Abstract
Aim
The aim of this study is to assess if left bundle branch pacing (LBBP) can preserve physiological cardiac synchrony and deliver favorable hemodynamic effects.
Methods
Consecutive patients undergoing dual chamber pacemaker implantation for sick sinus syndrome (SSS) and a normal cardiac function with a narrow QRS complex were recruited for the study. Electrocardiogram and echocardiographic examinations were performed during ventricular pacing‐on and native‐conduction modes. The QRS duration (QRSd), systolic dyssynchrony index (SDI), and the standard deviation of time‐to‐peak contraction velocity in left ventricular (LV) 12 segments (Tsd‐12‐LV) were measured to evaluate LV synchrony. The stroke volume (SV) and the degree of atrioventricular valvular regurgitation were also assessed.
Results
A total of 40 patients underwent LBBP, while another 38 patients underwent right ventricular septum pacing (RVSP) as control group. Baseline characteristics were similar between the two groups. With LBBP, the paced QRSd was slightly wider than the intrinsic QRSd (101.03 ± 8.79 ms vs 91.06 ± 14.17 ms, P < .0001) while the LV mechanical synchrony during LBBP pacing mode was similar to that of native‐conduction mode (SDI, 3.14 ± 2.49 vs 2.70 ± 1.68, P = 0.129; Tsd‐12‐LV, 26.43 ± 15.55 vs 25.61 ± 16.07, P = .671) in the LBBP group. The LV synchrony in the LBBP group was superior to the RVSP group significantly. No significant differences in SV (64.08 ± 16.97 mL vs 65.45 ± 18.68 mL, P = .241) or the degree of atrioventricular valvular regurgitation were noted between LBBP capture and native‐conduction modes.
Conclusion
LBBP could preserve satisfactory LV synchrony and result in favorable hemodynamic effects.
Keywords: cardiac mechanical synchrony, echocardiography, hemodynamic effects, left bundle branch pacing, physiological pacing, right ventricular septum pacing, sick sinus syndrome
1. INTRODUCTION
Conventional right ventricular pacing (RVP) is deemed as the standard treatment for patients with bradycardia arrhythmias. However, long‐term RVP can cause cardiac electromechanical asynchrony, impair cardiac contractility, and increase risk of death and hospitalization due to heart failure. 1 , 2 , 3 In recent years, His bundle pacing (HBP) has been established as a relatively mature method for physiological pacing because it preserves more physiological electromechanical activation of the left ventricle (LV). 4 , 5 , 6 , 7 However, HBP is technically challenging due to its anatomical location and high capture threshold. 8 In 2017, Huang et al 9 reported a new pacing method, termed as “left bundle branch pacing (LBBP),” in a patient with heart failure with left bundle branch block. Since then, several studies have demonstrated that LBBP is a feasible technique, with a low capture threshold and narrow paced QRS duration (QRSd). 10 , 11 , 12 However, research on cardiac synchrony of LBBP is limited, 11 , 13 and therefore more specific and reliable data are needed to evaluate the physiology of LBBP. In present study, we aimed to determine if LBBP could preserve electrical and mechanical synchrony and improve hemodynamic effects.
2. METHODS
2.1. Patients and ethical considerations
This was an observational study. Patients diagnosed with sick sinus syndrome (SSS) and normal cardiac function with a narrow QRS complex (QRSd < 120 ms) who were referred for dual‐chamber pacemaker implantation at Xiamen Cardiovascular Hospital, Xiamen University between February 2018 and May 2019 were recruited consecutively in this study. The exclusion criteria were atrioventricular block, cardiomyopathy, old myocardial infarction, congenital heart disease, valvular heart disease, and insufficient image quality. The study protocol was approved by the Research Ethics Committee of Xiamen Cardiovascular Hospital, Xiamen University. Our study was carried out in accordance with the Declaration of Helsinki and approval guidelines from the ethics committee. Informed consent was obtained from each patient before participation.
2.2. Pacemaker and lead implantation
LBBP was achieved by a transventricular‐septal method described elsewhere. 14 , 15 The pacing leads (models 3830, Medtronic Inc, Minneapolis, MN) were positioned in the LV septal subendocardium of the LBB region. According to the electrical features described by Chen et al, 16 a successful LBBP in our study was defined as: (a) the paced QRS morphology by unipolar pacing demonstrated right bundle branch block pattern in lead V1, (b) the stimulus to peak left ventricular activation time (Stim‐LVAT) in lead V5 was less than 75 ms and there was no change with low and high output, and (c) the LBB capture threshold was below 1.5 V/0.4 ms. In the right ventricular septum pacing (RVSP) group, the pacing leads (models 5076, Medtronic Inc, Minneapolis, MN) were positioned in the right ventricular septum. In both groups, dual‐chamber pacemakers (Medtronic Inc) were implanted with the atrial pacing leads being implanted in the right auricular appendix.
2.3. Electrocardiographic measurements
Twelve‐lead surface electrocardiography (ECG) was recorded by the GE CardioLab Electrophysiology recording system (GE Healthcare Inc, Marlborough, MA) at 100 mm/s and the intracardiac electrogram from the tip electrode of 3830 lead was recorded in a unipolar fashion to recognize LBB potentials during the implantation procedure. Three parameters, the intrinsic QRSd, paced QRSd, and Stim‐LVAT were measured in sequence. The QRSd was measured from the first to last sharp vector of QRS complex crossing the isoelectric line on the 12 leads under the intrinsic rhythm and the ventricular pacing rhythm respectively for comparison. The paced QRSd was measured from the stimulus to the end of the last deflection of the QRS complex on 12 leads. The Stim‐LVAT was measured from the pacing stimulus to the peak of R‐wave in lead V5. In addition, the PV interval was also measured, from the LBB potential, which is the high‐frequency signal before the QRS complex, to the onset of the QRS complex.
The electrocardiographic recording and measurements were performed and analyzed by two independent and experienced ECG specialists. Three continuous QRS complexes were measured and the averaged values were reported.
2.4. Echocardiographic measurement
Transthoracic echocardiographic examinations were performed 3 days after implantation using color Doppler ultrasonic diagnostic apparatus (EPIQ 7C, Philips Medical Systems) equipped with an X5‐1 transducer. Data were collected under two pacing modes in sequence: the AAI mode, which was defined as “native‐conduction mode”; and the DDD mode with short AV delay to ensure complete capture of RVSP or LBBP and avoid fusion with native‐conduction, which was defined as “ventricular pacing‐on mode (LBBP capture or RVSP capture).” The ventricular pacing parameters was programmed to unipolar pacing with an output of 3.5 V/0.4 ms. In both modes, several parameters including Doppler variables, two‐dimensional echocardiographic (2DE) measurement data, real‐time three‐dimensional echocardiographic (RT‐3DE) measurement data, the severity of mitral regurgitation (MR), and tricuspid regurgitation (TR) were assessed according to the latest American Society of Echocardiography guidelines. 17 , 18 , 19 In the tissue Doppler image (TDI) mode, the frame rate and speed range were optimized, and five consecutive cardiac cycle images of the apical four‐chamber view were acquired. The sampling volume was strip‐shaped with a fixed width of 5 mm.
2.5. LV mechanical synchrony assessment
LV mechanical synchrony was assessed using RT‐3DE and TDI. Semiautomated contour tracing software (3DQ adv, Philips Medical System) was used for regional time volume curves. The standard deviation of time from the QRS starting point to the minimum systolic volume (Tmsv) of 16 left ventricular segments (SD‐Tmsv‐16) as well as timing and displacement bull's eye diagrams obtained from it were analyzed. The LV systolic dyssynchrony index (SDI) was defined as the SD‐Tmsv‐16 corrected by the RR interval. 20 The normal reference value for SDI is ≤ 2.7%. 21 The TDI data were analyzed on an independent workstation (QLAB version 10.8; Philips Medical Systems) with software SQ (Philips Medical System). Offline analysis automatically depicted the velocity, strain rate, and stress curves for each segment. When the intraventricular synchrony was analyzed, the sampling volume was placed in the 12 segments of LV (except for the apex segments), and the time‐to‐peak contraction velocity (Ts) of each segment was measured. The averages of data obtained from three cardiac cycles were used for statistical analysis. The standard deviation of Ts in the 12 segments (Tsd‐12‐LV) was also used to evaluate LV systolic synchrony; Tsd‐12‐LV more than 33 ms was defined as intraventricular dyssynchrony. 22
2.6. Hemodynamics effects assessment
Hemodynamic parameters recorded in this study are illustrated below. The parameter, degree of valvular regurgitation, was classified as trace, mild, moderate, and severe according to previously established guidelines. When the degree of valvular regurgitation was greater than trace, the vena contracta width (VCW) and the ratio of the regurgitation jet area to the atrial area from MR and TR were measured. Left ventricular filling time (LVFT) was measured from the beginning of the mitral E‐wave to the end of the A‐wave. The LVFT/RR interval was calculated to evaluate atrioventricular dyssynchrony. RT‐3DE datasets were analyzed by automated contour tracing software (HeartModel; Philips Medical System) to obtain LV parameters, including left ventricular end‐systolic volumes (LVESV), left ventricular end‐diastolic volumes (LVEDV), stroke volume (SV), and ejection fraction (EF). SV was also assessed by the velocity time integration (VTI) method.
All echocardiographic examinations and measurements were performed by the same sophisticated technician and analyzed later by two independent experienced investigators.
2.7. Statistical analyses
The Shapiro‐Wilk normal test was performed first. Continuous variables were expressed as mean ± standard deviation and categorical variables were expressed as percentages. Differences in mean values between two groups or two modes (native‐conduction mode and ventricular pacing‐on mode) were compared by Student t test for continuous variables. The χ 2 test was used for categorical data. Software SPSS 22.0 (SPSS Inc, Armonk, NY) was used to statistically analyze the data for detection and observation. The P value of less than .05 was considered statistically significant.
3. RESULTS
3.1. Participants
Between February 2018 and May 2019, a total of 136 patients were diagnosed with SSS‐accepted pacing therapy in our hospital, Xiamen Cardiovascular Hospital, Xiamen University. Eight patients underwent pacemaker pulse generator replacements and eight patients underwent single chamber pacemaker implantations and hence were excluded while the remaining 120 patients had dual‐chamber pacemaker implanted. Among the 120 patients, LBBP was attempted in 52 patients and successful in 47 patients, with the success rate of 90.4%. Those that failed LBBP, received RVSP instead. The remaining 68 patients underwent RVSP as planned. Finally, 78 patients were enrolled in this study after excluding patients who had an intrinsic QRS more than 120 ms, or were accordant with other exclusive criterions, or refused to participate in the study. Among the participants, 40 patients (mean age 65.93 ± 9.99 years; 33% male) underwent LBBP, and 38 patients (mean age 68.61 ± 9.83 years; 37% male) underwent RVSP (Figure 1). Baseline demographics, past medical history, and lead parameters were similar between the two groups, except for the proportion of patients with hypertension. Baseline characteristics are shown in Table 1.
Figure 1.
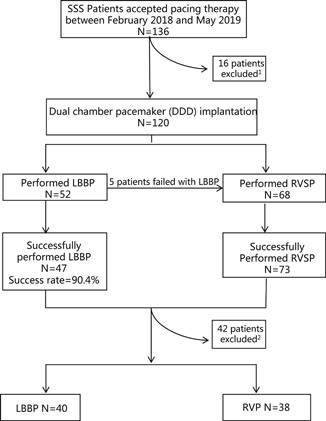
Flow chart of patients diagnosed with SSS who accepted pacing therapy in our study. 1 Reasons for exclusion: pacemaker replacements (n = 8), single chamber pacemaker implantations (n = 8) 2 ; Reasons for exclusion: intrinsic QRS > 120 ms, or were accordant with our exclusive criterions, or the patients refused to take part in the study (n = 42). LBBP, left bundle branch pacing; RVSP, right ventricular septum pacing; SSS, sick sinus syndrome
Table 1.
Patient baseline characteristics
| Parameters | LBBP (n = 40) | RVSP (n = 38) | P |
|---|---|---|---|
| Age, y | 65.93 ± 9.99 | 68.61 ± 9.83 | .242 |
| Male, n (%) | 13 (33%) | 14 (37%) | .687 |
| BSA, m2 | 1.63 ± 0.15 | 1.62 ± 0.27 | .833 |
| Baseline QRSd, ms | 91.06 ± 14.17 | 83.75 ± 14.82 | .076 |
| Hypertension, n (%) | 14 (35%) | 23 (61%) | .024 |
| DM, n (%) | 4 (10%) | 9 (24%) | .105 |
| CAD, n (%) | 5 (13%) | 10 (26%) | .122 |
| Pacing threshold (V/0.4 ms) | 0.49 ± 0.22 | 0.62 ± 0.18 | .120 |
| Sensing amplitude, mv | 11.74 ± 5.36 | 9.81 ± 3.42 | .080 |
Abbreviations: BSA, body surface area; CAD, coronary artery disease; DM, diabetes mellitus; LBBP, left bundle branch pacing; RVSP, right ventricular septum pacing; QRSd, QRS duration.
3.2. Synchronization parameters
In the RVSP group, the paced QRSd were prominently wider and LV mechanical synchronization parameters were significantly larger in the RVSP capture mode than those in the native‐conduction mode (P < .0001), which represented a poor electrical and mechanical synchrony resulting from RVSP. However in the LBBP group, the paced QRSd in LBBP capture was only slightly wider than that in the native‐conduction mode (101.03 ± 8.79 ms vs 91.06 ± 14.17 ms, P < .0001), and no significant differences were detected in the LV mechanical synchronization parameters of the LBBP capture and native‐conduction modes (P ≥ .05). Compared with the RVSP capture mode, the paced QRSd and LV mechanical synchronization parameters in LBBP capture mode were significantly shorter and smaller (Figures 2 and 3). The QRSd and LV mechanical synchronization parameters in LBBP and RVSP groups are summarized in Table 2.
Figure 2.
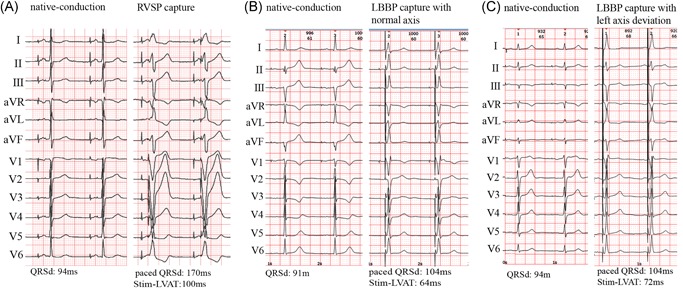
Comparison of QRSd of RVSP, LBBP with normal axis, and LBBP with left axis deviation. The 12‐lead surface ECGs of RVSP, LBBP with normal axis, and LBBP with left axis deviation in three patients. QRSd of RVSP mode was obvious wider than that in native‐conduction mode (A). QRSd of LBBP with normal axis (B) or with left axis deviation (C) was slightly wider than that in native‐conduction mode. ECG, electrocardiography; LBBP, left bundle branch pacing; QRSd, QRS durations; RVSP, right ventricular septum pacing; Stim‐LVAT, the interval from the pacing stimulus to the peak of the R‐wave
Figure 3.
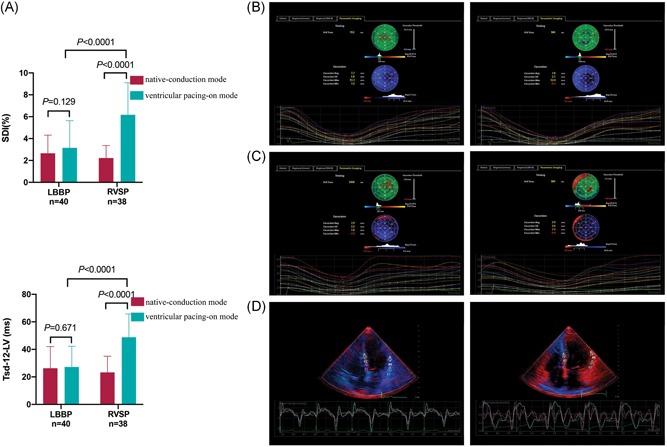
Comparison of LV mechanical synchrony between LBBP and RVSP groups. LV mechanical synchronization parameters (SDI or Tsd‐12‐LV) in LBBP capture were significantly shorter compared with RVSP capture mode (A). Similar LV synchrony was showed in native‐conduction (B, left) and LBBP capture modes (B, right) by color coded bull's eye maps in a patient with SSS. Poorer synchrony was showed in RVSP capture mode (C, right) compared with native‐conduction mode (C, left) in another patient with SSS. TDI showed that the time of peak myocardial contraction velocity in the LV segments was relatively concentrated in one LBBP patient (D, left) but was discrete in the other RVSP patient (D, right). LBBP, left bundle branch pacing; LV, left ventricular; RVSP, right ventricular septum pacing; SDI, systolic dyssynchrony index; SSS, sick sinus syndrome; Tsd‐12‐LV, the standard deviation of the time‐to‐peak contraction velocity in the 12 segments
Table 2.
QRSd and LV mechanical synchronization parameters in LBBP and RVSP groups
| Parameters | LBBP (n = 40) | RVSP (n = 38) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Native‐conduction | LBBP capture | P | Native‐conduction | RVSP capture | P | ||||
| Electrocardiogram parameters | |||||||||
| QRSd, ms a | 91.06 ± 14.17 | 101.03 ± 8.79 | .000 | 90.07 ± 17.48 | 141.77±12.01* | .000 | |||
| Paced QRSd, ms b | NA | 121.17 ± 8.45 | … | NA | 148.92±13.42** | … | |||
| Echocardiographic parameters | |||||||||
| RR interval, ms | 945.61 ± 120.48 | 919.78 ± 92.95 | .114 | 943.97 ± 91.84 | 946.71 ± 82.53 | .681 | |||
| SD‐Tmsv‐16, ms | 26.45 ± 16.83 | 28.85 ± 22.38 | .394 | 20.87 ± 10.32 | 59.05 ± 27.54 | .000 | |||
| SDI (%) | 2.70 ± 1.68 | 3.14 ± 2.49 | .129 | 2.22 ± 1.14 | 6.17±2.94*** | .000 | |||
| Tsd‐12‐LV, ms | 25.61 ± 16.07 | 26.43 ± 15.55 | .671 | 23.95 ± 11.22 | 50.22 ± 14.98 **** | .000 | |||
Note: LBBP capture vs RVSP capture.
Abbreviations: LBBP, left bundle branch pacing; LV, left ventricle; QRSd, QRS duration; RVSP, right ventricular septum pacing; SD‐Tmsv‐16, the standard deviation of time from the QRS starting point to the minimum systolic volume of 16 LV segments; SDI, LV systolic dyssynchrony index; Tsd‐12‐LV, the standard deviation of time‐to‐peak contraction velocity in LV 12 segments.
QRSd was measured from the first to last sharp vector of QRS complex crossing the isoelectric line 12 lead electrocardiogram.
Paced QRSd was measured from stimulus to the end of the last QRS complex deflection in the 12 lead electrocardiogram.
P < .0001.
P < .0001.
P < .0001.
P < .0001.
Among the 40 patients who underwent LBBP, 25 patients (62.5%) presented a normal axis of paced QRS and 15 patients (37.5%) had left axis deviation with ventricular pacing. LBB potentials were recorded in 32 patients (80%) and were absent in 8 patients. The duration of PV interval was 18.24 ± 6.23 ms. According to the paced QRS axis and existence of LBB potentials, the LBBP group was divided into four different subgroups as follows: axis between − 30° and + 90° (axis normal), axis between − 30° and − 90° (left axis deviation) with LBB potential (potential + ) and without LBB potential (potential−) subgroups. There were no statistically significant differences in paced QRSd, Stim‐LVAT, and LV mechanical synchronization parameters between the potential + and potential‐ subgroups (Figures 4, 5, 6). Nevertheless, the subgroup of left axis deviation had longer Stim‐LVAT (64.07 ± 4.57 vs 59.42 ± 6.14, P = .023) and mildly larger LV mechanical synchronization parameters than those with normal axis (SDI, 3.17 ± 1.24 vs 2.64 ± 1.61, P = .305; Tsd‐12‐LV, 32.48 ± 16.07 vs 24.13 ± 13.89, P = .100). The QRSd and LV mechanical synchronization parameters in subgroups are shown in Figures 5 and 6.
Figure 4.
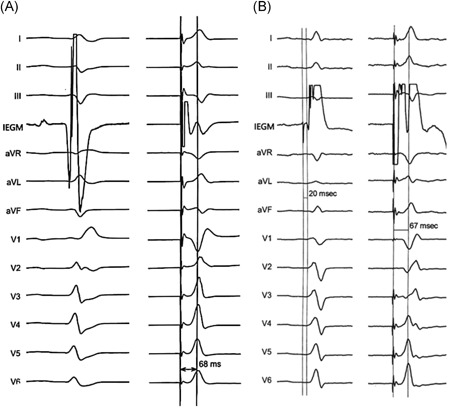
The Stim‐LVAT of tip‐paced ECGs in patients with LBBP with and without LBB potential. One patient without LBB potential (A, left), had Stim‐LVAT of 68 ms (A, right). The other patient recorded with LBB potential (B, left), had Stim‐LVAT of 67 ms (B, right). ECG, echocardiography; LBB, left bundle branch; Stim‐LVAT, the interval from the pacing stimulus to the peak of the R‐wave
Figure 5.
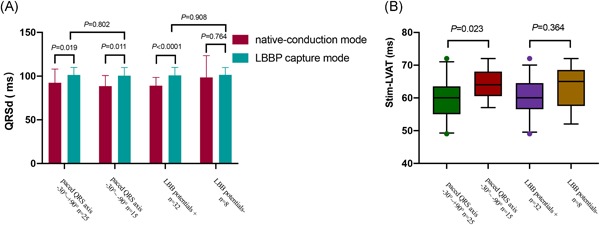
Comparison of QRSd (A) and Stim‐LVAT (B) between subgroups divided by paced QRS axis and LBB potential in LBBP group. LBBP, left bundle branch pacing; QRSd, QRS duration; Stim‐LVAT, the interval from the pacing stimulus to the peak of the R‐wave
Figure 6.
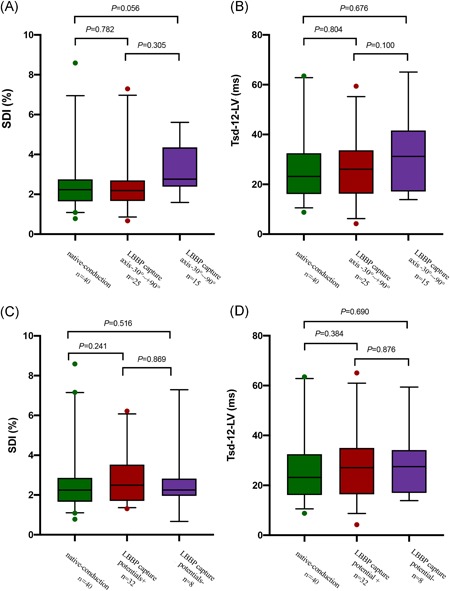
Comparison of LV mechanical synchrony between subgroups divided by paced QRS axis and LBB potential in LBBP group. Subgroup of left axis deviation had slightly greater SDI and Tsd‐12‐LV than the normal axis subgroup, but without statistically significance (A,B).There were no differences in SDI or Tsd‐12‐LV between the potential+ and potential− subgroups (C,D). ECG, echocardiography; LBB, left bundle branch; LBBP, left bundle branch pacing; LV, left ventricle; SDI, systolic dyssynchrony index; Tsd‐12‐LV, the standard deviation of time‐to‐peak contraction velocity in LV 12 segments
3.3. Early change in hemodynamics
There was no statistically significant difference in the SV measured by the VTI method between the native‐conduction mode and the LBBP capture mode (65.45 ± 18.68 vs 64.08 ± 16.97, P = 0.241); however, the SV was significantly reduced in the RVSP capture mode compared with the native‐conduction mode (63.51 ± 13.72 vs 66.43 ± 14.33, P = .005). LVEDV, LVESV, and SV measured by the RT‐3DE and LVFT/RR interval decreased in both ventricular pacing‐on groups (Table 3), while the decrease in the RVSP group were more prominent. Neither LBBP capture mode nor RVSP capture mode had significant change in EF. Regarding the degree of MR and TR, there were no differences between the native‐conduction or LBBP capture modes. However, worse MR (jet area/LAA, 4.75 ± 7.02 vs 8.26 ± 12.97, P = .050) and TR (VCW, 0.33 ± 0.28 vs 0.33 ± 0.28, P = .005; jet area/LAA, 18.20 ± 14.27 vs 21.54 ± 16.22, P = .005) were observed in the RVSP capture mode (Table 3).
Table 3.
Early change of hemodynamics in LBBP and RVSP groups
| Parameters | LBBP (n = 40) | RVSP (n = 38) | ||||
|---|---|---|---|---|---|---|
| native‐conduction | LBBP capture | P | native‐conduction | RVSP capture | P | |
| 2DE measurement data and Doppler variables | ||||||
| LA, mm | 37.20 ± 4.27 | 37.20 ± 4.09 | 1.000 | 35.55 ± 4.92 | 35.79 ± 4.86 | .163 |
| LVFT/RR interval (%) | 49.42 ± 5.66 | 46.93 ± 7.41 | .038 | 50.28 ± 4.57 | 48.30 ± 5.94 | .002 |
| LVEDD, mm | 48.73 ± 5.23 | 48.53 ± 5.44 | .500 | 47.39 ± 5.09 | 46.97 ± 5.00 | .125 |
| LVESD, mm | 30.75 ± 4.59 | 30.45 ± 4.71 | .172 | 29.05 ± 4.90 | 29.16 ± 4.87 | .652 |
| EF (M‐mode, %) | 65.44 ± 7.84 | 66.12 ± 7.02 | .151 | 68.84 ± 8.15 | 68.29 ± 7.70 | .143 |
| SV (VTI, mL) | 65.45 ± 18.68 | 64.08 ± 16.97 | .241 | 66.43 ± 14.33 | 63.51 ± 13.72 | .005 |
| RT‐3DE measurement data | ||||||
| LVEDV, mL | 98.73 ± 22.58 | 94.39 ± 18.82 | .015 | 92.13 ± 21.93 | 85.61 ± 22.28 | .011 |
| LVESV, mL | 42.96 ± 13.30 | 41.21 ± 11.55 | .035 | 39.53 ± 16.34 | 34.95 ± 11.25 | .033 |
| EF (3D, %) | 56.48 ± 6.65 | 55.95 ± 6.26 | .259 | 59.98 ± 7.26 | 58.88 ± 6.19 | .060 |
| SV (3D, mL) | 55.60 ± 13.35 | 53.13 ± 10.93 | .030 | 54.72 ± 12.38 | 51.42 ± 12.10 | .004 |
| MR degree | ||||||
| MR VCW (cm) | 0.26 ± 0.26 | 0.28 ± 0.26 | .338 | 0.16 ± 0.22 | 0.22 ± 0.29 | .128 |
| MR jet area/LAA (%) | 11.61 ± 12.00 | 12.41 ± 13.38 | .485 | 4.75 ± 7.02 | 8.26 ± 12.97 | .050 |
| TR degree | ||||||
| TR VCW, cm | 0.35 ± 0.33 | 0.34 ± 0.31 | .288 | 0.33 ± 0.28 | 0.37 ± 0.30 | .005 |
| TR jet area/RAA (%) | 13.54 ± 12.33 | 13.41 ± 12.20 | .893 | 18.20 ± 14.27 | 21.54 ± 16.22 | .005 |
Abbreviations: 2DE, two‐dimensional echocardiographic; 3D, three‐dimensional; EF, ejection fraction; LA, left atrium; LAA, left atrial area; LBBP, left bundle branch pacing; LVEDD, left ventricular end‐diastolic diameter; LVEDV, left ventricular end‐diastolic volume; LVESD, left ventricular end‐systolic diameter; LVESV, left ventricular end‐systolic volume; LVFT, left ventricular filling time; MR, mitral regurgitation; RT‐3DE, real‐time three‐dimensional echocardiographic; RAA, right atrial area; RVSP, right ventricular septum pacing; SV, stroke volume; TR, tricuspid regurgitation; VCW, vena contracta width; VTI, velocity time integral.
4. DISCUSSION
The present study evaluates the acute effects of LBBP on QRSd, echocardiographic measures of ventricular dyssynchrony, and MR and TR. We performed a comparative analysis of LBBP to intrinsic rhythm within the same patient as well as RVSP pacing in a different cohort of patients. The results confirmed that LBBP could preserve satisfactory electrical and mechanical LV synchrony and lead to favorable hemodynamic effects.
RVP is the traditional method for permanent pacing; however, clinical disadvantages of RVP, such as cardiac electromechanical asynchrony, impaired contractile force, increased risk of death, and hospitalization due to heart failure, have been recognized for decades. 1 , 2 , 3 , 23 In recent years, research on the His bundle‐Purkinje system pacing has emerged and developed. Studies have suggested that HBP is a relatively mature method for physiological pacing. 4 , 5 , 6 , 7 However, lead placement for HBP is technically challenging due to its anatomical location; besides long‐term capture thresholds of HBP have been found to be significantly higher than those of RVP. 8 LBBP is a new promising pacing technique. In this study, we investigated whether it could preserve satisfactory electromechanical synchrony and contribute to favorable hemodynamic effect.
The QRSd has been accepted as a surrogate for the evaluation of electrical synchrony, 24 with a narrow QRSd being associated with good ventricular synchrony. 25 During LBBP, the left ventricular His‐Purkinje system was swiftly recruited by advanced activation of the LBB, which results in better electrical synchrony and a shorter paced QRSd. As shown in our study, the QRSd was significantly shorter with LBBP compared with RVSP and only slightly prolonged compared with intrinsic conduction which is due to the delay activation of right ventricle. This finding was identical to other studies, 10 , 11 , 26 and confirmed that LBBP could preserve better electrical synchrony.
Previous studies have suggested that electric synchrony was associated with mechanical synchrony. 27 Although slightly wider paced QRSd had been observed in LBBP, the prolonged QRSd has no effect on LV mechanical synchrony measured by single‐photon emission computed tomography myocardial perfusion imaging in Hou's study. 11 His research showed that LV synchrony in the LBBP group was superior to that of the RVSP group and similar to that in HBP group. In the present study, we adopted echocardiography to evaluate LV mechanical synchrony. The Tmsv, as assessed by RT‐3DE, can objectively reflect the changes of myocardial motion in specific regions, without requiring geometric assumptions about the shape of the heart. Multiple studies have demonstrated that measurements of systolic dyssynchrony by RT‐3DE are practical and repeatable. 28 , 29 , 30 TDI technology is another feasible method for evaluating intraventricular synchrony evaluation. This technology is of high time resolution that enables quantitatively analyzing the myocardial mechanical motion of different segments and displaying speed‐time curves in real time. Both methods were applied in this study to enhance the credibility of results in the study.
Our study demonstrates that LBBP maintained a good LV mechanical synchrony that was similar to that of native conduction, and was significantly better than that of RVSP. This finding was consistent with Hou's results. 11 However, some other findings of the present study are not in agreement with Hou's study. In his study, patients with LBBP without LBB potentials had longer Stim‐LVAT (83.2 ± 16.8 ms vs 73.1 ± 11.3 ms, P = .03) and worse LV mechanical synchrony than those with potentials. In our study, Stim‐LVAT measurements were shorter than 75 ms in all patients of LBBP group, and the existence of LBB potentials was irrelevant with the duration of Stim‐LVAT, paced QRSd, or LV mechanical synchrony. In addition, the identification of LBB potential may be affected by many factors such as the direction of wavefront, electrode size, velocity of conduction, lead orientation, distance of the bundle branch, and signal of far field or near field. 31 Therefore, we cannot always record the potential during LBBP procedures. These results hint that Stim‐LVAT may be more important than LBB potentials in LBBP. On the other hand, the pacing region of LBBP is usually in the left bundle trunk or proximal branch, left anterior fascicle, and left posterior fascicle. Capture of left posterior fascicular branch may cause left axis deviation of the paced QRS. It has been reported that the abnormal paced QRS axis may cause cardiac function damage in RVP. 32 It is not yet clear whether the paced QRS axis deviation in LBBP affects cardiac synchrony. In our study, 37% patients in LBBP group presented left axis deviation. And the left axis deviation subgroup had a slightly longer Stim‐LVAT and poorer LV synchrony than the subgroup of normal axis, but the differences were not statistically significant. This may be due to the relatively small sample size and studies of larger sample sizes are still needed to verify this effect in the future.
Hemodynamic effects after implantation were evaluated in this study as well. According to our data, LBBP kept a similar SV to native‐conduction, while RVSP reduced SV significantly. This confirmed that good electromechanical synchrony can translate to satisfactory hemodynamic effects. The LVEDV, LVESV, and SV by 3DT‐RT decreased in the ventricular pacing‐on mode compared with the native‐conduction mode in both LBBP and RVSP groups. This may be due to the high sensitivity of the 3DE‐RT measuring method and relatively short AV interval adopted to avoid ventricular fusion beats. In terms of the AV interval, a short AV interval may reduce ventricular filling that was convinced by less LVFT/RR interval compared with native‐conduction mode. Therefore, the protocol applied in this study may affect the credibility of measured hemodynamic function. This effect should be considered when interpreting the results.
It has been proposed that the degree of regurgitation can also be considered as a predictor of prognosis, and mechanical dyssynchrony may increase the risk of secondary regurgitation. 33 In our study, RVSP increased the degrees of MR and TR compared with native conduction, Yet, no similar changes were observed in the LBBP group which may be a result of better mechanical synchrony maintained by LBBP.
In this study, the native‐conduction and the RVSP modes were both the control groups. Due to the self‐controlled design of this study, selection bias may be minimized. All patients enrolled in this study had normal cardiac function and a narrow QRSd, thus ventricular synchrony could be deemed as normal under the intrinsic rhythm. Therefore, the physiology of LBBP could be accurately evaluated by comparing with that of native‐conduction in this cohort. The LV synchrony between the LBBP and RVSP groups could be effectively compared simultaneously. In that case, we believed that the design applied in present research enhanced the credibility of our results.
5. LIMITATIONS
There are some limitations of this study. To begin with, we focused on intraventricular synchrony and compared the instant effects via different modes of activation. However, the interventricular synchrony and the function of the right ventricle were not assessed in this study. Besides, this was an instant hemodynamic study, thus the long‐term hemodynamic effects of LBBP remain uncertain. In addition, this study was a single‐center, nonrandomized, nonblind observational study, and has a relatively small sample size. Randomized multicenter studies with larger sample sizes, longer follow‐up periods, and a double‐blind design are beneficial for assessing the long‐term cardiac function of patients underwent LBBP and further proving our findings.
6. CONCLUSION
This study provides a detailed analysis of electromechanical synchrony and hemodynamic effects under native‐conduction, RVSP, and LBBP modes in patients with SSS with normal cardiac function. We confirmed that LBBP could preserve satisfactory LV synchrony leading to instantly favorable hemodynamic effects.
Supporting information
Supporting information
ACKNOWLEDGMENTS
We would like to thank Dr Weijian Huang (The First Affiliated Hospital of Wenzhou Medical University) for his technical assistance in LBBP procedure. We also thank Dr Parikshit S. Sharma (Division of Cardiology, Rush University Medical Center, Chicago, IL) and Dr Xin Zeng (Cardiac Ultrasound Laboratory, Massachusetts General Hospital, Boston, MA) for their diligent review of our manuscript.
Cai B, Huang X, Li L, et al. Evaluation of cardiac synchrony in left bundle branch pacing: Insights from echocardiographic research. J Cardiovasc Electrophysiol. 2020;31:560–569. 10.1111/jce.14342
Binni Cai and Xinyi Huang contributed equally to this study.
Disclosures: None.
REFERENCES
- 1. Cicchitti V, Radico F, Bianco F, Gallina S, Tonti G, De Caterina R. Heart failure due to right ventricular apical pacing: the importance of flow patterns. Europace. 2016;18:1679‐1688. [DOI] [PubMed] [Google Scholar]
- 2. Curtis AB, Worley SJ, Chung ES, Li P, Christman SA, John Sutton M St. Improvement in clinical outcomes with biventricular versus right ventricular pacing. J Am Coll Cardiol. 2016;67:2148‐2157. [DOI] [PubMed] [Google Scholar]
- 3. Pastore G, Zanon F, Baracca E, et al. The risk of atrial fibrillation during right ventricular pacing. Europace. 2016;18:353‐358. [DOI] [PubMed] [Google Scholar]
- 4. Abdelrahman M, Subzposh FA, Beer D, et al. Clinical outcomes of his bundle pacing compared to right ventricular pacing: results from the HBP registry. J Am Coll Cardiol. 2018;71:2319‐2330. [DOI] [PubMed] [Google Scholar]
- 5. Shan P, Su L, Zhou X, et al. Beneficial effects of upgrading to His bundle pacing in chronically paced patients with left ventricular ejection fraction <50. Heart Rhythm. 2018;15:405‐412. [DOI] [PubMed] [Google Scholar]
- 6. Sharma PS, Dandamudi G, Herweg B, et al. Permanent His‐bundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: a multicenter experience. Heart Rhythm. 2018;15:413‐420. [DOI] [PubMed] [Google Scholar]
- 7. Wang Y, Qian Z, Zhang J, Wu H, Hou X, Zou J. Effect of His bundle pacing on cardiac mechanical synchronization. Chin J Cardiac Arrhythmias. 2018;22:117‐122. [Google Scholar]
- 8. Vijayaraman P, Chung MK, Dandamudi G, et al. His bundle pacing. J Am Coll Cardiol. 2018;72:927‐947. [DOI] [PubMed] [Google Scholar]
- 9. Huang W, Su L, Wu S, et al. A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol. 2017;33:1736.e1‐1736.e3. [DOI] [PubMed] [Google Scholar]
- 10. Chen K, Li Y, Dai Y, et al. Comparison of electrocardiogram characteristics and pacing parameters between left bundle branch pacing and right ventricular pacing in patients receiving pacemaker therapy. Europace. 2019;21:673‐680. [DOI] [PubMed] [Google Scholar]
- 11. Hou X, Qian Z, Wang Y, et al. Feasibility and cardiac synchrony of permanent left bundle branch pacing through the interventricular septum. EP Europace. 2019;21:1694‐1702. [DOI] [PubMed] [Google Scholar]
- 12. Zhang W, Huang J, Qi Y, et al. Cardiac resynchronization therapy by left bundle branch area pacing in heart failure patients with left bundle branch block. Heart Rhythm. 2019;16:1783‐1790. [DOI] [PubMed] [Google Scholar]
- 13. Hasumi E, Fujiu K, Nakanishi K, Komuro I. Impacts of left bundle/peri‐left bundle pacing on left ventricular contraction. Circ J. 2019;83:1965‐1967. [DOI] [PubMed] [Google Scholar]
- 14. Huang W, Chen X, Su L, Wu S, Xia X, Vijayaraman P. A beginner's guide to permanent left bundle branch pacing. Heart Rhythm. 2019;16:1791‐1796. 10.1016/j.hrthm.2019.06.016 [DOI] [PubMed] [Google Scholar]
- 15. Chen K, Li Y. How to implant left bundle branch pacing lead in routine clinical practice. J Cardiovasc Electrophysiol. 2019;30:2569‐2577. 10.1111/jce.14190 [DOI] [PubMed] [Google Scholar]
- 16. Chen X, Wu S, Su L, Su Y, Huang W. The characteristics of the electrocardiogram and the intracardiac electrogram in left bundle branch pacing. J Cardiovasc Electrophysiol. 2019;30:1096‐1101. 10.1111/jce.13956 [DOI] [PubMed] [Google Scholar]
- 17. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;16:233‐270. [DOI] [PubMed] [Google Scholar]
- 18. Lang RM, Badano LP, Tsang W, et al. EAE/ASE recommendations for image acquisition and display using three‐dimensional echocardiography[J]. 2012, 13(1):3‐46. [DOI] [PubMed]
- 19. Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303‐371. [DOI] [PubMed] [Google Scholar]
- 20. Soliman OI, van Dalen BM, Nemes A, et al. Quantification of left ventricular systolic dyssynchrony by real‐time three‐dimensional echocardiography. J Am Soc Echocardiogr. 2009;22:232‐239. [DOI] [PubMed] [Google Scholar]
- 21. Kleijn SA, Aly MF, Knol DL, et al. A meta‐analysis of left ventricular dyssynchrony assessment and prediction of response to cardiac resynchronization therapy by three‐dimensional echocardiography. Eur Heart J Cardiovasc Imaging. 2012;13:763‐775. [DOI] [PubMed] [Google Scholar]
- 22. Zhong X, Xia D, Huang L. Evaluation of ventricular myocardial motion synchronization by tissue veiocity imaging before and after dual chamber pacemaker technology. Chin J Ultrasound Med. 2012;28:1002‐1005. [Google Scholar]
- 23. Bhatia V, Arora P, Gupta A, Bhandari S, Kaul U. Intraventricular flow patterns during right ventricular apical pacing. Open Heart. 2019;6(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pang BJ, Kumar S, Tacey MA, Mond HG. Capturing the His‐Purkinje system is not possible from conventional right ventricular apical and nonapical pacing sites. Pacing Clin Electrophysiol. 2014;37:724‐730. [DOI] [PubMed] [Google Scholar]
- 25. Sandhu R, Bahler RC. Prevalence of QRS prolongation in a community hospital cohort of patients with heart failure and its relation to left ventricular systolic dysfunction. Am J Cardiol. 2004;93:244‐246. [DOI] [PubMed] [Google Scholar]
- 26. Vijayaraman P, Panikkath R, Mascarenhas V, Bauch TD. Left bundle branch pacing utilizing three dimensional mapping. J Cardiovasc Electrophysiol. 2019;30:3050‐3056. 10.1111/jce.14242 [DOI] [PubMed] [Google Scholar]
- 27. Kirk JA, Kass DA. Electromechanical dyssynchrony and resynchronization of the failing heart. Circ Res. 2013;113(6):765‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gorcsan J 3rd, Abraham T, Agler DA, et al. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting—a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr. 2008;21:191‐213. [DOI] [PubMed] [Google Scholar]
- 29. Yang L, Wang J, Wang Y, Zhang X. Results of performing or not cardiac resynchronization therapy for live assessment of LV function and mechanical systole synchrony using gated myocardial perfusion imaging and real‐time three‐dimensional echocardiography. Seven months follow‐up. Hell J Nucl Med. 2017;20:247‐250. [DOI] [PubMed] [Google Scholar]
- 30. Ojala T, Mathur S, Vatanen A, Sinha MD, Jahnukainen K, Simpson J. Repeatability and agreement of real time three‐dimensional echocardiography measurements of left ventricular mass and synchrony in young patients. Echocardiography. 2014;32:522‐527. [DOI] [PubMed] [Google Scholar]
- 31. Josephson ME, Anter E. Substrate mapping for ventricular tachycardia: assumptions and misconceptions. JACC Clin Electrophysiol. 2015;1(5):341‐352. [DOI] [PubMed] [Google Scholar]
- 32. Kim SH, Oh YS, Nam GB, et al. Paced qrs axis as a predictor of pacing‐induced left ventricular dysfunction. J Interv Card Electrophysiol. 2014;41(3):223‐229. [DOI] [PubMed] [Google Scholar]
- 33. Cipriani M, Lunati M, Landolina M, et al. Prognostic implications of mitral regurgitation in patients after cardiac resynchronization therapy. Eur J Heart Fail. 2016;18:1060‐1068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


