Abstract
Objective:
Shilajit is a pale-brown to blackish-brown organic mineral substance available from Himalayan rocks. We demonstrated that in type I obese humans, shilajit supplementation significantly upregulated extracellular matrix (ECM)–related genes in the skeletal muscle. Such an effect was highly synergistic with exercise. The present study (clinicaltrials.gov ) aimed to evaluate the effects of shilajit supplementation on skin gene expression profile and microperfusion in healthy adult females.
Methods:
The study design comprised six total study visits including a baseline visit (V1) and a final 14-week visit (V6) following oral shilajit supplementation (125 or 250 mg bid). A skin biopsy of the left inner upper arm of each subject was collected at visit 2 and visit 6 for gene expression profiling using Affymetrix Clariom™ D Assay. Skin perfusion was determined by MATLAB processing of dermascopic images. Transcriptome data were normalized and subjected to statistical analysis. The differentially regulated genes were subjected to Ingenuity Pathway Analysis (IPA®). The expression of the differentially regulated genes identified by IPA® were verified using real-time polymerasechain reaction (RT-PCR).
Results:
Supplementation with shilajit for 14 weeks was not associated with any reported adverse effect within this period. At a higher dose (250 mg bid), shilajit improved skin perfusion when compared to baseline or the placebo. Pathway analysis identified shilajit-inducible genes relevant to endothelial cell migration, growth of blood vessels, and ECM which were validated by quantitative real-time polymerasechain reaction (RT-PCR) analysis.
Conclusions:
This work provides maiden evidence demonstrating that oral shilajit supplementation in adult healthy women induced genes relevant to endothelial cell migration and growth of blood vessels. Shilajit supplementation improved skin microperfusion.
Keywords: Shilajit, dietary supplementation, skin perfusion, ECM, aging
Introduction
Shilajit is a resinous blackish-brown sticky tar-like herbomineral exudate that seeps from sedimentary rocks of steep mountainous regions and has reported medicinal properties (1, 2). Although geographic and environmental factors determine the composition of shilajit (1, 3), chemical characterization of shilajit has revealed the presence of three major components as represented by dibenzo-α-pyrones (DBPs, also known as urolithins in free form as well as conjugated with chromoproteins), fulvic acid with DBP core nucleus, and humic acid (2, 3). Shilajit and its active constituents have been reported to possess an array of pharmacological properties including adaptogenic, antioxidant, anti-inflammatory, immunomodulatory, anti-diabetic, and neurological properties (4, 5).
Skin aging is characterized by wrinkles, dryness, laxity, thinning, irregular pigmentation, and loss of elasticity (6). Decrease in dermal thickness and vascularity is a hallmark of cutaneous aging (7). Aging is associated with decreased cutaneous perfusion (8). Dietary supplements show promise in preventing and managing serious health conditions. The present study was aimed at determining the effect of supplementing with a standardized shilajit extract on skin gene expression profile and related function.
Materials and methods
Shilajit (PrimaVie® Shilajit) capsules (125 mg referred to as S125, 250 mg referred to as S250) and placebo were provided by Natreon, Inc.. PrimaVie® Shilajit (U.S. patents: US 6,869,612, and 6,558,712) is a purified and standardized shilajit extract and contains at least 60% fulvic acid and equivalents with high levels of DBPs and DBP chromoproteins (9, 10, 18). Each capsule contained standard components including gelatin, microcrystalline cellulose, croscarmellose sodium, fumed silicon-dioxide, and magnesium stearate as excipients, which are of national formulary grade.
Study subjects and experimental design
Study protocols (clinicaltrials.gov NCT02762032) and materials were approved by the Western Institutional Review Board. Written informed consent was collected from all subjects before participation in the study. Female subjects aged between 30 and 65 were included in the study. Three groups (each with n = 15) of subjects were randomized (www.random.org). Supplement randomization was done at study visit 1 and distribution of the supplements were done at each study visit. During each visit, imaging and skin assessment were performed. Group 1 received placebo capsules; Groups 2 and 3 received 125 mg or 250 mg capsules of shilajit bid, respectively. Oral supplementation was continued for 14 weeks and six assessment visits were performed during the duration of study. The study design comprised six visits—visit 1: baseline; visits 2 through 6: after 2, 4, 8, 12, and 14 weeks of oral supplement of shilajit, respectively. During the study visit, the dietary and medical history and medications were recorded. Digital photographs of the face (left, right, and front views) were taken using a DSLR camera (Nikon D80 with 55–300mm lens) with neutral expressions without skin makeup. Noninvasive measurements such as transepidermal water loss (TEWL), hydration, elasticity, and dermascopic image were recorded with Dermalab (cyberDERM, Inc.) (11, 12). During visits 2 and 6, a skin biopsy of the left inner upper arm was taken. Review of adverse events and supplement count/compliance were performed. Any self-reported deviations were documented. Subjects using medications for cardiovascular disease–related disorders (hydrochlorothiazide, aspirin, steroids, ACE inhibitors, beta-blockers, and statins) were excluded from the study. Pregnant females and individuals receiving treatment for being immunocompromised were also not included in the study. The demographics of participating subjects are presented in Table 1.
Table 1.
Subject Demographics
| Parameters | Values |
|---|---|
| Total Subjects | 45 |
| Age (years) | 42.09 ± 1.17 |
| Body weight (kg) | 76.24 ± 2.75 |
| Body mass index (kg/m2) | 29.52 ± 1.42 |
| Race | |
| Caucasian | 38 |
| African American | 6 |
| Asian | 1 |
Dermascopic image processing
A MATLAB (Mathworks Inc.) program code was developed for this study Supplemental data 1. Images from the dermascopic imaging system were processed to multicolor coded images which were used for the detection of skin microperfusion (13–15). Regions of interest (ROIs) were traced and signal intensity was computed. Two-dimensional (2-D) ‘trapz()’ MATLAB function algorithm was used to calculate the area under the curve (AUC) by integrating intensity units over area of interest, which is a measure of total energy over the ROI.
Safety monitoring
No adverse effect was reported that was directly related to the dietary supplement.
Skin biopsy collection
Biopsy site was the left inner arm. Biopsy specimens were taken with a 3 mm punch from the upper inner left arm at week 2 and week 14. Wound care materials and care instructions were provided to the subjects. Due to practical limitations, biopsies could not be collected on visit 1. Biopsy specimens were processed for GeneChip® analysis and mRNA expression using quantitative real-time polymerase chain reaction (RT-PCR).
GeneChip® probe array analyses
GeneChip® analysis was done using Affymetrix Clariom™ D Assay as described previously (16–19) to identify sets of genes differentially expressed in the skin samples at different visits. Briefly, total RNA was isolated using the mirVana Isolation Kit as per the manufacturer’s protocol (Thermo Fisher Scientific) (18, 20, 21). RNA integrity was assessed using the Agilent 2100 Bioanalyzer. The isolated RNA was used to generate ss-cDNA using the GeneChip® WT PLUS reagent kit. Biotin-labeled ss-cDNA was hybridized, washed, and stained on the Affymetrix Fluidics Station 450 according to the manufacturer’s protocol and scanned with the Affymetrix GeneChip Scanner 3000 7 G (16–18) The expression data have been submitted to Gene Expression Omnibus (GEO) at NCBI (http://www.ncbi.nlm.nih.gov/geo/; series accession number GSE114170). Data files were generated and processed with Affymetrix software and Expression Console. Differentially expressed genes were identified using a two-class t test where significance level was set at p < 0.05 with Benjamini-Hochberg correction for false discovery rate (16, 18). Significantly differentially regulated coding genes were subjected to functional analysis using Ingenuity® Pathway Analysis as previously described (22).
Validation of microarray results using quantitative RT-PCR
For gene expression, total cDNA was synthesized using the SuperScript III First Strand Synthesis System (Thermo Fisher Scientific) (23). Candidate genes were verified by RT-PCR by using SYBR green-I and primers as previously described using β-actin as a housekeeping gene (20, 21, 24–27).
Statistical analysis
Data analysis was performed in a blinded fashion. Since fold-change values used for statistical analysis were highly skewed and non-normal, the values were transformed using natural logarithm. The transformed values were then used for all subsequent analyses. Paired two-tailed t tests were used to determine significant differences across baseline (or visit 2 for RT-PCR) and final visit (visit 6) for each subject in all the groups: Placebo, S125, and S250. Next, to detect the efficiency of the treatment with respect to the placebo, two-sample one-tailed t tests were used on the log-transformed fold-change values from the final visit in each group. p < 0.05 was considered statistically significant.
Results
Shilajit supplementation increased skin microperfusion
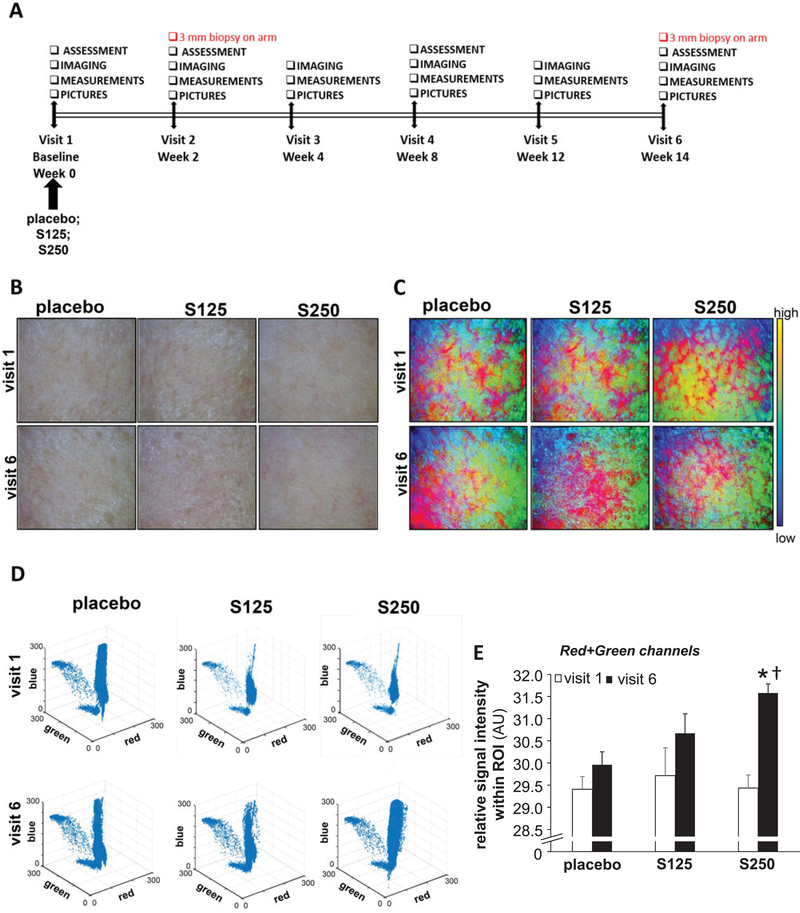
Dermascopic images were used to determine whether shilajit supplementation (Figure 1A) had any effect on skin blood microperfusion. Increased reddish hue of the skin as detected using MATLAB color-coded images indicated improved skin microperfusion (Figure 1B-E). Interestingly, oral Shilajit increased skin redness at a 250 mg dose. However, shilajit did not influence skin perfusion at a 125 mg dose (Figure 1).
Figure 1.
Shilajit improves skin microperfusion. A, Study design. B, Dermascopic images of the cheek. C, MATLAB multicolor coded dermascopic images. D, 3-D scatterplot of the Visible Bands of MATLAB processed dermascopic images. E, The sum of the area under the curve of red and green channels were plotted graphically. The intensity of the red and green channels was calculated from the multicolor images processed by MATLAB software from the raw dermascopic images. S125 represents shilajit 125 mg and S250 represents shilajit 250 mg. Data are mean ± SEM (n = 13). *p < 0.05 compared to the baseline visit. †p < 0.05 compared to placebo.
Transcriptome profiling of skin following oral shilajit supplementation
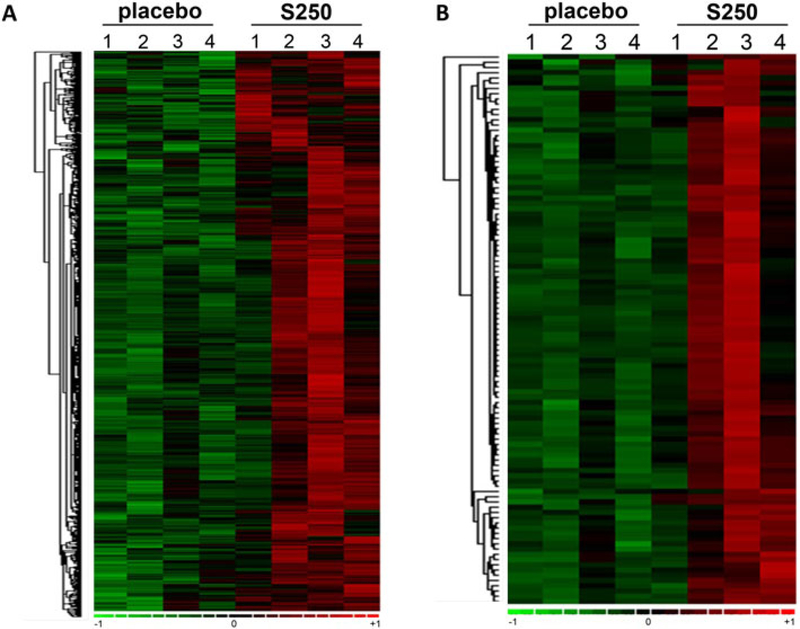
Skin samples were collected at visit 2 and visit 6. RNA extraction and target labeling were done, and GeneChip® data analysis was performed using Affymetrix Clariom™ D Assay (28) as described previously (16, 17) to determine the changes in the transcriptome of skin in response to oral shilajit supplementation. The high-resolution array design contains more than 6.0 million probes including coding transcripts and non-coding transcripts of which 70% cover exons for coding transcripts, and the remaining 30% cover exon-exon splice junctions and non-coding transcripts (28). A total of ~ 5000 annotated probe sets were differentially (p < 0.05) regulated following 14-week supplementation (250 mg bid) as compared to placebo (Figure 2).
Figure 2.
Heat map illustrating cluster of transcripts sensitive to shilajit supplementation (250 mg bid). Shilajit-sensitive transcripts were subjected to hierarchical clustering. A, Heat map illustrating cluster of transcripts that were upregulated upon shilajit supplementation. B, Heat map (top 100 candidates) demonstrating cluster of transcripts that were upregulated upon shilajit supplementation.
Pathway analysis and validation using RT-PCR
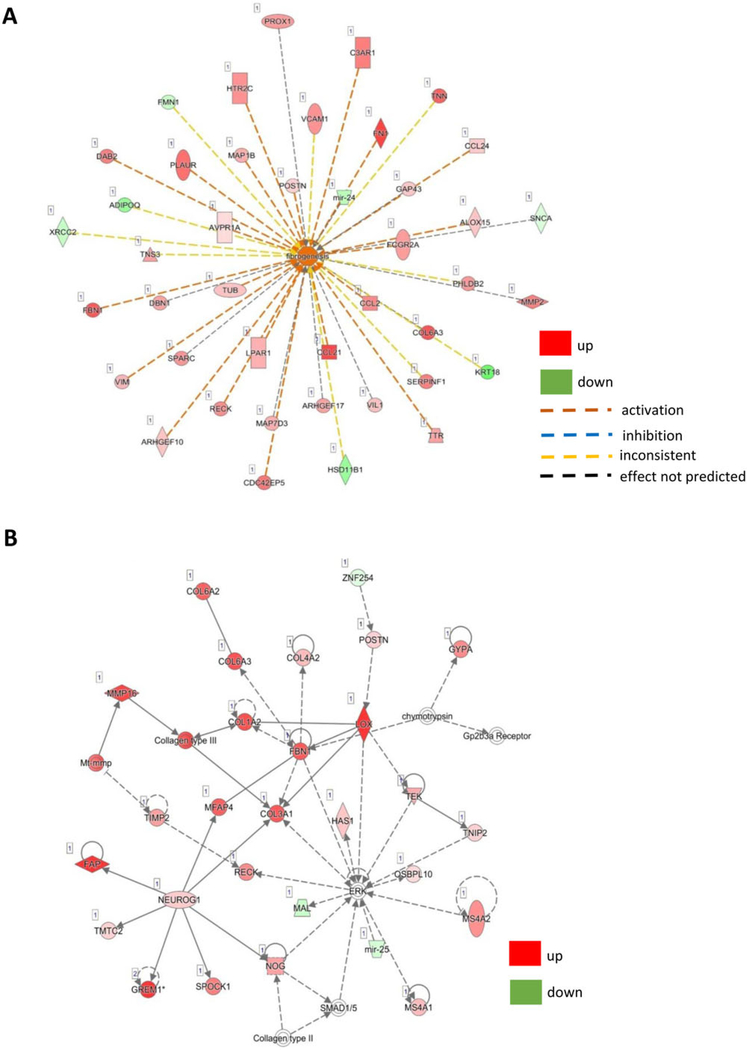
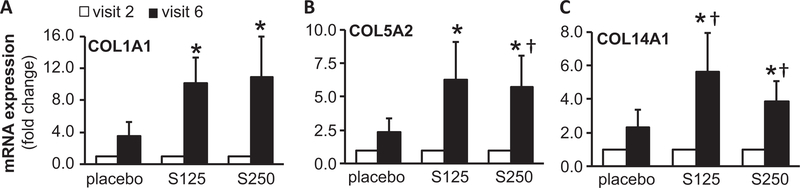
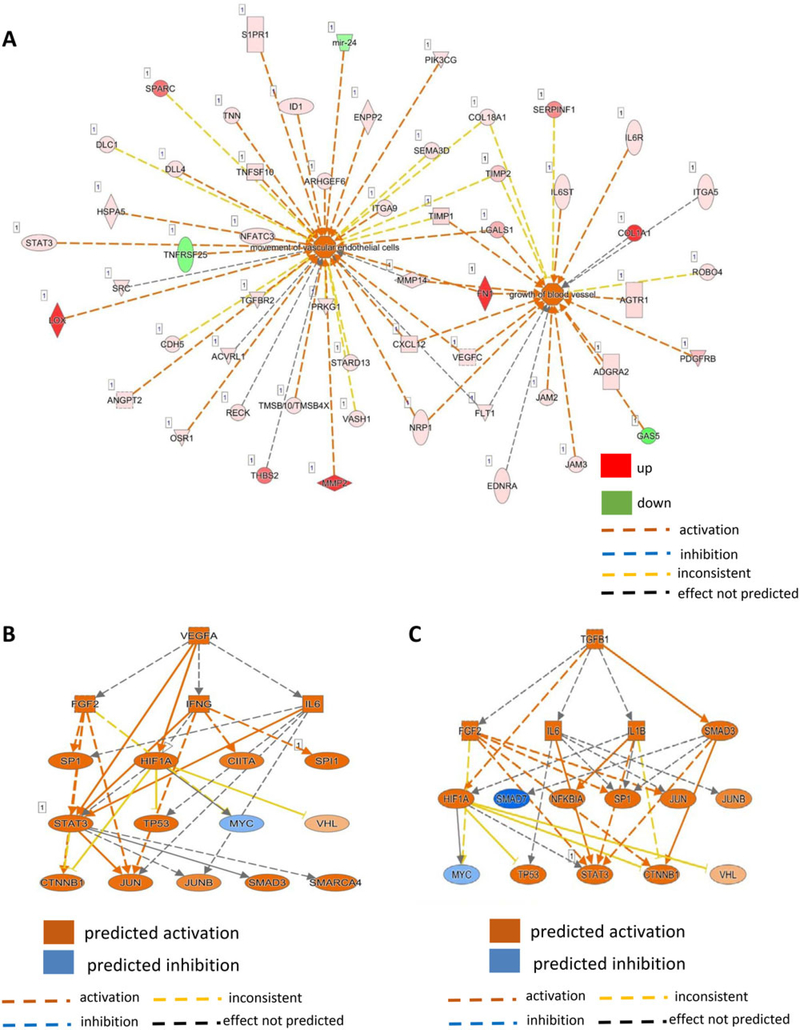
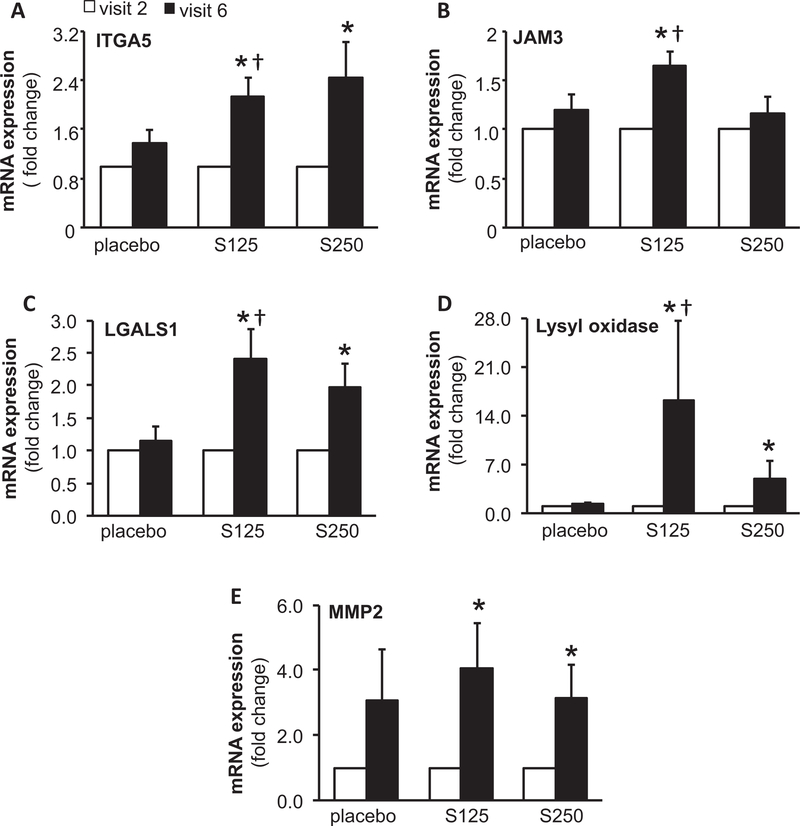
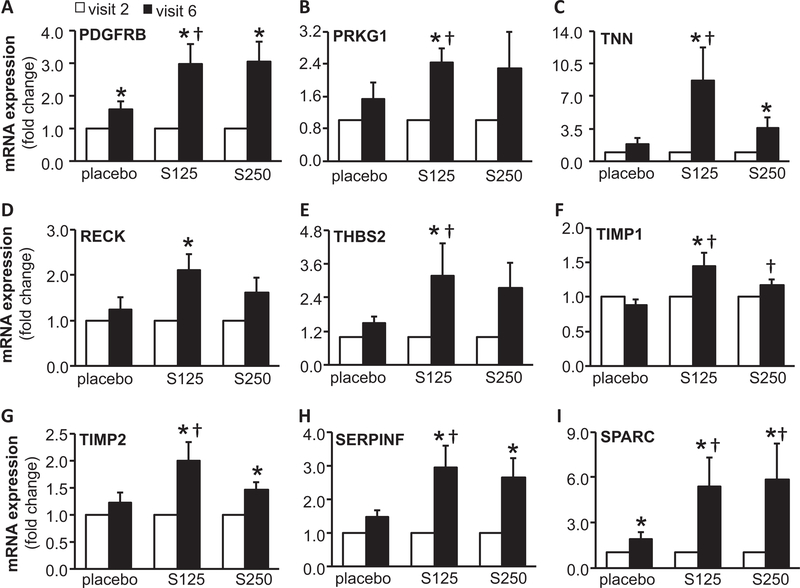
Ingenuity® Pathway Analysis (IPA®) is a powerful analysis and search tool that assists in evaluating the significance of “omics” data identifying novel mechanistic pathways. IPA analysis identified an extracellular matrix (ECM)–related cluster of probe sets that was significantly upregulated in visit 6 of the shilajit-supplemented group as compared to visit 6 of the placebo group (Figure 3). Among these upregulated genes, Col1A1, Col5A2, and Col14A1 were found to be significantly increased following shilajit supplementation as verified using quantitative RT-PCR (Figure 4). In addition, IPA analysis between visit 2 and visit 6 of the shilajit-supplemented group (250 mg bid) revealed upregulated genes involved in growth of blood vessels and movement of vascular endothelial cells upon shilajit supplementation (Figure 5A). TGFb1 and VEGFA path of vascularization was induced by shilajit supplementation (Figure 5B and C). These genes involved in the growth of blood vessels and endothelial cell migration included ITGA5, JAM3, LGALS1, LOX, MMP2, PDGFRB, PRKG1, RECK, SERPINF, SPARC, THBS2, TIMP1, TNN, and TIMP2. The expression of these genes was verified using quantitative RT-PCR (Figures 6 and 7) and was found to be upregulated in response to shilajit supplementation.
Figure 3.
A and B, Ingenuity pathway analysis (IPA) showing that the supplementation of shilajit induces ECM-related genes.
Figure 4.
RT-PCR validation of ECM-related genes following oral shilajit supplementation. Expression levels of selected genes identified by IPA were independently verified using quantitative real-time PCR. S125 represents shilajit 125 mg and S250 represents shilajit 250 mg. Data are mean ± SEM (n = 10–13). *p < 0.05 compared to visit 2. †p < 0.05 compared to placebo.
Figure 5.
Ingenuity pathway analysis (IPA) showing that the supplementation of shilajit increases genes involved in the (A) movement of endothelial cells and growth of blood vessels through the (B) VEGFA and (C) TGFβ1 pathway.
Figure 6.
RT-PCR validation of genes related to movement of endothelial cells following oral shilajit supplementation. Expression levels of selected genes identified by IPA were independently verified using quantitative real-time PCR. S125 represents shilajit 125 mg and S250 represents shilajit 250 mg. Data are mean ± SEM (n = 10–13). *p < 0.05 compared to visit 2. †p < 0.05 compared to placebo.
Figure 7.
RT-PCR validation of genes related to growth of blood vessels following oral shilajit supplementation. Expression levels of selected genes identified by IPA were independently verified using quantitative real-time PCR. S125 represents shilajit 125 mg and S250 represents shilajit 250 mg. Data are mean ± SEM (n = 10–13). *p < 0.05 compared to visit 2. †p < 0.05 compared to placebo.
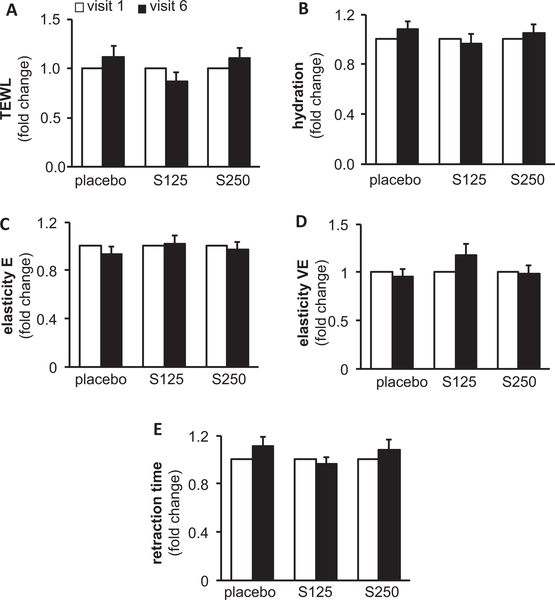
Shilajit supplementation did not adversely affect skin properties
Digital macrophotography and dermascopic imaging were performed on both left and right cheeks using the DermaLab Combo® device to determine the effect of shilajit supplementation on the properties of the skin. Trans-epidermal water loss (TEWL), an index for skin barrier function, surface electrical capacitance for skin hydration, and skin elasticity for tissue stiffness were measured to assess the effect of shilajit supplementation on skin. Analysis of TEWL revealed that there was no difference in the skin integrity in the treated groups compared to the placebo group (Figure 8), indicating that the supplement intake did not affect the barrier function of the skin. Similarly, there was no significant difference in the hydration, elasticity, viscoelasticity, and retraction time (Figure 8), establishing that shilajit supplementation did not adversely affect the quality of the skin. Thus, shilajit supplementation was safely tolerated.
Figure 8.
Shilajit is safe for facial skin. A, TEWL; B, hydration; C, elasticity (E); D, viscoelasticity (VE); and E, retention time was measured using Dermalab combo®. S125 represents shilajit 125 mg and S250 represents shilajit 250 mg. Data are mean ± SEM (n = 13–14).
Discussion
Skin microcirculation has a major thermoregulatory function, a nutritional role, and implications in cutaneous aging (29). A decrease in dermal vascularity is commonly encountered during cutaneous aging (7). Compromised circulation in the skin may cause cosmetic defects like unattractive skin tone and discoloration (8). The aged skin suffers from compromised sympathetic reflex as manifested by inability to vasoconstrict or vasodilate in response to changes in environmental temperature (30). In the cosmetic market, nutraceuticals play a major role (31–34). However, the scientific literature on the mechanism of action of such off-the-shelf nutraceuticals is scanty (35–37). Human studies must test not only efficacy but also safety of nutraceuticals because use of inappropriate supplements is known to pose risk to human health (38, 39). In this work, supplementation of shilajit to middle aged women for 14 weeks did not result in any reported adverse effects. Furthermore, basic skin function tests demonstrated no adverse effect of shilajit on skin health in middle-aged women. Our findings are consistent with previous findings that have demonstrated the safety of shilajit (40, 41).
The dietary supplement L-arginine improved microperfusion of the rat skin (42). Consistently, transdermal delivery of L-arginine improved cutaneous blood flow in the feet of diabetic patients (43). Significant improvement of reddish coloration of the face under standardized testing conditions provided the first line of evidence suggesting that shilajit may improve skin microcirculation. Follow-up on this line of finding was performed by collection of skin biopsy and unbiased query of the human skin transcriptome. Additional validation of the findings of such screening was performed using quantitative polymerase chain reaction (PCR). Ingenuity pathway analyses of GeneChip® data identified genes related to endothelial cell migration and growth of blood vessels, being significantly induced in response to shilajit supplementation. Both of these functions are known to be directly responsible for skin angiogenesis (44). Statistical treatment of large data sets such as those acquired by GeneChip® include conservative measures to manage risks of false discovery (16, 18, 45). Using such conservative analyses and quantitative PCR analyses, it was noted that shilajit was effective in the induction of such genes. Angiogenesis is a well-regulated physiological process that includes blood vessel formation followed by timely regression (46, 47). Consistent with that notion, the current work reports the induction of angiogenic mediators as well as anti-angiogenic pathways that are necessary for regression. Interestingly, the application of rigorous quantitative PCR revealed that shilajit was more effective at the lower dosage studied (125 mg bid). Such dose–effect response is not uncommon, as reported previously (48–51).
ECM provides scaffold support as well as biochemical cues for skin cells to grow and is recognized as a major determinant of skin health (52). Age-related decay of ECM accounts for skin thinning and wrinkles (7). In the current study, upregulation of the ECM genes, Col1A1, Col5A2, and Col14A1 was statistically significant upon shilajit supplementation. Collagens constitute a major component of skin ECM and provide structural support as well as molecular signals to resident cells (27, 52). A recent study addressing the mechanism of action of shilajit in improving human skeletal muscle adaptation to exercise provided first evidence on shilajit-induced expression of ECM-related genes (18). Shilajit supplementation significantly increased ECM-related gene expression in overweight/class I obese human subjects. is evident across independent studies that when taken orally Of interest, exercise and shilajit were synergistic in shilajit modifies organ ECM composition. In the skeletal upregulating collagen and other ECM proteins (18). Thus, it muscle, ECM is known to play an essential role in the development, maintenance, and regeneration of the organ well as ECM-related genes is of particular significance (53, 54). The observation that shilajit induced genes involved because these two functional domains of tissue biology are in growth of blood vessels and endothelial cell migration as known to be directly related (55, 56).
Conclusion
The current study provides interesting insight into the mechanism of action of shilajit on human skin health. The findings of this work therefore lays the rationale for further mechanistic studies addressing shilajit-inducible signaling pathways that are common to both vascular and ECM function.
Supplementary Material
Acknowledgment
Parts of this work were supported by an R01 NS085272 and research grant by Natreon Inc, NJ, USA.
Funding
Parts of this work were supported by an R01 NS085272 and research grant by Natreon Inc, NJ, USA.
Footnotes
Supplemental material for this article can be accessed at https://doi.org/10.1080/07315724.2018.1564088
References
- 1.Agarwal SP, Khanna R, Karmarkar R, Anwer MK, Khar RK. Shilajit: a review. Phytother Res. 2007;21(5):401–405. [DOI] [PubMed] [Google Scholar]
- 2.Wilson E, Rajamanickam GV, Dubey GP, Klose P, Musial F, Saha FJ, Rampp T, Michalsen A, Dobos GJ. Review on shilajit used in traditional Indian medicine. J Ethnopharmacol. 2011; 136(1):1–9. [DOI] [PubMed] [Google Scholar]
- 3.Schepetkin Igor A., Xie Gang, Jutila Mark A., and Quinn Mark T.. Complement-fixing Activity of Fulvic Acid from Shilajit and Other Natural Sources. Phytother Res. 2009; 23(3): 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stohs SJ. Safety and efficacy of shilajit (mumie, moomiyo). Phytother Res. 2014;28(4):475–479. [DOI] [PubMed] [Google Scholar]
- 5.Stohs SJ, Singh K, Das A, Roy S, Sen CK. Energy and Health Benefits of Shilajit. Sustained Energy for Enhanced Human Functions and Activity. 2017;28:187–204. [Google Scholar]
- 6.Konno M, Hamabe A, Hasegawa S, Ogawa H, Fukusumi T, Nishikawa S, Ohta K, Kano Y, Ozaki M, Noguchi Y, Sakai D, Kudoh T, Kawamoto K, Eguchi H, Satoh T, Tanemura M, Nagano H, Doki Y, Mori M, Ishii H. Adipose-derived mesenchymal stem cells and regenerative medicine. Develop Growth Differ. 2013;55(3):309–318. doi: 10.1111/dgd.12049. [DOI] [PubMed] [Google Scholar]
- 7.Amirkhani MA, Shoae-Hassani A, Soleimani M, Hejazi S, Ghalichi L, Nilforoushzadeh MA. Rejuvenation of facial skin and improvement in the dermal architecture by transplantation of autologous stromal vascular fraction: a clinical study. Bioimpacts. 2016;6(3):149–154. doi: 10.15171/bi.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waller JM, Maibach HI.. Age and skin structure and function, a quantitative approach (I): blood flow, pH, thickness, and ultrasound echogenicity. Skin Res Technol. 2005;11(4):221–235. [DOI] [PubMed] [Google Scholar]
- 9.Ghosal S, Reddy JP, Lal VK. Shilajit I: chemical constituents. J Pharm Sci. 1976;65(5):772–773. [DOI] [PubMed] [Google Scholar]
- 10.Meena H, Pandey HK, Arya MC, Ahmed Z. Shilajit: A panacea for high-altitude problems. Int J Ayurveda Res. 2010;1(1):37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed NS, Ghatak S, El Masry MS, Gnyawali SC, Roy S, Amer M, Everts H, Sen CK, Khanna S. Epidermal E-Cadherin Dependent beta-Catenin Pathway Is Phytochemical Inducible and Accelerates Anagen Hair Cycling. Mol Ther. 2017;25(11): 2502–2512. doi: 10.1016/j.ymthe.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghatak S, Chan YC, Khanna S, Banerjee J, Weist J, Roy S, Sen CK. Barrier Function of the Repaired Skin Is Disrupted Following Arrest of Dicer in Keratinocytes. Mol Ther. 2015; 23(7):1201–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silveira M, Nascimento JC, Marques JS, Marcal ARS, Mendonca T, Yamauchi S, Maeda J, Rozeira J. Comparison of Segmentation Methods for Melanoma Diagnosis in Dermoscopy Images. Ieee J Sel Topics Signal Process. 2009;3(1):35–45. [Google Scholar]
- 14.Barata C, Celebi ME, Marques JS. Improving Dermoscopy Image Classification Using Color Constancy. Ieee J Biomed Health Inform. 2015;19(3):1146–1152. [DOI] [PubMed] [Google Scholar]
- 15.Yu LQ, Chen H, Dou Q, Qin J, Heng PA. Automated Melanoma Recognition in Dermoscopy Images via Very Deep Residual Networks. Ieee Trans Med Imag. 2017;36(4):994–1004. [DOI] [PubMed] [Google Scholar]
- 16.Roy S, Khanna S, Rink C, Biswas S, Sen CK. Characterization of the acute temporal changes in excisional murine cutaneous wound inflammation by screening of the wound-edge transcriptome. Physiol Genomics. 2008;34(2):162–184. doi: 10.1152/physiolgenomics.00045.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roy S, Patel D, Khanna S, Gordillo GM, Biswas S, Friedman A, Sen CK. Transcriptome-wide analysis of blood vessels laser captured from human skin and chronic wound-edge tissue. Proc Natl Acad Sci USA. 2007;104(36):14472–14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das A, Datta S, Rhea B, Sinha M, Veeraragavan M, Gordillo G, Roy S. The Human Skeletal Muscle Transcriptome in Response to Oral Shilajit Supplementation. J Med Food. 2016;19(7): 701–709. doi: 10.1089/jmf.2016.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das A, Ghatak S, Sinha M, Chaffee S, Ahmed NS, Parinandi NL, Wohleb ES, Sheridan JF, Sen CK, Roy S. Correction of MFG-E8 Resolves Inflammation and Promotes Cutaneous Wound Healing in Diabetes. JI. 2016;196(12):5089–5100. doi: 10.4049/jimmunol.1502270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das A, Ganesh K, Khanna S, Sen CK, Roy S. Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. J Immunol. 2014;192(3): 1120–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das A, Datta S, Roche E, Chaffee S, Jose E, Shi L, Grover K, Khanna S, Sen CK, Roy S. Novel mechanisms of Collagenase Santyl Ointment (CSO) in wound macrophage polarization and resolution of wound inflammation. Sci Rep. 2018;8(1):1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh K, Pal D, Sinha M, Ghatak S, Gnyawali SC, Khanna S, Roy S, Sen CK. Epigenetic Modification of MicroRNA-200b Contributes to Diabetic Vasculopathy. Mol Ther. 2017;25(12): 2689–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Ghatak S, El Masry MS, Das A, Liu Y, Roy S, Lee RJ, Sen CK. Topical Lyophilized Targeted Lipid Nanoparticles in the Restoration of Skin Barrier Function following Burn Wound. Mol Ther. 2018; 26(9):2178–2188. doi: 10.1016/j.ymthe.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinha M, Sen CK, Singh K, Das A, Ghatak S, Rhea B, Blackstone B, Powell HM, Khanna S, Roy S. Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue. Nat Commun. 2018;9(1):936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barki KG, Das A, Dixith S, Ghatak PD, Mathew-Steiner S, Schwab E, Khanna S, Wozniak DJ, Roy S, Sen CK. Electric Field Based Dressing Disrupts Mixed-Species Bacterial Biofilm Infection and Restores Functional Wound Healing. Ann Surg. 2019;269(4):756–766.. doi: 10.1097/SLA.0000000000002504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganesh K, Das A, Dickerson R, Khanna S, Parinandi NL, Gordillo GM, Sen CK, Roy S. Prostaglandin E(2) induces oncostatin M expression in human chronic wound macrophages through Axl receptor tyrosine kinase pathway. J Immunol. 2012; 189(5):2563–2573. doi: 10.4049/jimmunol.1102762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Masry MS, Chaffee S, Das Ghatak P, Mathew-Steiner SS, Das A, Higuita-Castro N, Roy S, Anani RA, Sen CK. Stabilized collagen matrix dressing improves wound macrophage function and epithelialization. Faseb J. 2018;33(2):2144–2155;fj201800352R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Affymetrix. http://www.affymetrix.com/catalog/prod760002/AFFY/Human-Transcriptome-Array-2.0#1_1, accesses on August 09, 2015 [cited 2015 Aug 09].
- 29.Kobayashi H, Tagami H. Distinct locational differences observable in biophysical functions of the facial skin: with special emphasis on the poor functional properties of the stratum corneum of the perioral region. Int J Cosmet Sci. 2004;26(2): 91–101. doi: 10.1111/j.0412-5463.2004.00208.x. [DOI] [PubMed] [Google Scholar]
- 30.Holowatz LA, Thompson-Torgerson C, Kenney WL. Aging and the control of human skin blood flow. Front Biosci (Landmark Ed). 2010;15718–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nusgens BV, Humbert P, Rougier A, Colige AC, Haftek M, Lambert CA, Richard A, Creidi P, Lapiere CM. Topically applied vitamin C enhances the mRNA level of collagens I and III, their processing enzymes and tissue inhibitor of matrix metalloproteinase 1 in the human dermis. Journal of Investigative Dermatology. 2001;116(6): 853–859. doi: 10.1046/j.0022-202x.2001.01362.x. [DOI] [PubMed] [Google Scholar]
- 32.Guillerme JB, Couteau C, and Coiffard L, Applications for Marine Resources in Cosmetics. Cosmetics. 2017;4:35. [Google Scholar]
- 33.Lee J, Koo N, Min DB. Reactive oxygen species, aging, and antioxidative nutraceuticals. Comp Rev Food Sci Food Safety. 2004; 3(1):21–33. doi: 10.1111/j.1541-4337.2004.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 34.Villasenor IM, Simon MKB, Villanueva AMA. Comparative potencies of nutraceuticals in chemically induced skin tumor prevention. Nutrition and Cancer-an International Journal. 2002; 44(1):66–70. doi: 10.1207/S15327914NC441_9. [DOI] [PubMed] [Google Scholar]
- 35.Bernal J, Mendiola JA, Ibanez E, Cifuentes A. Advanced analysis of nutraceuticals. Journal of Pharmaceutical and Biomedical Analysis. 2011;55(4):758–774. doi: 10.1016/j.jpba.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 36.Baumann L. Skin ageing and its treatment. J Pathol. 2007;211(2): 241–251. [DOI] [PubMed] [Google Scholar]
- 37.Perez-Sanchez A, Barrajon-Catalan M. Herranz-Lopez E, Micol V. Nutraceuticals for Skin care: a comprehensive review of human clinical studies. Nutrients. 2018;10(4): 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Notaroberto P, T BM. issues concerning safety of topical cosmetics and nutraceuticals, in Daily Routine in Cosmetic Dermatology, Issa M. and Tamura B, Editors., New York: Springer; 2017 [Google Scholar]
- 39.Nohynek GJ, Antignac E, Re T, Toutain H. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol Appl Pharmacol. 2010;243(2):239–259. doi: 10.1016/j.taap.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Biswas TK, Pandit S, Mondal S, Biswas SK, Jana U, Ghosh T, Tripathi PC, Debnath PK, Auddy RG, Auddy B. Clinical evaluation of spermatogenic activity of processed Shilajit in oligospermia. Andrologia. 2010;42(1):48–56. doi: 10.1111/j.1439-0272.2009.00956.x. [DOI] [PubMed] [Google Scholar]
- 41.Sharma P, Jha J, Shrinivas V, Dwivedi LK, Suresh P, Sinha M. Shilajit: evalution of its effects on blood chemistry of normal human subjects. Anc Sci Life. 2003;23(2):114–119. [PMC free article] [PubMed] [Google Scholar]
- 42.Ohta F, Takagi T, Sato H, Ignarro LJ. Low-dose L-arginine administration increases microperfusion of hindlimb muscle without affecting blood pressure in rats. Proc Natl Acad Sci U S A. 2007;104(4):1407–1411. doi: 10.1073/pnas.0610207104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fossel ET. Improvement of temperature and flow in feet of subjects with diabetes with use of a transdermal preparation of Larginine: a pilot study. Diabetes Care. 2004;27(1):284–285. doi: 10.2337/diacare.27.1.284. [DOI] [PubMed] [Google Scholar]
- 44.Norton KA, Popel AS. Effects of endothelial cell proliferation and migration rates in a computational model of sprouting angiogenesis. Scientific Reports. 2016;6:36992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng C, Pounds S. False discovery rate paradigms for statistical analyses of microarray gene expression data. Bioinformation. 2007;1(10):436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fried LE, Arbiser JL. Application of angiogenesis to clinical dermatology. Adv Dermatol. 2008;2489–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gnyawali SC, Barki KG, Mathew-Steiner SS, Dixith S, Vanzant D, Kim J, Dickerson JL, Datta S, Powell H, Roy S, Bergdall V, Sen CK. High-resolution harmonics ultrasound imaging for non-invasive characterization of wound healing in a pre-clinical swine model. PLoS One. 2015;10(3):e0122327. doi: 10.1371/journal.pone.0122327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tapas AR, Sakarkar DM, Kakde RB. Flavonoids as Nutraceuticals: A Review. Tropical Journal of Pharmaceutical Research. 2008;7(3): 1089–1099. [Google Scholar]
- 49.Nigam N, Prasad S, Shukla Y. Preventive effects of lupeol on DMBA induced DNA alkylation damage in mouse skin. Food and Chemical Toxicology. 2007;45(11):2331–2335. doi: 10.1016/j.fct.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Afaq F, Mukhtar H. Botanical antioxidants in the prevention of photocarcinogenesis and photoaging. Exp Dermatol. 2006;15(9): 678–684. doi: 10.1111/j.1600-0625.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 51.Buonocore D, Lazzeretti A, Tocabens P, Nobile V, Cestone E, Santin G, Bottone MG, Marzatico F. Resveratrol-procyanidin blend: nutraceutical and antiaging efficacy evaluated in a placebocontrolled, double-blind study. Clin Cosmet Investig Dermatol. 2012;5159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xue M, Jackson CJ. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care (New Rochelle). 2015;4(3):119–136. doi: 10.1089/wound.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buck CA, Horwitz AF. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3179–205. [DOI] [PubMed] [Google Scholar]
- 54.Purslow PP. The structure and functional significance of variations in the connective tissue within muscle. Comp Biochem Physiol, Part A Mol Integr Physiol. 2002;133(4):947–966. [DOI] [PubMed] [Google Scholar]
- 55.Neve A, Cantatore FP, Maruotti N, Corrado A, Ribatti D. Extracellular matrix modulates angiogenesis in physiological and pathological conditions. Biomed Res Int. 2014;2014:756078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eming SA, Hubbell JA. Extracellular matrix in angiogenesis: dynamic structures with translational potential. Exp Dermatol. 2011;20(7):605–613. doi: 10.1111/j.1600-0625.2011.01309.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.