Abstract
While prominent models of suicidal behavior emphasize the hypothalamic- pituitary-adrenal (HPA) axis dysregulation, studies examining its role have yielded contradictory results. One possible explanation is that suicide attempters are a heterogeneous group and HPA axis dysregulation plays a more important role only in a subset of suicidal individuals. HPA axis dysregulation also plays a role in impulsivity and aggression. We hypothesize subgroups of attempters, based on levels of impulsivity and aggression, will differ in HPA axis dysregulation. We examined baseline cortisol, total cortisol output, and cortisol reactivity in mood disordered suicide attempters (N = 35) and non-attempters (N = 37) during the Trier Social Stress Test. Suicide attempters were divided into four subgroups: low aggression/low impulsivity, high aggression/low impulsivity, low aggression/high impulsivity, and high aggression/high impulsivity. As hypothesized, attempters and non-attempters did not differ in any cortisol measures while stress response differed based on impulsivity/aggression levels in suicide attempters, and when compared to non-attempters. Specifically, attempters with high impulsive aggression had a more pronounced cortisol response compared with other groups. This is the first study to examine the relationship between cortisol response and suicidal behavior in impulsive aggressive subgroups of attempters. These findings may help to identify a stress responsive suicidal subtype of individuals.
Keywords: Suicide, Cortisol, Stress, TSST, Impulsivity, Aggression
1. Introduction
Suicide is a major cause of death in the United States, with over 47,000 lives lost in the annually (https://webappa.cdc.gov/sasweb/ncipc/mortrate.html). The stress-diathesis model of suicide implicates the role of stress in suicidal behavior (Mann, 2003; Mann et al., 2006; Turecki et al., 2012; Oquendo et al., 2014; Rubinstein, 1986). The production of the stress hormone, cortisol, triggered by stress-induced activation of the hypothalamic–pituitary–adrenal (HPA) axis, is thought to be a key. But it remains unclear how stress influences vulnerability to suicidal behavior (van Heeringen, 2012) and which suicidal individuals are more susceptible to stressors. Furthermore, to date, research examining the role of the HPA axis in suicidal behavior has yielded inconsistent and conflicting results suggesting possible subtypes of suicidal individuals-stress reactive and non-stress reactive.
HPA axis dysregulation is typically examined in four ways: baseline levels of cortisol as assessed by a single measure typically under controlled conditions, total output of cortisol in response to a stressor, cortisol responsivity in response to a stressor or a pharmacological intervention. Several studies found an association between low baseline cortisol levels and suicidal behavior (Keilp et al., 2016; Lindqvist et al., 2008; McGirr et al., 2011; Melhem et al., 2016). However, other studies have found that suicidal behavior was associated with elevated baseline levels (Chatzittofis et al., 2013), or that no relationship was present (Eisenlohr-Moul et al., 2018; O’Connor et al., 2017).
A dynamic way to assess reactivity to stress is through a standardized laboratory stress paradigm, such as the Trier Social Stress Test (TSST). The TSST measures stress response through changes in cortisol levels during a laboratory-induced social stressor (Kirschbaum et al., 1993). Two measures of cortisol response are typically derived from the TSST: total output and cortisol responsivity. Total output includes baseline differences and cortisol response during the stress task. Measurement of cortisol responsivity examines cortisol reactivity to the stressor while controlling for baseline group differences. Few studies have investigated the relationship between stress response and suicidal behavior using stress paradigms, but, again the results have been inconsistent (Eisenlohr-Moul et al., 2018; Giletta et al., 2015; Melhem et al., 2016; O’Connor et al., 2017). One study found that young adult suicide attempters who had a parent with a mood disorder had lower total cortisol output during TSST compared with non-attempters and normal control subjects but found no differences in cortisol reactivity (Melhem et al., 2016). Similarly, a study using an alternate stress paradigm, the Maastricht Acute Stress Test (MAST), that includes cold pressor and mental arithmetic tasks, found that suicide attempters had lower total cortisol output compared with the controls, but not compared with current suicidal ideators who never attempted suicide and, similar to Melhem et al. (2016), they found no differences in cortisol reactivity (O’Connor et al., 2017). In a transdiagnostic sample of adolescent females, Eisenlohr-Moul et al. (2018) reported blunted cortisol response during the TSST in those with a history of lifetime suicide attempts compared with those with history of lifetime suicidal ideation but not behavior and with non-ideators non-attempters. All three studies did not control for the number of depressed individuals in the attempter groups compared with the non-attempter groups. It has been widely reported that the HPA axis is implicated in depression (Pariante and Lightman, 2008; Zorn et al., 2017) and, therefore, their findings may have been partially confounded by diagnosis.
While depression as a confound may account for the discrepant findings of HPA axis dysfunction in suicide attempters, another explanation for these inconsistencies is that different patterns of HPA axis reactivity may exist in different subsets of suicidal individuals. Interestingly, Giletta et al. (2015) identified three groups of cortisol stress-response patterns in adolescent females at risk for suicidality: hyporesponsive, normative, and hyperresponsive. They found that individuals in the hyperresponsive group were more likely to report a lifetime history of suicidal ideation at baseline and three months follow-up. Furthermore, impulsivity tended to interact with cortisol responsivity and suicidal ideation. Along these lines, we have postulated that there are at least two suicidal subtypes: stress responsive and non-stress responsive (Bernanke et al., 2017). In our model, the stress responsive subtype is proposed to be more influenced by psychosocial stressors, more likely to respond to those stressors with suicidal thinking and more likely to have reactive (impulsive) aggression. On the other hand, suicidal individuals with proactive aggression are thought to have a hyporesponsivity to stress. Evidence for these suicidal subtypes is supported by studies demonstrating patterns of suicidal thinking – suicidal ideation can be highly variable or more persistent (Witte et al., 2006) and the association of these patterns with stress responsivity (Rizk et al., 2018). Both variable and persistent ideation can result in suicidal behavior (Miranda et al., 2014; Witte et al., 2006). And it is well-known that suicidal behavior itself can be planned or impulsive (Chaudhury et al., 2016; Lopez-Castroman et al., 2016).
Impulsivity and aggression have been associated with both suicidal behavior and HPA axis dysfunction (Mann et al., 1999; McGirr and Turecki, 2007; Melhem et al., 2007; Turecki et al., 2012). Although often subsumed into a single dimension of ‘impulsive aggression’ in research, the two are distinct constructs. Impulsiveness is defined as spontaneous, poorly planned or situationally inappropriate behaviors that may or may not include aggressive behaviors, and aggression, in turn, may be premeditated or impulsive. One study using the combined construct found that youth high in reactive aggression had heightened cortisol response following a stress test, while those high in deliberate aggression or non-aggression did not (Lopez-Duran et al., 2009). Suicidality was not assessed. Other studies confirm the relationship between aggression, impulsivity, and cortisol levels (Poustka et al., 2010; Van Bokhoven et al., 2005). Thus, individuals high in impulsivity and aggression are more reactive to environmental stressors and given that impulsivity and aggression are associated with HPA axis dysfunction, suicidal individuals with elevated impulsivity and aggression may represent a stress responsive subtype of suicidal behavior.
In this study, we propose that stress responsiveness to the TSST in mood disordered suicide attempters will depend on the level of participants’ impulsivity and aggression. Further, we expect that there will be no difference in baseline cortisol levels, cortisol total output and cortisol reactivity between mood disordered attempters and mood disordered non-attempters. We hypothesize that 1. suicide attempters high in impulsivity and aggression will have a more pronounced response to the TSST, as measured by cortisol levels, compared to suicide attempters with lower levels of impulsivity and aggression; 2. baseline cortisol levels will not differ among the suicide attempter groups; and 3. total output will not differ among the suicide attempter groups given that we expect no baseline differences and total output is influenced by baseline levels.
2. Methods
2.1. Participants
Seventy-two participants were enrolled in a study of mood disorder and suicidal behavior after receiving a detailed description of study procedures and providing written informed consent. The study was approved by the Institutional Review Board at the New York State Psychiatric Institute. Participants were recruited through advertisements and postings. Individuals with a history of psychotic disorders or current substance dependence were excluded. Demographic and clinical information is provided in Table 1. The mean age of participants was 31.9 ± 10.4. The sample was predominantly female and Caucasian with an average of 2 years of college education. All had a diagnosis of mood disorder: 81% (58) Major Depressive Disorder (MDD); 15% (11) Bipolar Disorder; 3% (2) Dysthymic Disorder; and 1% (1) Depressive Disorder Not Otherwise Specified. Approximately half of the sample (49%, N = 35) had a history of at least 1 suicide attempt.
Table 1.
Demographic and clinical characteristics of attempters and non-attempters.
| Variables | Overall Sample | Attempters (N = 35) | Non-Attempters (N = 37) | Statistic X2 | p value | |||
| n | % | n | % | n | % | |||
| Female | 57 | 79% | 30 | 86% | 27 | 73% | 1.98 | 0.1833 |
| Psychotropic Medication | 33 | 46% | 15 | 43% | 18 | 49% | 0.24 | 0.6220 |
| Smoking Status | 18 | 26% | 9 | 26% | 9 | 25% | 0.02 | 0.8881 |
| Mean | SD | Mean | SD | Mean | SD | t | ||
| Age | 31.88 | 10.42 | 31.00 | 11.22 | 32.80 | 9.59 | −0.73 | 0.4678 |
| Education (yrs) | 14.43 | 2.00 | 14.68 | 1.65 | 14.18 | 2.31 | 1.01 | 0.3156 |
| BMI | 26.33 | 6.62 | 25.19 | 6.37 | 27.33 | 6.77 | 1.30 | 0.1999 |
| CTQ Emotional Abuse | 12.40 | 6.02 | 11.77 | 5.74 | 14.44 | 6.82 | −1.17 | 0.2495 |
| CTQ Physical Abuse | 10.17 | 5.93 | 9.83 | 5.63 | 11.33 | 7.09 | −0.66 | 0.5105 |
| CTQ Sexual Abuse | 8.10 | 6.16 | 7.40 | 5.69 | 10.44 | 7.42 | −1.31 | 0.1976 |
| CTQ Emotional Neglect | 13.01 | 6.23 | 12.88 | 6.19 | 13.44 | 6.73 | −0.24 | 0.8137 |
| CTQ Physical Neglect | 8.54 | 4.19 | 7.97 | 3.47 | 10.44 | 5.88 | −1.20 | 0.2572 |
| BGAH | 19.93 | 5.79 | 18.84 | 6.07 | 21.18 | 5.37 | −1.71 | 0.0913 |
| BIS | 55.06 | 18.11 | 55.00 | 17.82 | 54.66 | 19.76 | 0.08 | 0.9385 |
| HDRS-17 | 10.97 | 6.48 | 10.08 | 6.59 | 11.91 | 6.33 | −1.20 | 0.2330 |
| BDI | 17.40 | 11.11 | 15.38 | 11.05 | 19.67 | 10.91 | −1.63 | 0.1076 |
| BHS | 8.01 | 5.56 | 6.41 | 5.02 | 10.00 | 5.63 | −2.76 | 0.0075* |
| ALS | 68.70 | 41.85 | 71.46 | 44.42 | 59.83 | 32.81 | 0.72 | 0.4740 |
Abbreviations: BMI, Body Mass Index; CTQ, Childhood Trauma Questionnaire; BGAH, Brown-Goodwin Lifetime History of Aggressive Behavior; BIS, Barratt Impulsivity Scale; HDRS-17, 17-Item Hamilton Depression Rating Scale; BDI, Beck Depression Inventory; BHS, Beck Hopelessness Scale; ALS, Affective Lability Scale.
p< 0.01.
2.2. Assessments
Trained masters and doctoral level clinicians administered all baseline assessments. Lifetime Axis I DSM-IV diagnoses were established for all participants using the Structured Clinical Interview for DSM-IV(Spitzer et al., 1995). Axis II diagnoses were assessed using the Structured Clinical Interview for DSM-IV Personality Disorders (First et al., 1995). Suicide attempt history was obtained using the Columbia Suicide History Form (Oquendo et al., 2003). Inter-rater reliability was found to be high between assessors for both the SCID and the Columbia Suicide History Form (ICC=0.97 for Columbia Suicide History Form and ICC= 0.86 for the SCID-I).
The Barratt Impulsivity Scale (BIS) (Barratt, 1985), a self-report scale, was used to measure impulsivity. Aggression was assessed with the Brown-Goodwin Lifetime History of Aggressive Behavior (BGAH) (Brown and Goodwin, 1986). Inter-rater reliability was found to be high between assessors for the BGAH (ICC=0.94) and internal consistency for BIS has been documented as high for general psychiatric patients (Cronbach’s alpha=0.83) (Barratt, 1985).
Possible confounding effects of clinical measures known for their association with HPA axis reactivity have been examined. These include history of childhood adversity (Heim et al., 2008), current depression severity (Meador-Woodruff et al., 1990), hopelessness (Engstrom et al., 1997; Juruena et al., 2009), and affective instability (Braquehais et al., 2012).
Childhood histories of abuse and neglect were obtained using the Childhood Trauma Questionnaire (CTQ) (Bernstein et al., 1994). Cronbach’s alpha for the CTQ subscales ranged from 0.79 to 0.94, indicating high internal consistency and has also demonstrated good test-retest reliability over a 2- to 6-month interval (intraclass correlation = 0.88), which has shown good internal reliability and validity (Bernstein et al., 1994).
Depression severity was measured with the clinician-administered 17-item Hamilton Depression Rating Scale (HDRS-17) (Hamilton, 1960) and the self-report Beck Depression Inventory (BDI) (Beck et al., 1996). HDRS has shown internal reliability ranging from 0.46 to 0.97, an interrater reliability of 0.82 to 0.98, and a test–retest reliability of 0.81 to 0.98 across 70 different studies (reviewed in (Bagby et al., 2004)). Internal consistency of BDI has been reported to be high (Cronbach’s alpha = 0.88) (Beck and Steer, 1984).
Hopelessness was assessed with the Beck Hopelessness Scale (BHS) (Beck et al., 1974), which showed high internal consistency (Cronbach’s alpha=0.93) (Beck et al., 1974) and reliability ranging from 0.83 to 0.86 in psychiatric populations (Durham, 1982). Affect instability was measured using the Affective Lability Scale (ALS) (Moore et al., 1997), which showed high internal consistency with Cronbach’s Alpha scores ranging between 0.82–0.92 in different populations (Aas et al., 2015).
2.3. Trier Social Stress Test (TSST)
The TSST is a well-established procedure used to study psychological and physiological indices of stress response (Kirschbaum et al., 1993). In this version, the participant was asked to give a five-minute speech about him or herself, followed by 9 min of a speeded mental arithmetic task. The test administrator and a confederate, both dressed in white lab coats observed the participant with neutral expressions, responding “Please continue,” when there was a long pause. The procedure was performed late afternoon for each participant to improve the strength of signal to baseline ratio due to the TSST procedure (Kudielka et al., 2004). Saliva samples were obtained at several time points over the course of the TSST: 10 min before the procedure (baseline) and then at four intervals after baseline: 15, 20, 30, and 40 min. Subjective mood ratings were collected using the Profile of Mood States (POMS) (McNair et al., 1971/1981) at −10, 15, and 40 min. Prior to beginning the TSST procedures, individuals were given a 30-min rest period in a private, quiet room.
2.4. Salivary samples collection and assay
Saliva was collected via the Sarstedt Salivette Synthetic Swab saliva collection system (Catalogue # 51.1534.500 Sarstedt, Newton, NC 28658, USA). Participants were required to leave the cotton swab in their mouth for 3–5 min to obtain an adequate sample. Swabs were then sealed in the plastic cover tube of the salivette, and placed in a −20° freezer within two hours. Samples were shipped on dry ice to the New York State Psychiatric Institute’s central reference laboratory in Orangeburg, NY where they were assayed.
Salivary cortisol was measured by radioimmunoassay. Primary antibodies raised against cortisol-3-O-carboxymethyloxime-BSA and iodine labeled cortisol were purchased from MP Biomedicals. Cortisol standards were purchased from Sigma Chemical, Anti rabbit globulin serum in conjunction with polyethylene glycol was used for separation of the bound and free fractions. All samples and standards were analyzed in duplicate. The intra and inter-assay coefficient of variation was 4.7% at 1 ng/mL and 7.4% at 0.25 ng/mL, respectively.
2.5. Group comparisons
Participants were grouped based on suicide attempt history and impulsivity and aggression scores. Suicide attempters (n = 35) and non-attempters (n = 37) were compared on demographic and clinical characteristics (Table 1). The suicide attempters had a mean of 2.81 attempts ± 1.90; attempt lethality (possible range=0–9) of the most serious attempt was moderate (M = 2.96 ± 1.40). The minimally detectable effect size for the attempter vs. non-attempter two-group comparison (N = 35 vs. N = 37) is Cohen’s d = 0.67, a moderate effect size. Participants were also divided into subgroups based on levels of impulsivity and aggression. Once a decision to use cutoffs on psychiatric scales is taken, there are a few options for choosing them. The optimal choice would be to choose “theoretical cutoffs”- in our example, if there were an agreement among professionals what constitutes “high” aggression and “high” impulsivity on the relevant scales, these would work best. Unfortunately, no such agreement exists currently for the BGAH and BIS. Another option would be to select the cutoffs based on the scales’ association with the dependent variable of interest- cortisol response- so that the cutoff would coincide with an incline in the curve, or a change in the strength of association of cortisol response vs. aggression, and cortisol response vs. impulsivity, respectively, if such a point exists and is unique. Since we have two predictors of interest, this would mean solving an optimization problem in 3 dimensions. Based on the relatively small suicide attempter sample size (n = 37), the accuracy of estimation for such points (if they exist) would be low. Also, we had two secondary outcomes (total cortisol output and baseline cortisol) to compare. Weighing these drawbacks, we elected a third commonly used method- the median split (Iacobucci et al., 2015). Median split is sample-dependent, and thus we felt it is important to choose the medians from a larger sample of MDD patients and controls to give a fuller picture of aggression and impulsivity scores in the population. As the current sample was part of a larger study, it provided a natural choice of medians for the two scales of interest.
To determine if baseline cortisol levels, total output and responsivity differed in suicide attempters depending on their level of impulsivity and aggression, suicide attempters were divided into four groups based on median-split levels of impulsivity and aggression in a larger sample (N = 116) from the same study (some participants had clinical measures but did not do the TSST), as measured by the BIS (median in this sample = 52.5, cutoff=58) and BGAH (median in this sample = 20, cutoff=20). The breakdown is as follows: Low Aggression/Low Impulsivity (LowAgg/LoImp) (suicide attempters n = 9; non-attempters n = 15); High Aggression/Low Impulsivity (HiAgg/LoImp) (suicide attempters n = 12; non-attempters n = 5); Low Aggression/High Impulsivity (LoAgg/HiImp) (suicide attempters n = 6; non-attempters n = 9) and High Aggression/High Impulsivity (HiAgg/HiImp) (suicide attempters n = 8; non-attempters n = 8).
While our primary aim was to examine subtypes within suicide attempters, we also included non-attempter subgroups in our analysis. The four subgroups of attempters and non-attempters did not differ on any demographic or clinical variables other than impulsivity and aggression. We first compared cortisol measures between suicide attempters and non-attempters and then compared baseline cortisol level and cortisol levels during TSST among the four attempter groups: LoAgg/LoImp attempters, HiAgg/LoImp attempters, LoAgg/HiImp attempters, and HiAgg/HiImp attempters.
2.6. Statistical methods
Demographic and clinical characteristics between groups were tested using ANOVA to compare quantitative variables, including baseline cortisol levels, cortisol reactivity, and total cortisol output between the groups, with Tukey’s HSD post-hoc pairwise group comparison. Kruskal-Wallis test was used for count data or data with a skewed distribution, and chi-square test of independence, or Fisher’s exact test were used to compare categorical variables.
Three cortisol measures were computed: 1. baseline cortisol levels; 2. total output of cortisol (AUCg) as measured by measuring area under the curve (AUC) of the log-transformed values with respect to ground; and 3. cortisol reactivity (AUCi) assessed by measuring AUC of the log-transformed values, with respect to increase from participant’s baseline cortisol value. Salivary cortisol data were highly skewed and were consequently log transformed to reduce the effect of outliers. Outliers on the cortisol outcomes (the log-transformed baseline cortisol, the area under the (log)-cortisol curve calculated from the subject’s baseline, and from the ground (0)), were defined as values above Q3+1.5*IQR, or below Q1−1.5*IQR, where Q3 is the 3rd quartile (75th percentile), Q1 is the first quartile (25th percentile), and IQR= Q3−Q1 is the Inter Quartile Range; all calculated by group (for attempters vs. non-attempters, and attempter subgroups). Three outliers were detected in the baseline cortisol data and the analysis was repeated after removing them. Two outliers were detected in the AUC data for both measurement methods and the analysis was repeated after removing them. All models for baseline cortisol level as response were adjusted for age, but not for sex, as the latter was not correlated with this, or any other of the outcomes. We also planned to adjust the models for demographic factors/medication status associated with attempter/no-attempter status and attempter subgroup as covariates, but none were identified.
3. Results
3.1. Descriptive variables
Suicide attempters obtained higher scores on hopelessness, as measured by the BHS, than non-attempters (Table 1). There were no other differences on demographic or clinical variables. The number of attempts (LoAgg/LoImp attempters: Median=1.5, Inter Quartile Range: 1–3, LoAgg/HiImp attempters: Median=3, IQR: 2–6, HiAgg/LoImp attempters: Median=2.5, IQR: 1–4, HiAgg/HiImp attempters Median=2, IQR: 1.3, Kruskal-Wallis X2 = 3.16, df = 3, p = 0.367) and the lethality of attempts (LoAgg/LoImp attempters: Mean=2.88, SD=1.36, LoAgg/HiImp attempters: Mean=2.75,SD=0.96, HiAgg/LoImp attempters: Mean=2.78, SD=1.09, HiAgg/HiImp attempters Mean=2.96, SD=1.40; F = 0.01, df=3, 24; p = 0.998) did not differ among the attempter subgroups.
3.2. Clinical and demographic variables, baseline cortisol, total output and cortisol response
Childhood abuse and neglect scores did not correlate with baseline cortisol, total output or cortisol response (p>0.05). Age was negatively correlated with baseline cortisol levels (r = −0.27, t = −2.30, df = 70, p = 0.024), but not with total cortisol output or cortisol response. All other demographic and clinical measures, including medication status, body mass index (BMI) and current smoking status, did not correlate with baseline cortisol, total output or cortisol reactivity to TSST (Table 2), and therefore, were not included as covariates.
Table 2.
Correlation of clinical variables, baseline cortisol, total cortisol output, and cortisol response.
| CTQ Emotional Abuse | CTQ Physical Abuse | CTQ Sexual Abuse | CTQ Emotional Neglect | CTQ Physical Neglect | BGAH | BIS | HDRS-17 | BDI | BHS | ALS | Baseline Cortisol | AUCi | AUCg | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTQ Emotional Abuse | 1 | 0.63*** | 0.47** | 0.68*** | 0.58*** | 0.24 | 0.31 | 0.25 | 0.28 | 0.1 | 0.04 | −0.18 | 0.09 | −0.15 |
| CTQ Physical Abuse | 1 | 0.36* | 0.45** | 0.7*** | 0.07 | 0.18 | 0.18 | 0.1 | −0.16 | 0.11 | −0.13 | −0.11 | −0.26 | |
| CTQ Sexual Abuse | 1 | 0.3 | 0.4* | 0.01 | −0.08 | −0.11 | −0.07 | −0.03 | 0.05 | −0.27 | 0.09 | −0.27 | ||
| CTQ Emotional Neglect | 1 | 0.6*** | 0.17 | 0.2 | 0.37* | 0.31 | 0.29 | 0.25 | 0.04 | 0.04 | 0.09 | |||
| CTQ Physical Neglect | 1 | 0.07 | 0.04 | 0.22 | 0.13 | −0.05 | 0.07 | 0.01 | −0.09 | −0.07 | ||||
| BGAH | 1 | 0.37** | 0.18 | 0.09 | 0.25 | 0.25 | −0.16 | 0.25 | 0 | |||||
| BIS | 1 | 0.25* | 0.54*** | 0.36** | 0.48** | −0.05 | 0.17 | 0.07 | ||||||
| HDRS-17 | 1 | 0.6*** | 0.53*** | 0.31 | 0.01 | −0.01 | 0.01 | |||||||
| BDI | 1 | 0.64*** | 0.37* | 0 | 0.06 | 0.05 | ||||||||
| BHS | 1 | 0.29 | 0 | 0.09 | 0.07 | |||||||||
| ALS | 1 | 0.02 | −0.01 | 0.01 | ||||||||||
| Baseline Cortisol | 1 | −0.58** | 0.77*** | |||||||||||
| AUCi | 1 | 0.07 | ||||||||||||
| AUCg | 1 |
Abbreviations: CTQ, Childhood Trauma Questionnaire; BGAH, Brown-Goodwin Lifetime History of Aggressive Behavior; BIS, Barratt Impulsivity Scale; HDRS-17, 17-Item Hamilton Depression Rating Scale; BDI, Beck Depression Inventory; BHS, Beck Hopelessness Scale; ALS, Affective Lability Scale; AUCi, cortisol reactivity; AUCg, total cortisol output.
p< 0.01,.
p< 0.001,.
p< 0.0001.
Current baseline cortisol levels, total output, and cortisol response to the TSST were not statistically different between: 1. participants with or without a current MDD (baseline cortisol, in a model adjusted for age: t=−0.28, df=66, p = 0.777; cortisol output: t = 0.03, df=70, p = 0.974; cortisol response: t=−0.20, df=68, p = 0.845); 2. those with and without borderline personality disorder (baseline cortisol, adjusted for age: t = 1.02, df=66, p = 0.311; cortisol output t = 0.93, df=70, p = 0..354; cortisol response: t= −1.20, df=68, p = 0.234); or 3. those with and without bipolar disorder (baseline cortisol, model adjusted for age: t=−0.01, df=69, p = 0.886; cortisol output: t = 0.17, df=70, p = 0.869; cortisol response: t=−0.04, df=70, p = 0.971). We also examined the effects of psychotropic medication on outcomes. Nineteen of 72 participants were on psychotropic medication: 17 were on antidepressants (HiAgg/HiImp=2, LoAgg/HiImp=4, HiAgg/LoImp=4, LoAgg/LoImp=2, Non-attempter=5), 7 on antipsychotics (HiAgg/HiImp=2, LoAgg/HiImp=2, HiAgg/LoImp=1, Non-attempter=2), and 6 on mood stabilizers (HiAgg/HiImp=1, LoAgg/HiImp=2, HiAgg/LoImp=1, LoAgg/LoImp=1, Non-attempter=1). Some participants were on more than one type of medication. The proportion of participants on psychotropic medication did not differ significantly between attempters and non-attempters (Chisq = 2.19, df = 1, NS) or among the 5 impulsive-aggressive groups (Fisher’s exact test p = 0.181). Attempters were more likely to be on antidepressants than non-attempters (Chisq = 4.30, df = 1, p-value = 0.038), but did not differ on the proportion of participants on mood stabilizers (Fisher’s exact test p = 0.102) or antipsychotics (p = 0.254). The 5 groups did not differ on the proportion of participants on antidepressants (Fisher’s exact test p = 0.0541), antipsychotics (p = 0.075) and mood stabilizers (p = 0.081). Medication use of each type was not significantly associated with cortisol response or total cortisol output (all p>0.05). Baseline cortisol was significantly higher in those with any psychotropic medication use, adjusting for age (b = 0.14, SE=0.07, t = 2.05, df=66, p = 0.0441), mostly due to a trend for higher values in those on antidepressants (b = 0.14, SE=0.07, t = 1.91, df=66, p = 0.0607). Therefore, medication status was adjusted for in subsequent analyses. Consistent with our hypotheses, the 5-group differences in baseline cortisol and total cortisol output remained not significant after adjusting for medication status of any and each type (all p>0.05), while the difference in cortisol response stayed significant after adjustments (all p<0.05)
3.3. Baseline cortisol levels, total cortisol output, cortisol responsivity, suicidal behavior, and impulsivity and aggression
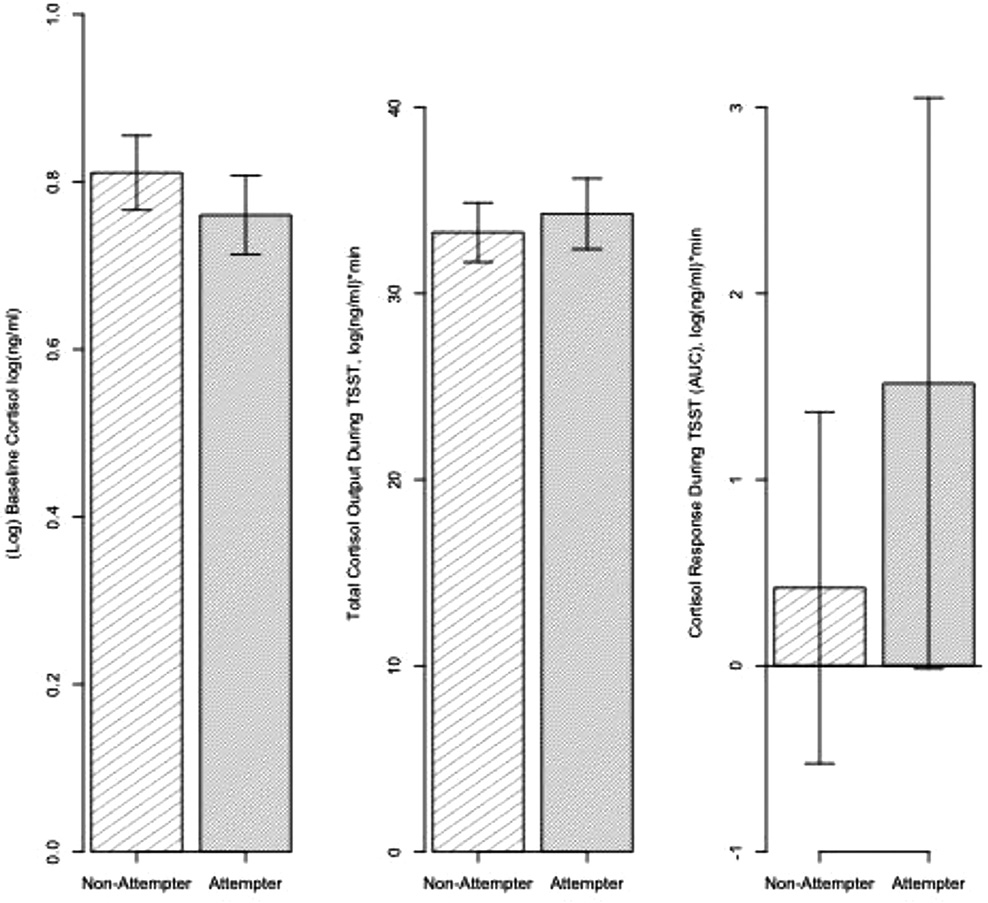
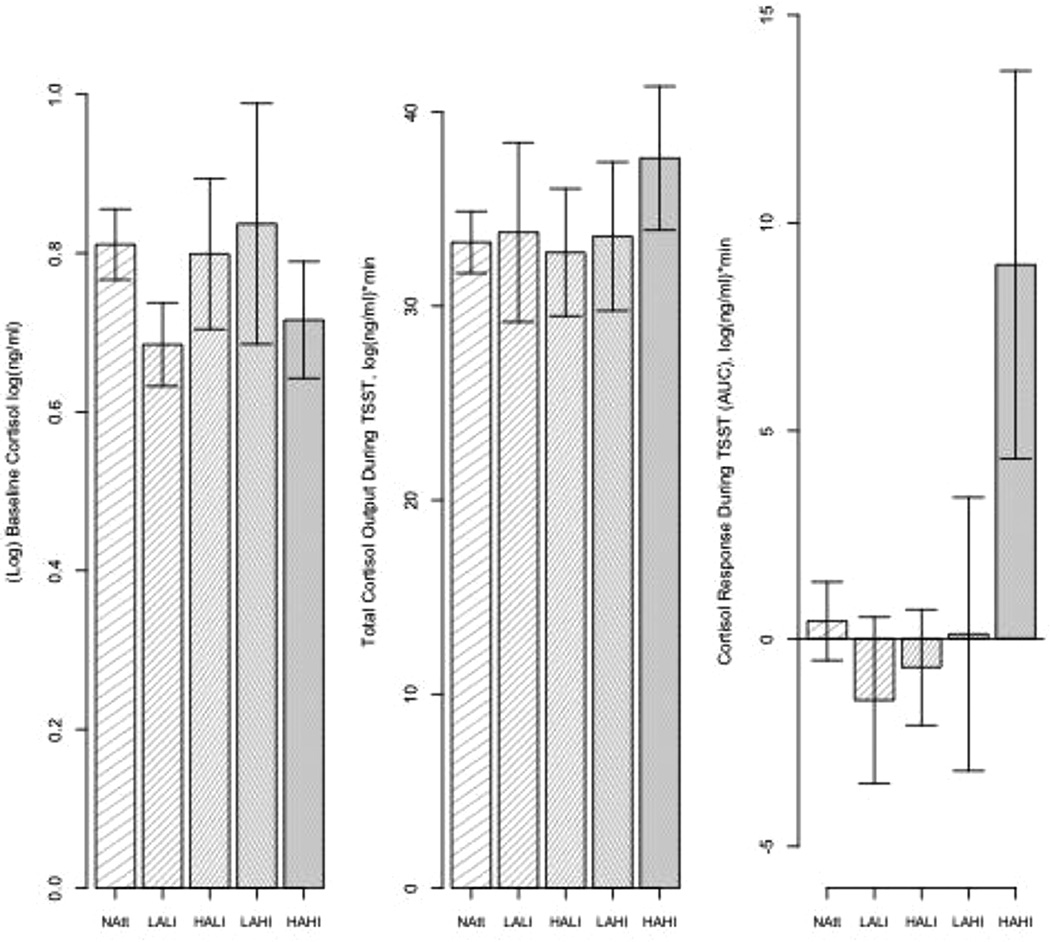
As expected, attempters did not differ from non-attempters with regard to baseline cortisol levels (t = 0.57, df=69, p = 0.572 after adjusting for age), total cortisol output (t=−0.41, df=70, p = 0.686), or cortisol reactivity to the TSST (t=−0.04, df=70, p = 0.971, without two outliers: t = 0.62, df=68, p = 0.539) (Fig. 1). Standardized effect sizes for the two group comparisons of the three cortisol measures were very small: for AUCi Cohen’s d = 0.12, for AUCg d = 0.09, for baseline cortisol, adjusted for age: Cohen’s f2<0.01. As hypothesized, with respect to cortisol reactivity in the five groups (LoAgg/LoImp attempters, LoAgg/HiImp attempters, HiAgg/LoImp attempters, HiAgg/HiImp attempters and non-attempters), there was a significant effect for group, both when including two outliers (F = 3.12, df=4, 67, p = 0.021), and when the two outliers were removed from analysis (F = 3.15, df=4, 65, p = 0.020) (Fig. 2). Post-hoc comparisons using Tukey’s HSD for multiple comparisons show differences between the HiAgg/HiImp attempters and LoAgg/LoImp attempters (Mean difference: 10.47, 95% CI: 0.95–20.00, p = 0.024), HiAgg/HiImp attempters and HiAgg/LoImp attempters (Mean difference: 9.69, 95% CI: 0.58–18.79, p = 0.03151), and HiAgg/HiImp attempters and non-attempters (Mean difference: 8.57, 95% CI: 0.91–16.23, p = 0.021) (Fig. 3). Fig. 3 shows the pattern of cortisol reactivity over time whereby, the HiAgg/HiImp group cortisol response rises sharply at 15 min post baseline in contrast to the other groups. There were no differences in baseline cortisol levels, adjusted for age, among the five groups with the outliers (F = 0.67, df=4, 66, p = 0.618) or after three outliers were removed from the data (F = 0.64, df=4, 63, p = 0.636). Also, there were no differences in total output among the five groups with outliers (F = 0.31, df=4,67, p = 0.869) or after removing two outliers (F = 1.08, df=4,67, p = 0.373). Standardized effect sizes for the 5-group comparisons were: baseline cortisol f2 =0.04 small effect size, AUCi: f2=0.19 medium effect size, and AUCg = f2=0.02 small effect size. For non-attempters, when they were partitioned into four aggressive-impulsive groups as described in the Methods section, no significant differences were found on any of the cortisol measures (AUCi: Cohen’s f2=0.04, F = 0.41, df=3,33, p = 0.7474; AUCg: f2=0.03, F = 0.34, df=3,33, p = 0.7999; baseline cortisol, in model adjusted for age: f2<0.01, F = 0.87, df=3,32, p = 0.4685; all small or very small effect sizes).
Fig. 1.
Baseline cortisol, total cortisol output, and cortisol response during TSST for attempters and non-attempters.
No differences between attempters and non-attempters in baseline cortisol, total cortisol output and cortisol response.
Fig. 2.
Baseline cortisol, total cortisol output, and cortisol response during TSST comparing non-attempters with suicide attempters categorized by levels of impulsive aggression and impulsivity.
HiAgg/HiImp suicide attempters demonstrate a heightened cortisol response to the TSST compared with all other suicide attempter groups and non-attempters. No differences among groups in baseline cortisol and total cortisol output.
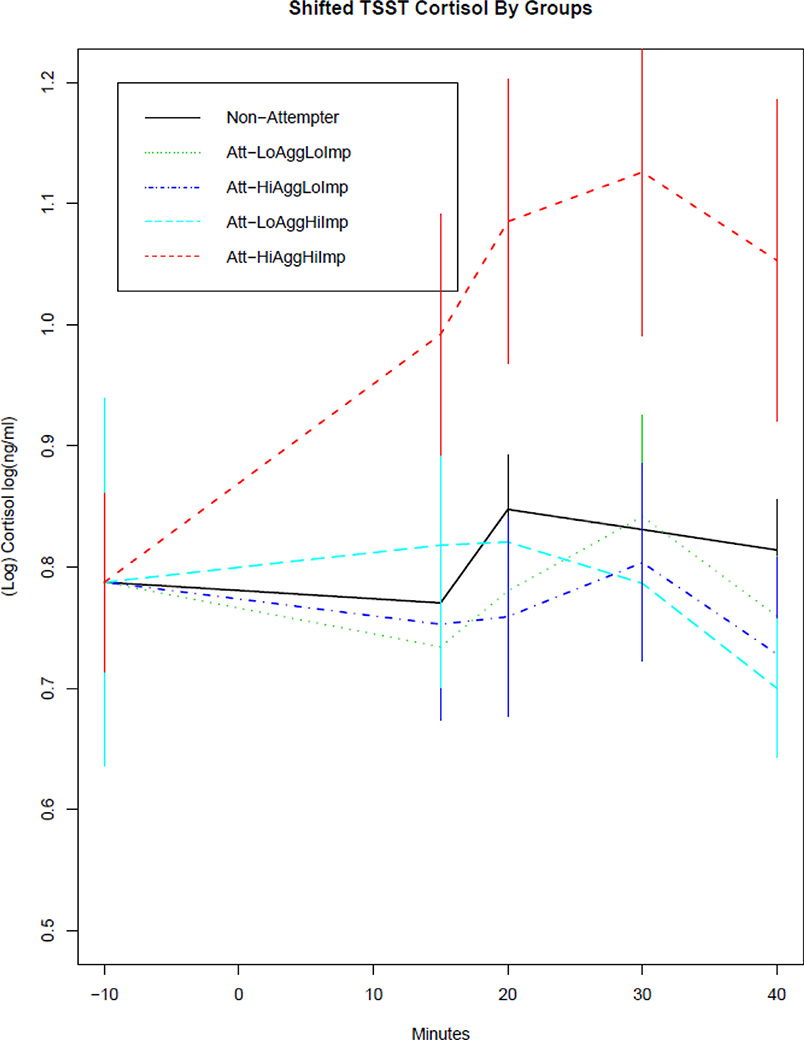
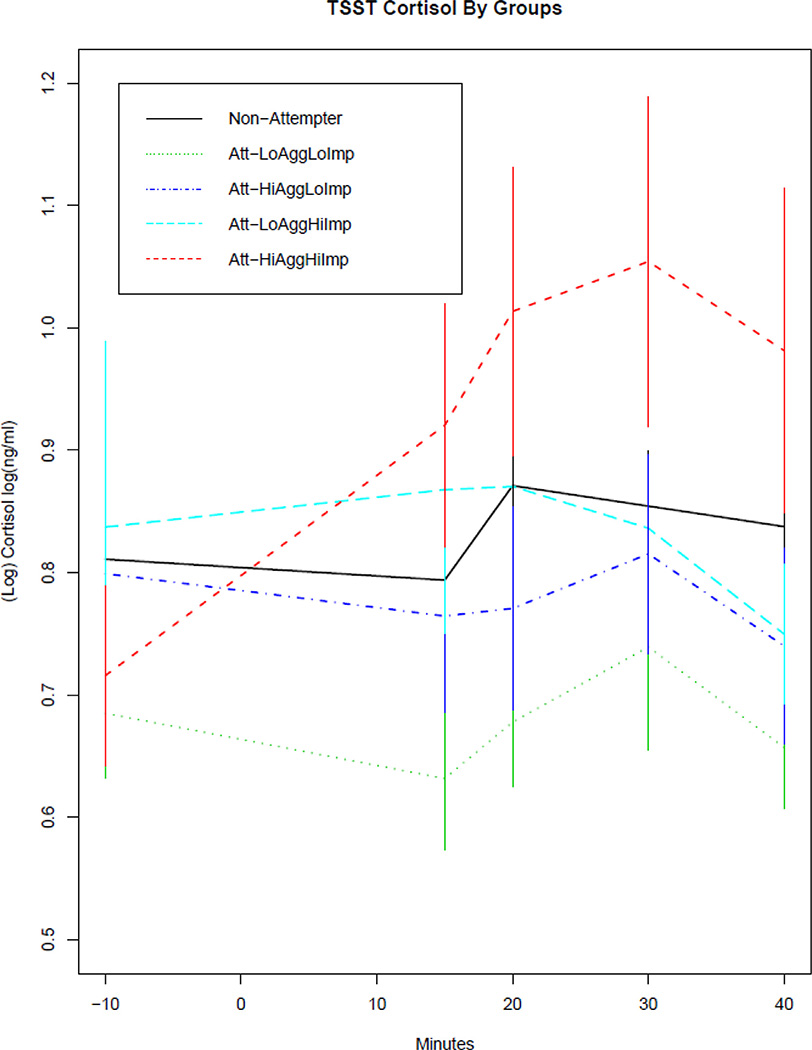
Fig. 3.
TSST cortisol response for four groups of impulsive aggressive suicide attempters and non-attempters.
Cortisol values during and after the TSST in the non-attempters, low aggression/low impulsivity attempters, high aggression/low impulsivity attempters, low aggression/high impulsivity attempters, and high aggression/high impulsivity attempters. The curves have been shifted to a common baseline value to illustrate cortisol response to TSST, regardless of baseline level.
Consistent with our hypotheses, the 5-group differences in baseline cortisol and total cortisol output remained not significant after adjusting for medication status of any and each type (all p>0.05), while the difference in cortisol response stayed significant after adjustments (all p<0.05). Additionally, adjusting for BHS, BDI, HDRS and sex did not change the significance of the group effect on TSST cortisol response (p = 0.030, p = 0.0217, p = 0.019, p = 0.024, respectively), and neither of the potential confounds were anywhere close to significant in the adjusted models (all ps>0.43). Finally, we compared the TSST response among the 4 impulsivity-aggression groups in the pooled sample. This analysis showed a significant effect of the group on TSST cortisol response (F = 3.23, df=3,68, p = 0.0277; or F = 3.34, df=3,64, p = 0.025 without outliers).
4. Discussion
To our knowledge, this study is the first to identify a relationship between stress responsivity and a subgroup of suicide attempters based on levels of impulsivity and aggression. These results may help to delineate stress responsive and non-stress responsive subtypes of suicide attempters. Furthermore, they may help to resolve the discrepant findings relating stress response and suicidal behavior. As expected, we found no differences in baseline cortisol, total cortisol output, or cortisol reactivity during a social stress test between mood disordered suicide attempters and mood disordered non-attempters. However, after separating suicide attempters based on levels of impulsivity and aggression, as hypothesized, significant differences in cortisol reactivity during the TSST emerged, with highly impulsive and aggressive suicide attempters demonstrating the strongest cortisol response to stress among subgroups. There were no differences in baseline cortisol levels or total cortisol output among the groups.
There are no prior studies of stress response in subgroups of suicide attempters, so we cannot compare our subgroup results to other studies. Furthermore, there has been limited TSST research examining the relationship between stress response and suicidal behavior. Results of previous studies (Eisenlohr-Moul et al., 2018; Giletta et al., 2015; Melhem et al., 2016; O’Connor et al., 2017) are discussed and reviewed in the context of our findings. With respect to comparison of suicide attempters and non-attempters, our finding of no difference in baseline cortisol levels in suicide attempters are consistent with some previous studies (Eisenlohr-Moul et al., 2018; O’Connor et al., 2017), but not with others reporting low baseline cortisol levels in suicide attempters (Keilp et al., 2016; Lindqvist et al., 2008; McGirr et al., 2011; Melhem et al., 2016). Similarly, our finding that there was no difference between attempters and non-attempters in cortisol reactivity is in agreement with previous TSST (Melhem et al., 2016; O’Connor et al., 2017) and pharmacological-based research (Roy, 1992). However, this finding is not consistent with Eisenlohr-Moul et al. (2018) who reported blunted cortisol responsivity to TSST in adolescent females with history of lifetime suicidal behavior compared with those without any history of lifetime suicidal ideation or behavior. The null difference in cortisol reactivity between suicide attempters and non-attempters in the current study is also divergent from some pharmacological-based research on HPA axis reactivity and suicidal behavior (Coryell and Schlesser, 2001; Fountoulakis et al., 2004; Jokinen and Nordström, 2009; Mann et al., 2006; Pfennig et al., 2005; Pitchot et al., 2008).
Melhem et al. (2016), examined the relationship between cortisol response during TSST and suicidal behavior in individuals with parental history of a mood disorder. They found that offspring who had made a previous suicide attempt had lower total cortisol output during TSST and lower baseline cortisol than offspring with suicide related behavior (i.e. interrupted attempts, aborted attempts, preparatory behavior or emergency evaluation for suicidality), offspring non-attempters, and normal control subjects (Melhem et al., 2016). Of note, it is difficult to compare our results to this study owing to sample differences such as diagnostic differences (50% of non-attempters in Melhem et al. (2016) were depressed as opposed to 100% in our study); Nevertheless, consistent with our findings, cortisol reactivity during TSST did not differ between attempters and non-attempters.
In another study, O’Connor et al. (2017) examined cortisol response to a different stress paradigm, the MAST, in suicide attempters, non-attempters with suicide ideation within the past 12 months and a control group with no history of suicide attempt or ideation and no present psychiatric illness. They found that the attempters had lower total cortisol output compared with the controls, but not compared with non-attempters with ideation in the past 12 months. This is consistent with our results that total cortisol output did not differ for attempters and non-attempters. Also in line with our findings, O’Connor et al. (2017) found no difference in cortisol reactivity during TSST or baseline cortisol levels between attempters and non-attempters. They did not examine impulsive aggression in their sample.
Another study examined cortisol response during TSST in adolescent girls with suicidal ideation (Giletta et al., 2015). While the study did not examine suicidal behavior, the results are supportive of suicidal subtypes. They found that participants fell into one of three categories of cortisol response during TSST: hyperactive response, normative response, or hypoactive response (Giletta et al., 2015). Those who had a hyperactive cortisol response to TSST were more likely to have a history of suicidal ideation. A hyperactive cortisol response was also related to increased risk for suicidal ideation at 3-month follow-up, even when controlling for past suicidal ideation. They did not examine impulsive aggression. Although this was an all-female adolescent sample, the findings are consistent with the notion that those who engage in or who are at high risk for suicidal behavior are a heterogeneous, rather than homogeneous, population. It may be that there are at least two subgroups of individuals at risk for suicidal behavior: those who are more reactive to environmental and social stressors, and thus have a more pronounced cortisol response (Bernanke et al., 2017; Giletta et al., 2015) and those who are be less reactive to stressors and exhibit a hypoactive or normative cortisol response (Bernanke et al., 2017; Giletta et al., 2015).
Our analyses comparing suicide attempters with non-attempters adds to this conflicting literature, but the most important finding in this study is that the relationship between cortisol response and suicidal behavior depends on the level of impulsivity and aggression. Our results may provide an explanation for the conflicting prior findings on stress response and suicidal behavior, as well as suggest an alternative framework for future research. The current study offers additional support for the notion that there may be at least two subtypes of suicidal individuals: stress responsive and non-stress responsive (Bernanke et al., 2017; Rizk et al., 2018). Future research should explore the role of impulsivity and aggression in suicidal individuals, as well as other bio-behavioral characteristics that may be associated with this heterogeneous population.
Our study does have some limitations. The sample size is relatively small. Larger samples are needed to replicate our findings. Our findings may not be generalizable to populations with different clinical and demographic characteristics. Specifically, our sample is confined to individuals with depression. Whether these findings hold in other diagnostic groups is unknown. Furthermore, because the study is cross-sectional, we cannot conclude that high impulsivity and aggression led to an altered stress response or that an altered stress response resulted in increased impulsivity and aggression. Finally, while our results indicate that only the high impulsivity/high aggression suicide attempter group differed from other groups by mounting a higher stress response, we cannot conclude that this group is “abnormal.” It could be that the other subgroups are impaired by not mounting a response to stress posed by the TSST. Future studies would benefit from incorporating a healthy control group, to understand how normal samples compare to the subgroups we identified. In conclusion, by gaining a better understanding of the different subtypes of suicidal behavior, it may help us move toward identifying more precise risk factors and biomarkers and ultimately reduce the number of lives lost to suicide.
Acknowledgments
Funding Sources
This research was supported in part by grants from the National Institute of Mental Health: [R01 MH109326; R01 MH61017; R01 MH062665].
Footnotes
Disclosures
Barbara Stanley, Maria Oquendo and J. John Mann receive royalties for commercial use of the Columbia Suicide Severity Rating Scale from the Research Foundation for Mental Hygiene.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psychres.2019.112486.
References
- Aas M, Pedersen G, Henry C, Bjella T, Bellivier F, Leboyer M, Kahn JP, Cohen RF, Gard S, Aminoff SR, Lagerberg TV, Andreassen OA, Melle I, Etain B, 2015. Psychometric properties of the affective lability scale (54 and 18-item version) in patients with bipolar disorder, first-degree relatives, and healthy controls. J. Affect. Disord. 172, 375–380. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Ryder AG, Schuller DR, Marshall MB, 2004. The hamilton depression rating scale: has the gold standard become a lead weight? Am. J. Psychiatry 161 (12), 2163–2177. [DOI] [PubMed] [Google Scholar]
- Barratt ES, 1985. Impulsiveness subtraits: arousal and information processing. Motiv. Emot. Personal. 5, 137–146. [Google Scholar]
- Beck AT, Steer RA, 1984. Internal consistencies of the original and revised beck depression inventory. J. Clin. Psychol. 40 (6), 1365–1367. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Beck depression inventory-II. San Antonio 78 (2), 490–498. [Google Scholar]
- Beck AT, Weissman A, Lester D, Trexler L, 1974. The measurement of pessimism: the hopelessness scale. J. Consult. Clin. Psychol. 42 (6), 861. [DOI] [PubMed] [Google Scholar]
- Bernanke JA, Stanley BH, Oquendo MA, 2017. Toward fine-grained phenotyping of suicidal behavior: the role of suicidal subtypes. Mol. Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J, 1994. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am. J. Psychiatry 151 (8), 1132–1136. [DOI] [PubMed] [Google Scholar]
- Braquehais MD, Picouto MD, Casas M, Sher L, 2012. Hypothalamic-pituitary-adrenal axis dysfunction as a neurobiological correlate of emotion dysregulation in adolescent suicide. World J. Pediatri. 8 (3), 197–206. [DOI] [PubMed] [Google Scholar]
- Brown GL, Goodwin FK, 1986. Human aggression and suicide. Suicide Life-Threat. Behav. 16 (2), 223–243. [DOI] [PubMed] [Google Scholar]
- Chatzittofis A, Nordström P, Hellström C, Arver S, Åsberg M, Jokinen J, 2013. CSF 5-HIAA, cortisol and DHEAS levels in suicide attempters. Eur. Neuropsychopharmacol. 23 (10), 1280–1287. [DOI] [PubMed] [Google Scholar]
- Chaudhury SR, Singh T, Burke A, Stanley B, Mann JJ, Grunebaum M, Sublette ME, Oquendo MA, 2016. Clinical correlates of planned and unplanned suicide attempts. J. Nerv. Ment. Dis. 204 (11), 806–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell W, Schlesser M, 2001. The dexamethasone suppression test and suicide prediction. Am. J. Psychiatry 158 (5), 748–753. [DOI] [PubMed] [Google Scholar]
- Durham TW, 1982. Norms, reliability, and item analysis of the hopelessness scale in general psychiatric, forensic psychiatric, and college populations. J. Clin. Psychol. 38 (3), 597–600. [DOI] [PubMed] [Google Scholar]
- Eisenlohr-Moul TA, Miller AB, Giletta M, Hastings PD, Rudolph KD, Nock MK, Prinstein MJ, 2018. HPA axis response and psychosocial stress as interactive predictors of suicidal ideation and behavior in adolescent females: a multilevel diathesis-stress framework. Neuropsychopharmacology 43 (13), 2564–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom G, Alling C, Gustavsson P, Oreland L, Traskman-Bendz L, 1997. Clinical characteristics and biological parameters in temperamental clusters of suicide attempters. J. Affect. Disord. 44 (1), 45–55. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 1995. The structured clinical interview for DSM-III-R personality disorders (SCID-II). Part I: description. J. Pers. Disord. 9 (2), 83–91. [Google Scholar]
- Fountoulakis KN, Iacovides A, Fotiou F, Nimatoudis J, Bascialla F, Ioannidou C, Kaprinis G, Bech P, 2004. Neurobiological and psychological correlates of suicidal attempts and thoughts of death in patients with major depression. Neuropsychobiology 49 (1), 42–52. [DOI] [PubMed] [Google Scholar]
- Giletta M, Calhoun CD, Hastings PD, Rudolph KD, Nock MK, Prinstein MJ, 2015. Multi-level risk factors for suicidal ideation among at-risk adolescent females: the role of hypothalamic-pituitary-adrenal axis responses to stress. J. Abnorm. Child Psychol. 43 (5), 807–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J. Neurol. Neurosurg. Psychiatr. 23 (1), 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB, 2008. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 33 (6), 693–710. [DOI] [PubMed] [Google Scholar]
- Iacobucci D, Posavac SS, Kardes FR, Schneider MJ, Popovich DL, 2015. Toward a more nuanced understanding of the statistical properties of a median split. J. Consum. Psychol. 25 (4), 652–665. [Google Scholar]
- Jokinen J, Nordström P, 2009. HPA axis hyperactivity and attempted suicide in young adult mood disorder inpatients. J. Affect. Disord. 116 (1), 117–120. [DOI] [PubMed] [Google Scholar]
- Juruena MF, Pariante CM, Papadopoulos AS, Poon L, Lightman S, Cleare AJ, 2009. Prednisolone suppression test in depression: prospective study of the role of HPA axis dysfunction in treatment resistance. Br. J. Psychiatry 194 (4), 342–349. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Stanley BH, Beers SR, Melhem NM, Burke AK, Cooper TB, Oquendo MA, Brent DA, Mann JJ, 2016. Further evidence of low baseline cortisol levels in suicide attempters. J. Affect. Disord. 190, 187–192. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K-M, Hellhammer DH, 1993. The ‘Trier social stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28 (1–2), 76–81. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Schommer NC, Hellhammer DH, Kirschbaum C, 2004. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology 29 (8), 983–992. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Isaksson A, Brundin L, 2008. Salivary cortisol and suicidal behavior—a follow-up study. Psychoneuroendocrinology 33 (8), 1061–1068. [DOI] [PubMed] [Google Scholar]
- Lopez-Castroman J, Nogue E, Guillaume S, Picot MC, Courtet P, 2016. Clustering suicide attempters: impulsive-ambivalent, well-planned, or frequent. J. Clin. Psychiatr 77 (6), e711–e718. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Olson SL, Hajal NJ, Felt BT, Vazquez DM, 2009. Hypothalamic pituitary adrenal axis functioning in reactive and proactive aggression in children. J. Abnorm. Child Psychol. 37 (2), 169–182. [DOI] [PubMed] [Google Scholar]
- Mann JJ, 2003. Neurobiology of suicidal behaviour. Nat. Rev. Neurosci. 4 (10), 819. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Currier D, Stanley B, Oquendo MA, Amsel LV, Ellis SP, 2006. Can biological tests assist prediction of suicide in mood disorders? Int. J. Neuropsychopharmacol. 9 (4), 465–474. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Waternaux C, Haas GL, Malone KM, 1999. Toward a clinical model of suicidal behavior in psychiatric patients. Am. J. Psychiatry 156 (2), 181–189. [DOI] [PubMed] [Google Scholar]
- McGirr A, Diaconu G, Berlim MT, Turecki G, 2011. Personal and family history of suicidal behaviour is associated with lower peripheral cortisol in depressed outpatients. J. Affect. Disord. 131 (1), 368–373. [DOI] [PubMed] [Google Scholar]
- McGirr A, Turecki G, 2007. The relationship of impulsive aggressiveness to suicidality and other depression-linked behaviors. Curr. Psychiatry Rep. 9 (6), 460–466. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF, 1971/1981. Profile of Mood States Manual. San Diego: Educational & Industrial Testing Service. [Google Scholar]
- Meador-Woodruff JH, Greden JF, Grunhaus L, Haskett RF, 1990. Severity of depression and hypothalamic-pituitary-adrenal axis dysregulation: identification of contributing factors. Acta Psychiatr. Scand. 81 (4), 364–371. [DOI] [PubMed] [Google Scholar]
- Melhem NM, Brent DA, Ziegler M, Iyengar S, Kolko D, Oquendo M, Birmaher B, Burke A, Zelazny J, Stanley B, 2007. Familial pathways to early-onset suicidal behavior: familial and individual antecedents of suicidal behavior. Am. J. Psychiatry 164 (9), 1364–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhem NM, Keilp JG, Porta G, Oquendo MA, Burke A, Stanley B, Cooper TB, Mann JJ, Brent DA, 2016. Blunted HPA axis activity in suicide attempters compared to those at high risk for suicidal behavior. Neuropsychopharmacology 41 (6), 1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Ortin A, Scott M, Shaffer D, 2014. Characteristics of suicidal ideation that predict the transition to future suicide attempts in adolescents. J. Child Psychol. Psychiatry 55 (11), 1288–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SR, Gresham LS, Bromberg MB, Kasarkis EJ, Smith RA, 1997. A self report measure of affective lability. J. Neurol. Neurosur. Psychiatry 63 (1), 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DB, Green JA, Ferguson E, O’Carroll RE, O’Connor RC, 2017. Cortisol reactivity and suicidal behavior: investigating the role of hypothalamic-pituitary-adrenal axis responses to stress in suicide attempters and ideators. Psychoneuroendocrinology 75, 183–191. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Halberstam B, Mann JJ, 2003. Risk factors for suicidal behavior. Stand. Eval. Clin. Pract. 22, 103–129. [Google Scholar]
- Oquendo MA, Sullivan GM, Sudol K, Baca-Garcia E, Stanley BH, Sublette ME, Mann JJ, 2014. Toward a biosignature for suicide. Am. J. Psychiatry 171 (12), 1259–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL, 2008. The HPA axis in major depression: classical theories and new developments. Trend. Neurosci. 31 (9), 464–468. [DOI] [PubMed] [Google Scholar]
- Pfennig A, Kunzel HE, Kern N, Ising M, Majer M, Fuchs B, Ernst G, Holsboer F, Binder EB, 2005. Hypothalamus-pituitary-adrenal system regulation and suicidal behavior in depression. Biol. Psychiatry 57 (4), 336–342. [DOI] [PubMed] [Google Scholar]
- Pitchot W, Scantamburlo G, Pinto E, Hansenne M, Reggers J, Ansseau M, Legros J-J, 2008. Vasopressin–neurophysin and DST in major depression: relationship with suicidal behavior. J. Psychiatr. Res. 42 (8), 684–688. [DOI] [PubMed] [Google Scholar]
- Poustka L, Maras A, Hohm E, Fellinger J, Holtmann M, Banaschewski T, Lewicka S, Schmidt MH, Esser G, Laucht M, 2010. Negative association between plasma cortisol levels and aggression in a high-risk community sample of adolescents. J. Neural Transm. 117 (5), 621–627. [DOI] [PubMed] [Google Scholar]
- Rizk MM, Galfalvy H, Singh T, Keilp JG, Sublette ME, Oquendo MA, Mann JJ, Stanley B, 2018. Toward subtyping of suicidality: brief suicidal ideation is associated with greater stress response. J. Affect. Disord. 230, 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, 1992. Hypothalamic-pituitary-adrenal axis function and suicidal behavior in depression. Biol. Psychiatry 32 (9), 812–816. [DOI] [PubMed] [Google Scholar]
- Rubinstein DH, 1986. A stress–diathesis theory of suicide. Suicide and Life-Threatening Behavior 16 (2), 182–197. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Gibbon M, Williams JB, 1995. Structured Clinical Interview For Axis I DSM-IV Disorders (SCID). American Psychiatric Association, Washington, DC. [Google Scholar]
- Turecki G, Ernst C, Jollant F, Labonté B, Mechawar N, 2012. The neurodevelopmental origins of suicidal behavior. Trend. Neurosci. 35 (1), 14–23. [DOI] [PubMed] [Google Scholar]
- Van Bokhoven I, Van Goozen SHM, Van Engeland H, Schaal B, Arseneault L, Seguin JR, Nagin DS, Vitaro F, Tremblay RE, 2005. Salivary cortisol and aggression in a population-based longitudinal study of adolescent males. J. Neural Transm. 112 (8), 1083–1096. [DOI] [PubMed] [Google Scholar]
- van Heeringen K, 2012. Stress-diathesis model of suicidal behavior. Neurobiol. Basis Suicide 51, 113. [Google Scholar]
- Witte TK, Fitzpatrick KK, Warren KL, Schatschneider C, Schmidt NB, 2006. Naturalistic evaluation of suicidal ideation: variability and relation to attempt status. Behav. Res. Ther. 44 (7), 1029–1040. [DOI] [PubMed] [Google Scholar]
- Zorn JV, Schur RR, Boks MP, Kahn RS, Joels M, Vinkers CH, 2017. Cortisol stress reactivity across psychiatric disorders: a systematic review and meta-analysis. Psychoneuroendocrinology 77, 25–36. [DOI] [PubMed] [Google Scholar]