Abstract
Objective:
To evaluate serum adiponectin levels and organization into multimers in women with polycystic ovary syndrome (PCOS) and assess relationships between adiponectin, glucose tolerance, and insulin resistance.
Design:
In vivo and in vitro study.
Setting:
Outpatient clinic at university and Veterans hospitals in the United States and university laboratory.
Patient(s):
Thirty-one obese women with PCOS and six age- and body mass index (BMI)-matched normal cycling control subjects.
Intervention(s):
All subjects studied in the fasting state.
Main Outcome Measure(s):
A 75-g oral glucose tolerance test (OGTT), hyperinsulinemic/euglycemic clamp, circulating adiponectin levels, adipocyte adiponectin content, and organization of adiponectin into multimeric forms.
Result(s):
Whole body insulin action (glucose disposal rate, 5.61 ± 2.90 vs. 8.79 ± 0.81 mg/kg/min, PCOS and control) and adiponectin levels (9.5 ± 0.7 7 vs. 17.4 ± 1 μg/mL, PCOS vs. control) were significantly reduced in the subjects with PCOS. There were significant correlations between glucose tolerance, insulin action, and circulating adiponectin levels in all subjects. The content of adiponectin protein was reduced in subcutaneous adipocytes from subjects with PCOS (252 ± 31 vs. 388 ± 58 arbitrary units/10 μg protein). Subjects with PCOS had less of their circulating adiponectin organized into high molecular weight (HMW) multimeric complexes. Glucose-intolerant subjects with PCOS also had less intracellular HMW adiponectin.
Conclusion(s):
Both circulating adiponectin levels and the portion present as the most active HMW form are reduced in PCOS, with differences related to the degree of glucose intolerance and insulin resistance.
Keywords: Polycystic ovary syndrome, adiponectin, insulin resistance, adipocytes
Polycystic ovary syndrome (PCOS) affects an estimated 4%–12% of women of reproductive age (1). In addition to oligomenorrhea and hyperandrogenism, insulin resistance and glucose intolerance are common clinical features of the syndrome (2). Women with PCOS often manifest multiple metabolic abnormalities such as impaired glucose tolerance, type 2 diabetes, dyslipidemia, hypercoagulable state, hypertension, and obesity. Obesity, particularly visceral obesity, affects more than 50% of women with PCOS in North America, and exacerbates the reproductive and metabolic manifestations of the syndrome (3).
Adipose tissue is now appreciated as an integral component of the endocrine system, releasing multiple factors termed adipokines, which impact insulin sensitivity (4). Among these adipokines is adiponectin, a protein hormone produced exclusively in adipose tissue whose circulating levels are strongly positively correlated with measures of insulin sensitivity (5, 6), being reduced in insulin resistant states such as obesity and type 2 diabetes (7, 8). In vivo and in vitro studies in animal and cell models have suggested that adiponectin can have insulin-sensitizing actions (9). Consistent with such actions are the observations that after interventions that improve insulin action, such as the administration of thiazolidinediones and weight loss, circulating adiponectin levels increase in some, but not all, situations (10-13). The assembly of adiponectin into multimolecular forms has also been postulated to play a role in the regulation of insulin sensitivity, with a high molecular weight complex being the most active form (14, 15).
Although the presence of insulin resistance in PCOS has been well documented, the impact of PCOS on circulating adiponectin levels remains unclear, reduced in some reports (16), and unaltered in others (17). Several groups have reported that the most important determinant of circulating adiponectin levels in PCOS is the absence or presence of insulin resistance (18-21). The multimerization state of adiponectin in PCOS is unknown. We hypothesize that, like type 2 diabetes, circulating levels of the most active forms of adiponectin will be lower in PCOS, independent of obesity.
Thus, we evaluated circulating adiponectin levels and multimerization status in subjects with PCOS compared with age- and body mass index (BMI)-matched control subjects. We also investigated circulating adiponectin levels in relation to the extent of glucose tolerance and insulin resistance in PCOS.
MATERIALS AND METHODS
Subjects
Thirty-one women with PCOS and six normal cycling, non-hirsute control subjects were studied. The diagnosis of PCOS was made on the basis of criteria established at the 1990 National Institutes of Health Conference on PCOS: [1] evidence of chronic anovulation or oligomenorrhea by clinical history; [2] clinical (Ferriman-Gallwey score ≥8) or biochemical evidence of hyperandrogenism; and [3] the exclusion of other conditions. Pregnancy was ruled out by a urine pregnancy test. Normal thyroid and PRL function were established by hormonal evaluation. Late-onset non-classic congenital hyperplasia was excluded by basal 17-OH P values of <300 ng/dL (22). Exclusionary medications included glucocorticoids, antiandrogens, or oral contraceptives (OC) within the previous 30 days, ovulation induction agents, antiobesity medications, or insulin sensitizing agents within the previous 60 days. Presence of polycystic ovaries (PCO) was also examined by transvaginal ultrasonography. The control subjects were screened by medical history, physical examination, laboratory evaluation, and transvaginal ultrasound to confirm their healthy status.
None of the subjects were affected by other significant unstable medical illness. The procedures used were in accordance with the guidelines of the Helsinki Declaration on Human Experimentation. The study was conducted in the Special Diagnostic and Treatment Unit of the Veterans Affairs Medical Center (San Diego, CA) and the General Clinical Research Center, University of CA, San Diego. The experimental protocol was approved by the appropriate institutional review boards. The purpose of the protocol was explained both to the research subjects with PCOS and the control subjects, and written informed consent was obtained from all subjects before beginning the study.
Protocol
All laboratory evaluations were performed in the early or midfollicular phase of the spontaneous menstrual cycle, except in subjects who did not have regular or predictable menses. All subjects underwent venous blood sampling after an overnight fast. All blood samples were immediately centrifuged, and the serum was stored at −70°C until analysis.
Hormonal Evaluation
Serum concentrations of T were measured by well-established RIA (23) with intra-assay coefficients of variation (CVs) less than 7%. Serum 17α-hydroxyprogesterone (17-OHP) and DHEAS were measured by RIA with intra-assay CVs less than 7% (Diagnostic Systems Laboratories, Webster, TX). Sex hormone-binding globulin (SHBG) was determined by the DSL 6300 kit with intra- and interassay CVs of 2.5% and 3.73%, respectively.
Oral Glucose Tolerance Test
Blood samples were obtained at baseline and at 30-minute intervals up to 2 hours after the ingestion of a 75-g glucose load. Glucose tolerance was evaluated using the criteria of the American Diabetes Association (24). Plasma glucose levels were determined by the glucose oxidase method with a YSI Glucose Analyzer (Yellow Springs, OH).
Quantitative Assay for Circulating Adiponectin
Circulating adiponectin was measured in serum using a commercially available radioimmunoassay kit (Linco, St. Louis, MO). According to the manufacturer’s instructions, samples were diluted before the assay. All samples were assayed in duplicate. The lower limit of detection with this assay was 2 μg/mL; the interassay and intra-assay CVs were 7% and 11%, respectively.
Hyperinsulinemic Euglycemic Clamp
In vivo insulin action was determined by performing a 3-hour hyperinsulinemic (300 mU/m2/min) euglycemic (5 mM) clamp, as described previously (25, 26). A high-dose clamp was used to ensure suppression of hepatic glucose production and to evaluate peripheral insulin action as we have previously shown (26). The whole body glucose disposal rate (GDR) in each subject was determined from the values obtained during the steady-state period, the average of the values between the 140th and 160th minute and the 160th and 180th minute (26).
Analysis of Adiponectin Multimerization
Analysis of the multimerization status of adiponectin was performed by Western blotting after sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions. Equal volumes of serum or amounts of adipocyte extract protein (discussed later) were combined 1:1 with Lamielli sample buffer (27), prepared without β-mercaptoethanol. Samples were kept at room temperature during preparation. Samples equivalent to 2 μL of serum or 10 μg of cell protein were size fractionated on 2%–8% PAGE gels in a tris-acetate system. Proteins were transferred to nitrocellulose membranes and blocked with 5% nonfat dried milk. Membranes were incubated with a monoclonal antibody against human adiponectin (BD Bioscience, San Diego, CA) at a 1:500 dilution for 16 hours at 4°C. The secondary antibody was an antimouse IgG conjugated with horseradish peroxidase (Amersham, Arlington Heights, IL). Detection was by enhanced chemiluminescence, followed by densitometric analysis. Quantitative densitometry was performed using ChemImager software (Alpha Innotech Corp., San Leandro, CA).
Adipose Tissue Biopsy and Preparation of Human Adipocytes
Adipose tissue was obtained by needle biopsy of the lower subcutaneous abdominal depot using a 5-mm side-cutting needle. The adipose tissue biopsy was performed before initiation of the hyperinsulinemic/euglycemic clamp procedure. Lidocaine (1%) was infiltrated in a square field fashion and the biopsy was taken from the center of the field. Isolated adipocytes were prepared by a modification (28) of the method of Rodbell (29). After digestion and filtration, the cells were washed twice in a buffer consisting of 150 mM NaCl, 5 mM KC1, 1.2 mM MgSO4, 1.2 mM CaC12, 2.5 mM NaH2PO4, 10 mM HEPES, 2 mM pyruvate, pH 7.4, supplemented with 4% bovine serum albumin (BSA). The cells were then washed twice in a buffer (HEPES washing salts [HWS]) consisting of: 116mM NaCl, 5 mM KCl, 0.5mM MgSO4, 0.7mM CaCl2, 25 mM HEPES, 5 mM glucose, 2% BSA, pH 7.4 and resuspended at ~5 x 104–10 x 104 cells/mL before cell extraction.
Adipocyte Extraction
Cells were concentrated by centrifugation (50 x g), then rapidly washed twice in 17°C, BSA-free HWS buffer, as described previously (30). A 2x-concentrated solubilization buffer was then added—final concentrations: 20 mM Tris-HCl, 145 mM NaCl, 10% glycerol, 5 mM EDTA, 1% Triton X-100, 0.5% NP-40, 200 μM sodium orthovanadate, 200 μM PMSF, 1 μM leupeptin, 10 mg/mL aprotinin, pH 7.5. After lysis/extraction for 30 minutes at 4°C with repeated vortexing, nonsolubulized material was removed by centrifugation at 14,000 x g (10 minutes, 4°C) and the cell extracts stored at −70°C before analysis.
Statistical Analysis
Statistical analyses were performed using unpaired Student’s t-test and linear regression analysis (Pearson coefficient). Multiple linear regression analysis was performed after log transformation of T levels to achieve normal distribution. Data are presented as mean ± SE with a P<.05 considered statistically significant.
RESULTS
Subject Characteristics
The basal clinical and hormonal characteristics of the PCOS and normal control subjects are given in Table 1. Because subjects with PCOS in our catchments area are predominantly obese (BMI ≥ 30), normal subjects with comparable obesity were also studied. The PCOS and control subjects were matched for age and BMI. There were no significant differences in waist circumference and waist-to-hip ratio between subject groups. As expected, subjects with PCOS had significantly higher Ferriman-Gallwey scores, higher levels of total T, DHEAS, and 17-OHP. The SHBG levels were lower only in subjects with PCOS who demonstrated an impaired glucose response (P=.05).
TABLE 1.
Clinical characteristics of subjects.
| PCOS | ||||
|---|---|---|---|---|
| Control (n = 6) | NGT (n = 13) | IGR (n = 18) | P value | |
| Age (year) | 32 ± 2 | 29 ± 1 | 29 ± 1 | NS |
| BMI (kg/m2) | 36.2 ± 2.8 | 34.7 ± 2.0 | 35.8 ± 1.8 | NS |
| WHR | 0.87 ± 0.06 | 0.90 ± 0.03 | 0.87 ± 0.03 | NS |
| Ferriman-Gallwey score | 3 ± 1 | 12 ± 2 | 14 ± 2 | <.05a |
| Total T (ng/dL) | 19.3 ± 2.9 | 37.2 ± 6.0 | 38.6 ± 4.3 | <.05a |
| DHEAS (μg/dL) | 115 ± 13 | 228 ± 21 | 250 ± 22 | <.05a |
| 17-OHP (ng/mL) | 1.20 ± 0.18 | 2.26 ± 0.28 | 1.77 ± 0.19 | <.05c |
| SHBG (μg/dL) | 0.55 ± 0.12 | 0.61 ± 0.06 | 0.42 ± 0.09 | <.05b |
Note: Results are average ± SE. NGT = normal glucose tolerant; IGR = impaired glucose response; BMI = body mass index; WHR = waist-to-hip ratio; 17-OHP = 17α-hydroxyprogesterone; SHBG = sex hormone-binding globulin; NS = not significant.
Both NGT and IGR vs. control.
P<.05 IGR vs. NGT and control.
P<.05 NGT vs. control.
Metabolic characteristics of the subjects are presented in Table 2. Responses to the oral glucose tolerance test (OGTT) revealed that 60% of our subjects with PCOS had a 2-hour glucose value of ≥ 140 mg/dL. Those individuals were designated as having an impaired glucose response (IGR). Subjects with PCOS with 2-hour glucose values <140 mg/dL are designated as normal glucose tolerant (NGT). There were no significant differences in glycosylated hemoglobin levels between subject groups, whereas fasting glucose in the NGT-PCOS group was marginally lower than in the normal controls, possibly due to the elevated insulin levels in these subjects.
TABLE 2.
Metabolic characteristics.
| PCOS | ||||
|---|---|---|---|---|
| Control (n = 6) | NGT (n = 13) | IGR (n = 18) | P value | |
| HbA1c (%) | 5.3 ± 0.1 | 5.2 ± 0.1 | 5.7 ± 0.2 | NS |
| Fasting glucose (mg/dL) | 97 ± 2 | 90 ± 2 | 95 ± 4 | <.05a |
| 2-h glucose (mg/dL) | 122 ± 4 | 113 ± 2 | 185 ± 7 | <.05a,b |
| Fasting insulin (μU/mL) | 10 ± 2 | 22 ± 3 | 36 ± 5 | <.05a,b |
Note: Results are average ± SE. NGT = normal glucose tolerant; IGR = impaired glucose response; HbA1c = glycosylated hemoglobin; NS = not significant.
P<.05 NGT vs. control by unpaired t-test.
P<.05 IGT vs. NGT and control.
Whole Body Insulin Action
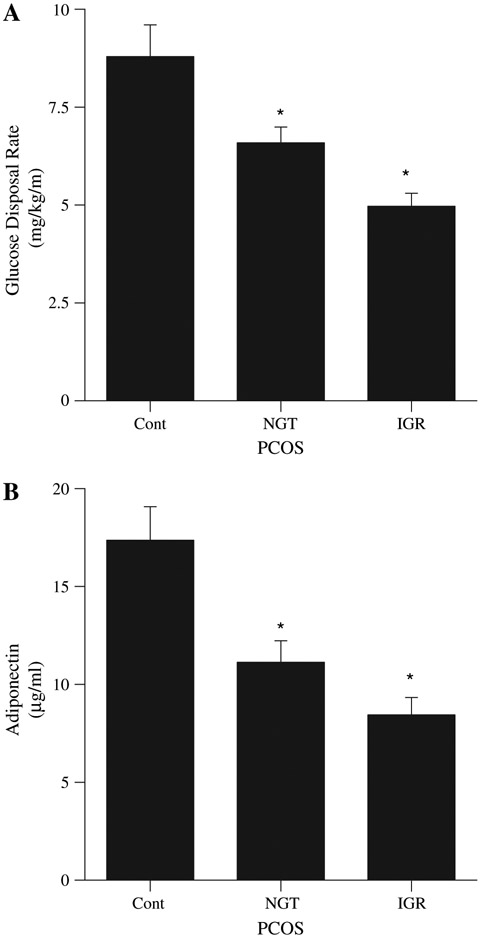
Comparisons of whole body insulin action, as calculated from the hyperinsulinemic euglycemic clamp procedure, revealed a significant decrease in glucose disposal rate in all subjects with PCOS compared with control subjects (5.61 ± 2.90 vs. 8.79 ± 0.81 mg/kg/min, P<.02). An impact of glucose intolerance on whole body insulin action was evident as indicated by statistically significant decreases in glucose utilization and whole body insulin action in subjects with PCOS compared with control, with the greatest impairment in whole body glucose disposal in the IGR-PCOS subjects (Fig. 1A). Based on this observation, we performed a correlation analysis of 2-hour glucose values with GDR and found a significant inverse correlation for all subjects combined (r = −0.547, P<.001). This correlation remained significant when evaluating GDR with the absolute increment in 2-hour glucose levels over fasting values (r = −0.496, P<.01). The relationship was the same for control and PCOS subjects when evaluated as separate groups.
FIGURE 1.
Whole body insulin action (insulin-stimulated glucose disposal rate) (A) and circulating adiponectin levels (B) in normal cycling control subjects (control [Cont], n = 6) and subjects with polycystic ovary syndrome (PCOS) categorized as normal glucose tolerant (NGT, n = 13), and impaired glucose response (IGR, n = 18). All values reported as average ±SEM. *P<.02 vs. control.
Circulating Adiponectin Levels
In all subjects with PCOS fasting circulating serum adiponectin levels were lower compared with those of normal controls (9.5 ± 0.7 vs. 17.4 ± 1.7 μg/mL, P<.005). As with GDR we noted a spectrum of adiponectin levels. When subjects with PCOS were categorized by glucose response to the OGTT, differences became more marked, revealing a significantly lower fasting adiponectin level in IGR-PCOS women compared with control subjects (8.4 ± 0.9 μg/mL, P<.001; Fig. 1B). In NGT-PCOS subjects circulating adiponectin levels were intermediate (11.13 ± 1.10, P<.01 vs. control, P=.065 vs. IGR).
To explore whether the presence of glucose intolerance was predictive of metabolic abnormalities, such as reduced adiponectin levels, a correlation analysis was performed. This revealed significant relationships between circulating adiponectin levels and two indicators of glucose tolerance: absolute 2-hour glucose values after oral glucose challenge (r = −0.3996, P<.02) and the increment in 2-hour glucose after OGTT (r = −0.418, P<.01) for all the subjects. There was also a statistically significant correlation between adiponectin and GDR (r = 0.3939, P<.05). There was no correlation between fasting glucose and circulating adiponectin levels (data not shown). With regard to the hyperandrogenemia of PCOS, there were no correlations between adiponectin and either total (r = −0.009, P=.96) or free T levels (r = −03339, P=.2239). Once again, the same relationships were present in control and PCOS subjects when evaluated separately. However, when multiple linear regression analysis was performed with adiponectin as the dependent variable and GDR and T as predictors, both GDR (standardized β = −0.444, P=.004) and total T (β = −0.477, P=.002) were identified as predictors of adiponectin levels.
Adiponectin Multimerization
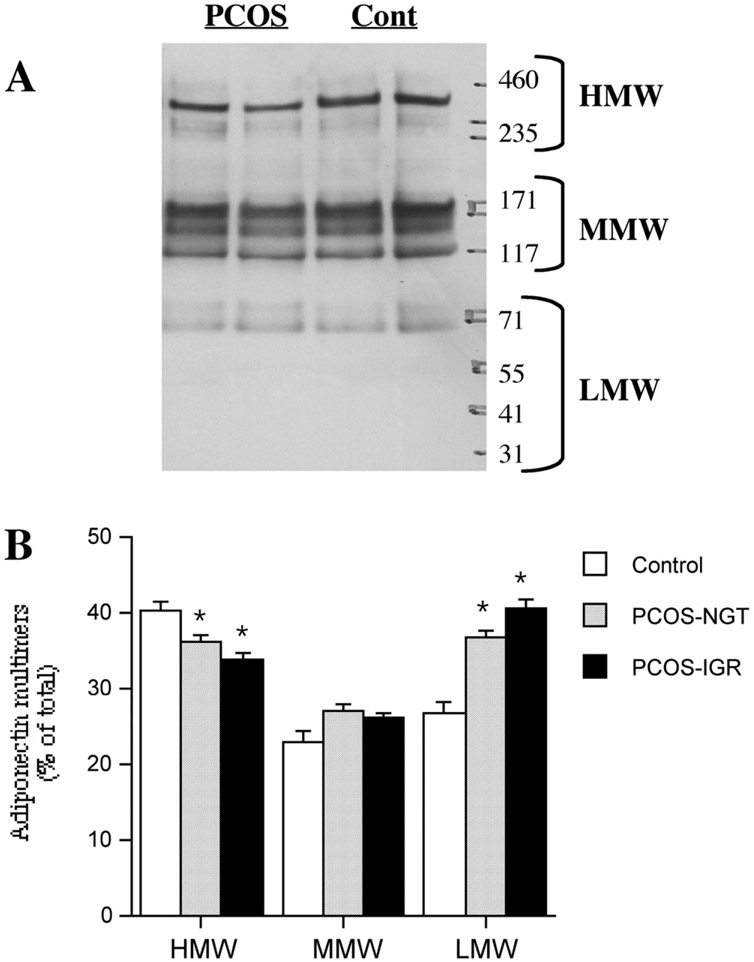
Adiponectin exists in the circulation as higher order forms, predominantly a hexamer and a larger multimer designated as the high molecular weight form (HMW) (14, 31). We evaluated the multimerization status of adiponectin in our subjects by gel electrophoresis under nonreducing conditions followed by Western blotting. With this method at least six immunoreactive forms of adiponectin were detected in the serum (Fig. 2A), a pattern similar to those seen by other investigators (31-33). Following the practice of other investigators, the different multimers were placed in three groups of high (HMW), medium (MMW), and low molecular weight (LMW) forms (Fig. 2A). The HMW forms could be converted in a temperature-dependent manner to MMW and LMW forms. High temperature (100°C) and chemical reduction (β-mercaptoethanol) were needed to convert all immunoreactive adiponectin to the ~32-kDa monomer (not shown).
FIGURE 2.
Distribution of adiponectin between multimers in the serum of control, normal glucose tolerant– polycystic ovary syndrome (NGT-PCOS) and impaired glucose response (IGR)-PCOS subjects. Equal amounts (2 μL) of serum loaded for each individual. (A) Representative Western blots of 2%−8% tris-acetate gels run under nonreducing conditions. (B) Quantitation of blots. Results are expressed as percent of the total densitometric signal for each individual, within each lane. Results are average ±SEM, n = 6 for control, 10 for NGT, 16 for IGR. *P<.05 vs. control (Cont). HMW = high molecular weight; MMW = medium molecular weight; LMW = low molecular weight.
Taking the sum of the signals within each individual lane as an indicator of the total adiponectin content of that sample showed that the values were highest in control subjects (8.3 ± 0.7 arbitrary units/2 μL serum), lowest in IGR-PCOS (4.7 ± 0.4, P<.001 vs. control) subjects, and intermediate in the NGT-PCOS group (5.7 ± 0.4, P<.01 vs. control). This was the same pattern seen with adiponectin levels determined by RIA (Fig. 1B). Several differences in the distribution of adiponectin between multimeric complexes were observed. The proportion of circulating adiponectin present in the highest molecular weight forms (HMW) was reduced in both groups of PCOS subjects compared to controls (Fig. 2B). This difference was also seen for the absolute amounts of the HMW form among groups (2.02, 1.23, and 0.98 arbitrary units for control, NGT, and IGR, respectively). Accordingly, both PCOS groups had proportionally more of their adiponectin in a lower molecular weight form (~90 kDa). Although total T was not associated with total adiponectin levels, there was an inverse relationship between total T and the percentage of circulating adiponectin in the HMW form (r .= −0.448, P<.05).
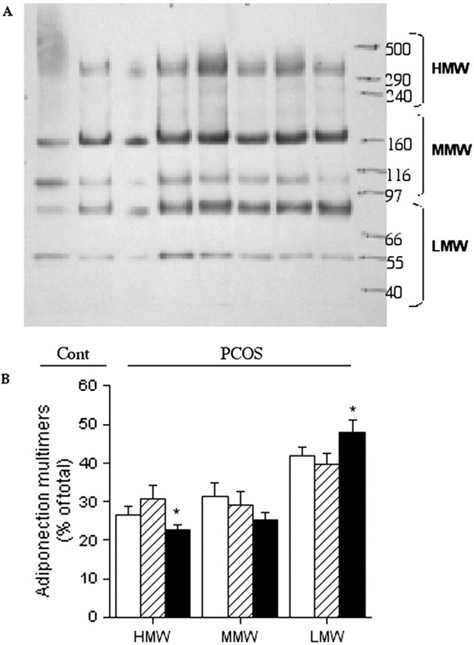
Because adiponectin is produced exclusively in adipocytes (34-36), we investigated the adipocyte content of adiponectin to determine the sources of the quantitative and qualitative differences seen in the circulation. The patterns of multimeric forms within adipocytes (Fig. 3A) differed from those observed in the circulation (Fig. 2A). This was true for all subjects. The largest HMW form was not detectable within adipocytes, whereas LMW forms were readily identifiable, with longer exposure of the blots, the ~32-kDa adiponectin monomer was observed in adipocytes but not seen in the serum. The total adipocyte content of adiponectin, determined by the sum of the signals for each sample, was similar in NGT (267 ± 58 arbitrary units/10 μg total cell protein) and IGR (247 ± 37 arbitrary units/10 μg total cell protein) subjects. When the data were pooled, PCOS adipocytes contained less adiponectin that control adipocytes (252 ± 31 vs. 388 ± 58 arbitrary units/10 μg total cell protein, P<.05). The IGR-PCOS adipocytes contained a lower proportion of the HMW form (P<.05) and more of the LMW form (Fig. 3B).
FIGURE 3.
Distribution of adiponectin between multimers in adipocytes of control, normal glucose tolerant– polycystic ovary syndrome (PCOS) and impaired glucose response-PCOS subjects. (A) Representative Western blots. Equal amounts of cell protein (10 mg) loaded for each individual. (B) Quantitation of blots. Results are expressed as percent of the total densitometric signal in each lane for each individual. Results are average ±SEM, n = 6 for control, 6 for NGT, and 16 for IGR. *P<.05 vs. control (Cont). HMW = high molecular weight; MMW = medium molecular weight; LMW = low molecular weight.
DISCUSSION
Adipokines are protein hormones secreted by adipose tissue that play an integral role in glucose and lipid metabolism (4). Among this family of proteins, adiponectin is potentially important in the control of energy metabolism and insulin action. Unlike other adipokines, such as leptin, circulating levels of adiponectin decrease with increasing adiposity (7). In addition, adiponectin levels are lower in insulin resistant states such as type 2 diabetes and obesity (7, 8) and have been shown in some studies to increase with improvement of insulin sensitivity and weight loss (10-13). In addition, several studies suggest that low adiponectin levels predict development of type 2 diabetes (5, 6, 37, 38). A strong sexual dimorphism has been found for circulating adiponectin levels, independent of the contributions of obesity or diabetes, with higher levels in women (14, 31, 39).
In PCOS, the roles of adiponectin and glucose intolerance, or insulin resistance, remains undefined. One might expect that with the increased prevalence of insulin resistance, glucose intolerance, and type 2 diabetes in PCOS, there might be a significant decrease in circulating adiponectin levels in women with this disorder. However, initial reports revealed conflicting results with regard to adiponectin levels in PCOS. Some investigators showed that circulating adiponectin levels were not different between subjects with PCOS and BMI-matched controls (16). However, other investigators have reported a significant decrease in adiponectin levels in obese women with PCOS compared to normal weight subjects with and without PCOS (17). More recently it has been observed that insulin sensitivity is an independent determinant of circulating adiponectin levels in individuals with PCOS (18-21).
In the current study, we showed that, when controlled for BMI, adiponectin is lower in subjects with PCOS compared to that observed in normal cycling women. In particular, the reduction in circulating adiponectin was correlated with the degree of glucose intolerance in women with PCOS. These findings suggest that although adiponectin levels are lower in women with PCOS, it is the degree of glucose intolerance and insulin resistance that is the strongest predictor of the decrement in circulating adiponectin levels, which would be in agreement with these recent publications. Such metabolic heterogeneity may account for some of the differences in the current literature regarding a relationship between PCOS and circulating adiponectin. Evidence suggests that the subcutaneous adipose tissue depot is a major source of adiponectin (40), therefore the lower adiponectin protein content of subcutaneous adipocytes from subjects with PCOS reported in the present study would contribute to the reduced circulating levels. This could be due to decreased synthesis but does not appear to involve sequestration of adiponectin in adipocytes.
Beyond associations with metabolism, there is a sexual dimorphism for adiponectin that has been seen in both rodents and humans after puberty, with significantly higher levels in women (39, 41). Both in vitro and in vivo studies have demonstrated that T treatment can suppress adiponectin release (39), which suggests that elevated T levels in subjects with PCOS may contribute to lower circulating adiponectin levels in these individuals. However, although our normal glucose tolerant and impaired glucose response PCOS subjects displayed equal elevations in total T (of approximately twofold) compared to control subjects, they differed in adiponectin levels. In addition, there were no correlations between total or free T and circulating adiponectin levels, as has been found by several other investigators (17, 19). Thus, it does not appear that T is necessarily the primary factor responsible for the lower adiponectin level in the impaired glucose response subjects, although multiple linear regression analysis indicates that insulin sensitivity (GDR) and T each contribute to adiponectin levels.
There are other possibilities for the differences in glucose tolerance and insulin action in subjects with PCOS. Circulating levels of total adiponectin reveal only part of the story. Although adiponectin is synthesized as a 32-kDa monomer, it rapidly multimerizes in the adipocyte to higher order forms and these multimers are released into the circulation (14, 31). The major circulating forms include a trimer, the building block for higher order forms, a hexamer, and high molecular weight forms (HMW) that may contain as many as six hexamers (14, 15). In vivo and in vitro studies indicate that the highest order forms exert the greatest influence on glucose and lipid metabolism (42). In fact, it is the ratio of HMW forms to total adiponectin in the circulation that has the strongest association with glucose tolerance (43) and tracks closely with improvements in insulin sensitivity after thiazolidinedione treatment (42). Interestingly, both human and rodent females have a greater proportion of the HMW form in their circulation compared to their male counterparts (14, 31, 43). Meanwhile, T treatment can also reduce secretion from adipocytes of the HMW form (44). The current results would be consistent with that observation, as the NGT and IGR PCOS groups with similar average T levels display similar reductions in circulating HMW adiponectin.
In summary, the current results indicate that there are both quantitative (lower total levels) and qualitative (fewer HMW forms) differences in adiponectin in individuals with PCOS, which are independent of obesity. That the differences from normal control subjects are greatest in the most insulin resistant/glucose intolerant subjects strengthens the importance of the specific nature as well as absolute amounts of adiponectin in regulating insulin sensitivity. Thus, adiponectin synthesis/secretion and organization into multimeric forms may each represent targets for therapeutic intervention in PCOS.
Acknowledgments:
We thank Susan Phillips, M.D., of the University of California, San Diego (UCSD) and Deborah Oh, M.D., Ph.D., of the VA San Diego Healthcare System (VASDHS) and UCSD for assistance with the determination of adiponectin multimers, Sunder R. Mudaliar, M.D., and Sunita Baxi, M.D., of VASDHS, Janet Yazdi, M.D., of UCSD for the recruitment and care of subjects, Paivi Burke, R.N., of VASDHS for nursing assistance, and Leslie Carter, B.S., of UCSD and VASDHS for technical assistance.
Supported by the Department of Veterans Affairs, the VA San Diego Healthcare System, the American Diabetes Association (T.P.C., R.R.H.), American Diabetes Association Mentor-based Fellowship (R.R.H.), NIH ROI-DK-258291 (R.R.H.), National Institute of Child Health and Human Development/National Institutes of Health through cooperative agreement (U54 HD 12303-20) as part of the Specialized Cooperative Centers Program in Reproduction Research (R.J.C.), Takeda Pharmaceuticals North America (R.R.H.), and grant MO1 RR-00827 from the General Clinical Research Branch, Division of Research Resources, National Institutes of Health.
Footnotes
Presented at 64th Scientific Sessions of the American Diabetes Association, Orlando, Florida, June 4–8, 2004.
REFERENCES
- 1.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endo Metabol 1998;83:3078–82. [DOI] [PubMed] [Google Scholar]
- 2.Chang RJ, Nakamura RM, Judd HL, Kaplan SA. Insulin resistance in nonobese patients with polycystic ovarian disease. J Clin Endocrinol Metab 1983;57:356–9. [DOI] [PubMed] [Google Scholar]
- 3.Cattrall FR, Healy DL. Long-term metabolic, cardiovascular and neoplastic risks with polycystic ovarian syndrome. Best Pract Res Clin Obstet Gynaecol 2004;18:803–12. [DOI] [PubMed] [Google Scholar]
- 4.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004;89:2548–56. [DOI] [PubMed] [Google Scholar]
- 5.Daimon M, Oizumi T, Saitoh T, Kameda W, Hirata A, Yamaguchi H, et al. Decreased serum levels of adiponectin are a risk factor for the progression to type 2 diabetes in the Japanese population: the Funagata study. Diabetes Care 2003;26:2015–20. [DOI] [PubMed] [Google Scholar]
- 6.Snehalatha C, Mukesh B, Simon M, Viswanathan V, Haffner SM, Ramachandran A. Plasma adiponectin is an independent predictor of type 2 diabetes in Asian Indians. Diabetes Care 2003;26:3226–9. [DOI] [PubMed] [Google Scholar]
- 7.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 2001;86:1930–5. [DOI] [PubMed] [Google Scholar]
- 8.Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes 2001;50:1126–33. [DOI] [PubMed] [Google Scholar]
- 9.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Medicine 2001;7:941–6. [DOI] [PubMed] [Google Scholar]
- 10.Hirose H, Kawai T, Yamamoto Y, Taniyama M, Tomita M, Matsubara K, et al. Effects of pioglitazone on metabolic parameters, body fat distribution, and serum adiponectin levels in Japanese male patients with type 2 diabetes. Metabolism 2002;51:314–7. [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki Y, Mahankali A, Wajcberg E, Bajaj M, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on circulating adiponectin levels and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 2004;89:4312–9. [DOI] [PubMed] [Google Scholar]
- 12.Yu JG, Javorschi S, Henever AL, Kruszynska YT, Norman RA, Sinha M, et al. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese and type 2 diabetic subjects. Diabetes 2002;51:2968–74. [DOI] [PubMed] [Google Scholar]
- 13.Phillips S, Ciaraldi T, Kong A, Bandukwala R, Aroda V, Carter L, et al. Modulation of circulating and adipose tissue adiponectin levels by anti-diabetic therapy. Diabetes 2003;52:667–74. [DOI] [PubMed] [Google Scholar]
- 14.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem 2003;278:9073–85. [DOI] [PubMed] [Google Scholar]
- 15.Tsao T-S, Tomas E, Murrey HE, Hug C, Lee DH, Ruderman NB, et al. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J Biol Chem 2003;278:50810–7. [DOI] [PubMed] [Google Scholar]
- 16.Orio F, Palomba S, Cascella T, Milan G, Mioni R, Pagano C, et al. Adiponectin levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2003;88:2619–23. [DOI] [PubMed] [Google Scholar]
- 17.Panidis D, Kourtis A, Farmakiotis D, Mouslech T, Rousso D, Koliakos G. Serum adiponectin levels in women with polycystic ovary syndrome. Hum Repro 2003;18:1790–6. [DOI] [PubMed] [Google Scholar]
- 18.Seplilian V, Nagamani M. Adiponectin levels in women with polycystic ovary syndrome and severe insulin resistance. J Soc Gynecol Invest 2005;12:129–34. [DOI] [PubMed] [Google Scholar]
- 19.Sieminska L, Marek B, Kos-Kudla B, Niedziolka D, Kajdaniuk D, Nowak M, et al. Serum adiponectin in women with polycystic ovarian syndrome and its relation to clinical, metabolic and endocrine parameters. J Endocrinol Invest 2004;27:528–34. [DOI] [PubMed] [Google Scholar]
- 20.Spranger J, Mohlig M, Wegewitz U, Ristow M, Pfeiffer AFH, Schill T, et al. Adiponectin is independently associated with insulin sensitivity in women with polycystic ovary syndrome. Clin Endocrinol 2004;61:738–46. [DOI] [PubMed] [Google Scholar]
- 21.Xita N, Georgiou I, Chatzikyriakidou A, Vounatsou M, Papassotiriou G-P, Papassotiriou I, et al. Effect of adiponectin gene polymorphism on circulating adiponectin and insulin resistance indexes in women with polycystic ovary syndrome. Clin Chem 2005;51:416–23. [DOI] [PubMed] [Google Scholar]
- 22.Lane DE. Polycystic ovary syndrome and its differential diagnosis. Obstet Gynecol Surv 2006;61:125–35. [DOI] [PubMed] [Google Scholar]
- 23.Coffler MS, Patel KD, M.H., Yoo RY, Malcom PJ, Chang RJ. Enhanced granulosa cell responsiveness to follicle-stimulating hormone during insulin infusion in women with polycystic ovary syndrome treated with pioglitazone. J Clin Endo Metabol 2003;88:5624–31. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2004;27:S5–10. [DOI] [PubMed] [Google Scholar]
- 25.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J. Physiol 1979;237:E214–23. [DOI] [PubMed] [Google Scholar]
- 26.Thornburn AW, Gumbiner B, Bulacan F, Wallace P, Henry RR. Intracellular glucose oxidation and glycogen synthase activity are reduced in non-insulin dependent (type II) diabetes independent of impaired glucose uptake. J Clin Invest 1990;85:522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;27:680–5. [DOI] [PubMed] [Google Scholar]
- 28.Ciaraldi TP, Kolterman OG, Scarlett JA, Kao M, Olefsky JM. Role of glucose transport in the post-receptor defect of non-insulin dependent diabetes mellitus. Diabetes 1982;31:1016–22. [DOI] [PubMed] [Google Scholar]
- 29.Rodbell M Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem 1964;239:375–80. [PubMed] [Google Scholar]
- 30.Thies RS, Molina JM, Ciaraldi TP, Freidenberg GR, Olefsky JM. Insulin-receptor autophosphorylation and endogenous substrate phosphorylation in human adipocytes from control, obese, and NIDDM subjects. Diabetes 1990;39:250–9. [DOI] [PubMed] [Google Scholar]
- 31.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, et al. Impaired multimerization of human adiponectin mutants associated with diabetes: molecular structure and multimer formation of adiponectin. J Biol Chem 2003;278:40352–63. [DOI] [PubMed] [Google Scholar]
- 32.Peake PW, Kriketos AD, Campbell IV, Shen Y, Charlesworth JA. The metabolism of isoforms of human adiponectin: studies in human subjects and in experimental animals. Eur J Endocrinol 2005;153:409–17. [DOI] [PubMed] [Google Scholar]
- 33.Bodles A, Banga A, Rasouli N, Ono F, Kern PA, Owens RJ. Pioglitazone increases secretion of high molecular weight adiponectin from adipocytes. Am J Physiol Endocrinol Metab 2006;291:E1100–5. [DOI] [PubMed] [Google Scholar]
- 34.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (adipose most abundant gene transcript 1). Biochem Biophys Res Commun 1996;221:286–9. [DOI] [PubMed] [Google Scholar]
- 35.Das K, Lin Y, Wilden E, Zhang Y, Scherer PE. Chromosomal localization, expression patters, and promoter analysis of the mouse gene encoding adipocyte-specific secretory protein Acrp30. Biochem Biophys Res Commun 2001;280:1120–9. [DOI] [PubMed] [Google Scholar]
- 36.Scherer PE, Williams S, Fogliano MF, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 1995;270:26746–9. [DOI] [PubMed] [Google Scholar]
- 37.Bb Duncan, Schmidt MI, Pankow JS, Bang H, Couper D, Ballantyne CM, et al. Adiponectin and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 2004;53:2473–8. [DOI] [PubMed] [Google Scholar]
- 38.Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PT, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 2002;360:57–8. [DOI] [PubMed] [Google Scholar]
- 39.Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, et al. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes 2002;51:2734–41. [DOI] [PubMed] [Google Scholar]
- 40.Fisher FM, McTernan PG, Valsamakis G, Chetty R, Harte AL, Anwar AJ, et al. Differences in adiponectin protein expression: effect of fat depots and type 2 diabetic status. Horm Metab Res 2002;34:650–4. [DOI] [PubMed] [Google Scholar]
- 41.Bottner A, Kratzsch J, Muller G, Kapellen TM, Bluher S, Keller E, et al. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endo Metabol 2004;89:4053–61. [DOI] [PubMed] [Google Scholar]
- 42.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity J. Biol Chem 2004;279:12152–62. [DOI] [PubMed] [Google Scholar]
- 43.Aso Y, Yamamoto R, Wakabayashi S, Uchida T, Takayanagi K, Takebayashi K, et al. Comparison of serum high-molecular weight (HMW) adiponectin with total adiponectin concentrations in type 2 diabetic patients with coronary artery disease using a novel enzyme-linked immunoabsorbent assay to detect HMW adiponectin. Diabetes 2006;55:1954–60. [DOI] [PubMed] [Google Scholar]
- 44.Xu A, Chan KW, Hoo RLC, Wang Y, Tan KCB, Zhang J, et al. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem 2005;280:18073–80. [DOI] [PubMed] [Google Scholar]