Abstract
Background
Surfactant replacement therapy has been proven beneficial in the prevention and treatment of neonatal respiratory distress syndrome (RDS). The deficiency of surfactant or surfactant dysfunction may contribute to respiratory failure in a broader group of disorders, including meconium aspiration syndrome (MAS).
Objectives
To evaluate the effect of surfactant administration in the treatment of late preterm and term infants with meconium aspiration syndrome.
Search methods
We searched The Cochrane Library (Issue 4, 2006), MEDLINE and EMBASE (1985 to December 2006), previous reviews including cross‐references, abstracts, conference and symposia proceedings, expert informants, and journal handsearching, without language restrictions. We contacted study authors for additional data.
We ran an updated search in November 2014 and searched the following sites for ongoing or recently completed trials: www.clinicaltrials.gov; www.controlled‐trials.com; and www.who.int/ictrp.
Selection criteria
Randomised controlled trials which evaluated the effect of surfactant administration in late preterm and term infants with meconium aspiration syndrome are included in the analyses.
Data collection and analysis
We extracted data on clinical outcomes including mortality, treatment with extracorporeal membrane oxygenation (ECMO), pneumothorax, duration of assisted ventilation, duration of supplemental oxygen, intraventricular haemorrhage (any grade and severe IVH), and chronic lung disease. We conducted data analyses in accordance with the standards of the Cochrane Neonatal Review Group.
Main results
Four randomised controlled trials met our inclusion criteria. The meta‐analysis of four trials (326 infants) showed no statistically significant effect on mortality [typical risk ratio (RR) 0.98, 95% confidence interval (CI) 0.41 to 2.39; typical risk difference (RD) ‐0.00, 95% CI ‐0.05 to 0.05]. There was no heterogeneity for this outcome (I² = 0% for both RR and RD). The risk of requiring extracorporeal membrane oxygenation was significantly reduced in a meta‐analysis of two trials (n = 208); [typical RR 0.64, 95% CI 0.46 to 0.91; typical RD ‐0.17, 95% CI ‐0.30 to ‐0.04; number needed to treat for an additional beneficial outcome (NNTB) 6, 95% CI 3 to 25]. There was no heterogeneity for RR (1² = 0%) but moderate heterogeneity for RD (I² = 50%). One trial (n = 40) reported a statistically significant reduction in the length of hospital stay (mean difference ‐8 days, 95% CI ‐14 to ‐3 days; test for heterogeneity not applicable). There were no statistically significant reductions in any other outcomes studied (duration of assisted ventilation, duration of supplemental oxygen, pneumothorax, pulmonary interstitial emphysema, air leaks, chronic lung disease, need for oxygen at discharge or intraventricular haemorrhage).
Authors' conclusions
In infants with MAS, surfactant administration may reduce the severity of respiratory illness and decrease the number of infants with progressive respiratory failure requiring support with ECMO. The relative efficacy of surfactant therapy compared to, or in conjunction with, other approaches to treatment including inhaled nitric oxide, liquid ventilation, surfactant lavage and high frequency ventilation remains to be tested.
Plain language summary
Surfactant for meconium aspiration syndrome in term and late preterm infants
Lay title: Surfactant treatment for infants who have inhaled meconium into the lungs in or around the time of birth
Review question: Does the administration of surfactant improve lung function and lead to better clinical outcomes in infants born at or near term who have inhaled meconium in or around the time of birth?
Background: The lungs of newborn babies can be damaged by meconium aspiration syndrome. Meconium aspiration syndrome is caused when a stressed baby passes a bowel movement while still in the womb and then breathes some of this material into the lungs. Pulmonary surfactant, the complex combination of chemicals that line the surface of the lung, may be altered or inactivated in babies who have meconium aspiration. It is thought that treatment with additional surfactant might help overcome this damage.
Study characteristics: Four randomised controlled trials enrolling 326 infants met our inclusion criteria.
Results: This review of trials found that surfactant can prevent worsening of breathing difficulties and reduce the need for heart‐lung bypass therapy in some babies suffering from meconium aspiration syndrome .
Background
Description of the condition
The deficiency of surfactant or surfactant dysfunction may contribute to respiratory failure in a broad group of disorders, including meconium aspiration syndrome (MAS). Meconium inhibits the surface tension‐lowering properties of surfactant (Chen 1985; Moses 1991). Instillation of meconium into the airways of term animals leads to acute mechanical obstruction and worsening pulmonary mechanics and gas exchange (Chen 1985; Tran 1980; Tyler 1978). A significant reduction in lung compliance, an increase in expiratory lung resistance and increased functional residual capacity can be demonstrated (Tran 1980). Investigators have postulated that the changes in compliance associated with meconium aspiration result from displacement of surfactant by free fatty acids (Clark 1987). In animals with experimentally induced meconium aspiration, treatment with large doses of animal‐derived surfactant extract improves compliance and ventilation (Sun 1993).
Description of the intervention
Surfactant replacement therapy has been proven beneficial in the prevention and treatment of neonatal respiratory distress syndrome (RDS) (Soll 1992). Respiratory distress syndrome is due to a primary deficiency in the production and release of pulmonary surfactant. Surfactant therapy has been shown to improve oxygenation, decrease the need for ventilatory support, and improve clinical outcome in infants with RDS. Surfactant‐treated infants have a reduced mortality and a decreased incidence of pneumothorax.
Uncontrolled studies of surfactant treatment in infants with MAS suggest that surfactant may be of benefit in MAS. In a pilot study of seven infants with MAS treated with surfactant, all seven demonstrated an improvement in respiratory failure (Auten 1991). Khammash 1993 treated 20 infants with severe MAS. Infants received an intratracheal dose of bovine surfactant extract (100 mg phospholipid/kg). Improvement in oxygenation index (OI) and arterial/alveolar ratio (a/A pO₂) were noted in 75% of the treated infants in the six hours following surfactant instillation. None of the treated infants required further experimental therapy, including extracorporeal membrane oxygenation (ECMO).
Other approaches to prevent or treat MAS include amnioinfusion (infusion of saline into the amniotic cavity), oronasopharyngeal suctioning of meconium‐stained neonates before delivery and the use of surfactant lavage in infants with the diagnosis of MAS.
In a systematic review of amnioinfusion in women with meconium‐stained fluid, Hofmeyr 2010 found no significant reduction in the primary outcomes of MAS, perinatal death or severe morbidity, and maternal death or severe morbidity. However, some benefits were reported in a subgroup analysis including studies performed at facilities where perinatal surveillance was limited.
Vain 2004 assessed the effectiveness of intrapartum suctioning for the prevention of MAS in a large multicentre randomised controlled trial. The primary outcome was the incidence of MAS. No significant difference between treatment groups was seen in the incidence of MAS, in mortality, or in the duration of ventilation, oxygen treatment, and hospital care. The authors concluded that routine intrapartum oropharyngeal and nasopharyngeal suctioning of term‐gestation infants born through meconium‐stained amniotic fluid does not prevent MAS. These findings led to changes in clinical practice, with routine suctioning of the oropharynx and the nasopharynx currently not recommended (AAP 2006).
Why it is important to do this review
This systematic review evaluates randomised controlled trials that studied the effect of bolus surfactant administration for the treatment of term and late preterm infants with MAS. This updates the previous review Surfactant for meconium aspiration syndrome in full term/near term infants (El Shahed 2007).
Studies that utilised dilute surfactant solutions to lavage meconium from the airways are not included in this review (Hahn 2013).
Several other Cochrane reviews evaluate surfactant in the treatment of respiratory disorders in neonates. Most of these reviews focus on infants with or at risk of RDS. Systematic reviews include reviews of surfactant in the prevention (Soll 1998; Soll 2010) and treatment (Seger 2009; Soll 1998) of RDS, reviews that compare animal‐derived products to synthetic products (Soll 2001), and reviews that evaluate newer protein‐containing synthetic surfactants (Pfister 2007; Pfister 2009).
Other reviews compare timing of treatment (Bahadue 2012; Rojas‐Reyes 2012; Stevens 2007), surfactant dosing (Soll 2009), methods of surfactant instillation (Abdel‐Latif 2011a; Abdel‐Latif 2011b: Abdel‐Latif 2012) or the use of surfactant in conditions other than RDS including surfactant for pulmonary haemorrhage in neonates (Aziz 2012) and surfactant for bacterial pneumonia in late preterm and term infants (Tan 2012).
Objectives
To evaluate the effect of surfactant administration in the treatment of late preterm and term infants with meconium aspiration syndrome (MAS).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials comparing surfactant treatment to routine management of late preterm and term infants with MAS.
Types of participants
Late preterm and term infants with MAS (modified from the previous review, which planned to include only term infants).
Types of interventions
Intratracheal administration of surfactant versus placebo or no therapy. We have not included studies that utilised dilute surfactant solutions to lavage meconium from the airways.
Types of outcome measures
For the update of this review, the following primary and secondary outcomes were selected:
Primary outcomes
Mortality
Secondary outcomes
Treatment with extracorporeal membrane oxygenation (ECMO);
Pneumothorax;
Pulmonary interstitial emphysema;
Air leaks (pneumothorax, pneumomediastinum, pulmonary interstitial emphysema);
Duration of assisted mechanical ventilation (days);
Duration of supplemental oxygen (days);
Need for supplemental oxygen at discharge;
Chronic lung disease (defined as need for oxygen therapy at 28 days or 36 weeks postmenstrual age);
Intraventricular haemorrhage (any grade);
Severe IVH (grade III ‐ IV);
Duration of hospital stay (days).
Additional outcomes for the update in 2014:
Death or chronic lung disease at 28 days;
Death or chronic lung disease at 36 weeks postmenstrual age;
Neurodevelopmental follow‐up.
Search methods for identification of studies
For the previous review in 2007, we searched The Cochrane Library (Issue 4, 2006) in December 2006. We searched MEDLINE (OVID, 1966 to December 2006) using the following strategy: (exp Pulmonary Surfactants/ or surfactan:.mp. or Surface‐Active Agents/ or (surfactan: adj2 lavage:).mp.) and (Meconium Aspiration Syndrome/ or Meconium/). We searched EMBASE (OVID, 1980 to 2006 Week 06), using the following strategy: (Lung Surfactant/ or exp Surfactant/ or (surfactan: adj2 lavage:).mp. or surfactan:.mp.) and (Meconium or Aspiration/ or meconium/).
We searched previous reviews and cross‐references, and abstracts published in Pediatric Research or electronically from Pediatric Academic Societies meetings from 2000 to December 2006, without any language restrictions.
In November 2014 we updated the electronic searches. See: Appendix 1. In addition, we searched for ongoing or recently completed trials in the following clinical trials registries (www.clinicaltrials.gov; www.controlled‐trials.com; and www.who.int/ictrp).
Data collection and analysis
We used the standard methods of the Cochrane Neonatal Review Group, as documented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
For each included study, we collected information regarding the method of randomisation, blinding, drug intervention, stratification, and whether the trial was single‐ or multicentre. We noted information regarding trial participants, including gestational age criteria, birth weight criteria, cause of respiratory failure, severity of respiratory failure, and postnatal age at the time of treatment. We extracted information on clinical outcomes, including mortality, treatment with ECMO, pneumothorax, pulmonary interstitial emphysema, chronic lung disease, duration of assisted ventilation, duration of supplemental oxygen, need for supplemental oxygen at discharge, duration of hospital stay, and intraventricular haemorrhage (any grade and grades III and IV). We contacted investigators or study sponsors for clarification or provision of data not specifically noted in the original report. For the update in 2007, two review authors (AS, AO) independently evaluated all studies, abstracted the data onto extraction forms and compared and agreed the abstracted data. One review author (AS) entered the data into RevMan 4.2.9 and the other review author (AO) checked the data for accuracy. Unpublished information on the subgroup of infants with MAS obtained from Lotze at al (Lotze 1998) included in the original review were entered unchanged. Unpublished information regarding the multicentre trial conducted in Chile and previously published in abstract form was obtained from the authors (Maturana 2005) and the data from the unpublished report were entered into RevMan 5.3.
Assessment of risk of bias in included studies
We have used the standard review methods of the CNRG (About the CNRG) to assess the methodological quality of included studies.
For the 2014 update of the review, two review authors (AO, AS) assessed the following areas and completed a 'Risk of bias' table for each included study; see Characteristics of included studies.
Selection bias (random sequence generation and allocation concealment).
For each included study, we categorised the risk of selection bias as:
Random sequence generation:
Low risk ‐ adequate (any truly random process, e.g. random number table; computer random number generator); High risk ‐ inadequate (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); Unclear risk ‐ no or unclear information provided.
Allocation concealment:
Low risk ‐ adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); High risk ‐ inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); Unclear risk ‐ no or unclear information provided.
Performance bias
For each included study, we categorised the methods used to blind study personnel to knowledge of which intervention a participant received. (As our study population consisted of neonates they would all be blinded to the study intervention).
Low risk ‐ adequate for personnel (a placebo that could not be distinguished from the active drug was used in the control group); High risk ‐ inadequate ‐ personnel aware of group assignment; Unclear risk ‐ no or unclear information provided.
Detection bias
For each included study, we categorised the methods used to blind outcome assessors to knowledge of which intervention a participant received. (As our study population consisted of neonates they would all be blinded to the study intervention). Blinding was assessed separately for different outcomes or classes of outcomes. We categorised the methods used with regards to detection bias as:
Low risk ‐ adequate; follow‐up was performed with assessors blinded to group; High risk ‐ inadequate; assessors at follow‐up were aware of group assignment; Unclear risk ‐ no or unclear information provided.
Attrition bias
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods with respect to the risk of attrition bias as: Low risk ‐ adequate (fewer than 10% missing data); High risk ‐ inadequate (more than 10% missing data); Unclear risk ‐ no or unclear information provided.
Reporting bias
For each included study, we described how we investigated the risk of selective outcome reporting bias and what we found. We assessed the methods as: Low risk ‐ adequate (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported); High risk ‐ inadequate (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); Unclear risk ‐ no or unclear information provided (e.g. the study protocol was not available).
Other bias
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as: Low risk ‐ no concerns of other bias raised; High risk ‐ concerns raised about multiple checking of the data with the results made known to the investigators, difference in number of participants enrolled in abstract and final publications of the paper; Unclear ‐ concerns raised about potential sources of bias that could not be verified by contacting the study authors.
Where necessary, we planned to explore the impact of the level of bias through undertaking sensitivity analyses (Higgins 2011).
Measures of treatment effect
The statistical methods included (typical) risk ratio (RR), (typical) risk difference (RD), number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) for dichotomous outcomes, and mean difference (MD), all reported with 95% confidence intervals (CI). We used a fixed‐effect model for meta‐analysis.
Unit of analysis issues
The unit of randomisation and the unit of analysis was in all cases the individual infant.
Dealing with missing data
We intended to contact the authors of all published studies if clarification was required, or to provide additional information. In the case of missing data, we intended to describe the number of participants with missing data in the Main results section. We present results only for the available participants. We intended to discuss the implications of missing data in the Discussion section of the review.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis (Higgins 2003). If we had identified substantial heterogeneity, we would have explored it by prespecified subgroup analysis and sensitivity analysis. We used the following cut‐offs for the degree of heterogeneity; < 25%, no heterogeneity; 25 to 49%, low heterogeneity; 50 to 74%, moderate heterogeneity and ≥ 75% high heterogeneity (Higgins 2011).
Assessment of reporting biases
If available, we planned to obtain the study protocols of all included studies so that we could compare outcomes reported in the protocol to those reported in the findings for each of the included studies. We would have investigated reporting and publication bias by examining the degree of asymmetry of a funnel plot (if at least 10 trials were available for a given outcome). Where we suspected reporting bias (see selective reporting in Assessment of risk of bias in included studies), we would have attempted to contact study authors to provide missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we would have explored the impact of including such studies in the overall assessment of results by conducting a sensitivity analysis.
Data synthesis
We analysed the data using Review Manager 5 software (RevMan 2014). We conducted a fixed‐effect Mantel‐Haenszel meta‐analysis for combining data where trials examined the same intervention and we judged the trial populations and methods to be sufficiently similar.
Subgroup analysis and investigation of heterogeneity
If sufficient data were available, we had planned to explore potential sources of clinical heterogeneity through the following a priori subgroup analyses: (i) studies done with and without availability of inhaled nitric oxide; (ii) studies done with and without availability of extracorporeal membrane oxygenation (ECMO).
Sensitivity analysis
If sufficient data were available, we had planned to explore methodological heterogeneity through the use of sensitivity analyses. We planned to perform these through including trials of higher quality, based on the presence of any of the following: adequate sequence generation, allocation concealment, and fewer than 10% lost to follow‐up.
Results
Description of studies
Results of the search
Seventeen potential studies were identified, of which four are included in the review.
Included studies
For details, see the table Characteristics of included studies.
We include four studies in this review:
Findlay 1996 is a single‐centre study performed in the USA:
Objective: To determine whether high‐dose surfactant therapy improves the pulmonary morbidity of term infants ventilated for meconium aspiration syndrome (MAS).
Population: Term newborn infants with MAS, diagnosed by the presence of meconium below the vocal cords at birth with or without characteristic chest radiographic findings, who needed ventilator support before six hours of age with a fractional inspired oxygen (Fi0₂) level of 0.5 or more, mean airway pressure of 7 cm of H₂O or more and arterial/alveolar (a/A) pO₂ ratio of 0.22 or less.
Intervention: Infants in the study group received up to four doses of 150 mg (6ml)/kg beractant (Survanta), installed every six hours by continuous infusion for 20 minutes via a side hole endotracheal tube adapter. Infants in the control group received 6 ml/kg air placebo.
Outcomes: Primary outcomes included decrease in Oxygen Index (OI), increase in a/A pO₂ ratio and decrease in the need for respiratory support (mean airway pressure (MAP), ventilation days). Secondary outcomes included the need for ECMO, incidence of air leaks, duration of oxygen therapy, discharge with supplemental oxygen, and mortality at less than 28 days of life.
Lotze 1998 is a multicentre study performed in the USA:
Objective: To determine whether surfactant (beractant) administration to term newborns in respiratory failure and at risk of requiring extracorporeal membrane oxygenation (ECMO) treatment would significantly reduce the incidence of severe complications through 28 days of age and the need for ECMO.
Population: Infants weighing 2000 gm or more with gestational ages of 36 weeks or greater with respiratory failure secondary to MAS, sepsis or idiopathic persistent pulmonary hypertension of newborn (requiring FiO₂ 1.00 with OI of 15 to 39).
Intervention: Infants were randomly assigned to receive either four doses of beractant 100 mg/kg or air placebo before ECMO treatment and four additional doses during ECMO, if ECMO was required (only infants with MAS are included in this analysis and the data were provided by the authors).
Outcomes: Need for ECMO and incidence of severe complications (haemorraghic, neurologic, pulmonary, renal, cardiovascular, infectious, metabolic and technical) during the first 28 days of age or at discharge.
The Chinese Collaborative Study (Chinese Study Group 2005) is a multicentre study performed in China:
Objective: To evaluate the safety and efficacy of exogenous surfactant replacement therapy for MAS in term and late preterm neonates.
Population: Term and late preterm neonates with MAS (diagnosis based on the presence of meconium in the airways with or without meconium‐stained amniotic fluid at delivery, typical chest x‐ray findings, onset of respiratory distress, and abnormal blood gas findings indicating respiratory failure and acidosis), birth weight greater than 2500 gm, postnatal age less than 36 hours, a/A pO₂ ratio less than 0.22, OI greater than 15 and need for mechanical ventilation for one to two hours without improvement.
Intervention: The infants in the surfactant group received an initial dose of porcine lung‐derived surfactant (Curosurf) at 200 mg/kg, with repeated doses of 200, 100 and 100 mg/kg given at 6 to 12 hourly intervals to a maximum of four doses if OI increased by more than two from baseline. The control group received the standard care without a placebo.
Outcomes: The primary outcomes were a reduction of OI to less than 10 and an increase of the pretreatment a/A pO₂ ratio of 100% over baseline 24 hours after surfactant treatment. The secondary outcomes were duration of mechanical ventilation, incidence of complications and survival to discharge from hospital.
Maturana 2005 is a multicentre study performed in Chile:
Objective: To evaluate the use of up to three doses of surfactant administered as a bolus (150 mg/kg) versus placebo to reduce the number of days on mechanical ventilation in term infants with moderate to severe MAS.
Population: Term newborns more than 37 weeks of gestation with moderate to severe MAS (defined as the presence of meconium‐stained amniotic fluid with or without evidence of meconium in the lower airway, abnormal x‐ray consistent with MAS and respiratory insufficiency defined as an oxygen requirement of 50% or more in an oxyhood to achieve saturation of greater than 90% or PaO₂ more than 50 mmHg if the infant was not ventilated, or an OI more than eight if the infant was on mechanical ventilation.
Intervention: Infants were randomly assigned to receive either 150 mg /kg/dose (6ml) of Survanta or an equivalent amount of air as placebo every six hours for total of three doses if they remained intubated.
Outcomes: The primary outcome was days on mechanical ventilation. Secondary outcomes included days requiring oxygen therapy with a target arterial oxygen saturation of more than 90%, air leaks (pneumothorax, pneumomediastinum, interstitial emphysema), persistent pulmonary hypertension (PPHN), OI after two hours following the first treatment dose, and mortality before discharge.
Notes: We obtained from the first author an unpublished manuscript of the study that included an additional four randomised infants (three infants in the surfactant group and one in the control group) compared to the published abstract. In the analyses we report on 28 infants in the surfactant group and 29 in the air‐placebo group as per the additional information we received from the authors.
Excluded studies
We excluded 14 studies from the analysis. These are detailed in the table Characteristics of excluded studies, with reasons for their exclusion.
Risk of bias in included studies
Randomised controlled trials that evaluate the effect of bolus surfactant administration in term or late preterm infants with MAS are included in the analysis. We discuss specific methodologic issues below:
Randomisation: The four included studies allocated treatments by randomisation. In Maturana 2005 the randomisation scheme was computer‐generated. The Collaborative Chinese Study (Chinese Study Group 2005) and Maturana 2005 used sealed randomisation envelopes. Findlay 1996 did not report on the method of randomisation, but stated that physicians and nurses caring for the infants were unaware of the infants' assignment groups. Lotze 1998 used a central randomisation service and stratified infants by primary diagnosis and disease severity.
Blinding of treatment: In Findlay 1996 the attending staff were unaware of treatment assignment. In Lotze 1998, the dosing investigator was prohibited from participating in any other aspects of infants' care and from revealing the treatment assignment. In the Chinese Collaborative Study (Chinese Study Group 2005), staff were not blinded to treatment groups. In Maturana 2005, the assigned treatment was administered by a person not involved in the direct infant care and was given behind a screen. The number of infants enrolled in the trial differed between the published abstract (Maturana 2005) and the information obtained from the first author (three additional infants in the surfactant group and one additional infant in the control group). Differences noted between abstracts and full reports may indicate elements of bias/poor data quality control, possibly including any of the following methodological issues: multiple examination of the data; changes in the definitions of outcomes; no prespecified sample size; closure of participant recruitment when statistical significance has been reached for the outcome under study, and other sources of bias (Walia 1999).
Blinding of outcome assessment: Outcomes were assessed by staff members unaware of treatment assignment in three of the four studies (Findlay 1996; Lotze 1998; Maturana 2005).
Exclusion after randomisation: In Chinese Study Group 2005, 66 infants were enrolled and five infants (four in the surfactant group and one in the control group) were excluded from the final analysis because of violation of the entry criteria. In Lotze 1998 all 330 randomised infants were accounted for (168 of these infants were enrolled on the basis of MAS, and the remaining infants on the basis of PPHN or sepsis). Two infants were later withdrawn from the study when parental consent was withdrawn. Their limited data were subsequently excluded from analysis. The diagnosis on which their enrolment was based and whether or not they had MAS was not reported.
Effects of interventions
SURFACTANT THERAPY versus PLACEBO OR NO TREATMENT (COMPARISON 1):
PRIMARY OUTCOME:
Mortality (Outcome 1.1):
All four studies enrolling 326 infants reported on mortality. Surfactant had no statistically significant effect on mortality [typical risk ratio (RR) 0.98, 95% confidence interval (CI) 0.41 to 2.39; typical risk difference (RD) ‐0.00, 95% CI ‐0.05 to 0.05] (Analysis 1.1). Heterogeneity of treatment effect for this outcome was low (I² = 0%) for both RR and RD.
1.1. Analysis.
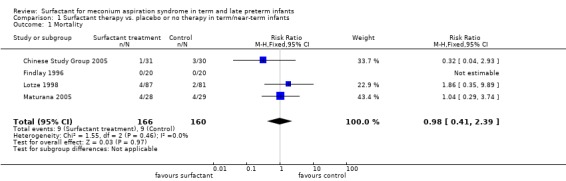
Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 1 Mortality.
SECONDARY OUTCOMES:
Treatment with extracorporeal membrane oxygenation (ECMO) (Outcome 1.2):
Two studies enrolling 208 infants reported on treatment with ECMO. Surfactant statistically significantly reduced treatment with ECMO [typical RR 0.64, 95% CI 0.46 to 0.91; typical RD ‐0.17, 95% CI ‐0.30 to ‐0.04; Number needed to treat for an additional beneficial outcome (NNTB) 6, 95% CI 3 to 25]. (Analysis 1.2) Heterogeneity of treatment effect for this outcome was moderate for RR (I² = 50%) and low for RD (I² = 0%).
1.2. Analysis.
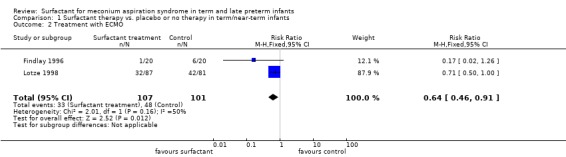
Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 2 Treatment with ECMO.
Pneumothorax (Outcome 1.3):
Three studies enrolling 269 infants reported on the occurrence of pneumothorax. Surfactant did not statistically significantly reduce the occurrence of pneumothorax (typical RR 0.82, 95% CI 0.39 to 1.73; typical RD ‐0.02, 95% CI ‐0.08 to 0.05) (Analysis 1.3). Heterogeneity of treatment effect for this outcome was moderate for RR (I² = 50%) and high for RD (I² = 75%).
1.3. Analysis.
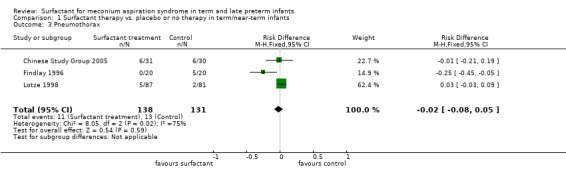
Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 3 Pneumothorax.
Pulmonary interstitial emphysema (Outcome 1.4):
One study enrolling 61 infants reported on the occurrence of interstitial emphysema. Surfactant had no statistically significant effect on pulmonary interstitial emphysema (RR 0.55, 95% CI 0.18 to 1.70; RD ‐0.10, 95% CI ‐0.30 to 0.09) (Analysis 1.4). Tests for heterogeneity were not applicable.
1.4. Analysis.
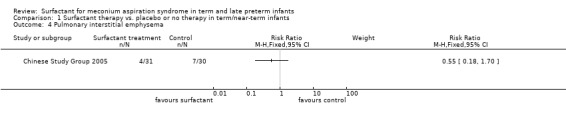
Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 4 Pulmonary interstitial emphysema.
Air leaks (pneumothorax, pneumomediastinum, interstitial emphysema) (Outcome 1.5):
One study enrolling 57 infants reported on a combination of air leaks. Surfactant did not have a statistically significant effect on air leaks (RR 1.04, 95% CI 0.23 to 4.71; RD 0.00, 95% CI ‐0.16 to 0.16) (Analysis 1.5). Tests for heterogeneity were not applicable.
1.5. Analysis.
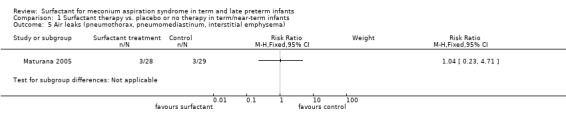
Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 5 Air leaks (pneumothorax, pneumomediastinum, interstitial emphysema).
Duration of assisted mechanical ventilation (days) (Outcome 1.6):
Three studies enrolling 158 infants reported on duration of assisted mechanical ventilation. Mechanical ventilated was stated as the outcome in all three studies, but whether or not this included continuous positive airway pressure was not indicated. Surfactant had no statistically significant effect on the duration of assisted ventilation (MD 0.60 days, 95% CI ‐0.41 to 1.62) (Analysis 1.6). Heterogeneity of treatment effect for this outcome was moderate to high (I² = 73%).
1.6. Analysis.

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 6 Duration of assisted mechanical ventilation (days).
Duration of supplemental oxygen (days) (Outcome 1.7):
Two studies enrolling 97 infants reported on duration of supplemental oxygen. Surfactant did not statistically significantly reduce the duration of supplemental oxygen (MD 0.40, 95% CI ‐2.83 to 3.64) (Analysis 1.7). Heterogeneity of treatment effect for this outcome was high (I² = 88%).
1.7. Analysis.
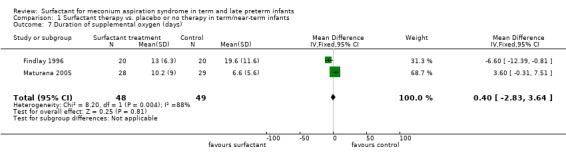
Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 7 Duration of supplemental oxygen (days).
Need for supplemental oxygen at discharge (Outcome 1.8):
One study enrolling 40 infants reported on the need for oxygen at discharge. Surfactant had no statistically significant effect on need for supplemental oxygen at discharge (RR 0.75, 95% CI 0.32 to 1.77; RD ‐0.10, 95% CI ‐0.39 to 0.19) (Analysis 1.8). Tests for heterogeneity were not applicable.
1.8. Analysis.
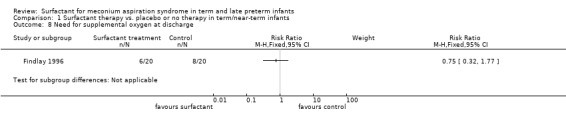
Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 8 Need for supplemental oxygen at discharge.
Chronic lung disease (age at diagnosis not stated) (Outcome 1.9):
One study enrolling 168 infants reported on chronic lung disease. Surfactant had no statistically significant effect on chronic lung disease (RR 0.47, 95% CI 0.12 to 1.80; RD ‐0.04, 95% CI ‐0.11 to 0.03) (Analysis 1.9). Tests for heterogeneity were not applicable.
1.9. Analysis.
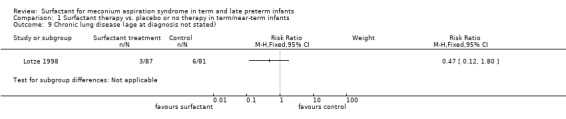
Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 9 Chronic lung disease (age at diagnosis not stated).
Intraventricular haemorrhage (any grade) (Outcome 1.10):
Two studies enrolling 229 infants reported on the incidence of intraventricular haemorrhage (any grade). Surfactant had no statistically significant effect on intraventricular haemorrhage (any grade) (typical RR 0.67, 95% CI 0.31 to 1.46; typical RD ‐0.04, 95% CI ‐0.12 to 0.04) (Analysis 1.10). Heterogeneity of treatment effect for this outcome was low to moderate (RR, I² = 47%) and moderate (RD, I² = 51%).
1.10. Analysis.
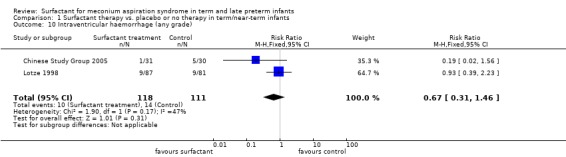
Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 10 Intraventricular haemorrhage (any grade).
Severe intraventricular haemorrhage (grades III and IV) (Outcome 1.11):
One study enrolling 168 infants reported on the incidence of severe intraventricular haemorrhage (grades III and IV). Surfactant had no statistically significant effect on severe intraventricular haemorrhage (grades III and IV) (RR 2.79, 95% CI 0.30 to 26.31; RD 0.02, 95% CI ‐0.02 to 0.07) (Analysis 1.11).Tests for heterogeneity were not applicable.
1.11. Analysis.
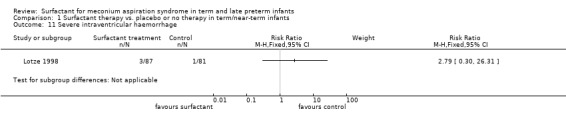
Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 11 Severe intraventricular haemorrhage.
Duration of hospital stay (days) (Outcome 1.12):
One study enrolling 40 infants reported on the duration of hospital stay. Surfactant statistically significantly reduced the duration of hospital stay (MD ‐8 days, 95% CI ‐14 to ‐3) (Analysis 1.12). Tests for heterogeneity were not applicable.
1.12. Analysis.
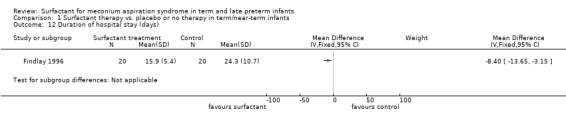
Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 12 Duration of hospital stay (days).
Additional outcomes for the update in 2014:
Death or chronic lung disease at 28 days: outcome not reported.
Death or chronic lung disease at 36 weeks postmenstrual age: outcome not reported.
Neurodevelopmental follow‐up: outcome not reported.
Discussion
Deficiency or dysfunction, or both, of pulmonary surfactant may contribute to respiratory failure in a broad group of disorders including pneumonia, meconium aspiration syndrome (MAS), and adult respiratory distress syndrome. We identified four randomised controlled trials that studied the effect of surfactant therapy in term and late preterm infants with MAS. Three of the studies were placebo‐controlled using air as the placebo, and in these three studies the outcomes were assessed blinded to group of allocation (Findlay 1996; Lotze 1998; Maturana 2005). In the fourth study, the clinical staff were not blinded to group allocation (Chinese Study Group 2005). The sample sizes of the studies were small with 40, 57, 61, and 168 infants enrolled (Findlay 1996; Maturana 2005; Chinese Study Group 2005; Lotze 1998) respectively. The number of infants enrolled in Maturana 2005 differed between the published abstract and the information obtained from the author. There were four more infants included in the report that we obtained from the authors.
Surfactant treatment did not have a statistically significant effect on the primary outcome of mortality. In the meta‐analysis of the results from two studies (Findlay 1996; Lotze 1998), surfactant treatment resulted in a statistically and clinically important reduction in the need for extracorporeal membrane oxygenation (ECMO) treatment, with a number needed to treat for an additional beneficial outcome (NNTB) of 6 (95% CI 3 to 25). ECMO treatment was not available for the units in the Chinese Collaborative study (Chinese Study Group 2005), nor in the study from Chile (Maturana 2005). The one study (Findlay 1996) that reported on duration of hospital stay demonstrated a reduction in hospital days. There were no other statistically significant reductions in any of the other important clinical outcomes (duration of assisted ventilation, duration of supplemental oxygen, air leaks, chronic lung disease, duration of assisted ventilation, need for supplemental oxygen at discharge and intraventricular haemorrhage). The trends for all respiratory tract‐associated outcomes favoured the use of surfactant.
A number of investigators have attempted to treat MAS with dilute surfactant solutions used as a lavage to wash residual meconium from the airway (Dargaville 2011; Ibara 1995; Lam 1999; Ogawa 1996). Wiswell 2002 enrolled 22 infants [15 surfactant (Surfaxin) and 7 control]. There were non‐significant trends for surfactant‐lavaged infants to be weaned from mechanical ventilation earlier (mean of 6.3 vs. 9.9 days, respectively), as well as to have a more rapid decline in their oxygenation index (OI) compared with control infants. Since the last update of this review, Dargaville 2011 has published a randomised controlled trial of lavage with two dilute bovine surfactants in the treatment of MAS. Sixty‐six infants were randomised, with one ineligible infant excluded from the analysis. In this study, fewer infants who underwent lavage died or required ECMO (10% compared with 31% in the control group). However, surfactant lavage did not alter the duration of respiratory support (median duration in the lavage group 5.5. days and in the control group 6.0 days). Randomised comparisons are warranted of surfactant bolus versus surfactant lavage therapy in MAS.
Current evidence indicates that amnioinfusion prior to birth or suctioning of the oropharynx/nasopharynx prior to the delivery of the shoulders do not prevent MAS from occurring. At the present time, the two most promising interventions appear to be treatment with surfactant or surfactant lavage. As few infants have been studied to date, further research is warranted, possibly using a three‐armed trial with 1) surfactant administration, 2) surfactant lavage and 3) a control group receiving air.
Clinical experience indicates that persistent pulmonary hypertension of the newborn (PPHN) is one of the major causes of death in infants with MAS (Hsieh 2004). There is evidence that meconium injury may directly trigger postnatal release of vasoconstrictors such as ET‐1, TXA2, and prostaglandin E2 (PGE2), which play a role in the development of pulmonary hypertension (Soukka 1998).
Infants with MAS and PPHN are usually treated with oxygen, conventional or high frequency mechanical ventilation or both, inotropic support, induction of alkalosis, and sedation. When these measures fail, ECMO has been shown to improve the outcome (UK Collab 1996). Inhaled nitric oxide (iNO) is frequently used for the treatment of newborns with severe pulmonary hypertension and respiratory failure. Consequently, increasing clinical and experimental evidence suggest that exogenous NO, given by inhalation, selectively reduces pulmonary vasoconstriction and improves oxygenation in a variety of pathological conditions of the newborn lungs, including meconium aspiration (Neonatal iNO 1997; Van Meurs 2003). In recent experimental data, Aaltonen 2007 demonstrated that iNO in MAS is associated with diminished pulmonary hypertensive response as well as decreased DNA oxidation and neuronal damage in hippocampal tissue that may potentially have significant adverse long‐term effects on the developmental status of the affected newborns.
ECMO procedures are complex because they require systemic anticoagulation and major vessel cannulation. Studies of iNO therapy for PPHN have shown rapid improvement in oxygenation, reducing the need for ECMO therapy without affecting the mortality (Christou 2000; Clark 2000). Finer 2000 showed that iNO treatment improves oxygenation in approximately 50% of term or late preterm neonates with hypoxaemic respiratory failure, and reduces the combined end point of death or the need for ECMO therapy (risk ratio 0.73) as compared with control subjects. However, lack of an early response to iNO treatment within a few hours in infants who are referred for ECMO therapy and younger age at the time of presentation may indicate the need for ECMO therapy in at least 50% of those with hypoxic respiratory failure (Fakioglu 2005).
Authors' conclusions
Implications for practice.
The results of this systematic review provide some support for the use of surfactant treatment in meconium aspiration syndrome (MAS). In infants with MAS leading to moderate to severe respiratory failure, surfactant administration will decrease the number of infants treated with extracorporeal membrane oxygenation (ECMO). This may have implications especially in resource‐poor settings where ECMO is not available. In the only study reporting on the duration of hospital stay, this outcomes was significantly reduced.
Implications for research.
Although surfactant therapy may be of use in severe MAS, the efficacy of surfactant therapy compared to other approaches including inhaled nitric oxide, liquid ventilation, and high frequency ventilation remains to be tested. Other approaches to surfactant therapy, including the use of surfactant lavage, may prove to be effective in the treatment of MAS. Trials that compare surfactant treatment to surfactant lavage and air (control) would be appropriate. The findings of this review need to be confirmed in randomised controlled trials of appropriate size.
What's new
| Date | Event | Description |
|---|---|---|
| 28 January 2020 | Amended | Arne Ohlsson deceased. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 30 November 2014 | New citation required but conclusions have not changed | No new studies were identified for inclusion. Three new studies were identified but excluded (Dargaville 2011; Gadzinowski 2008; Lin 2014). Conclusions not changed. |
| 30 June 2013 | New search has been performed | This review updates the existing review Surfactant for meconium aspiration syndrome in term and late preterm infants (El Shahed 2007). |
| 30 June 2011 | New search has been performed | This review updates the existing review "Surfactant for meconium aspiration syndrome in term and late preterm infants" published in the Cochrane Database of Systematic Reviews El Shahed 2007. One excluded study was added Dargaville 2011. No changes to conclusions. |
| 27 February 2008 | Amended | Converted to new review format. |
| 16 May 2007 | New citation required and conclusions have changed | Substantive amendment |
| 16 May 2007 | New search has been performed | This is an update of the review "Surfactant for meconium aspiration syndrome in full term infants" published in The Cochrane Library, Issue 2, 2002 (Soll 2002). The authorship of this review has been changed to: El Shahed AI, Dargaville P, Ohlsson A, Soll RF. Since this review was first published, two additional trials were identified. The increase in sample size has allowed for greater precision for some of the treatment effects. Surfactant treatment does not appear to have an effect on mortality, but does reduce the need for treatment with extracorporeal membrane oxygenation (ECMO). Surfactant treatment may reduce respiratory related outcomes and hospital stay in term/near‐term infants with meconium aspiration syndrome. |
Acknowledgements
For the original review we would like to thank Ms Elizabeth Zola, PharmD, for providing diagnosis‐specific data from the Survanta in Term Infants Study Group. .
For the 2007 update of this review we thank Ms Elizabeth Uleryk, Chief Librarian, the Hospital for Sick Children, Toronto, Ontario, Canada, for developing the search strategy for the literature retrieval. We also thank Dr Andres Maturana for providing us with unpublished data from his study, previously published in abstract form only
For the current update, Ms Yolanda R Brosseau, Managing Editor, Cochrane Neonatal Review Group and Ms Colleen M Ovelman, Trial Search Co‐ordinator and Webmaster, Cochrane Neonatal Review Group, conducted the literature searches in November 2014. One of the authors (AO) conducted searches of clinical trails registries in July 2013 and this was updated again in November 2014.
Appendices
Appendix 1. 2011 Search Strategy
PubMed
(Pulmonary Surfactants OR surfactan* OR Surface‐Active Agents) AND (Meconium Aspiration Syndrome OR Meconium) AND ((infant, newborn[MeSH] OR newborn OR neon* OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])) AND (("2006"[PDat] : "3000"[PDat]))
Cinahl
( (Pulmonary Surfactants OR surfactan* OR Surface‐Active Agents) AND (Meconium Aspiration Syndrome OR Meconium) ) and ( ( infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW) AND ( randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) )
Limiters ‐ Published Date from: 20070101‐20111231
Cochrane Central Register of Controlled Trials
(infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)and ("Pulmonary Surfactant" OR surfactan* OR "Surface‐Active Agents") and ("Meconium Aspiration Syndrome" OR Meconium), from 2007 to 2011
Embase
1 (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (607975)
2 (human not animal).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (11910500)
3 (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (1266847)
4 (Pulmonary Surfactant or surfactan* or Surface‐Active Agents).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (51503)
5 (Meconium Aspiration Syndrome or Meconium).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (6386)
6 1 and 2 and 3 and 4 and 5 (80)
7 limit 6 to yr="2007 ‐Current" (21)
Clinicaltrials.gov
terms: (infant OR newborn) AND (Pulmonary Surfactant OR surfactant OR Surface‐Active Agents) AND (Meconium Aspiration Syndrome OR Meconium)
Controlled‐trials.com
terms: (infant OR newborn) AND (Pulmonary Surfactant OR surfactant OR Surface‐Active Agents) AND (Meconium Aspiration Syndrome OR Meconium)
Data and analyses
Comparison 1. Surfactant therapy vs. placebo or no therapy in term/near‐term infants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 4 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.41, 2.39] |
| 2 Treatment with ECMO | 2 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.46, 0.91] |
| 3 Pneumothorax | 3 | 269 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.08, 0.05] |
| 4 Pulmonary interstitial emphysema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5 Air leaks (pneumothorax, pneumomediastinum, interstitial emphysema) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6 Duration of assisted mechanical ventilation (days) | 3 | 158 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.41, 1.62] |
| 7 Duration of supplemental oxygen (days) | 2 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐2.83, 3.64] |
| 8 Need for supplemental oxygen at discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9 Chronic lung disease (age at diagnosis not stated) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10 Intraventricular haemorrhage (any grade) | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.31, 1.46] |
| 11 Severe intraventricular haemorrhage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12 Duration of hospital stay (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chinese Study Group 2005.
| Methods | Multicentre (19 centres) study in China. Study period not stated. Blinding of randomisation: Yes Blinding of treatment: No Complete follow‐up: Yes Blinding of outcome measure: No | |
| Participants | Term and late preterm neonates with MAS, BW > 2500 gm, postnatal age < 36 hrs, a/A pO₂ ratio < 0.22, OI > 15 and needed mechanical ventilation for 1 to 2 hrs without improvement. No lethal congenital anomalies, IVH grade II ‐ IV, Apgar score < 3 at 10 minutes or clinically unstable. | |
| Interventions | Sixty‐one term infants with severe MAS were randomly assigned to either a surfactant or a control group within 36 h after birth. The infants in the surfactant group (n=31) received an initial dose of porcine lung‐derived surfactant (Curosurf) at 200 mg/kg, and repeated doses of 200, 100 and 100 mg/kg were given at 6 to 12 h intervals to a maximum of four doses if oxygenation index (OI) deteriorated by > 2 from baseline. | |
| Outcomes | PRIMARY: Reduction of OI to < 10 and an increase of the pretreatment a/A pO₂ ratio of 100% over baseline 24 hrs after surfactant treatment. SECONDARY: Duration of mechanical ventilation, incidence of complications and survival to discharge from hospital. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information presented |
| Allocation concealment (selection bias) | Low risk | Surfactant or control therapy was randomly assigned by the randomisation centre staff according to sequentially numbered randomisation cards, provided in sealed randomisation envelopes, based on an expected total enrolment of 64 participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Surfactant administration was not conducted in a blind manner because that would have required a separate dosing team for each clinic centre. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff were aware of group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 66 infants were enrolled and 5 infants (4 in the surfactant group and 1 in the control group were excluded from the final analysis because of violation of the entry criteria). |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we cannot ascertain if there were any deviations from the protocol. |
Findlay 1996.
| Methods | Single‐centre study in the US. Study period not stated. Blinding of randomisation: Yes Blinding of treatment: Yes Complete follow‐up: Yes Blinding of outcome measure: Yes | |
| Participants | Term infants with MAS, requiring assisted ventilation, supplemental oxygen > 50%, MAP > 7cm H₂0, a/A pO₂ ratio < 0.22, age < 6 hrs and no major congenital anomaly. | |
| Interventions | The treatment group (n = 20) received modified bovine surfactant extract (Survanta 150 mg/kg), repeated at 6‐hr intervals for a maximum of 4 doses, infused intratracheally via a side port adaptor over 20 mins. The control group (n = 20) received air placebo. | |
| Outcomes | PRIMARY: Improvement in OI, improvement in a/A pO₂ ratio SECONDARY: Pneumothorax, need for ECMO, duration of assisted ventilation, duration of oxygen therapy, mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | No specific information provided but the investigators and the physicians and nurses caring for the infants were unaware of the infants' assignment groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The investigators and the physicians and nurses caring for the infants were unaware of the infants' assignment groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigators and the physicians and nurses caring for the infants were unaware of the infants' assignment groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all enrolled infants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we cannot ascertain if there were any deviations from the protocol. |
Lotze 1998.
| Methods | Multicentre (44 centres) study in the US. Study period September 1 1992 to October 23 1995.
Blinding of randomisation: Yes (central randomisation)
Blinding of treatment: Yes (dosing investigators)
Complete follow‐up: Yes
Blinding of outcome measurement: Yes Stratification: primary diagnosis disease severity (oxygenation index) |
|
| Participants | Infants > 2000 gm, gestational age > 36 weeks, age < 120 hrs with MAS, PPHN or sepsis and severe respiratory failure but without any major congenital anomalies or IVH > Grade I. | |
| Interventions | The treatment group (n = 87) received modified bovine surfactant extract (Survanta, 100 mg/kg) or air placebo (up to 4 doses prior to ECMO and 4 additional doses if ECMO was required). The control group (n = 81) received air placebo. | |
| Outcomes | PRIMARY: Need for ECMO, severe complications, mortality. | |
| Notes | Only infants with MAS are included in this review. Data for this group were obtained by the authors of the first version of this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation. Computer‐generated random numbers Stratification: primary diagnosis disease severity (oxygenation index). |
| Allocation concealment (selection bias) | Low risk | The treatment assignments were made by having the pharmacist or dosing investigator at each site report the primary diagnosis and mean entry oxygen index for each participant to a central randomisation centre, Bio‐Pharm Clinical Services, Inc., which issued a participant number and treatment assignment on the basis of a computer‐generated random number. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study treatments were administered by dedicated dosing investigators at each site. The dosing investigators shielded the infant with drapes or a screen during treatment, and all other personnel left the immediate bedside area during the dosing procedure. The dosing investigator took the same amount of time to prepare and administer either treatment, and when treatment was complete, all supplies were stored in a locked area. Dosing investigators were prohibited from participating in any other aspect of the infants care and from revealing the treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | With the exception of dosing, the infant's clinical care during the 28 days of the study was provided by clinical investigators who were unaware of the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 330 randomised infants accounted for (168 of these infants were enrolled on the basis of MAS, and the remainder on the basis of PPHN or sepsis) . Two infants were later withdrawn from the study when consent was withdrawn. Their limited data were subsequently excluded from analysis. The diagnosis on which their enrolment was based was not stated. |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we cannot ascertain if there were any deviations from the protocol |
Maturana 2005.
| Methods | Multicentre (13 centres) study in Chile between March 2001 and June 2003. Blinding of randomisation: Yes Blinding of treatment: Yes Complete follow‐up: Yes Blinding of outcome measure: Yes | |
| Participants | Term infants ≥ 37 weeks of gestation with moderate to severe MAS and respiratory insufficiency within the first 12 hrs after birth. | |
| Interventions | The treatment group (n = 28) received 150 mg /kg/dose (6 ml) of Survanta every 6 hours for a total of 3 doses if they remained intubated. The control group (n = 29) received an equivalent amount of air as placebo. | |
| Outcomes | PRIMARY: Days of mechanical ventilation. SECONDARY: Days requiring oxygen therapy with a target arterial oxygen saturation > 90%, air leaks, PPHN, OI 2 hrs after the first treatment dose and mortality before discharge. | |
| Notes | We obtained unpublished data from Dr A Maturana. In the unpublished manuscript there were 3 more infants enrolled in the surfactant group and 1 more infant enrolled in the control group. We report the outcomes as per the unpublished report, not the referenced abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation scheme using blocks of 4 (as per unpublished manuscript). |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes stratified by centre |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A placebo (air) was used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomised (as per unpublished manuscript). |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we cannot ascertain if there were any deviations from the protocol. |
a/A pO₂ ratio = arterial/alveolar oxygen tension ratio BW = birth weight ECMO = extracorporeal membrane oxygenation IVH = Intraventricular haemorrhage MAP = mean airway pressure MAS = meconium aspiration syndrome OI = oxygen index PPHN = persistent pulmonary hypertension of the neonate
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Auten 1991 | Sequential case study; no control group |
| Blanke 1993 | Not randomised, no control group. As a part of a West German multicentre study (424 participants, 16 hospitals) 10 term neonates ventilated because of severe meconium aspiration syndrome were treated with 1 to 4 doses of 50 mg/kg/BW of a bovine surfactant (Alveofact). Before treatment respiratory distress was severe (median FiO₂: 1.0, median MAD 9.9 mmHg, median OI: 20). Acute improvement ("responders") was shown in 4 participants. All infants survived. Time of mechanical ventilation was 6 to 26 days. High frequency ventilation was applied in 2 non‐responders, ECMO in 1. |
| Chang 2003 | Retrospective review; treatment with bronchoalveolar lavage with dilute surfactant preparation Chang 2003 retrospectively reviewed the charts of all term infants with a diagnosis of MAS who had an oxygenation index (OI) > 20 during a 2‐year period. Tracheobronchial lavage was performed with a dilute surfactant suspension (5 mg/mL or 10 mg/mL) to reach a total dose of 60 to 70 mg/kg of phospholipid, administered in aliquots of 2 mL. The records of 22 patients were reviewed, of whom 12 had undergone lavage. These infants were subdivided into low‐concentration (surfactant concentration, 5 mg/mL; n = 6) and high‐concentration (surfactant concentration, 10 mg/mL; n = 6) subgroups. There were no significant differences in demographic characteristics between the 2 subgroups. The lavaged infants had a significantly higher arterial partial pressure of oxygen (PaO₂) 24 hours after lavage than the infants without lavage (178.3 mm Hg vs 80.6 mm Hg, P < 0.05). The incidence of pneumothorax (1/12 vs 7/10, P < 0.05) and requirement for inhaled nitric oxide (5/12 vs 9/10, P < 0.05) were significantly lower in the lavaged group. All infants tolerated the procedure well except for 2 with transient complications. There were no significant differences in duration of lavage, response and complications between subgroups lavaged at low and high surfactant concentration. |
| Dargaville 2011 | Bronchoalveolar lavage with dilute surfactant preparation Dargaville 2011 evaluated whether lung lavage with surfactant changes the duration of mechanical respiratory support or other outcomes in meconium aspiration syndrome (MAS). Randomised controlled trial that enrolled ventilated infants with MAS. Infants randomised to lavage received two 15‐mL/kg aliquots of dilute bovine surfactant instilled into, and recovered from, the lung. Control subjects received standard care, which in both groups included high frequency ventilation, nitric oxide, and, where available, ECMO. 66 infants were randomised, with 1 ineligible infant excluded from analysis. Median duration of respiratory support was similar in infants who underwent lavage and control subjects (5.5 vs. 6.0 days, P = .77). Requirement for high frequency ventilation and nitric oxide did not differ between the groups. Fewer infants who underwent lavage died or required ECMO: 10% (3/30) compared with 31% (11/35) in the control group (odds ratio, 0.24; 95% confidence interval, 0.060 to 0.97). Lavage transiently reduced oxygen saturation without substantial heart rate or blood pressure alterations. Mean airway pressure was more rapidly weaned in the lavage group after randomisation. |
| Diniz 1995 | Not randomised |
| Gadzinowski 2008 |
Gadzinowski 2008 compared the effectiveness of surfactant treatment either by bolus or surfactant lung lavage followed by inhaled nitric oxide (iNO) therapy in infants with MAS complicated by persistent pulmonary hypertension (PPHN). 13 infants with diagnosis of MAS and PPHN were first treated with conventional respiratory support. Then between 2 and 22 hrs of life they were randomised either to bolus surfactant treatment (n = 6) or surfactant lung lavage (SLL, n = 7) treatment. Then all infants were treated with iNO therapy. The groups were compared with regard to their clinical course: changes in PaO₂, FiO₂, MAP, OI, A‐a oxygen gradient, duration of iNO therapy, length of ventilation and hospitalisation. Complications and mortality were also compared. The results showed that infants treated with SLL had significant improvements in oxygenation, decreases in MAP and A‐a gradients. But there were no significant differences in duration of ventilation, iNO treatment, length of hospitalisation or complications. In conclusion these data show no advantage of SLL therapy over bolus surfactant treatment in infants with MAS complicated by PPHN. |
| Halliday 1996 | Retrospective, not randomised |
| Hung 2006 | Historical controls Hung 2006 assessed the effects of lavage with a small volume of dilute surfactant in neonates with MAS and compared the results with those of historical controls treated with larger volumes. Eleven newborns with MAS were treated using 20 ml of dilute surfactant at a phospholipid concentration of 10 mg/ml (SVL group). Results were compared with those of 9 infants previously treated with large‐volume lavage (LVL group), using 40 ml of dilute surfactant, 5 mg/ml. Measures of oxygenation, including mean PaO₂, oxygenation index, and arterial/alveolar 0₂ ratio, showed no significant difference between the 2 groups. |
| Ibara 1995 | Saline lavage and surfactant replacement, not randomised |
| Khammash 1993 | Sequential case study; no control group |
| Lam 1999 | Not randomised, historical controls, surfactant used as lavage. Lam 1999 reported a pilot experience on the use of diluted bovine lung surfactant lipid extract solution (Survanta, Ross Laboratories, Ohio, USA) as a tracheobronchial lavage fluid for the treatment of infants with severe MAS. 6 consecutively recruited infants with severe MAS necessitating mechanical ventilation with an oxygen index of ≥ 15 within 6 hours of life during a 1½‐year period were treated with tracheobronchial lavage with 15 mL/kg of diluted surfactant solution (Survanta) at a phospholipid concentration of 5 mg/mL administered in 2‐mL aliquots. The outcome of treatment was assessed by comparison with 6 consecutive historic control infants with equally severe MAS of similar inclusion criteria retrospectively. The mean oxygen index, mean airway pressure, fraction of inspired oxygen, and arterial/alveolar oxygen tension ratio improved significantly within the first 48 hours after treatment in the lavage group. The duration of ventilation (mean ± SEM, 55.3 ± 4.6 hours vs 131 ± 60 hours) and oxygen therapy (mean ± SEM, 4.1 ± 0.5 days vs 20.8 ± 8.2 days) were also significantly reduced in the lavage‐treated group compared with the control group. All 6 infants in the lavage group survived without sequelae whereas there were 2 deaths in the control group. The process of administering the surfactant lavage was well tolerated with no air leak complications. |
| Lin 2014 | Bronchoalveolar lavage with dilute surfactant preparation Lin 2014 evaluated 136 full‐term infants with severe MAS who were admitted to the neonatal intensive care unit. Infants were randomly allocated to pulmonary surfactant (PS) lavage and PS injection groups. In the PS lavage group, infants were treated with endotracheal lavage using 3 to 5 mL of diluted PS (12 mg/mL) each time, and the PS injection group was given PS by intratracheal injection with an initial dose of 200 mg/kg. Blood gas, oxygenation index (OI), and PaO2/FiO2 of the two groups were evaluated before and 2, 12, 24, and 48 hours after the treatment, and the duration of mechanical ventilation, complication rate, and cure rate were compared between the two groups. |
| Lista 2006 | Case series Lista 2006 evaluated the efficacy and safety of bronchoalveolar lavage (BAL) with diluted porcine surfactant in mechanically ventilated term infants with severe acute respiratory distress syndrome (ARDS) due to MAS. Eight consecutive mechanically ventilated term infants with severe ARDS due to MAS underwent BAL with 15 mL/kg of diluted (5.3 mg phospholipid/mL) surfactant saline suspension (porcine surfactant, Curosurf). Treatment was administered slowly in aliquots of 2.5 mL. The mean age of neonates at treatment was 3.5 (range 1 ‐ 8) hours. Heart rate, systemic blood pressure and oxygen saturation were monitored continuously. Arterial blood gases were measured immediately before treatment, and again at 3 and 6 hours post‐treatment. Chest x‐rays were taken 6 and 24 hours after treatment. Radiological improvement was evident in all 8 infants 6 hours post‐treatment. Compared with pre‐BAL values, significant improvements (P < 0.05) in mean values for partial pressure of oxygen in arterial blood, partial pressure of carbon dioxide in arterial blood, pH, arterial/alveolar O₂ ratio and oxygenation index were documented at 3 and 6 hours after BAL. In all participants, tracheal fluids that had been meconium‐stained prior to BAL were clear of meconium after BAL. Only one infant required nitric oxide therapy for transient pulmonary hypertension. No adverse sequelae of treatment occurred during the study. |
| Ogawa 1996 | Bronchoalveolar lavage with dilute surfactant preparation for the treatment of meconium aspiration syndrome |
| Wiswell 2002 | Bronchoalveolar lavage with dilute surfactant preparation Wiswell 2002 compared treatment with bronchoalveolar lavage using dilute Surfaxin with standard therapy in a population of newborn infants with MAS. Inclusion criteria were 1) gestational age ≥ 35 weeks, 2) enrolment within 72 hours of birth, 3) diagnosis of MAS, 4) need for mechanical ventilation, and 5) an oxygenation index ≥ 8 and ≤ 25. Infants were randomised to either lavage with Surfaxin or standard care (2:1 proportion). In lavaged infants, a volume of 8 mL/kg dilute Surfaxin (2.5 mg/mL) was instilled into each lung over approximately 20 seconds followed by suctioning after 5 ventilator breaths. The procedure was repeated twice. The third and final lavage was with a more concentrated solution (10 mg/mL) of Surfaxin. |
ECMO = extracorporeal membrane oxygenation MAP = mean airway pressure MAS = meconium aspiration syndrome
Differences between protocol and review
Additional outcomes sought for the update in 2014: 1. Death or chronic lung disease at 28 days 2. Death or chronic lung disease at 36 weeks postmenstrual age 3. Neurodevelopmental follow‐up
However, these outcomes were not available in included studies.
Contributions of authors
Amr I El Shahed: Updated the search for articles, extracted data, reviewed results, and wrote the text of the review.
Peter Dargaville: Performed the original search for articles, extracted data, reviewed results, and edited the text of the review.
Arne Ohlsson: Updated the search for articles, extracted data, reviewed results, and wrote the text of the review.
Roger F. Soll: Performed the original search for articles, extracted data, reviewed results, and edited the text of the updated review
The November 2014 update was conducted centrally by the Cochrane Neonatal Review Group staff (Yolanda Brosseau, Diane Haughton, and Roger Soll). This 2014 update was reviewed, revised and approved by Amr I El Shahed and Arne Ohlsson.
Sources of support
Internal sources
Department of Paediatrics, Mount Sinai Hospital, Toronto, Canada.
External sources
The Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN275201100016C, USA.
Declarations of interest
Dr R. Soll has acted as a consultant and invited speaker for several of the pharmaceutical companies which manufacture or distribute surfactant preparations (Abbott Laboratories, Ross Laboratories, Chiesi Pharmaceuticals, Dey Laboratories, Burroughs Wellcome) but has not done so for over 6 years. Dr P. Dargaville has received support for basic science research in surfactant lavage studies from Abbott Australasia. Neither Dr El Shahed nor Dr Ohlsson have conflicts of interest to disclose.
Deceased
Edited (no change to conclusions)
References
References to studies included in this review
Chinese Study Group 2005 {published data only}
- Chinese Collaborative Study Group for Neonatal Respiratory Diseases. Treatment of severe meconium aspiration with porcine surfactant: a multicentre, randomized, controlled trial. Acta Paediatrica 2005;94(7):896‐902. [DOI] [PubMed] [Google Scholar]
Findlay 1996 {published data only}
- Findlay RD, Taeusch HW, Walther FJ. Surfactant replacement therapy for meconium aspiration syndrome. Pediatrics 1996;97(1):48‐52. [PubMed] [Google Scholar]
Lotze 1998 {published data only}
- Lotze A, Mitchell BR, Bulas DI, Zola EM, Shalwitz RA, Gunkel HJ, et al. Multicenter study of surfactant (beractant) use in the treatment of term infants with severe respiratory failure. Journal of Pediatrics 1998;132(1):40‐7. [DOI] [PubMed] [Google Scholar]
Maturana 2005 {unpublished data only}
- Maturana A, Torres‐Pereyra J, Salinas R, Astudillo P, Moya FR, The Chile Surf Group. A randomized trial of natural surfactant for moderate to severe meconium aspiration syndrome. Pediatric Academic Societies. 2005; Vol. 57:1545.
References to studies excluded from this review
Auten 1991 {published data only}
- Auten RL, Notter RH, Kendig, JW, Davis JM, Shapiro DL. Surfactant treatment of full‐term newborns with respiratory failure. Pediatrics 1991;87(1):101‐7. [PubMed] [Google Scholar]
Blanke 1993 {published data only}
- Blanke JG, Jorch G. Surfactant therapy in severe neonatal respiratory failure ‐ multicenter study ‐ II. Surfactant therapy in 10 newborn infants with meconium aspiration syndrome [Surfactanttherapie bei 10 Neugeborenem mit Mekoniumaspirationssyndrom]. Klinische Padiatrie 1993;205(2):75‐8. [DOI] [PubMed] [Google Scholar]
Chang 2003 {published data only}
- Chang HY, Hsu CH, Kao HA, Hung HY, Chang JH, Peng CC, Jim WT. Treatment of severe meconium aspiration syndrome with dilute surfactant lavage. Journal of the Formosan Medical Association 2003;102(5):326‐30. [PubMed] [Google Scholar]
Dargaville 2011 {published data only}
- Dargaville PA, Copnell B, Mills JF, Haron I, Lee JK, Tingay DG, et al. lessMAS Trial Study Group. Randomized controlled trial of lung lavage with dilute surfactant for meconium aspiration syndrome. Journal of Pediatrics 2011;158(3):383‐9. [DOI] [PubMed] [Google Scholar]
Diniz 1995 {published data only}
- Diniz EMA, Fiori RM. Curosurf therapy in severe meconium aspiration (MAS). Biology of the Neonate 1995;67:86. [Google Scholar]
Gadzinowski 2008 {published data only}
- Gadzinowski J, Kowalska K, Vidyasagar D. Treatment of MAS with PPHN using combined therapy: SLL, bolus surfactant and iNO. Journal of Perinatology 2008;28(Suppl 3):S56‐66. [DOI] [PubMed] [Google Scholar]
Halliday 1996 {published data only}
- Halliday HL, Speer CP, Robertson B. Treatment of severe meconium aspiration syndrome with porcine surfactant. European Journal of Pediatrics 1996;155(12):1047‐51. [DOI] [PubMed] [Google Scholar]
Hung 2006 {published data only}
- Hung HY, Jim WT, Hsu CH, Chang JH, Peng CC, Shih SL, et al. Small versus large volume dilute surfactant lavage for meconium aspiration syndrome.. Acta Paediatrica Taiwan 2006;47(4):181‐6. [PubMed] [Google Scholar]
Ibara 1995 {published data only}
- Ibara S, Ikenoue T, Murata Y, Sakamoto H, Saito T, Nakamura Y, et al. Management of meconium aspiration syndrome by tracheobronchial lavage and replacement of surfactant ‐TA. Acta Paediatrica Japonica 1995;37(1):64‐7. [DOI] [PubMed] [Google Scholar]
Khammash 1993 {published data only}
- Khammash H, Perlman M, Wojtulewicz J, Dunn M. Surfactant therapy in full‐term neonates with severe respiratory failure. Pediatrics 1993;92(1):135‐9. [PubMed] [Google Scholar]
Lam 1999 {published data only}
- Lam BCC, Yeung CY. Surfactant lavage for meconium aspiration syndrome: a pilot study. Pediatrics 1999;103:1014‐8. [DOI] [PubMed] [Google Scholar]
Lin 2014 {published data only}
- Lin XZ, Lai JD, Lan ZY, Lin YY. [Clinical effect of endotracheal lavage with porcine pulmonary surfactant in term neonates with severe meconium aspiration syndrome]. Zhongguo Dang Dai Er Ke Za Zhi (Chinese Journal of Contemporary Pediatrics) 2014;16(7):709‐13. [PUBMED: 25008878] [PubMed] [Google Scholar]
Lista 2006 {published data only}
- Lista G, Bianchi S, Castoldi F, Fontana P, Cavigioli F. Bronchoalveolar lavage with diluted porcine surfactant in mechanically ventilated term infants with meconium aspiration syndrome. Clinical Drug Investigation 2006;26(1):13‐9. [DOI] [PubMed] [Google Scholar]
Ogawa 1996 {published data only}
- Ogawa Y, Ohara Y, Itakura Y, et al. Bronchial lavage with surfactant solution for the treatment of meconium aspiration syndrome. J Jpn Med Soc Biol Interface 1996;26(Suppl):179‐87. [Google Scholar]
Wiswell 2002 {published data only}
- Wiswell TE, Knight GR, Finer NN, Donn SM, Desai H, Walsh WF, et al. A multicenter, randomized, controlled trial comparing surfaxin (lucinactant) lavage with standard care for treatment of meconium aspiration syndrome. Pediatrics 2002;109(6):1081‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Aaltonen 2007
- Aaltonen M, Soukka H, Halkola L, Jalonen J, Kalimo H, Holopainen IE. Inhaled nitric oxide treatment inhibits neuronal injury after meconium aspiration in piglets. Early Human Development 2007;83(2):77‐85. [DOI] [PubMed] [Google Scholar]
AAP 2006
- American Academy of Pediatrics, American Heart Association. Textbook of neonatal resuscitation. 5th Edition. Elk Grove Village: American Academy of Pediatrics, American Heart Association, 2006. [Google Scholar]
Abdel‐Latif 2011a
- Abdel‐Latif ME, Osborn DA. Laryngeal mask airway surfactant administration for prevention of morbidity and mortality in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD008309.pub2] [DOI] [PubMed] [Google Scholar]
Abdel‐Latif 2011b
- Abdel‐Latif ME, Osborn DA. Pharyngeal instillation of surfactant before the first breath for prevention of morbidity and mortality in preterm infants at risk of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD008311.pub2] [DOI] [PubMed] [Google Scholar]
Abdel‐Latif 2012
- Abdel‐Latif ME, Osborn DA. Nebulised surfactant in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD008310.pub2] [DOI] [PubMed] [Google Scholar]
Aziz 2012
- Aziz A, Ohlsson A. Surfactant for pulmonary haemorrhage in neonates. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD005254.pub2] [DOI] [PubMed] [Google Scholar]
Bahadue 2012
- Bahadue FL, Soll R. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD001456.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 1985
- Chen CT, Toung TJK, Rogers MC. Effect of intra‐alveolar meconium on pulmonary surface tension properties. Critical Care Medicine 1985;13(4):233‐6. [DOI] [PubMed] [Google Scholar]
Christou 2000
- Christou H, Marter LJ, Wessel DL, Allred EN, Kane JW, Thompson JE. Inhaled nitric oxide reduces the need for extracorporeal membrane oxygenation in infants with persistent pulmonary hypertension of the newborn. Critical Care Medicine 2000;28(11):3722‐7. [DOI] [PubMed] [Google Scholar]
Clark 1987
- Clark DA, Nieman GF, Thompson JE, Paskanik AM, Rokhar JE, Bredenberg CE. Surfactant displacement by meconium free fatty acids: An alternative explanation for atelectasis in meconium aspiration syndrome. Journal of Pediatrics 1987;110(5):765‐70. [DOI] [PubMed] [Google Scholar]
Clark 2000
- Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, et al. Low‐dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. New England Journal of Medicine 2000;342(7):469‐74. [DOI] [PubMed] [Google Scholar]
Fakioglu 2005
- Fakioglu H, Totapally BR, Torbati D, Raszynski A, Sussmane JB, Wolfsdorf J. Hypoxic respiratory failure in term newborns: clinical indicators for inhaled nitric oxide and extracorporeal membrane oxygenation therapy. Journal of Critical Care 2005;20(3):288‐93. [DOI] [PubMed] [Google Scholar]
Finer 2000
- Finer NN, Barrington KJ. Nitric oxide therapy for the newborn infant. Seminars in Perinatology 2000;24(1):59‐65. [DOI] [PubMed] [Google Scholar]
Hahn 2013
- Hahn S, Choi HJ, Soll R, Dargaville PA. Lung lavage for meconium aspiration syndrome in newborn infants. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD003486.pub2] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):556‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2010
- Hofmeyr GJ, Xu H. Amnioinfusion for meconium‐stained liquor in labour. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD000014.pub3] [DOI] [PubMed] [Google Scholar]
Hsieh 2004
- Hsieh TK, Su BH, Chen AC, Lin TW, Tsai CH, Lin HC. Risk factors of meconium aspiration syndrome developing into persistent pulmonary hypertension of newborn. Acta Paediatrica Taiwan 2004;45(4):203‐7. [PubMed] [Google Scholar]
Moses 1991
- Moses D, Holm BA, Spitale P, Liu M, Enhorning G. Inhibition of pulmonary surfactant function by meconium. American Journal of Obstetrics and Gynecology 1991;164(2):477‐81. [DOI] [PubMed] [Google Scholar]
Neonatal iNO 1997
- The Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full‐term and nearly full‐term infants with hypoxic respiratory failure. New England Journal of Medicine 1997;336:597‐604. [DOI] [PubMed] [Google Scholar]
Pfister 2007
- Pfister RH, Soll RF, Wiswell T. Protein containing synthetic surfactant versus animal derived surfactant extract for the prevention and treatment of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006069.pub3] [DOI] [PubMed] [Google Scholar]
Pfister 2009
- Pfister RH, Soll R, Wiswell TE. Protein‐containing synthetic surfactant versus protein‐free synthetic surfactant for the prevention and treatment of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD006180.pub2] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rojas‐Reyes 2012
- Rojas‐Reyes MX, Morley CJ, Soll R. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD000510.pub2] [DOI] [PubMed] [Google Scholar]
Seger 2009
- Seger N, Soll R. Animal derived surfactant extract for treatment of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007836] [DOI] [PubMed] [Google Scholar]
Sinclair 1992
- Sinclair J, Bracken M. Effective Care of the Newborn. Oxford University Press, 1992. [Google Scholar]
Singh 2011
Soll 1992
- Soll RF, McQueen MC. Respiratory Distress Syndrome. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992. [Google Scholar]
Soll 1997
- Soll RF, Ozek E. Prophylactic animal derived surfactant extract for preventing morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 1997, Issue 4. [DOI: 10.1002/14651858.CD000511] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soll 1998
- Soll RF. Synthetic surfactant for respiratory distress syndrome in preterm infants. Cochrane Database of Systematic Reviews 1998, Issue 3. [DOI: 10.1002/14651858.CD001149] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soll 2001
- Soll RF, Blanco F. Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD000144] [DOI] [PubMed] [Google Scholar]
Soll 2009
- Soll R, Ozek E. Multiple versus single doses of exogenous surfactant for the prevention or treatment of neonatal respiratory distress syndrome. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD000141.pub2] [DOI] [PubMed] [Google Scholar]
Soll 2010
- Soll R, Ozek E. Prophylactic protein free synthetic surfactant for preventing morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD001079.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soukka 1998
- Soukka H, Jalonen J, Kero P, Kaapa P. Endothelin‐1, arterial natriuretic peptide and pathophysiology of pulmonary hypertension in porcine meconium aspiration. Acta Paediatrica 1998;87(4):424‐8. [DOI] [PubMed] [Google Scholar]
Stevens 2007
- Stevens TP, Harrington EW, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003063.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 1993
- Sun B, Curstedt T, Song GW, Robertson B. Surfactant improves lung function and morphology in newborn rabbits with meconium aspiration. Biolology of the Neonate 1993;63(2):96‐104. [DOI] [PubMed] [Google Scholar]
Tan 2012
- Tan K, Lai NM, Sharma A. Surfactant for bacterial pneumonia in late preterm and term infants. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD008155.pub2] [DOI] [PubMed] [Google Scholar]
Tran 1980
- Tran N, Lowe C, Sivieri EM, Shaffer TH. Sequential effects of acute meconium obstruction on pulmonary function. Pediatric Research 1980;14(1):34‐8. [DOI] [PubMed] [Google Scholar]
Tyler 1978
- Tyler DC, Murphy J, Cheney FW. Mechanical and chemical damage to lung tissue caused by meconium aspiration. Pediatrics 1978;62(4):454‐9. [PubMed] [Google Scholar]
UK Collab 1996
- UK Collaborative ECMO Trial Group. UK collaborative randomized trial of neonatal extracorporeal membrane oxygenation. Lancet 1996;348(9020):75‐82. [PubMed] [Google Scholar]
Vain 2004
- Vain NE, Szyld EG, Prudent LM, Wiswell TE, Aguilar AM, Vivas NI. Oropharyngeal and nasopharyngeal suctioning of meconium‐stained neonates before delivery of their shoulders: multicentre, randomised controlled trial. Lancet 2004;364(9434):597‐602. [DOI] [PubMed] [Google Scholar]
Van Meurs 2003
- Meurs K, Rhine WD, Benitz WE. Meconium staining and the meconium aspiration syndrome. Fetal and neonatal brain injury. Mechanisms, management and the risks of practice. Cambridge University Press, 2003:636‐62. [Google Scholar]
Walia 1999
- Walia R, Ohlsson A. Differences between information provided in abstracts of randomized controlled trials in neonates submitted to the Annual Meeting of the American Pediatric Society and the Society for Pediatric Research (APS/SPR) and final publications ‐ implications for meta‐analyses. 7th Annual Cochrane Colloquium Abstracts. 1999.
Ward 2003
- Ward MC, Sinn JKH. Steroid therapy for meconium aspiration syndrome in newborn infants. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003485] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
El Shahed 2007
- Shahed AI, Dargaville PA, Ohlsson A, Soll R. Surfactant for meconium aspiration syndrome in full term/near term infants. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD002054.pub2] [DOI] [PubMed] [Google Scholar]
Soll 2000
- Soll RF, Dargaville P. Surfactant for meconium aspiration syndrome in full term infants. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002054] [DOI] [PubMed] [Google Scholar]


