Abstract
Transinfections of the maternally transmitted endosymbiont Wolbachia pipientis can reduce RNA virus replication and prevent transmission by Aedes aegypti, and also have the capacity to invade wild‐type populations, potentially reaching and maintaining high infection frequencies. Levels of virus transmission blocking are positively correlated with Wolbachia intracellular density. Despite reaching high densities in Ae. aegypti, transinfections of wAlbA, a strain native to Aedes albopictus, showed no blocking of Semliki Forest Virus in previous intrathoracic injection challenges. To further characterize wAlbA blocking in Ae. aegypti, adult females were intrathoracically challenged with Zika (ZIKV) and dengue viruses, and then fed a ZIKV‐containing bloodmeal. No blocking was observed with either virus when challenged by intrathoracic injection. However, when ZIKV was delivered orally, wAlbA‐infected females showed a significant reduction in viral replication and dissemination compared with uninfected controls, as well as a complete absence of virus in saliva. Although other Wolbachia strains have been shown to cause more robust viral blocking in Ae. aegypti, these findings demonstrate that, in principle, wAlbA could be used to reduce virus transmission in this species. Moreover, the results highlight the potential for underestimation of the strength of virus‐blocking when based on intrathoracic injection compared with more natural oral challenges.
Keywords: Aedes aegypti, Aedes albopictus, dengue virus arbovirus, pathogen blocking, Wolbachia, Zika virus
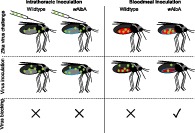
A transinfection of the wAlbA Wolbachia strain in Aedes aegypti blocks Zika virus transmission in saliva when virus is delivered orally via an infectious bloodmeal.
Significant Zika blocking by wAlbA was not observed when virus was delivered via intrathoracic injection, suggesting that the midgut barrier plays an important role in blocking.
Intrathoracic challenges can misrepresent virus blocking strength and may underestimate the true blocking potential of a strain.
Wolbachia are maternally‐transmitted alphaproteobacteria widespread among the phylum Arthropoda. These endosymbionts are obligately intracellular, comprising a large number of distinct strains distributed among a wide diversity of host species. Wolbachia strains are currently classified into a set of 16 phylogenetically distinct supergroups (A–Q) (Glowska et al., 2015; Gerth, 2016), with supergroups A and B containing strains capable of causing host reproductive parasitism (Casiraghi et al., 2005; Bordenstein et al., 2009; Zu Dohna et al., 2018).
Wolbachia are currently being deployed in the field as a vector control intervention. Certain Wolbachia strains cause a strong reduction in vector competence for RNA viruses, particularly when novel Wolbachia–host combinations are generated (Moreira et al., 2009; Bian et al., 2010; Kambris et al., 2010; Walker et al., 2011; Blagrove et al., 2012; van den Hurk et al., 2012; Joubert et al., 2016; Fraser et al., 2017; Ant et al., 2018). In the primary DENV vector Aedes aegypti, for example, Wolbachia transinfected lines have shown strong transmission blocking for the major arboviruses, including dengue (DENV) (Moreira et al., 2009; Walker et al., 2011; Frentiu et al., 2014; Ant et al., 2018), chikungunya (Moreira et al., 2009; van den Hurk et al., 2012), Zika (ZIKV) (Aliota et al., 2016; Dutra et al., 2016; Ant et al., 2018) and yellow fever (van den Hurk et al., 2012). Wolbachia density is generally higher, and tissue distribution broader, in novel transinfections compared with naturally occurring host–Wolbachia associations, and this is considered to the enhance the transmission blocking phenotype (Lu et al., 2012; Osborne et al., 2012; Chrostek et al., 2013; Martinez et al., 2014).
The host reproductive manipulations generated by some Wolbachia strains facilitates their population invasion and the maintenance of high infection frequencies. Cytoplasmic incompatibility (CI) is a sperm modification that results in sterility unless a compensatory Wolbachia rescue factor is present in the embryo. The coupling of CI rescue with maternal transmission generates a relative reproductive advantage for Wolbachia‐infected females, with frequency thresholds for population invasion largely determined by the balance between the fitness benefits of CI and any negative effects on life history (Turelli & Hoffmann, 1999; Jansen et al., 2008; Turelli, 2010; Hancock et al., 2011; Hancock et al., 2016).
The invasive arbovirus vector Aedes albopictus is naturally superinfected with the wAlbA (supergroup A) and wAlbB (supergroup B) Wolbachia strains, where wAlbA tends to exist at a low intracellular density relative to wAlbB (Dutton & Sinkins, 2004) and is hypothesized to have a longer evolutionary association with Ae. albopictus (Sinkins et al., 1995). A transinfection of both strains generated in Ae. aegypti revealed a reversal of the relative strain densities in this novel host, with wAlbA displaying broad tissue distribution and higher densities in somatic tissues compared with wAlbB, suggesting that the line would show strong virus inhibition (Ant et al., 2018). However, when wAlbA‐carrying females were challenged with Semliki Forest Virus (SFV) via thoracic injection, no reduction in viral genome copies was detected compared with Wolbachia‐free controls (Ant et al., 2018). In the present study, further characterization of the viral blocking capacity of wAlbA in Ae. aegypti is provided via challenge by intrathoracic injection with ZIKV and DENV viruses and oral feeding of ZIKV.
For the intrathoracic challenges, 30 5‐day old female mosquitoes from the wAlbA, wAu and wild‐type lines were injected with either DENV or ZIKV in the thorax using a pulled glass capillary and a Nanoject II (Drummond Scientific, Broomall, PA, U.S.A.) hand‐held microinjector. Injected mosquitoes were immediately transferred to an incubator set to 27 °C for recovery. DENV injected females were left for 10 days prior to RNA extraction and virus quantification by a quantitative reverse transcriptase‐polymerase chain reaction. ZIKV injected females were left for 7 days. DENV was serotype 2, New Guinea C strain, obtained from Public Health England culture collections. The concentration of injected DENV virus was 2.5 × 108 FFU/mL. ZIKV was strain MP1751, obtained from Public Health England culture collections. The concentration of injected ZIKV virus was 4.8 × 108 FFU/mL. The primers used to measure DENV genome copies were DENV‐NS5‐F: 5′‐ACAAGTCGAACAACCTGGTCCAT‐3′ and DENV‐NS5‐R: 5′‐GCCGCACCATTGGTCTTCT‐3. The primers used to measure ZIKV genome copies were ZIKV‐835: 5′‐TTGGTCATGATACTGCTGATTG‐3′ and ZIKV‐911c: 5′‐CCTTCCACAAAGTCCCTATTGC‐3′.
For the oral infections, 7‐day‐old wAlbA and wild‐type females were fed an infectious blood‐meal containing 1.4 mL of washed rabbit erythrocytes and 700 μL of viral suspension supplemented with ATP at a final concentration of 5 mm. The day before the infectious blood‐meal, batches of 65 females were isolated in feeding boxes and starved for 24 h. Mosquitoes were then exposed to the ZIKV NC‐2014‐5132 strain containing a final viral titre of 107 TCID50/mL.
For the infection, dissemination and transmission analysis, population batches of 30 wAlbA and 30 wild‐type mosquitoes were analysed at days 4, 7, 11, 14 and 21 post infection. To estimate infection, dissemination and transmission, mosquito bodies (thorax and abdomen), heads and saliva were analysed, respectively. To assess the transmission rate and transmission efficiency, mosquito saliva was collected from individual mosquitoes. Infection rate was determined by the proportion of mosquitoes with infected body (abdomen and thorax) among tested mosquitoes. The disseminated infection rate corresponds to the proportion of mosquitoes with infected heads among the previously detected infected mosquitoes (i.e. abdomen/thorax positive). The transmission rate refers to the proportion of mosquitoes with infectious saliva among mosquitoes with disseminated infection. Virus was titrated by plaque assay.
The capacity of the wAlbA strain to inhibit ZIKV and DENV in Ae. aegypti was assessed via virus intrathoracic injection and was compared with the blocking capacity of wAu, a strain with comparable intracellular densities but that had previously shown strong viral inhibition (Ant et al., 2018). Consistent with the findings for SFV, genome copies in wAlbA females did not differ significantly from Wolbachia‐uninfected wild‐type mosquitoes (for DENV P = 0.636, Wilcoxon rank sum; for ZIKV, P = 0.057, Wilcoxon rank sum). The wAu‐carrying line showed significantly reduced levels of both viruses (for DENV, P < 0.0001, Wilcoxon rank sum; for ZIKV, P < 0.00001, Wilcoxon rank sum), with 16 out of 24 (66.7%) ZIKV injected females containing no detectable virus compared with 100% virus positivity in wild‐type and wAlbA‐infected mosquitoes (Fig. 1).
Figure 1.
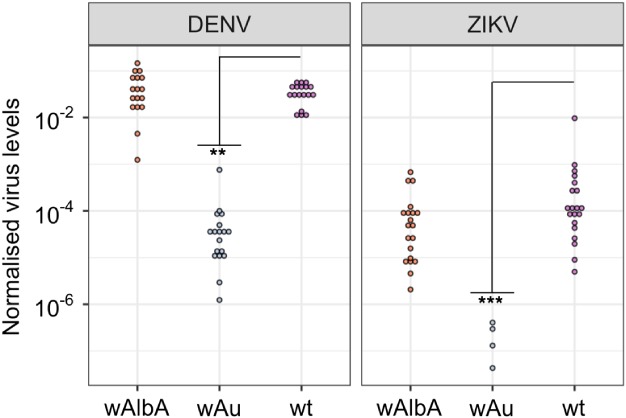
Dengue (DENV) and Zika (ZIKV) viral genome copies per host cell after thoracic injection into Wolbachia‐infected lines and wild‐type Aedes aegypti. Females were left for 10 days prior to total RNA extraction and virus quantification by a quantitative polymerase chain reaction. The amount of virus for each female was normalized to the RpS17 house‐keeping gene. Statistical analysis was performed using a Wilcoxon rank sum test with P < 0.05 considered statistically significant. [Colour figure can be viewed at http://wileyonlinelibrary.com].
To assess the blocking potential of the wAlbA strain when orally challenged, wAlbA‐carrying and wild‐type females were fed a blood meal containing ZIKV. Rates of ZIKV infection in whole females, viral dissemination to head, legs or wings, and the presence of infectious virus in saliva were assessed by plaque assay over a course of 21 days post infectious blood meal. Significant and consistent reductions were observed in the rates of whole female infectivity and virus dissemination in the wAlbA line compared with wild‐type, although these reductions were fairly modest, consistent with the low levels of blocking observed in the intrathoracic infections. However, the wAlbA infection in Ae. aegypti caused complete blocking of virus transmission (i.e. an absence of detectable virus in saliva), whereas wild‐type females were capable of transmitting infectious virus after 14 days post infectious blood meal (Fig. 2). Differences in transmission at day 14 were found to be significantly lower in wAlbA infected females (P < 0.05, Fisher's exact test).
Figure 2.
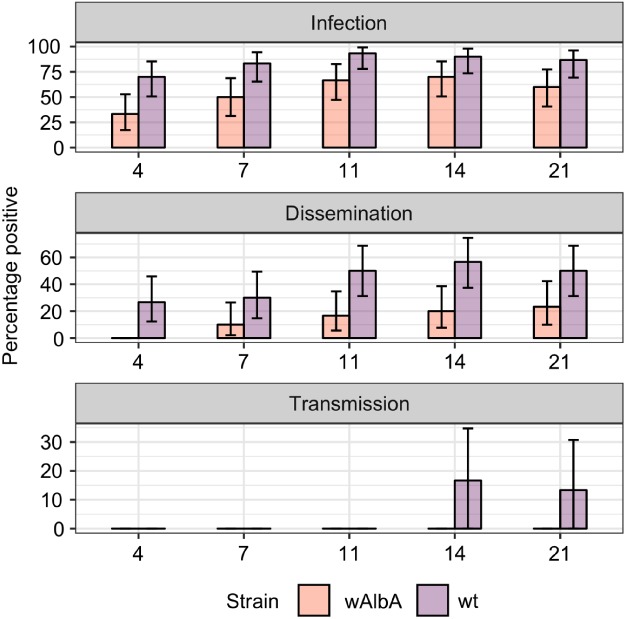
Percentage of females testing positive for Zika (ZIKV) infection, ZIKV dissemination to either the head, legs or wings, or ZIKV positivity in saliva measured by plaque assay. Each bar shows the percentage rates from 30 infected females of each strain with error bars showing the binomial 95% confidence intervals. Statistical testing was performed using a one‐tailed Fisher's exact test with P < 0.05 considered statistically significant. [Colour figure can be viewed at http://wileyonlinelibrary.com].
The difference in levels of virus inhibition between the intrathoracic and oral challenges highlights the biologically crucial role of the midgut and salivary gland barriers in Wolbachia‐mediated virus transmission blocking. Although present at lower densities than in salivary gland or ovary tissues, wAlbA is found in the cells of the Ae. aegypti midgut epithelium (Ant et al., 2018). The reduced dissemination of Zika virus to the legs or wings in the wAlbA line suggests that, even at a relatively low level, Wolbachia can limit the ability of ZIKV to cross the midgut barrier and escape into the haemolymph. Intrathoracic inoculation artificially bypasses the midgut cells, introducing high viral titres directly into the haemocoel. The data reported in the present study therefore highlight the importance of conducting oral challenges when assessing the potential of different Wolbachia strains as arbovirus control agents. Although technically easier to conduct, intrathoracic virus challenges alone can produce misleading results with respect to transmission‐blocking potential. Moreover, these findings illustrate the greater resolution achieved when virus blocking is characterized in terms of head dissemination, as well as virus titres in saliva, compared with whole‐body quantification. wAlbA can now be added to the small but growing list of Wolbachia strains that have been demonstrated to block transmission of ZIKV in Ae. aegypti and also have potential as tools for use in arbovirus control. Given the variability in blocking capacity and considering that comparative fitness effects can vary under different environmental conditions such as ambient temperature (Ulrich et al., 2016; Ross et al., 2017; Ant et al., 2018), it is important to create a range of Ae. aegypti transinfections with as many strains and therefore phenotypes as possible.
Acknowledgements
The authors declare that there are no disputes over the ownership of data presented in the present study and that all contributions have been appropriately attributed. The authors declare that they have no conflicts of interest.
References
- Aliota, M.T. , Peinado, S.A. , Velez, I.D. & Osorio, J.E. (2016) The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti . Scientific Reports, 6, 28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ant, T.H. , Herd, C.S. , Geoghegan, V. , Hoffmann, A.A. & Sinkins, S.P. (2018) The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti . PLoS Pathogens, 14, e1006815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian, G. , Xu, Y. , Lu, P. , Xie, Y. & Xi, Z. (2010) The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti . PLoS Pathogens, 6, e1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagrove, M.S. , Arias‐Goeta, C. , Failloux, A.B. & Sinkins, S.P. (2012) Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus . Proceedings of the National Academy of Sciences of the United States of America, 109, 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordenstein, S.R. , Paraskevopoulos, C. , Dunning Hotopp, J.C. et al (2009) Parasitism and mutualism in Wolbachia: what the phylogenomic trees can and cannot say. Molecular Biology and Evolution, 26, 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casiraghi, M. , Bordenstein, S.R. , Baldo, L. et al (2005) Phylogeny of Wolbachia pipientis based on gltA, groEL and ftsZ gene sequences: clustering of arthropod and nematode symbionts in the F supergroup, and evidence for further diversity in the Wolbachia tree. Microbiology, 151, 4015–4022. [DOI] [PubMed] [Google Scholar]
- Chrostek, E. , Marialva, M.S. , Esteves, S.S. et al (2013) Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenomic analysis. PLoS Genetics, 9, e1003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra, H.L. , Rocha, M.N. , Dias, F.B. , Mansur, S.B. , Caragata, E.P. & Moreira, L.A. (2016) Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host & Microbe, 19, 771–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton, T.J. & Sinkins, S.P. (2004) Strain‐specific quantification of Wolbachia density in Aedes albopictus and effects of larval rearing conditions. Insect Molecular Biology, 13, 317–322. [DOI] [PubMed] [Google Scholar]
- Fraser, J.E. , De Bruyne, J.T. , Iturbe‐Ormaetxe, I. et al (2017) Novel Wolbachia‐transinfected Aedes aegypti mosquitoes possess diverse fitness and vector competence phenotypes. PLoS Pathogens, 13, e1006751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentiu, F.D. , Zakir, T. , Walker, T. et al (2014) Limited dengue virus replication in field‐collected Aedes aegypti mosquitoes infected with Wolbachia . PLoS Neglected Tropical Diseases, 8, e2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerth, M. (2016) Classification of Wolbachia (Alphaproteobacteria, Rickettsiales): no evidence for a distinct supergroup in cave spiders. Infection, Genetics and Evolution, 43, 378–380. [DOI] [PubMed] [Google Scholar]
- Glowska, E. , Dragun‐Damian, A. , Dabert, M. & Gerth, M. (2015) New Wolbachia supergroups detected in quill mites (Acari: Syringophilidae). Infection, Genetics and Evolution, 30, 140–146. [DOI] [PubMed] [Google Scholar]
- Hancock, P.A. , Sinkins, S.P. & Godfray, H.C. (2011) Strategies for introducing Wolbachia to reduce transmission of mosquito‐borne diseases. PLoS Neglected Tropical Diseases, 5, e1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock, P.A. , White, V.L. , Ritchie, S.A. , Hoffmann, A.A. & Godfray, H.C. (2016) Predicting Wolbachia invasion dynamics in Aedes aegypti populations using models of density‐dependent demographic traits. BMC Biology, 14, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hurk, A.F. , Hall‐Mendelin, S. , Pyke, A.T. et al (2012) Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti . PLoS Neglected Tropical Diseases, 6, e1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen, V.A. , Turelli, M. & Godfray, H.C. (2008) Stochastic spread of Wolbachia . Proceedings of the Biological Sciences, 275, 2769–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert, D.A. , Walker, T. , Carrington, L.B. et al (2016) Establishment of a Wolbachia superinfection in Aedes aegypti mosquitoes as a potential approach for future resistance management. PLoS Pathogens, 12, e1005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambris, Z. , Blagborough, A.M. , Pinto, S.B. et al (2010) Wolbachia stimulates immune gene expression and inhibits plasmodium development in Anopheles gambiae . PLoS Pathogens, 6, e1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, P. , Bian, G. , Pan, X. & Xi, Z. (2012) Wolbachia induces density‐dependent inhibition to dengue virus in mosquito cells. PLoS Neglected Tropical Diseases, 6, e1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez, J. , Longdon, B. , Bauer, S. et al (2014) Symbionts commonly provide broad spectrum resistance to viruses in insects: a comparative analysis of Wolbachia strains. PLoS Pathogens, 10, e1004369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira, L.A. , Iturbe‐Ormaetxe, I. , Jeffery, J.A. et al (2009) A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium . Cell, 139, 1268–1278. [DOI] [PubMed] [Google Scholar]
- Osborne, S.E. , Iturbe‐Ormaetxe, I. , Brownlie, J.C. , O'Neill, S.L. & Johnson, K.N. (2012) Antiviral protection and the importance of Wolbachia density and tissue tropism in Drosophila simulans . Applied and Environmental Microbiology, 78, 6922–6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, P. , Wiwatanaratanabutr, I. , Axford, J. , White, V. , Endersby‐Harshman, N. & Hoffmann, A. (2017) Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathogens, 13, e1006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkins, S.P. , Braig, H.R. & O'Neill, S.L. (1995) Wolbachia superinfections and the expression of cytoplasmic incompatibility. Proceedings of the Biological Sciences, 261, 325–330. [DOI] [PubMed] [Google Scholar]
- Turelli, M. (2010) Cytoplasmic incompatibility in populations with overlapping generations. Evolution, 64, 232–241. [DOI] [PubMed] [Google Scholar]
- Turelli, M. & Hoffmann, A.A. (1999) Microbe‐induced cytoplasmic incompatibility as a mechanism for introducing transgenes into arthropod populations. Insect Molecular Biology, 8, 243–255. [DOI] [PubMed] [Google Scholar]
- Ulrich, J.N. , Beier, J.C. , Devine, G.J. & Hugo, L.E. (2016) Heat sensitivity of wMel Wolbachia during Aedes aegypti development. PLoS Neglected Tropical Diseases, 10, e0004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, T. , Johnson, P.H. , Moreira, L.A. et al (2011) The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature, 476, 450–453. [DOI] [PubMed] [Google Scholar]
- Zu Dohna, H. , Houry, C. & Kambris, Z. (2018) A comparative analysis of. Ecology and Evolution, 8, 1945–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]


