Abstract
Background
Women who have undergone surgical treatment for epithelial ovarian cancer (EOC) may develop menopausal symptoms due to immediate loss of ovarian function following surgery and chemotherapy. Women may experience vasomotor symptoms, sleep disturbance, difficulty concentrating, sexual dysfunction, vaginal symptoms and accelerated osteoporosis. Although hormone replacement therapy (HRT) is the most effective treatment to relieve these symptoms, its safety has been questioned for women with EOC.
Objectives
To assess the safety and efficacy of HRT for menopausal symptoms in women surgically treated for EOC.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 6), MEDLINE via Ovid (1946 to 12 June 2019) and Embase via Ovid (1980 to 2019, week 23). We also handsearched conference reports and trial registries. There was no language restriction.
Selection criteria
We included randomized controlled trials (RCTs) with participants of any age and menopausal status who had undergone surgery for EOC and, after diagnosis and treatment, used any regimen and duration of HRT compared with placebo or no hormone therapy. We also included trials comparing different regimens or duration of administration of HRT.
Data collection and analysis
Two review authors independently identified studies that met the inclusion criteria. They used Covidence to extract study characteristics, outcome data and to assess methodological quality of the included studies.
Main results
Our search strategy identified 2617 titles, of which 2614 titles were excluded. Three studies, involving 350 women, met our inclusion criteria. Two of the studies included pre and postmenopausal women, and the third only included premenopausal women. The overall age range of those women included in the studies was 20 to 89.6 years old, with a median follow‐up ranging from 31.4 months to 19.1 years. The geographical distribution of participants included Europe, South Africa and China. All stages and histological subtypes were included in two of the studies, but stage IV disease had been excluded in the third. The three included studies used a variety of HRT regimens (conjugated oestrogen with or without medroxyprogesterone and with or without nylestriol) and HRT administrations (oral, patch and implant), In all studies, the comparisons were made versus women who had not received HRT.
The studies were at low or unclear risk of selection and reporting bias, and at high risk of performance, detection and attrition bias. The certainty of the evidence was low for overall survival and progression‐free survival, and very low for quality‐of‐life assessment, incidence of breast cancer, transient ischaemic attack (TIA), cerebrovascular accident (CVA) and myocardial infarction (MI).
Meta‐analysis of these studies showed that HRT may improve overall survival (hazard ratio (HR) 0.71, 95% confidence interval (CI) 0.54 to 0.93; 350 participants, 3 studies; low‐certainty evidence). Quality‐of‐life assessment by use of the EORTC‐C30 questionnaire was performed only in one study. We are uncertain whether HRT improves or reduces quality of life as the certainty of the evidence was assessed as very low (mean difference (MD) 13.67 points higher, 95% CI 9.26 higher to 18.08 higher; 1 study; 75 participants; very low‐certainty evidence). Likewise, HRT may make little or no difference to progression‐free survival (HR 0.76, 95% CI 0.57 to 1.01; 275 participants, 2 studies; low‐certainty evidence).
We are uncertain whether HRT improves or reduces the incidence of breast cancer (risk ratio (RR) 2.00, 95% CI 0.19 to 21.59; 225 participants, 2 studies; very low‐certainty evidence); TIA (RR 5.00, 95% CI 0.24 to 102.42; 150 participants, 1 study; very low‐certainty evidence); CVA (RR 0.67, 95% CI 0.11 to 3.88; 150 participants, 1 study; very low‐certainty evidence); and MI (RR 0.20, 95% CI 0.01 to 4.10; 150 participants, 1 study; very low‐certainty evidence). The incidence of gallstones was not reported in the included studies.
Authors' conclusions
Hormone replacement therapy may slightly improve overall survival in women who have undergone surgical treatment for EOC, but the certainty of the evidence is low. HRT may make little or no difference to quality of life, incidence of breast cancer, TIA, CVA and MI as the certainty of the evidence has been assessed as very low. There may be little or no effect of HRT use on progression‐free survival. The evidence in this review is limited by imprecision and incompleteness of reported relevant outcomes and therefore the results should be interpreted with caution. Future well‐designed RCTs are required as this is an important area to women experiencing menopausal symptoms following surgical treatment for ovarian cancer, especially as doctors are often reluctant to prescribe HRT in this scenario. The evidence in this review is too limited to support or refute that HRT is very harmful in this population.
Plain language summary
Hormone replacement therapy for menopausal symptoms in women who have undergone surgical treatment for epithelial ovarian cancer
The issue Epithelial ovarian cancer (EOC) develops from the surface layer of the ovary. It is the eighth most common cancer and the seventh most common cause of death from cancer worldwide in women. The surgical treatment of EOC includes the removal of all visible tumour deposits in the abdomen; this usually includes both ovaries, the uterus (womb), omentum (fatty curtain that hangs from the stomach and transverse colon), and peritoneum, with or without the removal of lymph nodes or other organs. Women who were premenopausal before the procedure go on to experience the menopause as a result of the surgery. This may negatively affect their quality of life due to symptoms such as hot flushes, mood swings, change of sexual activity, vaginal dryness and loss of bone density. Around a quarter of women, especially younger women, will present with early‐stage disease and will be left with long‐term adverse health effects of a surgically induced menopause. In those women with advanced disease at diagnosis, quality of life is an important factor to consider, as their disease is life‐limiting.
Hormone replacement therapy (HRT) might be effective for postmenopausal symptoms, but there are serious concerns around the safety of this treatment. These concerns are not just related to cancer, but also to the heart, and they need to be balanced against the positive health effects of HRT for women with an early menopause. In recent years the safety of HRT has been questioned and doctors may be cautious in prescribing HRT for women who are experiencing surgically‐induced menopause after treatment for EOC.
The aim of the review To assess the safety and efficacy of hormone replacement therapy (HRT) for menopausal symptoms in women treated surgically for EOC.
What were the main findings? We searched for evidence of benefits and harms of HRT in EOC, up to June 2019. We identified three studies involving a total of 350 women. We found that HRT may improve overall survival and may make little or no difference to progression‐free survival. We are unsure about the effects on quality of life, incidence of breast cancer, transient ischaemic attack (also known as 'mini stroke'), cerebrovascular accident (stroke) and myocardial infarction (heart attack), as the certainty of the evidence was very low. There were no reports on the incidence of gallstones.
Quality of the evidence The certainty of the evidence was low to very low for all outcomes, mainly due to the small number of participants and low numbers of adverse events reported. The certainty of the evidence is also reduced due to the high risk of bias of the included studies, meaning their results might overestimate or underestimate the true effect of the treatment.
What were the conclusions? Hormone replacement therapy may improve the overall survival in women who are experiencing surgically induced menopause after treatment for EOC, but it may make little or no difference to survival without the disease getting worse. The overall certainty of these findings is low to very low, mainly due to a lack of information. This is a very important area for further research, which has the potential to make a big impact on many women.
Summary of findings
Summary of findings for the main comparison. Hormone replacement therapy (HRT) compared to no HRT for women who have undergone surgery for epithelial ovarian cancer (EOC).
| Hormone replacement therapy (HRT) compared to no HRT for epithelial ovarian cancer (EOC) | ||||||
| Patients: women of any age diagnosed with any stage of EOC who had surgical treatment, regardless of chemotherapy treatment Setting: multiple centres in the United Kingdom, Spain and Hungary, and single institutes in South Africa and China Intervention: HRT; oestrogen‐alone, and oestrogen combined with progestin, oestrogen agonist/antagonist, testosterone or tibolone in any dose and any route of administration Comparison: no HRT | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no HRT | Risk with HRT | |||||
| Overall survival | Study population | HR 0.71 (0.54 to 0.93) | 350 (3 RCTs) | ⊕⊕⊝⊝ LOW 1, 2 | The median follow‐up time in the three included studies was 31.4 months, 90 months and 19.1 years. | |
| 795 per 1,000 | 675 per 1,000 (575 to 771) | |||||
| Quality of life, general condition | The mean quality of life (general condition) in the no HRT group was 13.84 points. | The mean quality of life (general condition) in the HRT group was 13.67 points higher (9.26 higher to 18.08 higher) | ‐ | 75 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1, 2, 3 | The EORTC‐C30 questionnaire was used to evaluate this outcome; higher values correspond with improvement. |
| Progression‐free survival | Study population | HR 0.76 (0.57 to 1.01) | 275 (2 RCTs) | ⊕⊕⊝⊝ LOW 1, 2 | The median follow‐up time in the two studies was 90 months and 19.1 years. | |
| 773 per 1,000 | 676 per 1,000 (571 to 776) | |||||
| Incidence of breast cancer | Study population | RR 2.00 (0.19 to 21.59) | 225 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1, 4 | The median follow‐up time in the two studies was 31.41 months and 19.1 years. | |
| 8 per 1,000 | 17 per 1,000 (2 to 181) | |||||
| Incidence of transient ischaemic attack | Study population | RR 5.00 (0.24 to 102.42) | 150 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1, 2, 4 | The median follow‐up time in the study was 19.1 years. | |
| 7 per 1,000 | 33 per 1,000 (2 to 683) | |||||
| Incidence of cerebrovascular accident | Study population | RR 0.67 (0.11 to 3.88) | 150 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1, 2, 3 | The median follow‐up time in the study was 19.1 years. | |
| 40 per 1,000 | 27 per 1,000 (4 to 155) | |||||
| Incidence of myocardial infarction | Study population | RR 0.20 (0.01 to 4.10) | 150 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1, 2, 3 | The median follow‐up time in the study was 19.1 years. | |
| 27 per 1,000 | 5 per 1,000 (0 to 109) | |||||
| Incidence of gallstones | ‐ | ‐ | ‐ | ‐ | ‐ | The incidence of gallstones was not reported in the included studies. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; HR: hazard ratio | ||||||
| GRADE Working Group grades of evidence High‐certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate‐certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low‐certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low‐certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by one level due to limitations in study design 2 Downgraded by one level due to inconsistency of results 3 Downgraded by one level due to imprecision of results 4 Downgraded by two levels due to imprecision of results
Background
Description of the condition
One in 70 women will develop ovarian cancer (Fleming 2013) and its age‐standardized incidence rate (ASR) is 6.6 per 100,000 women (GLOBOCAN 2018). This is lower in Western Africa and higher in Northern Europe, varying from 3 to 13 per 100,000) (Fleming 2013). One in 100 women will die of the disease (Fleming 2013) and the ASR of mortality is 3.9 per 100,000 (GLOBOCAN 2018). The median age at diagnosis of epithelial ovarian cancer (EOC) is 60 years (Michaelson‐Cohen 2009). However, 40% of women affected are 30 to 60 years old and 3% to 17% are less than 40 years old (Ibeanu 2011; Michaelson‐Cohen 2009; Wen 2013). The majority of women with EOC (60% to 75%) have advanced stage disease at presentation and the overall five‐year survival for all stages is 45% (Ibeanu 2011; Singh 2010).
Debulking (removal) of any visible tumour (also known as cytoreductive surgery) is standard surgery for disease staging and treatment; it includes hysterectomy, bilateral salpingo‐oophorectomy and omentectomy with or without pelvic and para‐aortic lymphadenectomy (i.e. the removal of all visible lesions in the abdomen and pelvic cavity including the ovaries, uterus (womb) and omentum (fatty curtain that hangs from the stomach/transverse colon), with or without lymph nodes) (Fleming 2013). In young women with early‐stage disease or disease present in only in one ovary, fertility‐sparing surgery (where only the affected ovary is removed) may be appropriate. Removal of the remaining ovary is recommended once the woman has completed her family. Bilateral oophorectomy (the surgical removal of both ovaries), and the consequent the loss of ovarian function in premenopausal women, induces an immediate surgical menopause and may result in a range of symptoms including vasomotor symptoms, sleep depravation, difficulty with concentration, sexual dysfunction, vaginal symptoms and accelerated osteoporosis (Biliatis 2012; Hopkins 2004). Symptoms following a surgical menopause in younger women may be more intense than in the natural menopause because of sudden onset (Li 2012; Singh 2010; Wen 2013).
Studies have demonstrated that the origin of many high‐grade (HG) serous EOCs (and primary peritoneal cancer), the most common histological sub‐type of EOC, may be the fimbrial end of the fallopian tube (Leeper 2002). In this review, we use the terms 'EOC' and 'ovarian cancer' as umbrella terms to include primary peritoneal and fallopian tube HG serous cancers.
Description of the intervention
Hormone replacement therapy is the delivery of hormones, usually oestrogen with or without progesterone, to replace the normal ovarian production of hormones either due to ovarian failure (chemotherapy‐induced menopause) or after surgical removal of both ovaries (surgically induced menopause). HRT can be administrated in a variety of formulations and doses. It can be taken orally, vaginally, intranasally, as an implant, skin patch, cream or gel. The administration of HRT can be continuous (every day), sequential (for part of each month) or less frequently (Marjoribanks 2012). HRT can relieve menopausal symptoms and prevent heart disease and osteoporosis (Ibeanu 2011). However, HRT may have an effect on blood lipids levels and cause headaches, bloationg and breast tenderness (Marjoribanks 2012).
For women with a uterus, progesterone is required to prevent endometrial hyperplasia and malignancy. Women without a uterus can be given oestrogen‐only HRT. Combined continuous HRT — consisting of an oestrogen and a progestogen, taken daily without a break — can increase the risk of coronary events, venous thromboembolism, stroke, breast cancer, gallstones and death from lung cancer (Marjoribanks 2012). However, oestrogen‐only HRT following hysterectomy has not been shown to increase the incidence of breast cancer (Chlebowski 2015; Marjoribanks 2012).
How the intervention might work
Hormone replacement therapy (HRT) is a very effective treatment for the management of menopausal symptoms and the prevention of heart disease and osteoporosis (Ibeanu 2011). However, the effect of hormones is also dependent on the availability of oestrogen and progesterone receptors. Oestrogen and progesterone are mainly included in the regimen given to women experiencing menopausal symptoms. Oestrogens have an important role in reproductive development, bone homeostasis, cardiovascular remodelling and brain function (Hua 2018). Oestrogen has a role in cancer through binding with two types of oestrogen receptors (ER), namely ERα (an oncogene) and ERβ (a tumour suppressor gene which can be expressed in 40% to 60% of ovarian tumours) (Hua 2018). However, a meta‐analysis did not show any prognostic effect of ER levels on survival in women with ovarian cancer (Zhao 2013). It has been shown that progesterone and progesterone receptors (PRs) interact with oestrogen in order to promote a proliferative and pro‐survival response in breast cells. Conversley, progesterone inhibits the oestrogen‐driven growth in the uterus and ovary and protects the ovary from neoplastic transformation (Diep 2015); it has also been shown to have a significant association with survival (Luo 2017; Zhao 2013). BRCA1 and BRCA2 are oncosuppressor genes which, when mutated, increase the lifetime risk of developing breast cancer to between 45% and 80% and ovarian and fallopian tubal cancers to between 15% and 56%. This compares with a general population lifetime risk of developing ovarian cancer of 2% to 3% (Marchetti 2013), although those with BRCA germline mutations have a significantly improved overall survival compared to non‐BRCA mutation carriers (Zhong 2015). However, women with breast cancer who are BRCA1 (but not BRCA2) mutation carriers have significantly poorer survival (Zhong 2015).
Hormone replacement therapy has been associated with angiogenesis (Hopkins 2004) that may stimulate residual ovarian cancer cells of either microscopic or visible disease in women treated for EOC or induce new hormone‐dependent diseases, such as breast cancer (Hopkins 2004; Singh 2010). For these reasons, clinicians may be cautious in prescribing HRT for menopausal symptoms in women after surgery for EOC. Benefits in quality of life for each individual therefore have to be weighed against any theoretical risks. However, the prognosis of early‐stage EOC is good, with a low incidence of recurrence and mortality (less than 10% of women with recurrent disease in stage I EOC) (Lowe 2013) and prolonged survival. Due to these reasons, the risks of premature menopause, including osteoporosis, cardiovascular disease, venous thromboembolic disease and stroke, may outweigh the risk of HRT use. Advanced stage EOC has a poor prognosis with a higher incidence of recurrence and mortality (rate greater than 90% for stage IV) (Lowe 2013) and five‐year overall survival of less than 25% to 30% (Biglia 2015; Ibeanu 2011). Quality‐of‐life outcomes are therefore important for both those with early‐ and late‐stage disease.
Hormone replacement therapy can relieve menopausal symptoms among women with early‐stage ovarian cancer and improve the quality of life of those with advanced‐stage disease (Ursic‐Vrscaj 2001). Three retrospective studies have shown that HRT use was not associated with an increase in overall survival and tumour recurrence or a trend to decreased mortality (Eeles 1991; Ursic‐Vrscaj 2001; Wen 2013). In one study, women with serous type ovarian cancer who received HRT achieved better overall survival (Mascarenhas 2006). In addition, two small randomized controlled trials (RCTs) showed no adverse effects of HRT on survival (Guidozzi 1999; Li 2012). Conversely, studies of HRT use in women with no type of cancer have shown an increased risk of developing ovarian cancer (Zhou 2008). Therefore, the effectiveness of HRT on overall survival of women with ovarian cancer remains unclear.
Why it is important to do this review
In recent years the safety of HRT has been questioned. This has led to fewer women taking HRT, with the consequence that more women experience menopausal symptoms and the long‐term effects of menopause. Premenopausal women affected by ovarian cancer who have both ovaries removed experience an acute surgically or chemotherapy‐induced menopause, which can lead to more prominent menopausal symptoms. Disease‐specific survival is better for younger women compared to older women (age at diagnosis 30 years or younger, versus 30 to 60 years and 60 years or older) (Fleming 2013). Therefore a younger population of women are longer‐term survivors of EOC and are more likely to experience an early and possibly more symptomatic menopause (Li 2012; Singh 2010; Wen 2013).
Hormone replacement therapy may be helpful for treatment of menopausal symptoms, but a meta‐analysis of cohort and case‐control studies showed increase risk of developing ovarian cancer in women not previously diagnosed with ovarian cancer who were on HRT for more than 10 years (Zhou 2008). There have been three systematic reviews looking into the use of HRT in ovarian cancer after surgery (Hopkins 2004; Li 2015; Pergialiotis 2015). The first systematic review included one RCT (Guidozzi 1999) and two observational studies (Eeles 1991; Ursic‐Vrscaj 2001) and suggested that HRT was acceptable as supportive and symptomatic therapy and did not affect overall survival and disease‐free survival (Hopkins 2004). The second and third systematic reviews (in which the last search was conducted in March 2015) included four cohort studies (Eeles 1991; Mascarenhas 2006; Ursic‐Vrscaj 2001; Wen 2013) and two RCTs (Guidozzi 1999; Li 2012), and showed a favourable impact of HRT on overall survival, without increasing the risk of recurrence (Li 2015; Pergialiotis 2015).
The aim of this Cochrane review is to investigate the safety of HRT in women experiencing surgically induced menopause, both from the oncological perspective (recurrence and survival) and also in terms of quality of life. We hope that this review will facilitate counselling and informed decision making by women who seek advice and management following their cancer treatment and early and acute menopause. Currently, there is no clear evidence and opinions are conflicting.
Objectives
To assess the safety and efficacy of hormone replacement therapy (HRT) for menopausal symptoms in women surgically treated for epithelial ovarian cancer (EOC).
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) (excluding cluster‐randomized or cross‐over trials) comparing HRT of any regimen and duration of administration versus placebo or no hormone therapy, or trials comparing different regimens or duration of administration of HRT.
Types of participants
We included women of any age who were diagnosed with any stage of EOC and had surgical treatment, regardless of chemotherapy treatment.
Types of interventions
We included studies of HRT used after treatment for EOC. We did not study the aetiological effect of HRT used before diagnosis. We included any HRT (oestrogen alone or combined with progestin, oestrogen agonist/antagonist, progestin, or testosterone, and tibolone), of any regimen or duration of administration, compared with placebo or no hormone therapy. Due to pharmacokinetic differences between different regimens and durations of administration, we investigated the following three comparisons.
HRT versus placebo or no HRT
Different regimens of HRT
Different durations of HRT administration
Types of outcome measures
Primary outcomes
Overall survival: survival until death from all causes; survival was assessed from time of enrolment in the study.
Quality of life: defined as an individual's perception of life in the context of the culture and value systems (which also includes specific menopausal symptoms like hot flushes, night sweats, vaginal dryness, etc.) measured by any validated scale or questionnaire for quality of life.
Secondary outcomes
Progression‐free survival: survival until progression of disease. Survival was assessed from the time of enrolment in the study.
-
Adverse events
Incidence of breast cancer
Thromboembolic events (pulmonary embolism (PE), deep vein thrombosis (DVT), coronary event, myocardial infarction (MI), stroke, transient ischaemic accident (TIA), cerebrovascular accident (CVA)
Gallstones
Search methods for identification of studies
In consultation with the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Information Specialists, we searched for papers published in any language. When necessary, papers would have been translated.
Electronic searches
We searched the following electronic databases on 12 June 2019:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 6), in the Cochrane library (Appendix 1)
MEDLINE via Ovid (1946 to 12 June 2019) (Appendix 2)
Embase via Ovid (1980 to 2019 week 23) (Appendix 3)
Searching other resources
All relevant articles were identified on PubMed and using the 'related articles' feature; a further search was carried out for newly published articles. We searched the following registries for ongoing trials.
Metaregister (www.controlled‐trials.com/rct)
Physicians Data Query (www.cancer.gov/publications/pdq)
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/en/)
ClinicalTrails.gov (www.clinicaltrials.gov)
National Cancer Institute (www.cancer.gov/about‐cancer/treatment/clinical‐trials)
If we had identified any ongoing trials that had not been published, we would have approached the principal investigators and major co‐operative groups active in this area, to ask for relevant data.
We handsearched the citation lists of included studies, key textbooks, and previous systematic reviews, and contacted experts in the field to identify further reports of trials. We also handsearched conferences abstracts from the following sources.
Gynecologic Oncology (Annual Meeting of the American Society of Gynecologic Oncology)
International Journal of Gynecological Cancer (Annual Meeting of the International Gynecologic Cancer Society)
British Gynaecological Cancer Society (BGCS)
Annual Meeting of European Society of Gynaecological Oncology (ESGO)
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to a reference management database (Endnote). After duplicates were removed, we transferred these data to Covidence for study selection. Two review authors (NS and RB) examined the remaining references independently. We excluded those studies which clearly did not meet the inclusion criteria. We obtained copies of the full text of potentially relevant references. Two review authors (NS and RB) independently assessed the eligibility of the retrieved reports/publications. We resolved any disagreement through discussion and we consulted a third person (TL) for a final decision. We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table (Liberati 2009).
Data extraction and management
Two review authors (NS and KP) independently extracted study characteristics and outcome data from included studies to a piloted data collection form, using Covidence. We noted in the Characteristics of included studies table if outcome data were not reported in a usable way. We resolved disagreements by consensus or by involving a third person (RB). One review author (NS) transferred data into the Review Manager 5 (RevMan 2014) file. We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (KP) spot‐checked study characteristics for accuracy against the trial report.
For included studies, we extracted the following details.
Author, year of publication and journal citation (including language)
Country
Setting
Inclusion and exclusion criteria
Study design, methodology
-
Study population
Total number enrolled
Participant characteristics: age, menopausal status at diagnosis, performance status
Treatment: type of surgery and chemotherapy
Tumour stage, grade and types
-
Intervention details
All types of HRT: oestrogen alone or combined with progestin, oestrogen agonist/antagonist, progestin, or testosterone, and tibolone
Duration of administration of HRT in years
Route and doses of HRT
-
Comparison
Placebo or no treatment
Risk of bias in study (see Assessment of risk of bias in included studies)
Duration of follow‐up
Outcomes: for each outcome, we extracted the outcome definition. For adjusted estimates, we recorded variables adjusted for in analyses.
Results: we extracted the number of participants allocated to each intervention group, the total number analyzed for each outcome and number of dropouts, including reason for leaving the study.
Notes: funding for trial and notable conflicts of interest of trial authors.
Outcome data were extracted as follows.
For time‐to‐event data (survival and disease progression), we extracted the log of the hazard ratio (log(HR)) and its standard error from trial reports. For those studies that they did not report the HR, we attempted to estimate HR, the observed minus expected events (O‐E) and the variance (V) using formula according to Tierney 2007.
For dichotomous outcomes (e.g. adverse events or deaths) we extracted the number of participants in each treatment arm who experienced the outcome of interest and the number of participants assessed at endpoint, in order to estimate a risk ratio.
For continuous outcomes (e.g. quality‐of‐life measures), we extracted the final value and standard deviation of the outcome of interest and the number of participants assessed at endpoint in each treatment arm at the end of follow‐up, in order to estimate the mean difference between treatment arms and its standard error.
Where possible, all data extracted would be those relevant to an intention‐to‐treat analysis, in which participants would be analyzed in groups to which they were assigned. We noted the time points at which outcomes were collected and reported.
Assessment of risk of bias in included studies
Two review authors (NS and KP) applied the Cochrane 'Risk of bias' tool independently and resolved differences by discussion or by appeal to a third review author (EM). We judged each item as being at high, low or unclear risk of bias, as set out in the criteria provided by Higgins 2011 (and shown below), and provided a quote from the study report and/or a statement as justification for the judgement for each item in the 'Risk of bias' table. We summarized results in both a 'Risk of bias' graph and a 'Risk of bias' summary. When interpreting treatment effects and meta‐analyses, we took into account the risk of bias for the studies that contributed to that outcome. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
-
Random sequence generation
Low risk of bias: e.g. participants were assigned to treatments on basis of a computer‐generated random sequence or a table of random numbers
High risk of bias: e.g. participants were assigned to treatments on basis of date of birth, clinic ID number or surname, or there was no attempt to randomize participants
Unclear risk of bias: e.g. sequence generation not reported or the information was not available
-
Allocation concealment
Low risk of bias: e.g. where the allocation sequence could not be foretold
High risk of bias: e.g. the allocation sequence could be foretold by participants, investigators or treatment providers
Unclear risk of bias: e.g. allocation concealment was not reported
-
Blinding of participants and personnel
Low risk of bias if participants and personnel were adequately blinded
High risk of bias if participants were not blinded to the intervention that the participant received
Unclear risk of bias if this was not reported or unclear
-
Blinding of outcomes assessors
Low risk of bias if outcome assessors were adequately blinded
High risk of bias if outcome assessors were not blinded to the intervention that the participant received
Unclear risk of bias if this was not reported or unclear
-
Incomplete outcome data: we recorded the proportion of participants whose outcomes were not reported at the end of the study. We coded a satisfactory level of loss to follow‐up for each outcome as follows.
Low risk of bias if fewer than 20% of participants were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms
High risk of bias if more than 20% of participants were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms
Unclear risk of bias if loss to follow‐up was not reported
-
Selective reporting of outcomes
Low risk of bias: e.g. the study reported all outcomes specified in the protocol
High risk of bias: e.g. it was suspected that outcomes had been selectively reported
Unclear risk of bias: e.g. it was unclear whether outcomes had been selectively reported
-
Other biases
Low risk of bias: if we did not suspect any other source of bias and the trial appeared to be methodologically sound
High risk of bias: if we suspected that the trial had been prone to an additional bias
Unclear risk of bias: if we are uncertain whether an additional bias might had been present
Measures of treatment effect
We used the following measures of the effect of treatment.
For time‐to‐event data, we used the hazard ratio.
For dichotomous outcomes, we analyzed data based on the number of events and the number of people assessed in the intervention and comparison groups. We used these to calculate the risk ratio and 95% confidence interval (CI).
For continuous outcomes, we analyzed data based on the mean, standard deviation and number of people assessed for both the intervention and comparison groups to calculate mean difference between treatment arms, with a 95% CI. If the mean difference was reported without individual group data, we planned to use this to report the study results. If more than one study measured the same outcome using different tools, we planned to calculate the standardized mean difference and 95% CI using the inverse variance method in RevMan 2014.
Unit of analysis issues
If any trials with multiple treatment groups had been identified, we would have divided the ‘shared’ comparison group into the number of treatment groups and comparisons between each treatment group and treated the split comparison group as independent comparisons.
Dealing with missing data
We did not contact study authors to obtain missing data (participant, outcome or summary data). We reported on the levels of loss to follow‐up and assessed this as a source of potential bias. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis. We did not impute missing outcome data for the primary outcome.
Assessment of heterogeneity
We assessed the degree of heterogeneity among trials using I2 (Higgins 2003) and Chi2 statistics (Deeks 2001). We regarded heterogeneity to be substantial if I² was greater than 50% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi2 test for heterogeneity. If substantial heterogeneity was found, we would have used subgroup and sensitivity analyses to explore the causes of heterogeneity.
Assessment of reporting biases
If we identify more than ten studies in future updates of this review, we will examine funnel plots corresponding to meta‐analysis of the primary outcome to assess the potential for small‐study effects such as publication bias. We plan to assess funnel plot asymmetry visually, and if asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using Review Manager 5 (RevMan 2014). We used a fixed‐effect model for combining data if there was no substantial heterogeneity. If substantial statistical heterogeneity was detected, we used a random‐effects meta‐analysis to produce an overall summary and the results were presented as the average treatment effect with 95% CIs (DerSimonian 1986).
For time‐to‐event data, we pooled hazard ratios using the generic inverse variance facility of RevMan 2014.
For dichotomous outcomes, we calculated the risk ratio for each study and then pooled them.
For continuous outcomes, we pooled the mean differences between the treatment arms at the end of follow‐up, where all trials measured the outcome on the same scale; otherwise we planned to pool standardized mean differences.
When we were unable to pool the data statistically using meta‐analysis we conducted a narrative synthesis of results. We presented the major outcomes and results, organised by intervention categories according to the major types or aims (or both) of the identified interventions. Depending on the assembled research, we may also in future explore the possibility of organising the data by population. Within the data categories we will explore the main comparisons of the review.
We presented the overall quality of the evidence for each outcome listed below, using the GRADE approach, which took into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity, such as directness of results (Langendam 2013). We created a 'Summary of findings' table based on the methods described Chapter 12.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Schunemann 2011), using GRADEpro GDT. We used the checklist to maximize consistent GRADE decisions and the GRADE Working Group quality of evidence definitions (Meader 2014). We downgraded the evidence from 'high' quality by one level for serious limitations (or two levels for very serious limitations) for each outcome, and outlined our rationale in the footnotes.
High quality: further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: we were very uncertain about the estimate.
We included the following outcomes included in the 'Summary of findings' table.
Overall survival
Quality of life assessment
Progression‐free survival
-
Adverse events
Incidence of breast cancer
Incidence of thromboembolic events (DVT/ PE, stroke, MI)
Incidence of gallstones
Subgroup analysis and investigation of heterogeneity
We did not identify any substantial heterogeneity; therefore subgroup analysis were not conducted. In future updates, if more studies are included and substantial heterogeneity is identified, it will be of clinical interest to investigate the safety (risk and benefits) for the prespecified outcomes in this protocol for the following factors.
Menopausal status at diagnosis: premenopausal versus postmenopausal. If menopausal status is not extractable from studies, we will instead analyze by age (under 50 years versus 50 years or older).
Hysterectomy versus no hysterectomy.
Stage of cancer: stage I to II versus stage III to IV
Tumour types: endometrioid versus non‐endometrioid
Positive or negative oestrogen receptor, progesterone receptor or BRCA mutation status.
Sensitivity analysis
If necessary in any future update, we will use sensitivity analyses to assess the cause of substantial heterogeneity by omitting the studies with at a high risk of bias.
Results
Description of studies
Results of the search
We searched for references up to June 2019. We found 2617 references which met our search criteria, of which 29 were duplicates. Two review authors (NS and RB) independently screened titles and abstracts; the majority of references identified were not RCTs or the objective of study was to investigate the efficacy of hormones on therapeutic outcomes of ovarian cancer and not the effect of HRT. We reviewed 11 full‐text references and excluded eight as they did not meet the inclusion criteria. We identified three studies for inclusion (Eeles 2015; Guidozzi 1999; Li 2012) (see PRISMA flow diagram; Figure 1).
1.
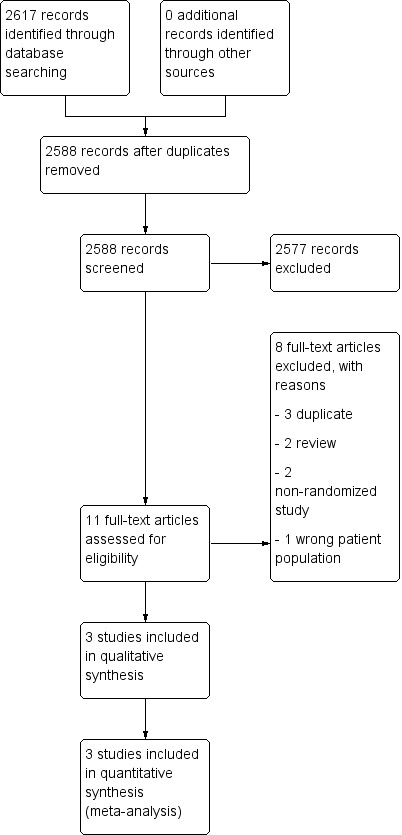
Study flow diagram.
Included studies
See Characteristics of included studies.
Study location and setting
One study (Eeles 2015) included women from multiple centres in the United Kingdom, Spain and Hungary, whereas Guidozzi 1999 and Li 2012 included single institutes in South Africa and China, respectively.
Participants
All women with a diagnosis of EOC had received cytoreductive surgery, chemotherapy, or both, and were well balanced across the two arms of the studies for demographic data. Eeles 2015 included 150 women with a median age of 58.7 years (and an age range of 29.3 to 89.6 years) and both pre and postmenopausal women were eligible for inclusion. Guidozzi 1999 included 130 women aged 59 years or younger, 44% of whom were between 56 and 59 years of age. Li 2012 included 90 women aged 45 years or younger (age range: 20 to 45 years).
The number of women that were lost to follow‐up were 0, 5 and 15 participants in Eeles 2015, Guidozzi 1999 and Li 2012, respectively. Two studies included all stages and histological types (Eeles 2015; Guidozzi 1999), whereas Li 2012 included only International Federation of Gynecology and Obstetrics (FIGO) stage I to III and serous and mucinous type. The majority of tumours were grade 3 in Eeles 2015 and grade 1 in Guidozzi 1999. There were no data on tumour grade in Li 2012. Participants were randomized to HRT versus no HRT arms using computer‐generated (Eeles 2015) or sealed‐envelope randomization (Guidozzi 1999; Li 2012).
The women that were excluded from studies were those who had conservative surgery to preserve their ovarian function, had history of hormone‐dependent malignancy with contraindications to hormone therapy (Eeles 2015), or had low malignant potential and had never taken hormone therapy (Guidozzi 1999).
None of the studies looked into the effect of BRCA status and HRT effects on survival in women with ovarian cancer. Oestrogen and progesterone receptors were investigated only in one study (Li 2012).
Interventions
The three included studies used a variety of HRT. Guidozzi 1999 used only conjugated oestrogen (0.625 mg/day). Li 2012 used combined HRT: conjugated oestrogen (0.625 mg/day) with medroxyprogesterone (4 mg/day) and nylestriol (2.5 mg/15 day) with medroxyprogesterone (4 mg/day). Eeles 2015 used oestrogen only: conjugated oestrogen, estradiol patch and estradiol implant, and combined with progesterone; conjugate oestrogen and norgestrel.
Outcomes
The median follow‐up time was 31.4 months, 90 months and 19.1 years for Li 2012, Guidozzi 1999 and Eeles 2015, respectively. There were participants who were lost to follow‐up: 3.8% in Guidozzi 1999 and 16.7% in Li 2012. These participants were excluded from the analysis and results. In Eeles 2015, where the median follow‐up was 19.1 years, 63.9% of participants discontinued hormone therapy; the most common being the presence of adverse effects or unknown reason.
There were participants who were assigned to HRT, but did not receive or discontinued HRT (65.5% in Eeles 2015 and 13.6 % in Guidozzi 1999). There were also participants who were assigned to no HRT, but who received HRT (10.7% in Eeles 2015 and 8.5% in Guidozzi 1999). However, all analyses were performed on an intention‐to‐treat basis for these studies.
Overall survival was the primary outcome in all three studies. The incidence of breast cancer was studied in two studies (Eeles 2015; Li 2012). Treatment adverse effects and quality of life (using the EORTC‐C30 questionnaire and GMU‐Gynae Index), were only studied in Li 2012. The EORTC‐C30 was used to assess five function domains (physical, emotional, social, role and cognitive), eight symptoms (fatigue, pain, nausea/vomiting, constipation, diarrhoea, insomnia, dyspnoea and loss of appetite) and global health/quality of life. The GMU‐Gynae Index assessed quality of sexual life (sexual difficulties, emotional exchange between the couple, regression of sexual life and sexual desire), symptoms of lower urinary tract infection (urethral burning and frequent urination), autonomic dysfunction (itchy skin, dry skin and formication). The incidence of gallstones was not reported in any study.
Dates of study, funding sources and declarations of interest
For Guidozzi 1999 the study period was four to seven years and included participants between January 1987 and June 1994. Eeles 2015 included participants between February 1990 and November 1995; and Li 2012 included participants between August 1999 and June 2003.
Funding was provided by their own institute in two studies(Guidozzi 1999; Li 2012); these studies did not provided details of any conflict of interest. Funding for Eeles 2015 was provided from multiple sources as this was a multicentre study; they declared conflicts of interest among the various authors.
Excluded studies
We excluded eight references from the full‐text review, of which three were duplicate reports (Guidozzi 1998; Guidozzi 1999a; Li 2008), two were non‐randomized studies (Bebar 2000; Ursic‐Vrscaj 2001), two were literature reviews (Guidozzi 2013; Lipkowitz 2015), and one was a study which included healthy postmenopausal women (Anderson 2003). See Characteristics of excluded studies.
Risk of bias in included studies
The details of risk of bias for the three included studies are shown in Figure 2 and Figure 3.
2.
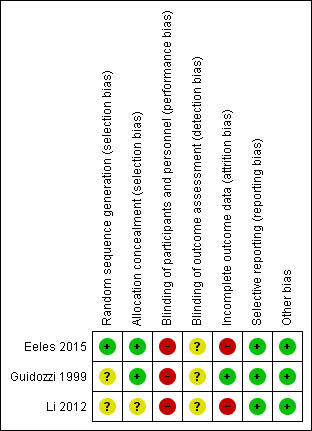
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
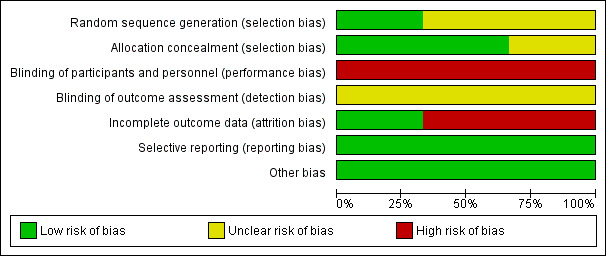
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Three studies used randomization for allocation; one study clearly described using computer‐generated random permuted blocks (Eeles 2015) and the other two did not mention the method used (Guidozzi 1999; Li 2012).
Two studies showed adequate allocation concealment using central randomization (Eeles 2015) or sealed opaque envelopes (Guidozzi 1999); and one study had insufficient information to justify a judgement of low or high risk of bias (Li 2012).
Blinding
All studies had a high risk of performance bias because the participants knew the HRT they were receiving and there was no placebo used. There was no information about the assessors and whether they knew the treatment group in all three studies; therefore we classified the risk of detection bias as unclear.
Incomplete outcome data
All three studies reported data on losses to follow‐up and non‐compliance (0% and 65.3%, respectively in Eeles 2015; and 3.8% and 31.1%, respectively in Guidozzi 1999); however, they analyzed their results on an intention‐to‐treat basis. Li 2012 excluded from analysis participants who were lost to follow‐up or non‐compliance to HRT within six months (16.7%).
Selective reporting
The study protocols were not available, however, the studies reported the results as detailed in their objectives.
Other potential sources of bias
The studies appeared to be free of other sources of bias.
Effects of interventions
See: Table 1
See Table 1: HRT versus no HRT for women who have undergone surgical treatment for EOC.
Primary outcomes
Overall survival
All three studies reported overall survival. The pooled result showed that HRT may improve overall survival in women who have had surgery for EOC (HR 0.71, 95% CI 0.54 to 0.93; three studies, 350 participants; low‐certainty evidence; Analysis 1.1).
1.1. Analysis.
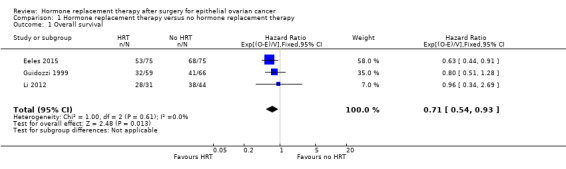
Comparison 1 Hormone replacement therapy versus no hormone replacement therapy, Outcome 1 Overall survival.
Quality of life
We are uncertain whether HRT improves or reduces the overall quality of life women who have had surgery for EOC (MD 13.67 points higher; 95% CI 9.26 higher to 18.08 higher; very low‐certainty evidence; one study, 75 participants; very low‐certainty evidence; Analysis 1.2).
1.2. Analysis.
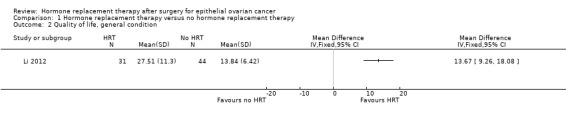
Comparison 1 Hormone replacement therapy versus no hormone replacement therapy, Outcome 2 Quality of life, general condition.
Secondary outcome
Progression‐free survival
The effect of HRT on progression‐free survival in women who have had surgery for EOC was assessed in two studies (Eeles 2015; Guidozzi 1999); the range where the actual effect may be (the "margin of error") indicated that it may make little or no difference (HR 0.76, 95% CI 0.57 to 1.01; two studies, 275 participants; low‐certainty evidence; Analysis 1.3).
1.3. Analysis.
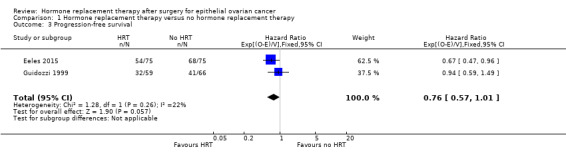
Comparison 1 Hormone replacement therapy versus no hormone replacement therapy, Outcome 3 Progression‐free survival.
Incidence of breast cancer
It is uncertain whether HRT increased the incidence of breast cancer. There were only three participants who developed breast cancer: two participants in the HRT arm, and one participant in the no HRT arm, in one study (Eeles 2015); and none in another study (Li 2012). The effect of HRT varied and it was possible that HRT made little or no difference as we are very uncertain of the results (RR 2.00, 95% CI 0.19 to 21.59; two studies, 225 participants; very low‐certainty evidence; Analysis 1.4).
1.4. Analysis.
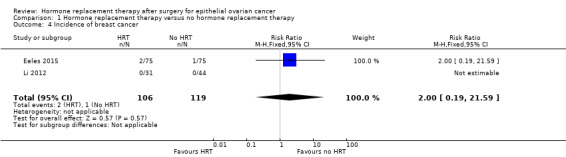
Comparison 1 Hormone replacement therapy versus no hormone replacement therapy, Outcome 4 Incidence of breast cancer.
Transient ischaemic attack (TIA)
It was very uncertain whether HRT increases the incidence of TIA. There were only two participants who developed TIA in Eeles 2015, both in the HRT arm (RR 5.00, 95% CI 0.24 to 102.42; one study, 150 participants; very low‐certainty evidence; Analysis 1.5).
1.5. Analysis.
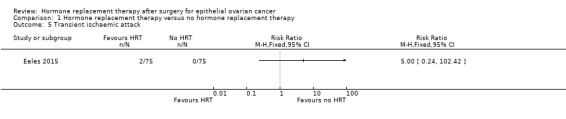
Comparison 1 Hormone replacement therapy versus no hormone replacement therapy, Outcome 5 Transient ischaemic attack.
Cerebrovascular accident (CVA)
It was uncertain whether HRT decreases the incidence of CVA. There were five participants who developed CVA in one study (Eeles 2015): two participants in the HRT arm and three participants in the no HRT arm (RR 0.67; 95% CI 0.11 to 3.88; one study, 150 participants; very low‐certainty evidence; Analysis 1.6).
1.6. Analysis.
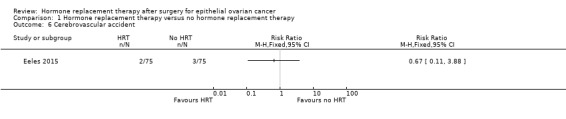
Comparison 1 Hormone replacement therapy versus no hormone replacement therapy, Outcome 6 Cerebrovascular accident.
Myocardial infarction (MI)
It was uncertain whether HRT decreases the incidence of MI. There were two participants in Eeles 2015 who developed MI, both in the no HRT arm (RR 0.20, 95% CI 0.01 to 4.10; one study, 150 participants; very low‐certainty evidence; Analysis 1.7).
1.7. Analysis.
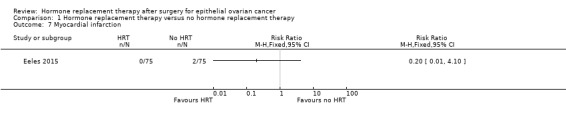
Comparison 1 Hormone replacement therapy versus no hormone replacement therapy, Outcome 7 Myocardial infarction.
Incidence of gallstone
The incidence of gallstone was not reported in any of the included studies.
Subgroup and sensitivity analyses
We did not perform subgroup or sensitivity analyses for any outcomes due to there only being a small number of trials and no substantial heterogeneity identified.
Discussion
Summary of main results
The evidence on the efficacy and safety of HRT versus no HRT in women who have undergone surgical treatment for EOC is limited; only three small studies met the inclusion criteria for our review and they provided low‐ to very low‐certainty evidence. Quality of life, measured using EORTC‐C30 scale, was presented only in one study (Li 2012). HRT may slightly improve overall survival in women who have had surgical treatment for EOC, but may make little or no difference to quality of life, progression‐free survival, incidence of breast cancer, transient ischaemic accident, cerebrovascular accident and myocardial infarction events.
Overall completeness and applicability of evidence
All published RCTs that were included in this meta‐analysis reported outcomes for all the outcomes of this review, except the incidence of gallstones. We found that HRT may slightly improve overall survival and quality of life in women after oncological treatment for EOC.
One of the included trials was conducted across European centres including the United Kingdom, Spain and Hungary (Eeles 2015), while the other two were single‐centre trials in South Africa (Guidozzi 1999) and Asia (Li 2012). All three studies reported on overall survival; two reported progression‐free survival (Eeles 2015; Guidozzi 1999) and incidence of breast cancer (Eeles 2015; Li 2012). Adverse effects were only reported in one study, the results for which had wide confidence intervals influenced by the small sample size (Eeles 2015).
The EORTC‐C30 questionnaire was used for quality‐of‐life assessments in Li 2012, and we selected only the domain of general condition in this questionnaire for analysis. EORTC‐C30 is a standardized questionnaire commonly used for people with cancer (Snyder 2013), not a menopause‐specific assessment. The Menopause Specific Quality of Life Questionnaire (MENQOL), World Health Organization Quality of Life (WHO QOL‐BREF), Greene Climacteric Scale, Utian Quality of Life (UQOL) Scale, Women's Health Questionnaire (WHQ), 36‐Item Short Form (SF‐36), MENCAV, Cervantes Scale, Cervantes Short‐Form Scale, and Menopause Rating Scale (MRS) are the questionnaires that can be used to specifically assess menopausal symptoms (Jenabi 2015).
Hormone replacement therapy may increase the risk of developing other diseases such as breast cancer, when combined hormonal therapy is used (Benkhadra 2015; Chlebowski 2015; Manson 2013), coronary heart disease (Rossouw 2002; Rossouw 2007), stroke (Manson 2013; Rossouw 2002), pulmonary embolism (Manson 2013; Rossouw 2002), deep vein thrombosis (Manson 2013), dementia (Manson 2013) and gallbladder disease (Manson 2013). Prolonged use of HRT and the use of combined HRT for women older than 60 years of age has been associated with higher risk of developing pulmonary embolism and lower risk of colorectal cancer. Women who have undergone hysterectomy who use oestrogen‐only HRT may have an increased risk of stroke and colorectal cancer, when older than 60 and 70 years of age, respectively (Manson 2013). The use of HRT in healthy postmenopausal women has been associated with increased risk of developing ovarian cancer (Rodrigguez 2001; Zhou 2008), of both serous (Cancer Epidemiology 2015; Mørch 2012; Shi 2015) and endometrioid types (Cancer Epidemiology 2015; Mørch 2012). Using HRT for more than five years is linked to increased risk of ovarian cancer (Zhou 2008) and this correlation is stronger for the serous type (Mørch 2012).
Hormone receptors, oestrogen receptors or progesterone receptors and BRCA mutation status are known prognostic factors in ovarian cancer ( Luo 2017; Shen 2017; Zhong 2015). The presence of oestrogen receptors has been associated with favourable overall survival in women with unclassified EOC in Europe, South America and Oceania. However, oestrogen receptors have not been associated with a longer progression‐free survival (Shen 2017). The presence of progesterone receptors has also been associated with favourable overall survival and progression‐free survival in European women with unclassified EOC (Luo 2017). Likewise, BRCA mutation has been correlated to better overall and progression‐free survival (Zhong 2015). However, no studies were identified that looked at the effect of HRT in women who have undergone surgical treatment for EOC with hormone receptors or BRCA mutation.
We did not identified heterogeneity of outcomes among the various studies. There was no studies comparing the different regimens and duration of HRT, although there were variations in the age and race of participants, stage and histological type of disease, regimen, dose and route of administration for HRT. This may indicate the generalisability of the application in clinical practice. However, the information on duration of HRT use, hormone receptor status and BRCA mutation was limited.
Quality of the evidence
Using the GRADE system, we estimated the certainty of the evidence to be low because of limitations in the design of the included studies design and inconsistency of results. Not all studies had used placebo in the control group; participants knew what hormone therapy they received and in some studies, there was loss to follow‐up greater than 20% (Li 2012). There were also differences in the outcomes; one study showed an effect of HRT use (Eeles 2015), whereas the others did not show the same effect (or only partly in one study (Guidozzi 1999)). The GRADE rating was further downgraded to very low‐certainty evidence due to imprecision of results, small sample sizes and wide 95% CIs.
Potential biases in the review process
We conducted a comprehensive search of the available evidence. Two review authors independently searched for studies following clear inclusion and exclusion criteria and identified eligible studies. Two review authors independently extracted data and evaluated risk of bias. It remains possible that studies may have not been reported or were missed and not included. Analysis of publication bias could not be performed in this review as the number of included studies is lower than ten.
Agreements and disagreements with other studies or reviews
A recent meta‐analysis on the effect of HRT in the general population showed that HRT did not affect the risk of death from cardiac events, stroke and cancer (breast, lung, colorectal or ovarian) (Benkhadra 2015). However, combined HRT use, but not oestrogen‐only HRT, was associated with an increased risk of mortality from breast cancer (Benkhadra 2015). This meta‐analysis examined younger women (who started HRT aged under 60 or within 10 years of menopause) separately and found that HRT use was associated with lower all‐cause mortality. The evidence on HRT in women experiencing natural menopause remains unclear. The findings of a systematic review and meta‐analysis (which included 310,329 women) showed that women experiencing early menopause (at less than 45 years of age) had overall higher incidence of coronary heart disease and mortality from coronary heart disease, cardiovascular disease and all‐cause mortality (Muka 2016). A large epidemiological study, the Black Women’s Health Study, investigated all‐cause mortality in 11,212 women with natural menopause and found that women aged less than 45 years who never used HRT had a higher mortality rate; this was not significant for those who ever used HRT (Li 2013). In another USA‐based epidemiological study of 11,287 women, all‐cause mortality was higher in women experiencing menopause under 45 years of age (Malek 2019). Over a 7.1 year follow‐up, those experiencing menopause under 45 years who had ever used HRT had a higher all‐cause mortality rate than those who experienced menopause over 45 years of age who had ever used HRT, although limitations include association of smoking with an early age of menopause. In addition, women who used HRT for more than five years, from around the age of 50, were found to have a higher risk of ovarian cancer, especially for serous and endometrioid types (Cancer Epidemiology 2015).
There have been three previous systematic reviews of the use of HRT in EOC after treatment. The first systematic review, Hopkins 2004, included one RCT (Guidozzi 1999) and two observational studies. The second and third systematic reviews (Li 2015; Pergialiotis 2015) included two RCTs (Guidozzi 1999; Li 2012) and four cohort studies. The result of all these systematic reviews showed that HRT may make little or no difference to overall and progression‐free survival. Our review included three RCTs (Eeles 2015; Guidozzi 1999; Li 2012) and the results suggest that HRT may be associated with a slight improvement in overall survival and quality of life (especially for general condition), but may make little or no difference to progression‐free survival. The results of adverse events of HRT were not reported in any of the three previous systematic reviews (Hopkins 2004; Li 2015; Pergialiotis 2015).
Authors' conclusions
Implications for practice.
Hormone replacement therapy (HRT) may improve overall survival in women who have undergone surgery for epithelial ovarian cancer (EOC). However, this is based on low‐certainty evidence and therefore should be interpreted with caution. We are very uncertain about the impact of HRT on progression‐free survival and incidence of adverse events such as breast cancer, transient ischaemic accident, cerebrovascular accident and myocardial infarction. The incidence of gallstones was not reported in any of the included studies. The evidence in this review is too limited to support or refute that HRT is very harmful in this population. Women and their doctors should therefore make decisions based on individual priorities and symptoms.
Implications for research.
Menopause can be the cause of severe symptoms. There is a lot of concern and confusion surrounding the use of HRT, due to the fear of potential risks and menopausal symptoms, and it is often neglected as clinicians are more likely to focus on cancer treatment and survival outcomes. Therefore, future well‐designed randomized controlled trials (RCTs) investigating the risks and benefits of HRT use in women treated for EOC and surgically induced menopause would be welcomed. These studies should have larger sample sizes and longer follow‐up of women with EOC and should look at both survival and quality‐of‐life outcomes. Future research should consider the age of women, duration of HRT use, hormone receptors status and BRCA mutation status. Moreover, specific measurements for quality‐of‐life assessments regarding menopausal symptoms should be mandated in future studies. This is significant for women with good prognosis, who may have a premature surgically induced menopause and subsequent health problems. It is also important for women with more advanced disease in whom quality of life is often overlooked, as the main focus is on cancer treatment and survival outcomes. Given that many of these women have life‐limiting disease, treatments that so far appear to have limited deleterious effect on survival, but may have significant effect on their well‐being, deserve more attention.
Acknowledgements
We would like to thank Jo Morrison for clinical and editorial advice, Jo Platt for designing and running the searches and Gail Quinn, Clare Jess and Tracey Harrison for their valuable contribution to the editorial process.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Group. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, National Insititute for Health Research (NIHR), National Health Service, or the Department of Health.
We thank the referees for many helpful suggestions and comments; these referees included Fani Kokka, Elly Brockbank, Ruth Payne and Nicolette Biglia.
Appendices
Appendix 1. CENTRAL search strategy
#1. MeSH descriptor: [Ovarian Neoplasms] explode all trees #2. ovar* near/5 (cancer* or neoplas* or tumor* or tumour* or carcinoma* or adenocarcinoma* or malignan*) #3. #1 or #2 #4. MeSH descriptor: [Hormone Replacement Therapy] explode all trees #5. hormone replacement or HRT #6. MeSH descriptor: [Estrogens] explode all trees #7. MeSH descriptor: [Estrogen Antagonists] explode all trees #8. estrogen* or oestrogen* #9. MeSH descriptor: [Progestins] explode all trees #10. MeSH descriptor: [Progesterone] explode all trees #11. progest* #12. MeSH descriptor: [Testosterone] explode all trees #13. testosterone #14. tibolone #15. #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 #16. #3 and #15
Appendix 2. MEDLINE Ovid search strategy
1. exp Ovarian Neoplasms/ 2. (ovar* adj5 (cancer* or neoplas* or tumor* or tumour* or carcinoma* or adenocarcinoma* or malignan*)).mp. 3. 1 or 2 4. exp Hormone Replacement Therapy/ 5. (hormone replacement therapy or HRT).ti,ab,kw. 6. exp Estrogens/ 7. exp Estrogen Antagonists/ 8. (estrogen* or oestrogen*).ti,ab,kw. 9. exp Progestins/ 10. exp Progesterone/ 11. progest*.mp. 12. exp Testosterone/ 13. testosterone.mp. 14. tibolone.mp. 15. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 16. 3 and 15 17. randomized controlled trial.pt. 18. controlled clinical trial.pt. 19. randomized.ab. 20. placebo.ab. 21. drug therapy.fs. 22. randomly.ab. 23. trial.ti. 24. groups.ab. 25. 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 26. (animals not (humans and animals)).sh. 27. 25 not 26 28. 16 and 27
Key: mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier fs=floating subheading ab=abstract sh=subject heading pt=publication type
Appendix 3. Embase search strategy
1. exp ovary tumor/ 2. (ovar* adj5 (cancer* or neoplas* or tumor* or tumour* or carcinoma* or adenocarcinoma* or malignan*)).mp. 3. 1 or 2 4. exp hormone substitution/ 5. (hormone replacement or HRT).mp. 6. exp estrogen/ 7. exp antiestrogen/ 8. (estrogen* or oestrogen*).mp. 9. exp progesterone/ 10. progest*.mp. 11. exp testosterone/ 12. testosterone.mp. 13. tibolone.mp. 14. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15. 3 and 14 16. crossover procedure/ 17. double‐blind procedure/ 18. randomized controlled trial/ 19. single‐blind procedure/ 20. random*.mp. 21. factorial*.mp. 22. (crossover* or cross over* or cross‐over*).mp. 23. placebo*.mp. 24. (double* adj blind*).mp. 25. (singl* adj blind*).mp. 26. assign*.mp. 27. allocat*.mp. 28. volunteer*.mp. 29. 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 30. 15 and 29
Key: mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier fs = floating subheading ab = abstract sh = subject heading pt = publication type
Data and analyses
Comparison 1. Hormone replacement therapy versus no hormone replacement therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 3 | 350 | Hazard Ratio (95% CI) | 0.71 [0.54, 0.93] |
| 2 Quality of life, general condition | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Progression‐free survival | 2 | 275 | Hazard Ratio (95% CI) | 0.76 [0.57, 1.01] |
| 4 Incidence of breast cancer | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.59] |
| 5 Transient ischaemic attack | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Cerebrovascular accident | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Myocardial infarction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Eeles 2015.
| Methods | Randomized controlled trial | |
| Participants |
Country: United Kingdom centres, Spain, Hungary Setting: the Clinical Trials and Statistics Unit at The Institute of Cancer Research had overall responsibility for trial co‐ordination, data collation, central statistical monitoring, and all final analyses. The patients were recruited from 17 United Kingdom centres, with the remaining 26 recruited from single centres in Spain (n = 14. 9%) and Hungary (n = 12.8%). Computer‐generated random permuted blocks were used and stratification by treating centres, menopausal status and FIGO stage to assign in a 1:1 ratio to receive HRT and no HRT, from Febuary 1990 to November 1995. Baseline characteristics: the 150 participants were randomized into HRT and no HRT (75 participants in each group). The median age was 58.7 years (range: 29.3 to 89.6 years). Most participants were 50 to 59 years old (33.3%), postmenopausal (77.3%), of serous type (39.3%), grade 3 (38.0%), FIGO stage III (54.7%), with no residual lesion (41.3%) and receiving single‐agent platinum (47.3%). Inclusion criteria: eligible participants were women who had been diagnosed with EOC (any FIGO stage) fewer than 9 months previously. Both premenopausal and postmenopausal women were eligible. Exclusion criteria: the women who needed to preserve ovarian function and had a history of hormone‐dependent malignancy or with any contraindications to HRT. |
|
| Interventions |
Intervention characteristics: HRT
Control: no HRT (N=75) The median time receiving hormone therapy was 1.14 years (IQR: 0.46 to 5.08 years). |
|
| Outcomes | The median follow‐up time was 19.1 years. There were participants who were assigned to HRT but did not receive or discontinued HRT during follow‐up (49 participants (65.3%)) and participants who were assigned to no HRT but received HRT during follow‐up (8 participants (10.7%)). However, all analyses were performed on an intention‐to‐treat basis. Death rate: 121 participants (81%) died: 53 (71%) in the HRT group and 68 (91%) participants in the no HRT group. Recurrent rate: 122 participants (81%) had recurrence: 54 (72%) in the HRT group and 68 (91%) in the no HRT group. Adverse event: the adverse event rate was low, with no statistically significant difference between two groups. The presence of TIA, CVA, MI, fracture and second primary cancer in the HRT group was 2.7%, 2.7%, 0%, 2.7%, and 5.3%, respectively; whereas in the no HRT group it was 0%, 4.0%, 2.7%, 5.3% and 4.0 %, respectively. Breast cancer was found two participants receiving HRT and one participant receiving no HRT. |
|
| Notes | Sponsorship source: supported by the Institute of Cancer Research (to RE and JMB), by core funding (C1491/A8895) to the Clinical Trials and Statistics Unit at the Institute of Cancer Research from Cancer Research UK, and by the National Institute for Health Research support (to RE) to the Biomedical Research Centre at The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust. The trial was sponsored by The Royal Marsden NHS Foundation Trust and was conducted in accordance with the principles of good clinical practice. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated random permuted blocks were used; stratification was by treating centre, menopausal status (pre versus post), and FIGO stage (I and II versus III and IV)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants were centrally randomly assigned in a 1:1 ratio to receive either AHT or no AHT (control). Independent random assignment was performed via telephone (or fax for international sites) at the Clinical Trials and Statistics Unit at The Institute of Cancer Research." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were due to start treatment within 2 weeks of random assignment and to continue their treatment for a minimum of 5 years, if tolerated. Treatment was non‐blinded, and no placebo was given to control‐group participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was no information on who were outcome assessors and whether they knew the treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was no participant loss to follow‐up but after random assignment for HRT, 3 (4%) participants denied receiving HRT and 46 (61.3%) participants discontinued HRT up to death or last follow‐up. |
| Selective reporting (reporting bias) | Low risk | Quote: "All analyses were performed on an intention‐to‐treat basis, and a two‐sided significance level of 5% and corresponding 95% CIs were used throughout. All analyses were performed with Stata13 (StataCorp, College Station, TX)." |
| Other bias | Low risk | We do not suspect any other source of bias. |
Guidozzi 1999.
| Methods | Randomized controlled trial | |
| Participants |
Country: South Africa Setting: Department of Obstetrics and Gynecology, Johannesburg Hospital and Medical School of the University of the Witwatersrand, Johannesburg, South Africa. Sealed opaque envelopes were used to random the participants in equal number of HRT and no HRT, from January 1987 to June 1994. Baseline characteristics: of the 130 participants included, there were participants who were lost to follow‐up: three participants in the HRT and two participants in the no HRT groups, therefore the total number of participants who were analysed were 59 participants in the HRT and 66 participants in the no HRT groups. The participants aged less than 59 years old were included. Most participants were 56 to 59 years old (44.0%), of serous type (68.0%), grade 1 (42.4%), FIGO stage III (67.2%), and had undergone optimal surgery (72.0%). Inclusion criteria: all participants were younger than 59 years old with invasive EOC treated with total abdominal hysterectomy, bilateral salpingo‐oophorectomy, omentectomy, and tumour debulking, following chemotherapy 6 cycles of cisplatin 100 mg/m2 and cyclophosphamide 500 mg/m2, then 2 cycles of cisplatin only and oral chlorambucil for 1 year thereafter. Exclusion criteria: participants with ovarian carcinoma of low malignant potential and those who had ever taken conjugated oestrogens were excluded. |
|
| Interventions |
Intervention characteristics: HRT
Control: no HRT (N=66) There were no data on length of time participants received HRT. |
|
| Outcomes | The median follow‐up time was 90 months. There were participants who were assigned to HRT but did not receive or discontinued HRT within 9 months (9 participants (13.6%)) and participants who were assigned to no HRT but received HRT (five participants (8.5%)). However, all analyses were performed on an intention‐to‐treat basis. Death rate: 73 participants (58.4%) died: 32 (54%) in the HRT group and 41 (62%) participants in the no HRT group. The median overall survival was 44 months in the HRT group and 34 months in the no HRT group. Recurrent rate: 73 participants (58.4%) had recurrence: 32 (54%) in the HRT group and 41 (62%) in the no HRT group. The median disease‐free interval was 34 months in the HRT group and 27 months in the no HRT group. |
|
| Notes | Sponsorship source: there were no data of funding source and conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All participants were randomised at the routine assessment consultation held 6 – 8 weeks postoperatively." Comment: no randomization method was identified. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization involved participant choice of a sealed opaque envelope from a predetermined equal number of similarly sealed opaque envelopes that contained directions for the randomisation to either continuous conjugated equine oestrogen replacement (ERT), consisting of Premarin 0.625 mg daily (Wyeth‐ Ayerst, Philadelphia, PA), or no supplementation (non‐ERT)." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Placebo tablets were not used, and prior to randomization all participants were fully counselled about the risks and benefits of oestrogen replacement as well as the aims and limitations of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information who were outcome assessor and whether they knew the treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One hundred thirty participants met the criteria and were randomised into their respective groups: 62 to post‐operative ERT and 68 to non‐ERT. Three participants in ERT group and two participants in non‐ERT group were lost to follow‐up, so that the final analysis involved 59 participants in ERT group and 66 participants in non‐ERT." |
| Selective reporting (reporting bias) | Low risk | Quote: "For the purposes of analysis, the participants remained in the treatment group to which they were originally allocated, irrespective of whether they elected to commence, stop taking, or refuse ERT, i.e., intention‐to‐treat analyses are reported." |
| Other bias | Low risk | We do not suspect any other source of bias. |
Li 2012.
| Methods | Randomized controlled trial | |
| Participants |
Country: China Setting: the Department of Gynecological Oncology of the Cancer Hospital of Guangxi Medical University, China. The envelope method were used to random the participants in equal number of HRT and no HRT, from August 1999 to June 2003. Baseline characteristics: of the 90 participants included, there were participants lost to follow‐up (14 participants in the HRT group and one participant in no HRT group). The total number of participants analyzed into HRT were 31 participants with an average age of 40.3 years (range: 20 to 45 years), and to no HRT, 44 participants with the average age of 42.9 years (range: 20 to 45 years). Most participants were of serous type (62.7%), FIGO stage III (72.0%); all participants received combined‐agent platinum. Inclusion criteria: participants with a diagnosis of ovarian cancer who had received cytoreductive surgery (total hysterectomy plus bilateral ovarian resection), followed by 6 to 8 courses of platinum‐based combination chemotherapy. The histological types included were mucinous and serous cystadenocarcinoma and FIGO stage I to III. Exclusion criteria: no data |
|
| Interventions |
Intervention Characteristics : HRT
Control: no HRT (N=44) The average of time receiving HRT was 28.7 months (range: 6 to 43 months). |
|
| Outcomes | The median follow‐up time was 31.4 months (range 10 to 43 months). There were participants who were assigned to HRT but were lost to follow‐up or discontinued HRT within 6 months (14 participants (31.1%)) and participants who were assigned to no HRT but lost to follow‐up (one participant (2.2%)), and were not included in the result and analysis. Survival period: the average survival period was 1108 ± 52 days and 1086 ± 43 days in the HRT and no HRT arms, respectively. There was no statistically significant difference. Quality of life: the EORTC‐C30 was used to assess functional (body, role, cognition, emotion and social), symptom (fatigue, pain, nausea and vomiting, shortness of breath, insomnia, loss of appetite, constipation and diarrhoea) and general health status. The GMU‐Gynae Index was used to assess sexual (sexual difficulties, emotional exchange between the couple, regression of sexual life and sexual desire), urinary tract (urethral burning and frequent urination) and autonomic dysfunction (itchy skin, dry skin and formication). There were statistically significant differences between the two groups for physical function, emotional function symptom sub‐scale, general health status, sexual behaviour and autonomic dysfunction. Additionally, the other objectives in this study were the expression of oestrogen and progesterone receptor using immunohistochemical stain in cancer tissues and levels of calcitonin and transforming growth factor using radioimmunoassay and enzyme‐linked immunosorbent assay in serum. The survival rate did not differ significantly in the expression of oestrogen and progesterone receptor and the level of serum transforming growth factor and calcitonin were not significantly different between the HRT and no HRT arms. |
|
| Notes |
Sponsorship source: a grant from the Provincial Research Project Funding of Guangxi, China (No. GSR 9817101). We contacted the author for more information on the quality‐of‐life assessment that they used EORTC‐C30 and GMU‐Gynae Index, but we did not receive any reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "At 20 days following cytoreductive surgery, 90 participants were randomly divided into a HRT group and a no HRT group (n = 45 each),using the envelope method." The description of the envelope method was insufficient to justify a judgement of low or high risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information available to permit a judgement of low or high risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants knew the HRT that they received and no placebo was used in the control group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was no information on who were outcome assessors and whether they knew the treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14 participants (31.1%) in the HRT group and one participant (2.2%) in the control group were lost to follow‐up or non‐compliance within 6 months. |
| Selective reporting (reporting bias) | Low risk | Survival data were analyzed using Kaplan‐Meier survival curves and the log‐rank test. The Student's t‐test was used to analyze averaged data, and the Chi2 test was used for ratio comparisons. All analyses were performed with SPSS.10 software (Statsoft, USA). P < 0.05 was considered to be statistically significant. |
| Other bias | Low risk | We do not suspect any other source of bias. |
CVA: cerebrovascular accident EOC: epithelial ovarian cancer FIGO: International Federation of Gynecology and Obstetrics HRT: hormone replacement therapy IQR: interquartile range MI: myocardial infarction N: number TIA: transient ischaemic attack
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anderson 2003 | Study in healthy postmenopausal women |
| Bebar 2000 | Non‐randomized study |
| Guidozzi 1998 | Duplicate |
| Guidozzi 1999a | Duplicate |
| Guidozzi 2013 | Review |
| Li 2008 | Duplicate |
| Lipkowitz 2015 | Review |
| Ursic‐Vrscaj 2001 | Non‐randomized study |
Differences between protocol and review
We excluded, from further subgroup analysis, women who underwent fertility‐preservation surgery (retention of one ovary). We could not compare regimens and durations of HRT administration because of the small number of participants. Adverse events of pulmonary embolism, deep vein thrombosis and gallstones were not reported in the included studies, however we aim to analyse these results including oestrogen and progesterone receptor status in future updates of this review if appropriate data become available. We added transient ischaemic attack and cerebrovascular accident to the adverse events, and changed the method for estimating the hazard ratio from standard error using methods of Parmar 1998 to using the Observed minus Expected events (O‐E) and the Variance (V) using formula according to Tierney 2007.
Contributions of authors
Protocol development: NS, EM, TL Selection of studies: NS, RB Assessing eligibility of studies: NS, RB; third person: TL Data extraction: NS, KP; third person: RB Data entry into Review Manager 5: NS, TL; spot‐check: KP Assessment of risk of bias: NS, RB; third person: EM Analysing data: NS, TL Drafting the final review: NS, RB, KP, TL, EM Updating the review: NS, RB, KP, TL, EM
Sources of support
Internal sources
Faculty of Medicine, Prince of Songkla University, Thailand.
External sources
Thailand Research Fund (Distinguished Research Professor Award), Thailand.
Declarations of interest
Nungrutai Saeaib: none known Krantarat Peeyananjarassri: none known Tippawan Liabsuetrakul: none known Rakchai Buhachat: none known Eva Myriokefalitaki: none known
New
References
References to studies included in this review
Eeles 2015 {published data only}
- Eeles RA, Morden JP, Gore M, Mansi J, Glees J, Wenxzl M, et al. Adjuvant hormone therapy may improve survival in epithelial ovarian cancer: results of the AHT Randomized Trial. Journal of Clinical Oncology 2015;33(35):4138‐44. [DOI: 10.1200/JCO.2015.60.9719] [DOI] [PubMed] [Google Scholar]
Guidozzi 1999 {published data only}
- Guidozzi F, Daponte A. Estrogen replacement therapy for ovarian carcinoma survivors: a randomized controlled trial. Cancer 1999;86(6):1013‐8. [DOI] [PubMed] [Google Scholar]
Li 2012 {published data only}
- Li L, Pan Z, Gao K, Zhang W, Luo Y, Yao Z, et al. Impact of post‐operative hormone replacement therapy on life quality and prognosis in patients with ovarian malignancy. Oncology Letters 2012;3(1):244‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Anderson 2003 {published data only}
- Anderson GL, Judd HL, Kaunitz AM, Barad DH, Beresford SA, Pettinger M, et al. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women's Health Initiative randomized trial. Journal of the American Medical Association 2003;290(13):1739‐48. [DOI] [PubMed] [Google Scholar]
Bebar 2000 {published data only}
- Bebar S, Ursic‐Vrscaj M. Hormone replacement therapy after epithelial ovarian cancer treatment. European Journal of Gynaecological Oncology 2000;21(2):192‐6. [PubMed] [Google Scholar]
Guidozzi 1998 {published data only}
- Guidozzi F. Hormone replacement therapy in patients with ovarian cancer. The 29th Congress of the South African Society of Obstetrics and Gynecology 8‐12 March 1998:XAOC ‐ LTE 1998; 29‐XPH ‐ N1998.
Guidozzi 1999a {published data only}
- Guidozzi F, Paponte A. Estrogen replacement therapy in ovarian cancer survivors. International journal of gynecological cancer 1999;9 Suppl 1 XPH ‐ Y(Suppl 1):5:Abstract A13. [Google Scholar]
Guidozzi 2013 {published data only}
- Guidozzi F. Estrogen therapy in gynecological cancer survivors. Climacteric 2013;16(6):611‐7. [DOI] [PubMed] [Google Scholar]
Li 2008 {published data only}
- Li L, Pan ZM, Chen XQ, Gao K, Zhang W, Luo Y, et al. Relationship between hormone therapy in women with ovarian malignancy and prognosis. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology] 2008;43(11):843‐8. [PubMed] [Google Scholar]
Lipkowitz 2015 {published data only}
- Lipkowitz S, Kohn EC. To treat or not to treat: the use of hormone replacement therapy in patients with ovarian cancer. Journal of Clinical Oncology 2015;33(35):4127‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ursic‐Vrscaj 2001 {published data only}
- Ursic‐Vrscaj M, Bebar S, Zakelj MP. Hormone replacement therapy after invasive ovarian serous cystadenocarcinoma treatment: the effect on survival. Menopause 2001;8(1):70‐5. [DOI] [PubMed] [Google Scholar]
Additional references
Benkhadra 2015
- Benkhadra K, Mohammed K, Nofal AA, Leon BC, Alahdab F, Faubion S, et al. Menopausal hormone therapy and mortality: a systematic review and meta‐analysis. Journal of Clinical Endocrinology & Metabolism 2015;100(11):4021‐8. [DOI] [PubMed] [Google Scholar]
Biglia 2015
- Biglia N, Bounous VE, Sgro LG, D’Alonzo M, Gallo M. Treatment of climacteric symptoms in survivors of gynaecological cancer. Maturitas 2015;82(3):296‐8. [DOI] [PubMed] [Google Scholar]
Biliatis 2012
- Biliatis I, Thomakos N, Rodolakis A, Akrivos N, Zacharakis D, Antsaklis A. Safety of hormone replacement therapy in gynaecological cancer survivors. Journal of Obstetrics and Gynaecology 2012;32:321‐5. [DOI] [PubMed] [Google Scholar]
Cancer Epidemiology 2015
- Collaborative Group on Epidemiological Studies of Ovarian Cancer. Menopausal hormone use and ovarian cancer risk: individual participant meta‐analysis of 52 epidemiological studies. Lancet 2015;385:1835‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chlebowski 2015
- Chlebowski RT, Rohan TE, Manson JE, Aragaki AK, Kaunitz A, Stefanick ML, et al. Breast cancer after use of estrogen plus progestin and estrogen alone; analyses of data from 2 Women's Health Initiative randomized clinical trials. Journal of the American Medical Association 2015;1(3):296‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London (UK): BMJ Publication Group, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Diep 2015
- Diep CH, Daniel AR, Mauro LJ, Knutson TP, Lange CA. Progesterone action in breast, uterine, and ovarian cancers. Journal of Molecular Endocrinology 2015;54(2):R31‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eeles 1991
- Eeles RA, Tan S, Wiltshaw E, Fryatt I, A'Hern RP, Shepherd JH, et al. Hormone replacement therapy and survival after surgery for ovarian cancer. British Medical Journal 1991;302:259‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleming 2013
- Fleming G, Seidman J, Lengyel E. Epithelial ovarian cancer. In: Barakat RR, Berchuck A, Markman M, Randall ME editor(s). Principles and Practice of Gynecologic Oncology. 6th Edition. Philadelphia (PA): Lippincott Williams & Wilkins, 2013:757‐847. [Google Scholar]
GLOBOCAN 2018
- International Agency for Research on Cancer and World Health Organization. Cancer Today. Estimated age‐standardized incidence rates (World) in 2018, worldwide, both sexes, all ages. Available from https://gco.iarc.fr/today (accessed 6 January 2019).
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;357:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook.
Hopkins 2004
- Hopkins ML, Fung MF, Le T, Shorr R. Ovarian cancer patients and hormone replacement therapy: a systematic review. Gynecologic Oncology 2004;92(3):827‐32. [DOI] [PubMed] [Google Scholar]
Hua 2018
- Hua H, Zhang H, Kong Q, Jiang Y. Mechanisms for estrogen receptor expression in human cancer. Experimental Hematology & Oncology 2018;7(24):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ibeanu 2011
- Ibeanu O, Modesitt SC, Ducie J, Gruenigen V, Agueh M, Fader AN. Hormone replacement therapy in gynecologic cancer survivors: why not?. Gynecologic Oncology 2011;122(2):447‐54. [DOI] [PubMed] [Google Scholar]
Jenabi 2015
- Jenabi E, Shobeiri F, Hazavehei SMM, Roshanaei G. Assessment of questionnaire measuring quality of life in menopausal women: a systematic review. Oman Medical Journal 2015;30(3):151‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schunemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;23(2):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leeper 2002
- Leeper K, Garcia R, Swisher E, Goff B, Greer B, Paley P. Pathologic findings in prophylactic oophorectomy specimens in high‐risk women. Gynecologic Oncology 2002;87(1):52‐6. [DOI] [PubMed] [Google Scholar]
Li 2013
- Li S, Rosenberg L, Wise LA, Boggs DA, LaValley M, Palmer JR. Age at natural menopause in relation to all‐cause and cause‐specific mortality in a follow‐up study of U.S. black women. Maturitas 2013;75(3):246‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2015
- Li D, Ding C‐y, Qiu L‐h. Postoperative hormone replacement therapy for epithelial ovarian cancer patients: a systematic review and meta‐analysis. Gynecologic Oncology 2015;139(2):355‐62. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lowe 2013
- Lowe KA, Chia VM, Taylor A, O'Malley C, Kelsh M, Mohamed M, et al. An international assessment of ovarian cancer incidence and mortality. Gynecologic Oncology 2013;130(1):107‐14. [DOI] [PubMed] [Google Scholar]
Luo 2017
- Luo H, Li S, Zhao M, Sheng B, Zhu H, Zhu X. Prognostic value of progesterone receptor expression in ovarian cancer: a meta‐analysis. Oncotarget 2017;8(22):36845‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malek 2019
- Malek AM, Vladutiu CJ, Meyer ML, Cushman M, Newman R, Lisabeth LD, et al. The association of age at menopause and all‐cause and cause‐specific mortality by race, postmenopausal hormone use, and smoking status. Preventive Medicine Reports 2019;15:100955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manson 2013
- Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. Journal of the American Medical Association 2013;310(13):1353‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marchetti 2013
- Marchetti C, Iadarola R, Palaia I, Donato V, Perniola G, Muzii L, et al. Hormone therapy in oophorectomized BRCA 1/2 mutation carriers. Menopause 2013;21(7):763‐8. [DOI] [PubMed] [Google Scholar]
Marjoribanks 2012
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD004143.pub4] [DOI] [PubMed] [Google Scholar]
Mascarenhas 2006
- Mascarenhas C, Lambe M, Bellocco R, Bergfeldt K, Riman T, Persson I, et al. Use of hormone replacement therapy before and after ovarian cancer diagnosis and ovarian cancer survival. International Journal of Cancer 2006;119(12):2907‐15. [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Michaelson‐Cohen 2009
- Michaelson‐Cohen R, Beller U. Managing menopausal symptoms after gynecological cancer. Current Opinion in Oncology 2009;21(5):407‐11. [DOI] [PubMed] [Google Scholar]
Muka 2016
- Muka T, Oliver‐Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all‐cause mortality: a systematic review and meta‐analysis. Journal of the American Medical Association 2016;1(7):767‐76. [DOI] [PubMed] [Google Scholar]
Mørch 2012
- Mørch LS, Løkkegaard E, Andreasen AH, Kjær SK, Lidegaard Ø. Hormone therapy and different ovarian cancers: a National Cohort Study. American Journal of Epidemiology 2012;175(12):1234‐42. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Pergialiotis 2015
- Pergialiotis V, Pitsouni E, Prodromidou A, Frountzas M, Perrea DN, Vlachos GD. Hormone therapy for ovarian cancer survivors: systematic review and meta‐analysis. Menopuase 2015;23(3):335‐42. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodrigguez 2001
- Rodriguez C, Patel AV, Calle EE, Jacob EJ, Thun MJ. Estrogen replacement therapy and ovarian cancer mortality in a large prospective study of US women. Journal of the American Medical Association 2001;285(11):1460‐5. [DOI] [PubMed] [Google Scholar]
Rossouw 2002
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Journal of the American Medical Association 2002;288(3):321‐33. [DOI] [PubMed] [Google Scholar]
Rossouw 2007
- Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. Journal of the American Medical Association 2007;297(13):1465‐77. [DOI] [PubMed] [Google Scholar]
Schunemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glaziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011.. Available from training.cochrane.org/handbook.
Shen 2017
- Shen Z, Luo H, Li S, Sheng B, Zhao M, Zhu H, et al. Correlation between estrogen receptor expression and prognosisin epithelial ovarian cancer: a meta‐analysis. Oncotarget 2017;8(37):62400‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shi 2015
- Shi LF, Wu Y, Li CY. Hormone therapy and risk of ovarian cancer in postmenopausal women: a systematic review and meta‐analysis. Menopause 2015;23(4):417‐24. [DOI] [PubMed] [Google Scholar]
Singh 2010
- Singh P, Oehler MK. Hormone replacement after gynaecological cancer. Maturitas 2010;65(3):190‐7. [DOI] [PubMed] [Google Scholar]
Snyder 2013
- Snyder CF, Blackford AL, Okuyama T, Akechi T, Yamashita H, Toyama T, et al. Using the EORTC‐QLQ‐C30 in clinical practice for patient management: identifying scores requiring a clinician's attention. Quality of Life Research 2013;22:2685‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. BioMed Central 2007;8:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wen 2013
- Wen Y, Huang H, Wu M, Shen K, Pan L. The safety of postoperative hormone replacement therapy in epithelial ovarian cancer patients in China. Climacteric 2013;16(6):673‐81. [DOI] [PubMed] [Google Scholar]
Zhao 2013
- Zhao D, Zhang F, Zhang W, He J, Zhao Y, Sun J. Prognostic role of hormone receptors in ovarian cancer: a systematic review and meta‐analysis. International Journal of Gynecological Cancer 2013;23(1):25‐33. [DOI] [PubMed] [Google Scholar]
Zhong 2015
- Zhong Q, Peng HL, Zhao X, Zhang L, Hwang WT. Effects of BRCA1‐ and BRCA2‐related mutations on ovarian and breast cancer survival: a meta‐analysis. Clinical Cancer Research 2015;21(1):211‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2008
- Zhou B, Sun Q, Cong R, Gu H, Tang N, Yang L, et al. Hormone replacement therapy and ovarian cancer risk: a meta‐analysis. Gynecologic Oncology 2008;108(3):641‐51. [DOI] [PubMed] [Google Scholar]


