Abstract
Introduction
The faecal immunochemical test detects blood in the faeces, reporting faecal haemoglobin quantitatively in micrograms of haemoglobin per gram of faeces. The aim of this pilot study was to determine the feasibility of using the faecal immunochemical test as a rule-out test in symptomatic patients at low and high risk of colorectal cancer.
Material and methods
Between November 2016 and October 2017, consecutive symptomatic patients within a multicultural part of London were recruited to perform a faecal immunochemical test prior to colonoscopy. Analysis was performed on the HM-JACKarc analyser.
Results
Faecal immunochemical test samples were returned by 298 patients who underwent colonoscopy. There was no significant variation in faecal haemoglobin levels by age, sex, ethnicity or deprivation. The overall detection rate for colorectal cancer was 100% at 2 µg/g and 92% at 10 µg/g. If a faecal haemoglobin threshold for investigation of 2 µg/g (ie detectable) or 10 µg/g had been employed, the number of colonoscopies would have been reduced by 70% and 84%, respectively, in all symptomatic patients. For low-risk patients, the sensitivity of the faecal immunochemical test for colorectal cancer at both thresholds of 2 µg/g or 10 µg/g remained 100%, with the number of colonoscopies reduced by 80% and 91%, respectively.
Conclusion
This study shows that the faecal immunochemical test is a promising technology that detected colorectal cancer in all high- or low-risk symptomatic patients in our cohort at a threshold of detectable faecal haemoglobin. Data from adequately powered cohort studies will elucidate the true diagnostic accuracy of the test and the rate and patterns of undetected colorectal cancer.
Keywords: FIT, Faecal immunochemical test, Colorectal neoplasms, Colonoscopy, Diagnostic accuracy, Diagnostic tests and procedures
Introduction
Colonoscopy remains the gold standard diagnostic test to exclude colorectal cancer. In the UK, the number of colonoscopies performed to investigate symptomatic patients referred urgently with suspected colorectal cancer on the two-week-wait pathway has risen year-on-year.1 Of these patients, 3–5% will be diagnosed with colorectal cancer.
Guidelines from the National Institute for Health and Care Excellence (NICE) in 2015 (NG12) broadened the criteria for referral to include ‘medium’ and ‘low-risk’ symptoms (Table 1).2 By investigating a larger cohort of symptomatic patients, NICE hoped that more patients with colorectal cancer would be diagnosed via the two-week-wait pathway at an earlier cancer stage, reducing the number of patients diagnosed through the emergency route with a more advanced stage and worse outcomes. However, this requires an even greater number of patients to undergo investigation, particularly with colonoscopy. In addition to the significant costs, it has added further strain on endoscopy units already struggling to meet demand within the NHS.3 The knock-on impact on capacity could reduce the provision of screening programmes such as bowel scope or bowel cancer screening, where the survival benefits of early stage cancer diagnosis are maximised.
Table 1.
Eligible symptoms stratified by risk criteria under successive National Institute for Health and Care Excellence guidelines.
| Symptoms | CG27 (2005)19 | NG12 (2015)2 | DG30 (2017)5 | Risk of cancer |
| Rectal bleeding: | ||||
| > 6 weeks (> 60 years) | ||||
| + diarrhoea for 6 weeks (> 40 years) | ||||
| Change in bowel habit (CIBH) > 6 weeks (> 60 years) | REFER | REFER | REFER | High: > 5% |
| Mass (any age) | ||||
| Iron deficiency anaemia (IDA) | ||||
| Abdo pain AND weight loss (> 40 years) | ||||
| Rectal bleeding (> 50) | ||||
| Rectal bleeding + (IDA/CIBH/weight loss, < 50 years) | REFER | REFER | Medium: 3–5% | |
| Iron deficiency anaemia (> 60 years) | ||||
| Change in bowel habit (> 60 years) | ||||
| Abdominal pain OR weight loss (> 50 years) | ||||
| Change in bowel habit (< 60 years) | Faecal occult blood test | FIT | Low: 1–3% | |
| Iron deficiency anaemia (< 60 years) | ||||
| Anaemia, non-iron deficiency anaemia (> 60 years) | ||||
| Other symptoms | FIT | ? |
One solution would be to use a simple and cheap test, ideally performed in primary care, which could reliably triage patients to referral for investigation or conservative management in primary care. The faecal immunochemical test (FIT) detects haemoglobin in faeces. A recent Health Technology Assessment has shown in its meta-analysis of five studies that FIT is a highly sensitive test for measuring faecal haemoglobin (f-Hb) levels, which are raised in colorectal cancer.4 Shortly afterwards, NICE released its diagnostic guidance (DG30) for low-risk symptomatic patients who did not meet the two-week-wait criteria.5 This recommended the use of FIT in primary care for these patients, and the reference to the use of faecal occult blood test in low-risk patients was removed from NICE guidance (NG12).2
At present, no direct evidence for FIT in low-risk patients in England exists. The Health Technology Assessment concluded that based on evidence in patients with high-risk symptoms, FIT could probably be used as a triage test for low-risk symptoms. The Health Technology Assessment called for further research into known variation of faecal haemoglobin levels by age, sex, deprivation and in different ethnic groups,6,7 particularly as most of the studies reviewed were performed in ethnically homogenous European populations.4
Croydon University Hospital serves an ethnically diverse population in South West London. We combined data from an initial service evaluation and subsequent ethics approved, prospective single-centre pilot study to determine the diagnostic accuracy of FIT to rule out colorectal cancer in symptomatic patients, including low-risk patients meeting the NICE criteria (DG30).5
Ethical approval for the study was received from the Health Research Authority: IRAS No 218404.
Materials and methods
The study was conducted according to STARD (Standards for Reporting of Diagnostic Accuracy Studies) guidelines.8 Specimen collection and handling, quality management and result handling were conducted and reported according to recent FITTER guidelines for studies on FIT (supplementary file, online only),9 using recommended analytical performance specifications.10 Ethics approval was granted locally and from the UK Health Research Authority (IRAS No 218404).
Patient selection
All symptomatic patients undergoing colonoscopy at Croydon University Hospital between November 2016 and October 2017 were eligible for recruitment into the study. These patients were offered a FIT by the endoscopy team when they collected their bowel preparation. If they accepted, they were given a patient information sheet, instruction brochure and written consent forms. Patients returned their sample and consent forms when attending for colonoscopy. One HM-JACKarc analytical system (Kyowa Medex/Alpha Labs) was used to analyse all samples. The analytical working range was 2–8000 µg Hb/g faeces (µg/g). The limit of detection of the assay is 2 µg/g and the limit of quantification was 10 µg/g. If the faecal haemoglobin concentration was below the limit of detection, it was classified as 0 µg/g for statistical analysis, and at 1 µg/g for logarithmic graphing.
The endoscopy department of Croydon University Hospital is accredited by the Joint Advisory Group (JAG) on gastrointestinal endoscopy. All colonoscopies were performed or supervised by JAG-accredited endoscopists, including middle-grade doctors, consultant gastroenterologists and surgeons and one nurse endoscopist. The unit’s polyp detection rate is 21–25%.
The laboratory staff were blinded to the colonoscopy results and the colonoscopists were blinded to the faecal haemoglobin results. Clinical and demographic details for each patient were entered and double checked by members of the research team. Clinical details underwent final verification by a senior colorectal registrar or consultant.
Data analysis
Colonoscopy indications were divided up into two-week-wait or non- two-week-wait referral symptoms. Two-week-wait symptoms were classified according to 2015 NICE (NG12) criteria for referral (Table 1).2 All patients with two-week-wait symptoms with ‘low-risk’ NG12 criteria were included in this cohort, as the London region recommended that these patients were referred under the two-week-wait pathway without testing by guaiac-based faecal occult blood tests. We then reclassified symptomatic patients into high–medium (NG12)2 and low-risk (DG30)5 groups as specified by NICE. The revised NG12 comprised only high–medium risk two-week-wait criteria, after low-risk symptoms were removed from it and absorbed into the wider spectrum of low-risk symptoms of DG30, including symptomatic patients referred routinely to a clinic.
Polyps were classified as high-risk adenomas if they were larger than 1cm, serrated adenomas in the right colon or contained high grade dysplasia.
Statistical analysis was performed using STATA and graphed using Microsoft Excel or STATA. Subgroup analysis was performed using analysis of variance for continuous variables and analysis of covariance for categorical variables.
Results
Over the study period, 298 symptomatic patients who underwent a complete colonoscopy were recruited. Kits were accepted by 800 patients and returned by 384 (48% return rate). Colonoscopy was performed for surveillance in 86 patients who were excluded. No adverse events were reported from patients undergoing FIT or colonoscopy. The period between date of sample and date of analysis averaged 10.1 days, during which the kits were refrigerated at the endoscopy unit and laboratory. Kits were analysed on average 1.4 days after receipt in the laboratory. The mean patient age was 60.6 years (range 20–90 years); 198 (51.4%) patients were female. The most common ethnic groups were white (62%), Asian (14%), ‘other’ ethnicity (12%) and black (9%), representative of the ethnic diversity of the Croydon population.
Of the 298 symptomatic patients, 160 underwent colonoscopy for symptoms that met the NG12 two-week wait criteria. A total of 138 patients (58 two-week-wait low-risk patients and 80 non-two-week-wait symptomatic patients) met the NICE DG30 criteria for low-risk symptoms. Symptomatic patients were frequently referred with more than one symptom. Of the 218 patients referred on the two-week-wait pathway, the most common referral criteria were iron deficiency anaemia or change in bowel habit in patients over 60 years (33%), change in bowel habit in patients under 60 years (18%) or rectal bleeding in patients over 50 years (16%).
At colonoscopy, 123 patients (41%) had normal colonoscopies, with no disease (benign or otherwise) found. Cancer was detected in 12 patients, HRA in 4 and inflammatory bowel disease in 11 patients (Table 2). The remaining patients had minor pathologies.
Table 2.
Incidence of cancer, inflammatory bowel disease, high-risk adenomas and normal findings at colonoscopy by colonoscopy indication. Low-risk NG12 symptoms and non-two-week-wait symptoms were reclassified as eligible for referral under DG30 guidance5 if the faecal immunochemical test was positive.
| Colonoscopy Indication | Patients (n) | Normal (%) | Cancer n (%) | Inflammatory bowel disease n (%) | High-risk adenoma n (%) |
| Two-week-wait criteria: | |||||
| All | 218 | 79 (36) | 10 (4.6) | 11 (5.1) | 4 (1.8) |
| High–medium risk (NG12) | 160 | 160 (34) | 8 (5) | 9 (5.6) | 4 (1.8) |
| Low risk (DG30) | 58 | 24 (41) | 2 (3.4) | 1 (1.7) | 0 |
| Non-two-week-wait symptoms (DG30) | 80 | 46 (58) | 2 (2.5) | 2 (2.5) | 0 |
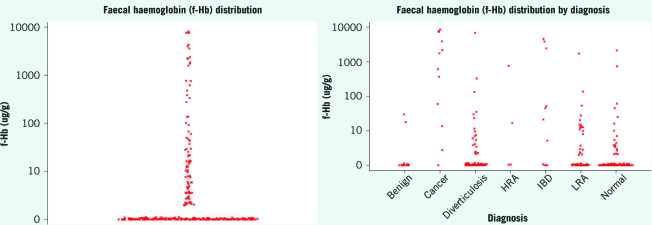
Faecal haemoglobin levels were below the limit of detection in 70% of patients and below 10 in 84% of patients (interquartile range < 2–3 µg/g; Fig 1a).
Figure 1.
(left) Faecal haemoglobin distribution (undetectable graphed at 1 µg Hb/g); (right) faecal haemoglobin distribution by diagnosis. Both graphs plotted on log(10) scales.
At a faecal haemoglobin cut-off threshold of 2 µg/g, FIT would have detected every colorectal cancer. A threshold of 10 µg/g would have detected 92% of colorectal cancer (one undetected colorectal cancer). FIT had similar diagnostic accuracy in patients meeting DG30 (low risk) or revised NG12 (medium–high risk) symptoms (Table 3). For the 138 patients in the DG30 group, at faecal haemoglobin thresholds of 2 µg/g or 10 µg/g, FIT detected every colorectal cancer. For the 160 patients in the NG12 group, FIT detected every colorectal cancer at a faecal haemoglobin threshold of 2 µg/g but missed one colorectal cancer at a threshold of 10 µg/g.
Table 3.
Sensitivity and specificity of the faecal immunochemical test for colorectal cancer by faecal haemoglobin (µg/g) threshold according to revised National Institute for Health and Care Excellence criteria (NG12 compared with DG30).
| Disease | Faecal haemoglobin (µg/g) | Sensitivity (%) | Specificity (%) | ||
| NG122 | DG305 | NG122 | DG305 | ||
| Cancer/inflammatory bowel disease/high-risk adenoma | Detectable (> 2) | 81.8 | 66.7 | 75.4 | 82.9 |
| > 10 | 72.7 | 66.7 | 89.9 | 94.6 | |
| Cancer | > 2 | 100.0 | 100 | 71.05 | 82.1 |
| > 10 | 87.5 | 100 | 84.2 | 93.3 | |
| Inflammatory bowel disease | > 2 | 88.9 | 33.3 | 70.9 | 80.0 |
| > 10 | 77.8 | 33.3 | 84.1 | 90.3 | |
| High-risk adenoma | > 2 | 40.0 | 50 | 67.4 | 80.2 |
| > 10 | 40.0 | 50 | 89.4 | 91.2 | |
Discussion
Our pilot study has shown that in the group of symptomatic patients tested, FIT could be safely used to rule out bowel cancer and reduce the number of patients undergoing colonoscopy or other colonic investigations. If patients with undetectable faecal haemoglobin (below a threshold of 2 µg/g) had been triaged to follow-up in primary care, the referral rate would have been reduced by 70% and no cancers would have been missed. At a higher threshold of 10 µg/g, the referral rate would have been reduced by 84%, but colorectal cancer would have been missed in one patient, although sensitivity remained high at 92%. FIT had 100% sensitivity for colorectal cancer in low-risk patients at both thresholds of 2 µg/g and 10 µg/g, with a reduction in colonoscopies up to 91%. We found that a significant number of patients referred with symptoms had no disease detected at colonoscopy. This occurs in 34% of patients referred with high-risk two-week-wait symptoms and rises to 58% for patients referred with non-two-week-wait symptoms. This group of patients in particular will benefit from a non-invasive rule out-test such as FIT.
In July 2017, NICE issued DG30 guidance encouraging the use of FIT in low-risk patients using a cut-off threshold of 10 µg/g.5 In our study, 138 patients met DG30 criteria, of whom four patients (2.9%) were diagnosed with colorectal cancer, all with faecal haemoglobin levels greater than 10 µg/g. Our study seems to support the guidance, inasmuch as no colorectal cancer was missed below 10 µg/g in patients meeting DG30 criteria. However, our results may not be truly representative as we only tested a small group of patients with low-risk symptoms who had been vetted for colonoscopy and who agreed to return FIT samples. There is a larger group of patients with low-risk symptoms who were triaged to other investigations such as computed tomography colonoscopy or flexible sigmoidoscopy, or those managed in primary care without referral. Furthermore, the risk of type II error is high in this small, underpowered pilot study. Nonetheless, in our results, FIT would have reduced the number of low-risk patients undergoing colonoscopy by up to 91% without missing colorectal cancer. However, as DG30 does not issue guidance as to the duration or type of symptoms that warrant testing with FIT, a potentially enormous group of patients with low-risk symptoms may be eligible. Indiscriminate use of FIT in patients without any predefined symptom criteria may generate an even larger number of referrals due to false positive FIT results (elevated faecal haemoglobin with no bowel disease) with a specificity that ranged from 82.1% at a cut-off of 2 µg/g to 91.3% at 19 µg/g. While a higher cut-off for low-risk patients would reduce false positive results (Table 4), it risks not detecting colorectal cancer. As conceded by the Health Technology Assessment, adequately powered data on FIT in low-risk patients are still lacking.4
Strengths and limitations
We would be cautious of the 100% sensitivity of FIT for colorectal cancer in this study, which has been replicated in some,11 but not all other studies using HM-JACKarc.12,13 We did not find significant variation in faecal haemoglobin values due to ethnicity, age or sex, but again this may be due to our study being underpowered. This may also explain why we found that faecal haemoglobin levels were higher (although not at a significance level of 0.05) with increasing deprivation, which has been reported previously in larger screening studies.14 Study recruitment was limited to a 48% return rate, but this is not dissimilar to the recruitment rates (35–54%) in other UK-based FIT research studies.12,16 Interestingly, the same problem was not encountered in a service improvement project with FIT in Nottingham, which recorded a much higher FIT return rate of 80.9%.17 Nonetheless, our study recruitment resulted in representation of Croydon’s ethnic diversity. The 4% incidence of colorectal cancer in the study population is in line with the 3–5% incidence in symptomatic patients predicted by NICE NG12 criteria.
One of the strengths of this study was that we used appropriate faecal haemoglobin cut-off points after calibration in the laboratory. Previous studies had set cut-off points of ‘detectable’ faecal haemoglobin at values greater than 0,13 and up to 7 µg/g.12 In practice, all values below 2 µg/g cannot be reliably distinguished from 0. Setting the definition of ‘detectable’ faecal haemoglobin at 2 µg/g in our study led to a much greater reduction in colonoscopies than previously published (67% vs 41.7%).14 While initial comparison studies between competing analysers have been published,18 further biochemical calibration studies are required to compare detectable and quantifiable faecal haemoglobin levels on multiple FIT analysers.
Comparison with existing literature
Our results in the multicultural community of south London suggest that FIT holds considerable promise as a rule-out test for colorectal cancer in symptomatic patients within a diverse population, in keeping with the studies on homogenous European populations,12,14 including those evaluated by the Health Technology Assessment and DG30.4,5 While the results are very encouraging, we are still cognizant of the limitations of the evidence base for FIT. The Health Technology Assessment and DG30 reviews are meta-analyses of small diagnostic accuracy studies on FIT (such as this one), which report a summary sensitivity and specificity but lack the requisite patient-level data to identify when or how colorectal cancer is not detected by FIT. There is still debate about which cut-off threshold to use, the optimum safety-netting pathway to minimise missed cancers, and whether FIT should be not be used with specific NICE referral criteria (eg iron deficiency anaemia).
Implications for research and practice
While FIT should not be expected to be a 100% accurate test, minimisation of missed cancers and delayed diagnosis will encourage patients and clinicians to use this test to its full potential. Large, adequately powered diagnostic accuracy studies are currently under way in England, which should provide answers to the above uncertainties. If used appropriately and under certain conditions that have yet to be defined, FIT offers the NHS the opportunity to revolutionise the management of symptomatic patients with suspected colorectal cancer. FIT could reduce the number of unnecessary colonoscopies and other bowel investigations, reduce costs and free up endoscopy capacity for bowel cancer screening. If the above conditions are identified, there is also the possibility that FIT may lead to early diagnosis, in that patients with symptoms may be willing to seek earlier medical advice knowing that they will undergo a simple and reliable test at home rather than referral for a colonoscopy in hospital.
Conclusion
In our ethnically diverse population, FIT detected all cases of colorectal cancer in symptomatic patients who had detectable faecal haemoglobin. FIT demonstrates potential to be used as a triage tool to reduce the number of patients referred for colonoscopy. Further data from the large cohort studies currently underway in England could help prevent missed cancers, if the patterns and causes of false negative results are identified.
Acknowledgements
We are grateful to Matthew Davis and the team at Alpha Laboratories, Carolyn Piggott and Cerin John at the Bowel Cancer Screening Southern Hub; Jyoti Ambhir, Ruth Woodfine and Toni Mitchell from Hammersmith Medical Research; Dr Michelle Chen from RM Partners; Carlene Parchment, Saidyousuf Ahmadi, Anthonette Dzakpasu-Amevor, Christine Nangendo and Olayinka Odunaiya from the NICE FIT team.
References
- 1.NHS England Waiting Times for Suspected and Diagnosed Cancer Patients: 2016–17 Annual Report. London: NHS; 2017. [Google Scholar]
- 2.National Institute for Health and Care Excellence Suspected Cancer: Recognition and Referral. NICE Guideline NG12. London: NICE; 2015. [PubMed] [Google Scholar]
- 3.Shenbagaraj L, Thomas-Gibson S, Stebbing J et al. Endoscopy in 2017: a national survey of practice in the UK. Frontline Gastroenterol 2019; : 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westwood M Coro-Ramos I, Lang S, et al. Faecal immunochemical tests to triage patients with lower abdominal symptoms for suspected colorectal cancer referrals in primary care: a systematic review and cost-effectiveness analysis. Health Technol Assess 2017; : 1–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence Quantitative Faecal Immunochemical Tests to Guide Referral for Colorectal Cancer in Primary Care. Diagnostics Guidance DG30. London: NICE; 2017. [PubMed] [Google Scholar]
- 6.Fraser CG, Rubeca T, Rpi S et al. Faecal haemoglobin concentrations vary with sex and age, but data are not transferable across geography for colorectal cancer screening. Clin Chem Lab Med 2014; : 1,211–1,216. [DOI] [PubMed] [Google Scholar]
- 7.Digby J, McDonald PJ, Strachan JA et al. Deprivation and faecal haemoglobin: implications for bowel cancer screening. Journal of medical screening 2014; : 95–97. [DOI] [PubMed] [Google Scholar]
- 8.Bossuyt PM, Reitsma JB, Bruns DE et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Radiology 2015; : 826–832. [DOI] [PubMed] [Google Scholar]
- 9.Fraser CG, Allison JE, Young GP et al. A Standard for Faecal Immunochemical TesTs for Haemoglobin Evaluation Reporting (FITTER). Ann Clin Biochem 2014; (Pt 2): 01–302. [DOI] [PubMed] [Google Scholar]
- 10.Fraser CG, Benton FC. Detection capability of quantitative faecal immunochemical tests for haemoglobin (FIT) and reporting of low faecal haemoglobin concentrations. Clin Chem Lab Med 2019; : 611–616. [DOI] [PubMed] [Google Scholar]
- 11.Godber IM, Todd LM, Fraser CG et al. Use of a faecal immunochemical test for haemoglobin can aid in the investigation of patients with lower abdominal symptoms. Clin Chem Lab Med 2016; : 595–602. [DOI] [PubMed] [Google Scholar]
- 12.Widlak MM, Thomas CL, Thomas MG et al. Diagnostic accuracy of faecal biomarkers in detecting colorectal cancer and adenoma in symptomatic patients. Aliment Pharmacol Ther 2017; : 354–363. [DOI] [PubMed] [Google Scholar]
- 13.Mowat C, Digby J, Strachan JA et al. Faecal haemoglobin and faecal calprotectin as indicators of bowel disease in patients presenting to primary care with bowel symptoms. Gut 2016; : 1,463–1,469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Digby J, Strachan JA, Fraser CG et al. Validation of the utility of a faecal immunochemical test for haemoglobin (FIT) in patients presenting to primary care with new bowel symptoms. United Eur Gastroenterol J 2017; (5 Suppl): P1111. [Google Scholar]
- 16.Turvill J, Mellen S, Jeffery L et al. Diagnostic accuracy of one or two faecal haemoglobin and calprotectin measurements in patients with suspected colorectal cancer. Scand J Gastroenterol 2018; : 1,526–1,534. [DOI] [PubMed] [Google Scholar]
- 17.Chapman C, Bunce J, Oliver S et al. Service evaluation of faecal immunochemical testing and anaemia for risk stratification in the 2-week-wait pathway for colorectal cancer. BJS Open 2019; : 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman C, Banerjea A, Ng O et al. PTU-076 The ‘getting fit’ project in Nottingham: a comparison of haemoglobin levels as measured by OC sensor and HM jack in two week wait referrals. Gut 2017; (Suppl 2): A88–A89. [Google Scholar]
- 19.National Institute for Health and Clinical Excellence Referral Guidelines for Suspected Cancer. Clinical Guideline CG27. London: NICE; 2005. [Google Scholar]