were used. The ophthalmic scissors, bent ophthalmic forceps, straight forceps, bent vessel forceps, gauze, medical cotton balls, and sutures were obtained from Harbin Medical Devices Co., Ltd. (Harbin, China).
Laboratory animals and grouping
Sixty 28-week-old healthy male Sprague-Dawley rats were provided by the Guangdong Laboratory Animal Center (Guangzhou, China), and were housed at a temperature of 25±1°C, a humidity of 50±3% and a 12-hour light and dark cycle. The frats were given free access to food and water. The rats were divided into three study groups the sham group (N=20), the model group (N=20), and the dexmedetomidine-treated group (N=20). The study sample size was determined by preliminary experiments (data not shown).
The rat model of sepsis-induced lung injury was established by cecal ligation and puncture in the model group and the dexmedetomidine group. Dexmedetomidine (10 μg/kg) was injected intraperitoneally at 10 min after anesthesia in the dexmedetomidine group. Cecal ligation was not performed in the sham group, but surgery was otherwise similar to the model group. At 12 h before the experiments, the rats were fasted but allowed water to drink. This study was approved by the Animal Ethics Committee of Guangxi University of Traditional Chinese Medicine (Approval No. GX20180201).
Establishment of the rat model of sepsis-induced lung injury
The rat model of sepsis-induced lung injury included cecal ligation and puncture, as previously described [10]. Following anesthesia with an intraperitoneal injection of 1% pentobarbital sodium (40 mg/kg), the abdominal hair was shaved using a razor and the rats were secured on an operating table, the abdomen was disinfected with iodine and covered with a sterile sheet. The rats were breathing spontaneously and the abdomen opened along the midline of the mid-lower abdomen to expose the cecum. The fecal contents of the cecum were squeezed to the distal end. The inferior margin of the cecum and the cecal artery were punctured with the 6/0 silk thread at the inferior margin of the cecum and the cecum was ligated. The ileum and cecal mesenteric vessels were not ligated. The dorsal distal and proximal ends of the cecum were punctured through-and-through once using an 18 F needle and fecal content was squeezed out. The cecum was then placed back into the abdominal cavity. The abdominal wall incision was sutured in layers. Then, sterile normal saline (5 mL/100 g) at 37°C was subcutaneously injected to resuscitate the rats. The rats without analgesia were placed on a warming blanket after surgery.
Survival rates following surgery and measurement of the body temperature
After surgery, the survival duration in the rats was observed. The anal temperature in each rat group was measured before surgery and at 24 h after surgery. The changes in body temperature were recorded and calculated as follows:
Lung histology
At 24 h following surgery, lung injury in the rat groups was assessed using histology of rat lung tissue sections that were stained with hematoxylin and eosin (H&E). After anesthesia with 5% isoflurane, the rats were euthanized by cervical dislocation, the left lung was isolated, blood was washed away three times with pre-cooled PBS, and the excess adipose tissues and connective tissues on the lungs were removed. The lung tissues were fixed with 10% formalin for 36 h, dehydrated with ethanol and xylene, and embedded in paraffin wax. Tissue sections were cut onto glass slides at 4 μm in thickness using a microtome. The lung tissue sections were deparaffinized with xylene, dehydrated with graded ethanols, stained with hematoxylin for 10 min, rinsed with tap water, differentiated with hydrochloric acid alcohol for 20 s, and rinsed again with tap water. After dehydration with 70% and 80% ethanol for 5 min, the sections were stained with eosin for 15 min, dehydrated with gradient ethanol, cleared with xylene, and sealed with neutral resin.
Histology of the rat lung tissue sections from rats in the study groups was evaluated by three experienced pathologists who were unaware of the assignment of the study groups. Lung tissue sections from each rat were observed in ten high-power visual fields, and the average value was recorded. A visual semiquantitative scoring system was used to evaluate the rat lung injury, as follows: 0, no injury; 1, <25% lung injury; 2, 25–50% lung injury; 3, 50–75% lung injury; 4, lung injury seen in the whole microscopic field. The total lung injury score was the sum of these scores.
Measurement of cell apoptosis in rat lung tissues using TUNEL staining
The apoptosis level of lung tissues was detected using a TUNEL apoptosis kit. The tissues were observed and photographed. The number of apoptotic cells in the lung tissues was calculated. The apoptotic cells showed yellow-green fluorescence, while the normal cells showed no fluorescence.
Measurement of inflammatory cytokines in rat lung tissues by ELISA
Levels of the inflammatory cytokines, TNF-α, IL-1β, and IL-10 in rat lung tissues in each group were determined using ELISA kits: The lung tissues were taken, lysed with an appropriate amount of RIPA lysis buffer, and diluted at 1: 100 using the sample diluent. The standards of TNF-α, IL-1β, and IL-10 at concentrations of 1000, 500, 250, 125, 62.5, 31.3, 15.6, and 0 pg/mL were prepared and used to determine the standard curves. The biotin-labeled anti-mouse TNF-α, IL-1β, and IL-10 antibody working solution and avidin-biotin-peroxidase complex (ABC) working solution were also prepared. ELISA was performed according to the manufacturer’s instructions. The standard curves were plotted, based on which the content of TNF-α, IL-1β, and IL-10 in each group was calculated.
Detection of cytokine protein expression by Western blot
The RIPA lysis buffer was used to extract the total protein in each group of lung tissues. The bicinchoninic acid (BCA) kit (Invitrogen, Carlsbad, CA, USA) was used to quantitate the protein concentration. The sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel was prepared, and equal amounts of loading buffer were added for electrophoresis. Protein samples were electrophoresed and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). After blocking with 5% dried skimmed milk powder, the membranes were incubated with the primary antibodies to caspase-3, Bcl-2, Bax, AMPK, SIRT1, NF-κB, FoxO3a, p-AMPK, p-SIRT1, p-NF-κB, p-FoxO3a, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) at 4°C overnight. The membrane was incubated with the secondary antibody after rinsing with the TBST buffer. Freshly-prepared electrochemiluminescence (ECL) solution was used for image development and analysis.
Statistical analysis
Data were analyzed using SPSS version 22.0 software (SPSS Inc., Chicago, IL, USA). Quantitative data were expressed as the mean±standard deviation (SD). Survival curves were plotted using the Kaplan-Meier method, and the log-rank test was used for analysis. Analysis of variance (ANOVA) was used for comparisons between groups. Bonferroni’s test was used for pairwise comparisons, and Welch’s method was used in cases with heterogeneity of variance. A P-value <0.05 was considered statistically significant.
Results
The survival rate of rats and change in body temperature
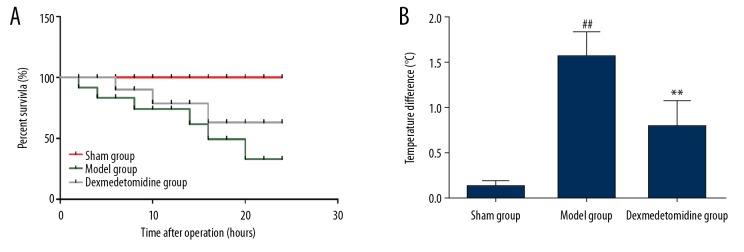
The rat model of sepsis-induced lung injury was established by cecal ligation and puncture. At 24 h following surgery, the survival rate of all rats was observed, and the anal temperature was measured before and after surgery. As shown in Figure 1, the survival rate of rats was significantly lower in the model group than in the sham group (35% vs. 100%; P<0.01). The survival rate of rats was significantly greater in the dexmedetomidine group compared with the model group (65% vs. 35%; P=0.006). The mean survival time (19.3 h vs.17.1 h) was significantly greater in the dexmedetomidine group compared with the model group. At 24 h following surgery, the body temperature was significantly increased in the model group compared with the sham group (P<0.05), while dexmedetomidine pretreatment significantly reduced the body temperature in the model group (P<0.05).
Figure 1.
Rat survival rates in the three study groups, the sham group, the model group, and the dexmedetomidine-treated group. (A) The survival rate of rats in each group, and (B) changes in body temperature. The survival rate of rats in the model group is significantly lower than that in the sham group and dexmedetomidine group, and the body temperature in the model group is significantly higher than that in the sham group and the dexmedetomidine group. ** P<0.01 compared with the model group, ## P<0.01 compared with the sham group.
Histology of the lung
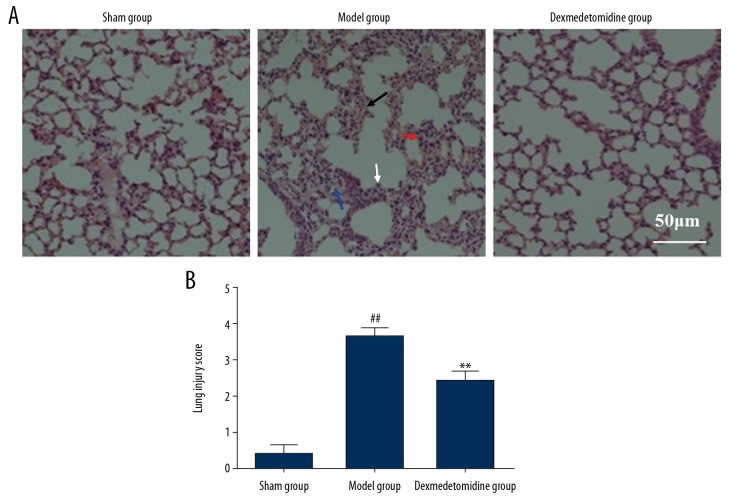
Following surgery to create the rat model of sepsis-induced lung injury, the left lung was removed from each rat. Lung injury was evaluated by light microscopy following routine staining with hematoxylin and eosin (H&E). As shown in Figure 2, there was increased alveolar edema (black arrow), alveolar fusion and hemorrhage in lung tissues (blue arrow), pulmonary septal thickening (white arrow), and inflammation (red arrow) in the lung parenchyma in the model group compared with the sham group. Pretreatment with dexmedetomidine significantly reduced the degree of inflammation in the lung parenchyma, reduced alveolar collapse, edema, and hemorrhage, and significantly reduced lung injury.
Figure 2.
Histological analysis of the rat lung tissues in the three study groups, the sham group, the model group, and the dexmedetomidine-treated group. (A) Lung injury was detected histologically. Hematoxylin and eosin (H&E). (Scale bar=50 μm). (B) Semiquantitative analysis of lung injury using histological scores. ** P<0.01 compared with the model group, ## P<0.01 compared with the sham group.
Cell apoptosis in lung tissues detected by TUNEL staining
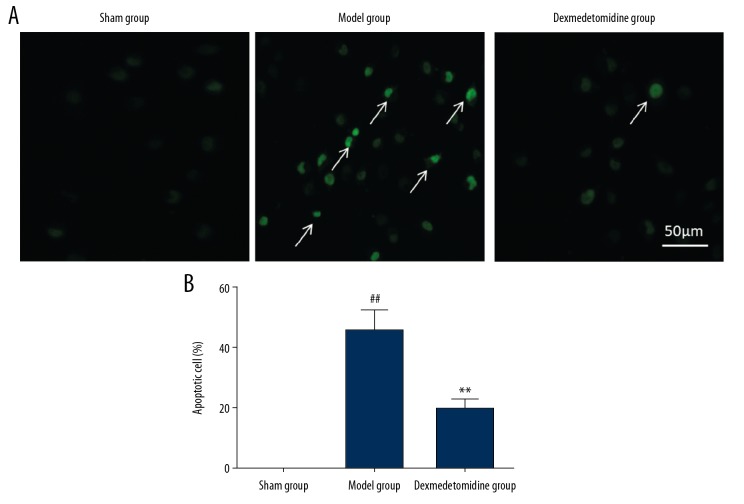
The TUNEL staining results showed that the model group had significantly increased numbers of TUNEL-positive apoptotic cells in the lung tissues compared with the sham group (P<0.01). Pretreatment with dexmedetomidine significantly reduced the number of TUNEL-positive cells in the lung tissues (P<0.01) and reduce the levels of cell apoptosis (Figure 3).
Figure 3.
(A, B) Cell apoptosis in the rat lung tissues in the three study groups, the sham group, the model group, and the dexmedetomidine-treated group, detected by TUNEL staining (A). Cell apoptosis levels in lung tissues in the model group are significantly higher than that in the sham group and the dexmedetomidine-treated group (B). ** P<0.01 compared with the model group, ## P<0.01 compared with the sham group.
Detection of inflammatory cytokines in the lung tissue by enzyme-linked immunosorbent assay (ELISA)
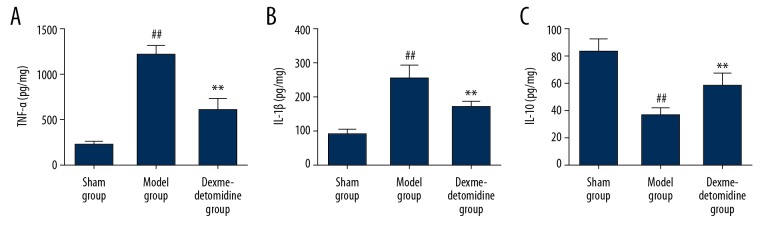
The ELISA results showed that the model group had significantly increased levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) (P<0.01) and significantly reduced levels of IL-10 in lung tissues compared with the sham group (P<0.01). Dexmedetomidine significantly reduced the TNF-α and IL-1β levels (P<0.01) and increased the content of IL-10 in lung tissues (P<0.01) (Figure 4).
Figure 4.
(A–C) The levels of inflammatory cytokines in lung tissues in the three study groups, the sham group, the model group, and the dexmedetomidine-treated group detected by enzyme-linked immunosorbent assay (ELISA). The levels of tumor necrosis factor-α (TNF-α) (A) and interleukin-1β (IL-1β) (B) in lung rat tissues is significantly higher in the model group compared with the sham group and the dexmedetomidine-treated group. ** P<0.01 compared with the model group, ## P<0.01 compared with the sham group.
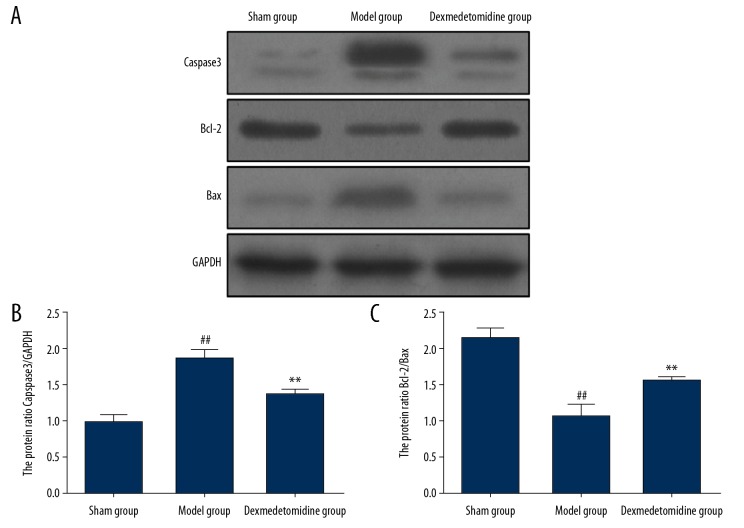
Expression levels of apoptosis-related proteins by Western blot
The expression of caspase-3 in the rat lung tissues in the model group was significantly increased compared with the sham group (P<0.01). Bcl-2/Bax expression levels in the model group were significantly reduced compared with the sham group (P<0.01). Dexmedetomidine significantly reduced the expression of caspase-3 in lung tissues (P<0.01) and increased the expression of Bcl-2/Bax (P<0.01) (Figure 5).
Figure 5.
Expression levels of apoptosis-associated proteins in lung tissues in the three study groups, the sham group, the model group, and the dexmedetomidine-treated group determined by Western blot. (A) In the model group, the expression of caspase-3 (B) in the lung tissues is significantly higher than that in the sham group and the dexmedetomidine-treated group, while Bcl-2/Bax (C) are significantly lower compared with the sham group and the dexmedetomidine-treated group. ** P<0.01 compared with the model group, ## P<0.01 compared with the sham group.
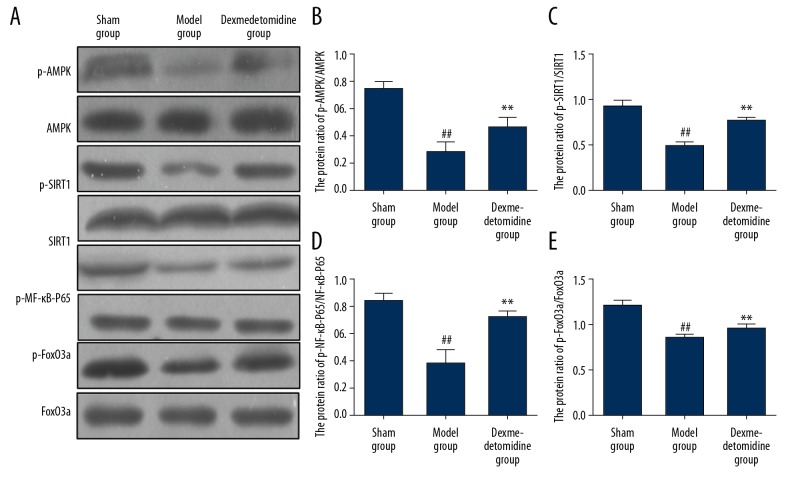
The effect of dexmedetomidine on the AMPK/SIRT1 signaling pathway
The expression levels of AMPK/SIRT1 signaling pathway-associated proteins in lung tissues were determined using Western blot. Expression levels of p-AMPK, p-SIRT1, p-NF-κB-P65, and p-FoxO3a in rat lung tissues were significantly reduced in the model group compared with the sham group (P<0.01). Dexmedetomidine significantly increased the expression levels of p-AMPK, p-SIRT1, p-NF-κB-P65, and p-FoxO3a in rat lung tissues (P<0.01) (Figure 6).
Figure 6.
The expression of AMPK/SIRT1 signaling pathway-associated proteins in lung tissues in the three study groups, the sham group, the model group, and the dexmedetomidine-treated group determined by Western blot (A). The expression levels of p-AMPK (B), p-SIRT1 (C), p-NF-κB-P65 (D), and p-FoxO3a (E) in lung tissues in the model group are significantly lower compared with the sham group and the dexmedetomidine-treated group. ** P<0.01 compared with the model group, ## P<0.01 compared with the sham group.
Discussion
Sepsis is systemic inflammation caused by infection and is characterized by persistent fever, leading to microcirculation damage, organ damage, and multi-organ failure [11]. Sepsis is extremely dangerous, and the mortality rate is high. Worldwide, according to the World Health Organisation (WHO), there are millions of deaths from sepsis each year [12]. Li et al. [13] showed that fever in patients with sepsis is a secondary reaction resulting from the release of endogenous pyrogens that include IL-1β and IL-6 from the activation of macrophages in vivo. These inflammatory cytokines also act on the hypothalamic thermoregulatory center, which is a protective response of the body to inflammation and infection.
The aim of the present study was to investigate the effects of dexmedetomidine in a rat model of sepsis-induced lung injury. After cecal ligation and puncture used to create the rat model, the survival rate in the model group was 35%, and the body temperature in the model group was significantly increased after surgery. There was significant mortality in the first 12 hours of injury, but the cause of early mortality was not limited to lung injury and may have included infective endocarditis and septic shock. In this study, pretreatment with dexmedetomidine significantly increased the survival rate and reduced the body temperature of the rats in the model of sepsis-induced lung injury.
Chen et al. [14] showed that high-dose dexmedetomidine significantly inhibited the release of IL-1β, IL-6, and TNF-α in vivo, reduced leukocyte infiltration, and inhibited the release of inflammatory cytokines. In the present study, dexmedetomidine significantly reduced the levels of IL-1β and TNF-α and increased the levels of IL-10 in lung tissues in the rat model of sepsis, and significantly reduced sepsis-induced lung injury. These findings are supported by those from a previous animal model study reported by Zhu et al. [15] who found that dexmedetomidine significantly reduced the inflammatory response in the lung, and inhibited the release of inflammatory cytokines, to reduce lung injury.
Dexmedetomidine is widely used clinically as a sedative and analgesic drug, but the anti-inflammatory and organ protective effects have only recently begun to be investigated [16]. Dexmedetomidine reduces the serum levels of inflammatory cytokines in patients after abdominal surgery in a dose-dependent manner, reducing the inflammatory response and reducing organ damage [17]. Zheng et al. [18] showed dexmedetomidine regulated multiple signaling pathways through G-protein-coupled receptors, and reduced focal ischemia-reperfusion injury in rats through activation of the PI3K/Akt and ERK1/2 pathways. Recent studies support that the AMPK/SIRT1 signaling pathway has a role in the pathogenesis of fatty liver, cardiovascular disease, and inflammation, and the increased phosphorylation of AMPK and SIRT1 proteins in tissues can effectively reduce apoptosis [19,20].
In the present study, the expression levels of p-AMPK and p-SIRT1 in lung tissues of rats in the model of sepsis-induced lung injury were significantly reduced, the AMPK/SIRT1 signaling pathway was inhibited, and the release of inflammatory cytokines in lung tissues was significantly increased, resulting in alveolar edema and collapse and lung tissue injury. Dexmedetomidine significantly increased the expressions of p-AMPK and p-SIRT1 in lung tissues of rats in the model of sepsis-induced lung injury, thereby promoting the phosphorylation of downstream NF-κB-P65 and FoxO3a, reducing the release of inflammatory cytokines and protecting lung tissues. NF-κB-P65, as a key cytokine, plays a crucial role in inflammation and apoptosis. Qu et al. [21] showed that the activation of NF-κB facilitated the release of anti-apoptotic factors in PC3 prostate carcinoma cells, reducing apoptosis. Also, Liu et al. [22] showed that activation of AMPK increased NF-κB activity and reduced the expression of inflammatory cytokines by phosphorylating SIRT1 and FoxO3a.
This study had several limitations. The study did not include the use of an inhibitor of either the AMPK/SIRT1 signaling pathway or its components, and their molecular mechanisms require further study. Also, in the rat model, dexmedetomidine was given as a pretreatment before the development of sepsis, and the effects of dexmedetomidine on the treatment of established sepsis and sepsis-induced lung injury remain to be studied.
Conclusions
This study aimed to investigate the effects of dexmedetomidine in a rat model of sepsis-induced lung injury and the role of the adenosine monophosphate-activated protein kinase (AMPK) gene and silent information regulator 1 (SIRT1) gene signaling pathway. In the rat model, dexmedetomidine reduced lung damage by activating the AMPK/SIRT1 signaling pathway and reduced the expression of inflammatory cytokines and cell apoptosis.
Footnotes
Source of support: Guangxi Medical and Health Appropriate Technology Development and Promotion Application Project (No. S2018029) and the Guangxi Natural Science Foundation Project (No. 2017GXNSFAA198217)
Conflict of interest
None.
References
- 1.Razazi K, Boissier F, Surenaud M, et al. A multiplex analysis of sepsis mediators during human septic shock: A preliminary study on myocardial depression and organ failure. Ann Intensive Care. 2019;9:64. doi: 10.1186/s13613-019-0538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mark-Christensen A, Kjaer MD, Ganesalingam S, et al. Increasing incidence of pelvic sepsis following ileal pouch-anal anastomosis for ulcerative colitis in Denmark: A nationwide cohort study. Dis Colon Rectum. 2019;62(8):965–71. doi: 10.1097/DCR.0000000000001404. [DOI] [PubMed] [Google Scholar]
- 3.Hou J, Chen Q, Wu X, et al. S1PR3 signaling drives bacterial killing and is required for survival in bacterial sepsis. Am J Respir Crit Care Med. 2017;196:1559–70. doi: 10.1164/rccm.201701-0241OC. [DOI] [PubMed] [Google Scholar]
- 4.Shen Y, Song J, Wang Y, et al. M2 macrophages promote pulmonary endothelial cells regeneration in sepsis-induced acute lung injury. Ann Transl Med. 2019;7:142. doi: 10.21037/atm.2019.02.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bitker L, Cutuli SL, Cioccari L, et al. Sepsis uncouples serum C-peptide and insulin levels in critically ill patients with type 2 diabetes mellitus. Crit Care Resusc. 2019;21:87–95. [PubMed] [Google Scholar]
- 6.Ge Y, Li Q, Nie Y, et al. Dexmedetomidine improves cognition after carotid endarterectomy by inhibiting cerebral inflammation and enhancing brain-derived neurotrophic factor expression. J Int Med Res. 2019;47:2471–82. doi: 10.1177/0300060519843738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng X, Guan W, Zhao Y, et al. Dexmedetomidine ameliorates lipopolysaccharide-induced acute kidney injury in rats by inhibiting inflammation and oxidative stress via the GSK-3beta/Nrf2 signaling pathway. J Cell Physiol. 2019;234(10):18994–9009. doi: 10.1002/jcp.28539. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Prieto CF, Gil-Ortega M, Vega-Martin E, et al. Beneficial effect of bariatric surgery on abnormal MMP-9 and AMPK activities: Potential markers of obesity-related CV risk. Front Physiol. 2019;10:553. doi: 10.3389/fphys.2019.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Lan Z. Polydatin attenuates potassium oxonate-induced hyperuricemia and kidney inflammation by inhibiting NF-kappaB/NLRP3 inflammasome activation via the AMPK/SIRT1 pathway. Food Funct. 2017;8:1785–92. doi: 10.1039/c6fo01561a. [DOI] [PubMed] [Google Scholar]
- 10.Jiang ZJ, Zhang MY, Fan ZW, et al. Influence of lncRNA HOTAIR on acute kidney injury in sepsis rats through regulating miR-34a/Bcl-2 pathway. Eur Rev Med Pharmacol Sci. 2019;23:3512–19. doi: 10.26355/eurrev_201904_17717. [DOI] [PubMed] [Google Scholar]
- 11.Zheng YH, Xiong B, Deng YY, et al. [Effects of allogeneic bone marrow mesenchymal stem cells on polarization of peritoneal macrophages in rats with sepsis]. Zhonghua Shao Shang Za Zhi. 2017;33:217–23. doi: 10.3760/cma.j.issn.1009-2587.2017.04.006. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi LU, Azevedo L, Bozza FA, et al. Availability of resources to treat sepsis in Brazil: A random sample of Brazilian institutions. Rev Bras Ter Intensiva. 2019;31:193–201. doi: 10.5935/0103-507X.20190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li N, Zhou H, Wu H, et al. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 2019;24:101215. doi: 10.1016/j.redox.2019.101215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Jiang Z, Zhou X, et al. Dexmedetomidine preconditioning protects cardiomyocytes against hypoxia/reoxygenation-induced necroptosis by inhibiting HMGB1-mediated inflammation. Cardiovasc Drugs Ther. 2019;33:45–54. doi: 10.1007/s10557-019-06857-1. [DOI] [PubMed] [Google Scholar]
- 15.Zhu YS, Xiong YF, Luo FQ, Min J. Dexmedetomidine protects rats from postoperative cognitive dysfunction via regulating the GABAB R-mediated cAMP-PKA-CREB signaling pathway. Neuropathology. 2019;39:30–38. doi: 10.1111/neup.12530. [DOI] [PubMed] [Google Scholar]
- 16.Abad-Gurumeta A, Gomez-Rios MA, Calvo-Vecino JM. Intravenous dexmedetomidine: Can it modulate the effects of inflammation, or is it only an antinociceptive agent? Minerva Anestesiol. 2019;85:226–28. doi: 10.23736/S0375-9393.18.13217-2. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Zhang A, Liu W, et al. Effects of dexmedetomidine on perioperative stress response, inflammation and immune function in patients with different degrees of liver cirrhosis. Exp Ther Med. 2018;16:3869–74. doi: 10.3892/etm.2018.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng B, Zhang S, Ying Y, et al. Administration of Dexmedetomidine inhibited NLRP3 inflammasome and microglial cell activities in hippocampus of traumatic brain injury rats. Biosci Rep. 2018;38 doi: 10.1042/BSR20180892. BSR20180892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Zhao C, Kong P, et al. Treatment with NAD(+) inhibited experimental autoimmune encephalomyelitis by activating AMPK/SIRT1 signaling pathway and modulating Th1/Th17 immune responses in mice. Int Immunopharmacol. 2016;39:287–94. doi: 10.1016/j.intimp.2016.07.036. [DOI] [PubMed] [Google Scholar]
- 20.Xue B, Yang Z, Wang X, Shi H. Omega-3 polyunsaturated fatty acids antagonize macrophage inflammation via activation of AMPK/SIRT1 pathway. PLoS One. 2012;7:e45990. doi: 10.1371/journal.pone.0045990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qu L, Zhu Y, Liu Y, et al. Protective effects of ginsenoside Rk3 against chronic alcohol-induced liver injury in mice through inhibition of inflammation, oxidative stress, and apoptosis. Food Chem Toxicol. 2019;126:277–84. doi: 10.1016/j.fct.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Zhang M, Zhou T, et al. Exendin-4 promotes the vascular smooth muscle cell re-differentiation through AMPK/SIRT1/FOXO3a signaling pathways. Atherosclerosis. 2018;276:58–66. doi: 10.1016/j.atherosclerosis.2018.07.016. [DOI] [PubMed] [Google Scholar]