Abstract
Very Small Embryonic-Like (VSEL) stem cells are a proposed pluripotent population, residing in adult tissues. VSELs have been described in multiple tissues including bone marrow, cord blood, and gonads. They exhibit multiple characteristics of embryonic stem cells including the ability to differentiate into cellular lineages of all three germ layers, including cardiomyocytes and vascular endothelial cells. However, their presence in adult solid organs such as heart in humans has not been established. VSELs are valuable source of stem cells for tissue regeneration and replacement of cells for turnover and usual wear-and-tear. The purpose of our study was to explore the existence of human VSELs (huVSELs) in human heart tissue and examine the changes in their prevalence with aging and cardiac disease. Human heart tissue, collected from healthy and ischemic heart disease subjects was examined for the prevalence of VSELS, defined as CD45−/CD133+/SSEA4+. Both epicardial and endocardial tissues were examined comparing VSEL numbers across different age groups. Our data confirm the existence of huVSELs in adult hearts with decreasing prevalence during aging.
This is the first evidence of huVSELs in adult cardiac tissue. Cardiac huVSELs could be further explored in future studies to characterize their primitive potential and therapeutic potential in regenerative studies.
Keywords: Very Small Embryonic Like Stem Cells, epicardium, endocardium, human heart, age
INTRODUCTION
Under steady-state conditions, adult cardiomyocytes undergo cell homeostasis which amounts to ~1% in young individuals and 0.5% in old individuals.1 The source of this renewal is poorly understood, and multiple studies have suggested the presence of small number of resident cardiac stem cells that can aid in this process.
Very small embryonic-like stem cells (VSELs) have been described in adult murine and human tissues.2, 3 Their presence has been implicated in tissue regeneration, and studies examining their transplantation after acute and chronic myocardial infarction demonstrated their therapeutic potential. Moreover, animal and human studies have shown that VSELs respond to cardiac injury with enhanced mobilization after myocardial infarction.4, 5 However, the presence of human (huVSELS) in cardiac tissue at steady state conditions has not been examined previously. To our knowledge, this is the first study to examine the existence of huVSELs in human heart tissue and the distribution of huVSELs with advancing age.
METHODS
We examined the frequency of human VSELs, defined as CD133+/SSEA4+/CD45− in the human epicardial and endocardial tissues obtained from the Gill Heart and Vascular Institute-Cardiovascular Biorepository. Our sample set included 18 subjects (age ranging from 9 to 76 years).
Immunohistochemistry.
Immunohistochemical assessments were carried out on frozen cardiac tissue. Briefly, sections were fixed in 4.2 % paraformaldehyde (BD Biosciences, San Jose CA) for 15 mins, followed by permeabilization and blocking with normal goat serum for 30 minutes at room temperature. Slides were incubated with primary antibodies (Abcam, Cambridge, MA): rabbit anti-CD133 (Catalog # ab19898; used at 1:25), rat anti-CD45 (Catalog # ab30446; used at 1:25), and mouse anti-SSEA-4 (Catalog # ab16287; used at 1:20). The sections were then washed with PBS-Tween, and then incubated with all three secondary antibodies (Abcam, Cambridge, MA) at room temperature for 30 minutes: goat anti-rabbit IgG (Alexa Fluor 488; Catalog # ab150081; used at 1:200), goat anti-rat IgG (Alexa Fluor 647; Catalog # ab150167; used at 1:200), goat anti-mouse IgG (Alexa Fluor 555; Catalog # ab150118; used at 1:200). The sections were finally incubated with 0.1% Sudan Black B (Sigma Aldrich, St. Louis, MO) for 30 minutes. ~20 adjacent areas were imaged at 40x magnification using Nikon Confocal Microscope A1 (Nikon, Tokyo, Japan) in the University of Kentucky Confocal Microscopy facility. Cell numbers were expressed as cells/high power field (HPF). Cell numbers are expressed as cells/HPF.
Statistical Analysis
Subject characteristics were reported as descriptive statistics with means, medians, standard deviations and ranges where appropriate. Cell numbers were expressed as mean ± SEM. We used unpaired Student t-test or analysis of variance (one-way or multiple comparisons) to estimate differences, as appropriate. We conducted stepwise linear regression models to assess the effect of clinical variables, such as age, smoking, coronary artery disease, hypertension, diabetes mellitus, hyperlipidemia and heart failure on the number of cardiac huVSELs. The effect sizes were expressed as coefficient of regression and their standard error (SE). Throughout the analyses, a p value less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 25 (SPSS Inc, New York, USA).
RESULTS
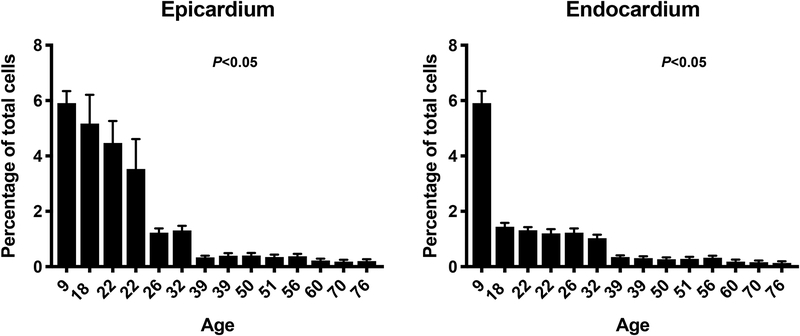
The study included heart samples from 18 patients with age ranging from 9 to 76 years. Study subject characteristics are summarized in table 1. We believe that our panel that focused on CD133 (a specific marker for VSELs), SSEA4 (marker of pluripotency) and lack of CD45 expression is specific for VSELs. We conducted additional studies using CD34 and we found that ~80% of CD45−/CD133+ cells express CD34+. Therefore, we elected to proceed with the final panel consisting of CD133, SSEA4 and CD45 for our immunohistochemistry studies. Our histological evaluation identified small number of VSELs in cardiac tissue in all subjects examined (Figure 1). VSELs were more prevalent in epicardial compared to endocardial tissue (1.81 ± 0.16 vs. 1.01 ± 0.07 cells/HPF, P < 0.001). Interestingly and in agreement with prior reports4, 6–8, the prevalence of VSELs was dramatically reduced with age reaching a nadir in subjects greater than 40 years (5.9 ± 0.4 cells/HPF at age of 9 years down to 0.2 ± 0.07 cells/HPF at the age of 76 years, P < 0.05). We performed Pearson correlation analysis on our data and found strong negative correlation between age and number of VSELs in both the epicardium (R= −0.4, P<0.001) and endocardium (R = −0.6, P<0.001). The reduction of huVSEL number in cardiac tissue with age was noticed irrespective of the presence of cardiac disease or underlying risk factors. Indeed, a stepwise regression analysis confirmed the significant correlation between age and the number of huVSELs both in the epicardium and endocardium after adjusting for confounding factors (Figure 2). We conducted additional analyses to explore the effect of baseline characteristics other than age on the number of huVSELs in the heart. Our linear stepwise regression models suggest that smoking and hypertension correlated negatively with the number of huVSELs in cardiac tissue after adjusting for age (Table 2).
TABLE 1.
Baseline characteristics of study subjects included in the study.
| Clinical characteristic | Percentage |
|---|---|
| Diabetes | 33% |
| Smoking | 50% |
| Hypertension | 44% |
| Hyperlipidemia | 28% |
| Coronary artery disease | 16% |
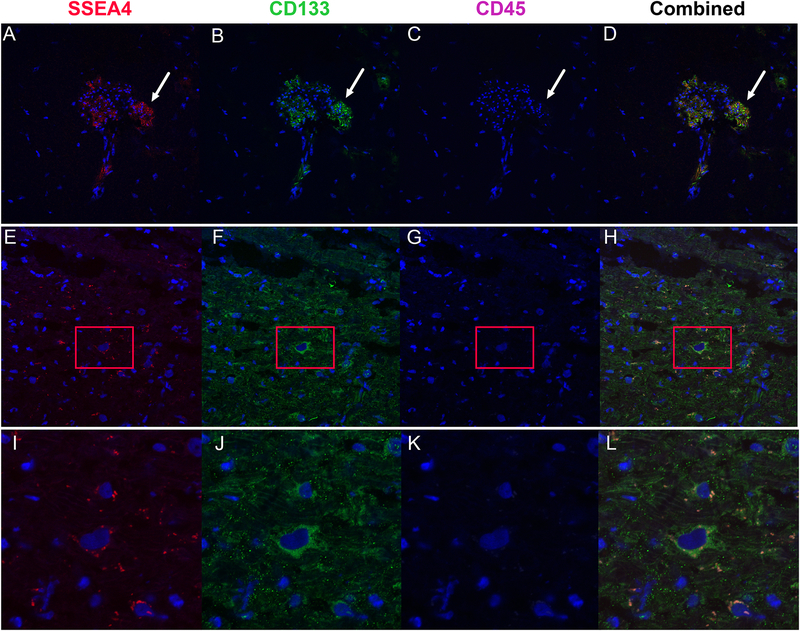
Figure 1.
Representative images demonstrating stem cells positive for SSEA4 (Panel A), CD133 (Panel B) and negative for CD45 (Panel C). Panel D represents the combined image confirming the colocalization of SSEA4 and CD133. The images demonstrate different morphology of VSELs in cardiac tissue. While some of VSELs were present in aggregates, Panels E-H demonstrate single VSEL cell in the myocardium and panels I-L demonstrate zoomed image of this cell.
Figure 2.
Bar graphs showing the percentage of SSEA4+/CD133+/CD45− cells in relationship to patient’s age in the epicardium and endocardium. The figure shows significant reduction in the percentage of stem cells enriched in VSELs with aging (P value for interaction between percentage of stem cells and age is <0.05).
Table 2.
Linear stepwise logistic regression model of cardiac huVSELs numbers.
| Model | Coefficient | SE of coefficient | Beta | P value |
|---|---|---|---|---|
| Endomyocardial huVSELs numbers | ||||
| Age | −0.09 | 0.01 | −0.42 | < 0.001 |
| Diabetes* | 0.10 | 0.31 | 0.01 | 0.71 |
| Smoking* | −0.70 | 0.31 | −0.09 | 0.02 |
| Hypertension* | −0.77 | 0.36 | 0.10 | 0.03 |
| Hyperlipidemia* | −0.08 | 0.40 | −0.01 | 0.85 |
| Heart failure* | 0.68 | .49 | 0.09 | 0.16 |
| Coronary artery disease* | −0.73 | 0.40 | −0.06 | 0.06 |
| Endomyocardial huVSELs numbers | ||||
| Age | −0.04 | 0.00 | −0.55 | < 0.001 |
| Diabetes* | −0.05 | 0.12 | −0.01 | 0.70 |
| Smoking* | −0.75 | 0.12 | −0.22 | < 0.001 |
| Hypertension* | −0.25 | 0.14 | −0.06 | 0.08 |
| Hyperlipidemia* | 0.13 | 0.14 | −0.02 | 0.41 |
| Heart failure* | 0.90 | .17 | 0.27 | < 0.001 |
| Coronary artery disease* | −0.30 | 0.15 | −0.07 | 0.05 |
These baseline characteristics were adjusted for age as a confounding factor.
DISCUSSION
Very small embryonic like (VSEL) stem cells were first described in 2007 as a rare population of small CD45−/Lin−/Sca1+ cells isolated from murine bone marrow and later in human umbilical cord blood (CD45−/CD133+/SSEA4+)2, 9. VSELs exhibit many characteristics of embryonic stem cells such as large nuclei with open-type chromatin (euchromatin) surrounded by a narrow rim of cytoplasm10. Murine VSEL cells have been proposed to express pluripotency genes such as OCT4 and Nanog. In vitro, they are capable of differentiating into all three germ-layer lineages. Therefore, VSELs represent an attractive source for stem cells for regenerative applications. Indeed, VSELs have been successfully employed in cardiac regenerative studies both in acute11 and chronic heart failure models12. Furthermore, clinical studies have demonstrated their therapeutic potential in patients with chronic heart failure13, 14.
The presence of human VSELs in solid organs such as the heart has not been fully explored. Our report is the first to describe a small population of huVSELs in human cardiac tissue of adult individuals. HuVSELs were identified in various regions of the heart including epicardium and endocardium but with higher prevalence in the epicardial tissue. This is in agreement with prior reports confirming the higher prevalence of cells expressing stem cell markers in the epicardium15–17. However, our data does not provide details on the origin of huVSELs in the heart and whether they are native to the human heart or have migrated from other tissues. Future mechanistic studies examining the origin and therapeutic potential of VSELs isolated from cardiac tissue are planned.
Aging has been linked to the reduction of stem cell content in different organs9, 18. Our prior studies have been in agreement with this phenomenon with reduction in the number of bone marrow VSELs with age under physiological conditions in animal models.8 Similarly, the number of circulating VSELs in response to acute myocardial infarction are significantly lower in older patients4. While the majority of the literature suggest a strong negative correlation between the number of stem cells and age, other studies have reached different conclusions. Sovalat et al. examined the number of circulating huVSELs in healthy volunteers and found no correlating with age which could be explained by the relative small number of circulating VSELs under physiological conditions.19 We suspect that the difference between these reports stems from the clinical scenario and that the number of circulating VSELs following injury reflect the reserve of VSELs which is affected by age. In addition to age, our studies suggest that smoking and hypertension correlated negatively with huVSELs content in cardiac tissue even after adjusting for age.
One of the limitations of our study is the reliance on immunohistochemistry to identify and quantify huVSELs. We have attempted flow cytometry of digested heart tissue but could not identify sufficient cells to reliably report their numbers. As previously reported, VSELs are small in size and usually reside in the debris gate on the forward/side scatter plot. After excluding dead cells and debris, the number of VSELs was small and was technically difficult to report them reliably. We are currently exploring other strategies to verify and quantify huVSELs in digested cardiac tissue. Another limitation is the fact that the majority of our samples were left ventricular apex. Therefore, we cannot exclude the presence of huVSELs niches in other areas of the heart such as left atrial appendage.
In conclusion, huVSELs defined as CD133+/SSEA4+/CD45− were identified for the first time in human cardiac tissue, in both epicardium and endocardium with being more prevalent in former mentioned. Their numbers decrease with age reaching a nadir by the age of forty. These cells exhibited characteristics similar to VSELs identified in other heart tissue and expressed the embryonic marker SSEA4. More studies are needed to explore the response of cardiac huVSELs to various cardiac pathologies and investigate their regenerative capacity in repairing heart tissue.
ACKNOWLEDGMENTS
Dr. Abdel-Latif is supported by the University of Kentucky COBRE Early Career Program (P20 GM103527) and the NIH Grant R01 HL124266.
We thank the University of Kentucky Biorepository core for their help collecting and curating the human cardiac tissue samples.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest Disclosures
None of the authors have conflicts of interest to disclose.
REFERENCES.
- 1.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kucia M, Halasa M, Wysoczynski M, et al. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: preliminary report. Leukemia. 2007;21:297–303. [DOI] [PubMed] [Google Scholar]
- 3.Ratajczak MZ, Zuba-Surma E, Wojakowski W, et al. Very small embryonic-like stem cells (VSELs) represent a real challenge in stem cell biology: recent pros and cons in the midst of a lively debate. Leukemia. 2014;28:473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdel-Latif A, Zuba-Surma EK, Ziada KM, et al. Evidence of mobilization of pluripotent stem cells into peripheral blood of patients with myocardial ischemia. Exp Hematol. 2010;38:1131–1142 e1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuba-Surma EK, Kucia M, Dawn B, et al. Bone marrow-derived pluripotent very small embryonic-like stem cells (VSELs) are mobilized after acute myocardial infarction. J Mol Cell Cardiol. 2008;44:865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhartiya D, Singh J. FSH-FSHR3-stem cells in ovary surface epithelium: basis for adult ovarian biology, failure, aging, and cancer. Reproduction. 2015;149:R35–48. [DOI] [PubMed] [Google Scholar]
- 7.Kucia M, Shin DM, Liu R, et al. Reduced number of VSELs in the bone marrow of growth hormone transgenic mice indicates that chronically elevated Igf1 level accelerates age-dependent exhaustion of pluripotent stem cell pool: a novel view on aging. Leukemia. 2011;25:1370–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuba-Surma EK, Wu W, Ratajczak J, et al. Very small embryonic-like stem cells in adult tissues-potential implications for aging. Mech Ageing Dev. 2009;130:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratajczak MZ, Ratajczak J, Suszynska M, et al. A Novel View of the Adult Stem Cell Compartment From the Perspective of a Quiescent Population of Very Small Embryonic-Like Stem Cells. Circ Res. 2017;120:166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuba-Surma EK, Kucia M, Abdel-Latif A, et al. Morphological characterization of very small embryonic-like stem cells (VSELs) by ImageStream system analysis. J Cell Mol Med. 2008;12:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawn B, Tiwari S, Kucia MJ, et al. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells. 2008;26:1646–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuba-Surma EK, Guo Y, Taher H, et al. Transplantation of expanded bone marrow-derived very small embryonic-like stem cells (VSEL-SCs) improves left ventricular function and remodelling after myocardial infarction. J Cell Mol Med. 2011;15:1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tendera M, Wojakowski W, Ruzyllo W, et al. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J. 2009;30:1313–1321. [DOI] [PubMed] [Google Scholar]
- 14.Wojakowski W, Kucia M, Zuba-Surma E, et al. Very small embryonic-like stem cells in cardiovascular repair. Pharmacol Ther. 2011;129:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lie-Venema H, van den Akker NM, Bax NA, et al. Origin, fate, and function of epicardium-derived cells (EPDCs) in normal and abnormal cardiac development. Scientific World Journal. 2007;7:1777–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winter EM, Gittenberger-de Groot AC. Epicardium-derived cells in cardiogenesis and cardiac regeneration. Cell Mol Life Sci. 2007;64:692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winter EM, Grauss RW, Hogers B, et al. Preservation of left ventricular function and attenuation of remodeling after transplantation of human epicardium-derived cells into the infarcted mouse heart. Circulation. 2007;116:917–927. [DOI] [PubMed] [Google Scholar]
- 18.Ratajczak MZ, Bartke A, Darzynkiewicz Z. Prolonged Growth Hormone/Insulin/Insulin-like Growth Factor Nutrient Response Signaling Pathway as a Silent Killer of Stem Cells and a Culprit in Aging. Stem Cell Rev. 2017;13:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sovalat H, Scrofani M, Eidenschenk A, et al. Human Very Small Embryonic-Like Stem Cells Are Present in Normal Peripheral Blood of Young, Middle-Aged, and Aged Subjects. Stem Cells Int 2016;2016:7651645. [DOI] [PMC free article] [PubMed] [Google Scholar]