Abstract
Background and Aim
Standardizing evaluative outcomes and their assessment facilitates comparisons between clinical studies and provides a basis for comparing direct effects of different treatment options. The aim of this study was to systematically review types of outcomes and measurement instruments used in studies regarding treatment options for slow‐transit constipation (STC) in adults.
Methods
In this systematic review of the literature, we searched MEDLINE, Embase, and PsycINFO from inception through February 2018, for papers assessing any STC treatment in adult patients. Outcomes were systematically extracted and categorized in domains using the conceptual framework of the Outcome Measures in Rheumatology filter 2.0. Outcome reporting was stratified by decade of publication, intervention, and study type.
Results
Forty‐seven studies were included in this systematic review. Fifty‐nine different types of outcomes were identified. The outcomes were structured in three core areas and 18 domains. The most commonly reported domains were defecation functions (94%), gastrointestinal transit (53%), and health‐care service use (51%). The most frequently reported outcomes were defecation frequency (83%), health‐related quality of life (43%), and adverse events and complications (43%). In 62% of the studies, no primary outcome was defined, whereas in two studies, more than one primary outcomes were selected. A wide diversity of measurement instruments was used to assess the reported outcomes.
Conclusion
Outcomes reported in studies on STC in adults are heterogeneous. A lack of standardization complicates comparisons between studies. Developing a core outcome set for STC in adults could contribute to standardization of outcome reporting in (future) studies.
Keywords: Core outcome set, OMERACT, Outcomes, Slow‐transit constipation
Introduction
Chronic constipation is a condition with a high reported prevalence ranging from 16% to 19.2% in Europe.1, 2 Functional, or idiopathic, constipation is a subtype of chronic constipation that is associated with no clear organic cause.3 The diagnosis of functional constipation is based on the Rome IV criteria.3, 4 These criteria define constipation as the presence of at least two symptoms of constipation, such as a low defecation frequency, straining, lumpy or hard stools, and a sensation of incomplete evacuation.4 Functional constipation can be classified in three (overlapping) subtypes: normal transit constipation, slow‐transit constipation (STC), and evacuation disorders.5, 6
Even though the 2013 medical position statement of the American Gastroenterological Association argues different treatment algorithms for patients with normal transit constipation or STC versus patients with evacuation disorders,6 clinical studies do not always distinguish between the various subtypes of functional constipation. Different treatment algorithms, however, may be associated with different relevant outcomes. Therefore, and also in order to facilitate epidemiological, etiological, pathophysiological, and therapeutic enquiry,5 it is relevant to take this subclassification into account when designing clinical studies.
This paper focuses on STC. STC is present in 15% to 42% of the patients diagnosed with functional constipation and is associated with slow transit of feces.3, 7 First treatment options for STC consist of (a combination of) lifestyle changes, behavioral treatment, pharmacological treatment, or retrograde colonic irrigation.3, 8, 9 Patients not responding to conservative treatment might undergo surgical interventions such as sacral neuromodulation, subtotal colectomy, or colostomy.7
In the scientific literature, there is no formal consensus on the types of outcomes to be assessed or measurement instruments to be used in clinical studies in patients with STC. Formalization could be achieved by the development of a core outcome set (COS). A COS is a minimal set of outcomes to be included in every (clinical) study regarding treatment for a certain health condition.10, 11, 12, 13 Implementation of a COS can standardize evaluative outcomes and their assessment and decrease the likelihood of outcome reporting bias.10, 12, 13 It also facilitates comparisons between the direct effects of different treatment options, which is of importance when supporting the development of treatment guidelines and policies.10
Databases of the International Consortium for Health Outcomes Measurement and Core Outcome Measures in Effectiveness Trials provide only one constipation‐related COS for clinical trials in childhood constipation (0–18 years).14 This COS, however, does not specifically focus on STC as a subtype of functional constipation and focuses on outcome assessment in children and adolescents. Therefore, we argue that an additional COS will contribute to standardizing outcome reporting in studies on STC in adult patients.
Before the actual development of a COS, a comprehensive overview of the existing types of outcomes is required.10, 11 Therefore, we systematically reviewed the literature and extracted the types of outcomes and measurement instruments reported in STC studies in adult patients. As our aim was to provide an overview of the existing types of outcomes in STC research, this review does not focus on the results of the included studies. This literature review could, in turn, provide a basis for future development of a COS for STC in adult patients.
Methods
Search strategy
The initial search was conducted in July 2016, followed by an updated search in February 2018. In the Supporting Information, the full search query is described. In short, the databases MEDLINE (PubMed), Embase (Ovid), and PsycINFO (EBSCO) were searched with a combination of disease terms (e.g. constipation, slow‐transit, and idiopathic), treatment terms (e.g. treatment, therapy, laxatives, biofeedback, and colectomy), and outcome terms (e.g. effectiveness and outcome). The search was restricted to English articles using human subjects. If MeSH terms or subject headings existed, they were included in the search. Although the review focuses on STC, a broad search strategy was used to ensure that all potentially relevant articles were identified. Subsequently, articles on other (sub)types of constipation were excluded during eligibility assessment.
Eligibility assessment
Titles and abstracts were retrieved and imported in Endnote X7. Duplicates were removed. Titles and abstracts were screened for eligibility by the first reviewer (A. R. for the initial search or S. H. for the update) using the criteria outlined in Table 1. The second reviewer (M. K.) screened a subset of 3% randomly selected titles and abstracts for eligibility. As within this subset there was complete agreement between the reviewers, duplicate title and abstract screening of the full sample of papers was not considered necessary. After title and abstract screening, full‐text articles were retrieved and screened for eligibility by the first reviewer (A. R. or S. H.).
Table 1.
Eligibility criteria in chronological inclusion order
| Population | Patients (humans) with slow‐transit constipation aged ≥ 18 years. If patients < 18 years were included, only outcomes assessed in participants ≥ 18 years were extracted |
| Intervention | Any treatment of constipation such as laxatives, biofeedback, physical exercise, subtotal colectomy, and sacral neuromodulation |
| Comparator | Any treatment of constipation such as laxatives, biofeedback, physical exercise, subtotal colectomy, and sacral neuromodulation or placebo, or a different patient group as comparator, or no control group |
| Outcome | Safety, efficacy, or effectiveness of constipation treatment, measured with any outcome |
| Study type | Case studies, cohort studies (prospective and retrospective), case–control studies, noncontrolled trials, cross‐over studies, and ([non]randomized controlled) trials |
| Language | English |
| Outcome reporting quality | Studies were excluded when it was unclear which outcomes were used and/or how outcomes were measured |
| Publication status | Published |
| Publication year | No restriction |
| Publication type | Full‐text articles (no conference abstracts) |
Quality assessment of outcome reporting
The eligibility criterion “outcome reporting quality” was evaluated through a quality assessment. When articles were eligible based on the full text, the quality of reporting of the outcomes and measurement instruments was assessed. Table 2 shows the criteria of the quality assessment. Articles were included in the systematic literature review if they scored ≥ 3 points (out of 4 points) in the quality assessment. The assessment was performed using a selection of appropriate criteria regarding outcome reporting from the following existing quality checklists: STROBE, TREND, CONSORT, and MINORS.15, 16, 17, 18
Table 2.
Three item assessment of reporting quality of outcomes and measurement instruments
| No. | Criterion | Yes | No |
|---|---|---|---|
| 1 |
Study outcomes and methods used to collect data are clearly described. All of the following criteria must be true: • The outcomes are clearly specified and defined. • It is clearly described how the outcomes are measured. • It is clearly described by whom the outcomes are reported. |
2 points | 0 points |
| 2 |
All outcomes reported in the Results section are specified in the Introduction or Methods section. If there are any outcomes reported in the Results section that are not specified in the Introduction/Methods section, 0 points are scored. |
1 point | 0 points |
| 3 |
Unambiguous explanation of outcomes and outcome measures throughout the article. The reporting of outcomes is consistent throughout the article. There is no unambiguous reporting that makes it confusing for the reader to assess what has been done. |
1 point | 0 points |
Data extraction
The first reviewer (A. R. or S. H.) conducted the primary data extraction. Extraction included data on population age, treatment option(s), primary and secondary outcomes, study type, and reporting and measurement methods of the outcomes. The second reviewer (M. K.) extracted data from a subset of 15% randomly selected articles to check the comprehensiveness of the primary data extraction. As full consensus was reached between the two reviewers for this subset, duplicate data extraction was not deemed necessary.
Classification of outcomes in domains
The Outcome Measures in Rheumatology (OMERACT) filter 2.0 was used to categorize the extracted primary and secondary outcomes. This conceptual framework follows the 1.0 version that has been used since 1998 to categorize outcomes in rheumatology.19 The 2.0 version has been developed for other health conditions and was successfully used in multiple systematic reviews.11, 20, 21, 22 The framework covers two concepts: impact of health conditions and pathophysiological manifestations. These concepts cover four core areas: life impact, resource use and economical impact, pathophysiological manifestations, and death. All outcomes reported in the included papers were classified in domains by two authors (A. R. and S. H.). Each domain was classified under one of the four core areas. When more than six outcomes were categorized into the same domain, the domain was split in subdomains based on grouping of clinical symptoms. Adverse events and complications were reported as an additional concept of the OMERACT 2.0 filter.11 See Figure 1 for the conceptual framework.
Figure 1.
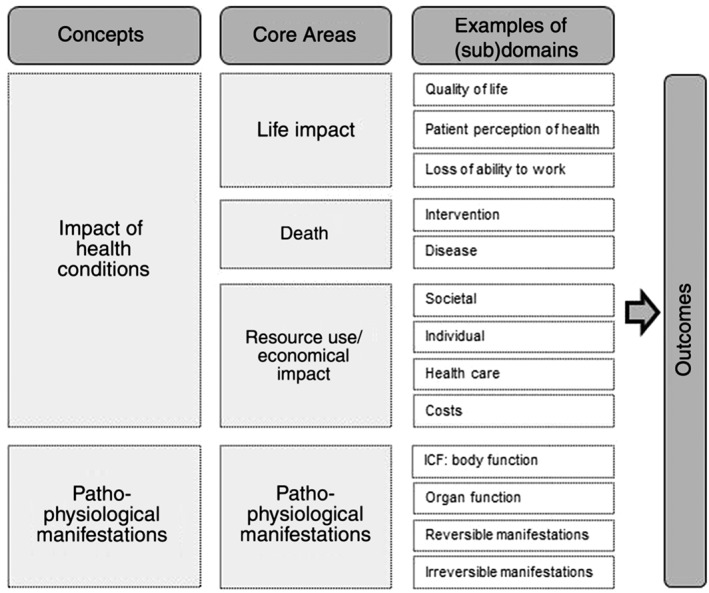
Conceptual framework OMERACT filter 2.0.11. International Classification of Functioning (ICF), Disability and Health
Analysis
Primary, as well as overall, outcome reporting was analyzed. Results were summarized using frequencies and percentages. The frequency of outcome domain reporting was calculated for all included papers and for various subgroups of papers. Subgroups were made according to decade of publication (inception–1996, 1997–2007, and 2008–2018), study type (case report/case series, uncontrolled trials, retrospective cohort study, and [non]randomized controlled trials), and intervention category (pharmacological agents, stimulation therapy, resectional surgical techniques, and other treatments). Analyses were conducted using Microsoft Excel 2016.
Results
Search results
MEDLINE, Embase, and PsycINFO were first searched in July 2016, identifying 1680 records (PubMed: 552; Embase: 1108; and PsycINFO: 20). The updated search in February 2018 revealed another 272 records (PubMed: 60; Embase: 205; and PsycINFO: 7), resulting in a total of 1952 records. After deducting duplicates, 1458 records were included in title and abstract screening, resulting in full‐text screening of 229 records. Full‐text screening followed by quality assessment of outcome reporting revealed 47 studies to be included. Figure 2 shows the PRISMA flow diagram.
Figure 2.
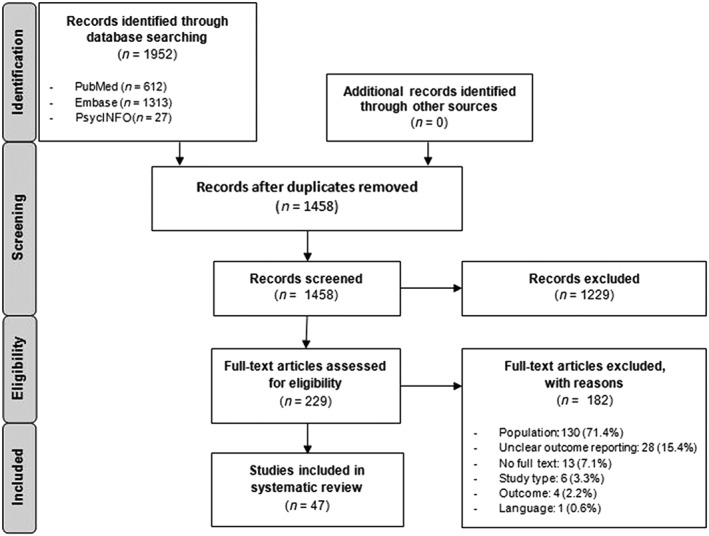
PRISMA flow diagram showing identification, screening, eligibility, and inclusion of studies on slow‐transit constipation in adult patients.
Study characteristics
In total, 47 articles published between 1989 and February 2018 were included. Table S1 lists the included studies.23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69 Characteristics of the included studies are summarized in Table 3. The majority of the studies were conducted in both adults and elderly (55%).28, 30, 32, 35, 36, 38, 39, 41, 42, 45, 47, 51, 52, 53, 54, 56, 57, 59, 60, 61, 62, 63, 65, 66, 67, 68 Twenty interventions were examined across the studies, classified in four categories. Most studies assessed the effect of stimulation therapy (34%),28, 29, 30, 32, 33, 38, 41, 43, 46, 49, 60, 61, 63, 65, 66, 69 followed by surgical resections (28%),31, 34, 39, 42, 45, 48, 50, 51, 52, 54, 58, 59, 62 pharmacologic agents (24%),23, 24, 26, 37, 40, 44, 47, 53, 55, 56, 64 and other treatment modalities (14%).25, 27, 35, 36, 57, 67, 68 Various diagnostic methods and thresholds to define STC were applied in the study populations. Thirty‐four (72%) studies used a radiopaque marker study to define STC, whereas four studies used colonic transit scintigraphy. Four studies combined both methods. A detailed overview can be found in Table S2.
Table 3.
Characteristics of the 47 included studies on any treatment options for adult patients with slow‐transit constipation
| n (%) | n (%) | ||
|---|---|---|---|
| Study type† | Intervention | ||
| Case report and case study | 17 (36) | Stimulation therapy§ | 16 (34) |
| Uncontrolled trial | 15 (32) | Resectional surgical techniques¶ | 13 (28) |
| Retrospective cohort study | 1 (2) | Pharmacologic agents†† | 11 (24) |
| (Non)randomized controlled trial | 14 (30) | Biofeedback‡‡ | 3 (6) |
| Fecal microbiota transplantation‡‡ | 2 (4) | ||
| Publication year | Abdominal wall massage‡‡ | 1 (2) | |
| 1989–1996 | 9 (19) | Body acupuncture‡‡ | 1 (2) |
| 1997–2007 | 15 (32) | ||
| 2008–2018 | 23 (49) | Comparator | |
| No control | 30 (64) | ||
| Population‡ | Placebo | 13 (28) | |
| Adults (18–64 years) | 20 (43) | Fiber | 1 (2) |
| Elderly (> 64 years) | 1 (2) | Conventional treatment | 1 (2) |
| Adults and elderly (18+ years) | 26 (55) | Control is different patient group | 1 (2) |
| Subtotal colectomy | 1 (2) |
The study type taxonomy of Shawhan et al.70 and Solomon and McLeod71 was adapted for comparative purposes of this study, informed by the book of Gerstman.72
The UN age classification was adapted for the purpose of this study.73
Includes sacral nerve stimulation/sacral neuromodulation (n = 10; 21%); transcutaneous/transabdominal electrical stimulation/interferential therapy (n = 2; 4%); percutaneous nerve stimulation (n = 2; 4%); external magnetic sacral dermatome stimulation (n = 1; 2%); and colonic electrical stimulation with intramuscular electrode placement (n = 1; 2%).
Includes total colectomy (n = 6; 13%); subtotal colectomy (n = 5; 11%); appendicostomy (n = 1; 2%); and a combination of techniques (n = 1; 2%).
Includes fiber (n = 3; 6%); prokinetic agents (n = 2; 4%); colchicine (n = 2; 4%); probiotics (n = 1; 2%); synbiotics (n = 1; 2%); oral vancomycin (n = 1; 2%); and misoprostol (n = 1; 2%).
The interventions biofeedback, fecal microbiota transplantation, abdominal wall massage, and body acupuncture form the fourth intervention category “other treatment modalities.”
Primary outcomes
In 29 (62%) studies, no distinction was made between primary and secondary outcomes. Sixteen studies defined one primary outcome: digestive functioning (n = 4),24, 44, 45, 47 improvement in constipation symptoms or defecation frequency (n = 3),27, 49, 57 constipation severity (n = 3),28, 55, 65 defecation frequency (n = 3),38, 60, 66 treatment success (n = 2),32, 68 and health‐related quality of life (n = 1).42 One study defined a composite primary outcome: treatment success defined in terms of both the clinical cure and the clinical improvement rate.64 Another study defined four primary outcomes: treatment success, clinical improvement rate, stool consistency, and safety endpoints.67 In total, eight different (combinations of) primary outcomes were defined. Of the studies defining a primary outcome, 50% were uncontrolled trials,27, 28, 32, 45, 47, 49, 65, 66, 67 39% were (non)randomized controlled trials,24, 38, 44, 55, 60, 64, 68 one case study,57 and one retrospective cohort study.42
Categorization of outcomes
In total, 59 different types of outcomes were assessed in the 47 included studies. On average, 6.9 (range 1–14) different outcomes were assessed per study. Of the 59 different types of outcomes, 23 outcomes were categorized in the concept impact of health conditions and 35 in the concept pathophysiological manifestations. Subsequently, the outcomes were categorized in three core areas and 18 domains. Complications and side effects were labeled as an additional concept. Outcomes within the core area death were not reported in any of the included studies. The most frequently reported domains are defecation functions, digestive functions, and health‐care service use in 94%, 53%, and 51% of the studies, respectively.
The most frequently reported types of outcomes are defecation frequency in 83% of the studies, followed by 43% of the studies reporting health‐related quality of life, adverse events and complications, and constipation severity.
Core area life impact
The core area life impact consists of eight domains: health‐related quality of life, treatment satisfaction, treatment success/improvement, self‐care, domestic life, interpersonal interactions and relationships, well‐being, and community, social, and civic life. In total, 64% of the studies reported 16 different types of outcomes in these domains.26, 27, 28, 31, 32, 33, 34, 35, 36, 39, 42, 43, 46, 47, 48, 49, 50, 51, 52, 54, 58, 59, 60, 61, 62, 63, 64, 66, 67, 68 Table 4a summarizes the domains, the types of outcomes, and the measurement instruments reported in the 47 included studies. The domains most frequently reported were health‐related quality of life (43%),28, 31, 32, 33, 34, 42, 46, 48, 49, 50, 51, 52, 58, 59, 60, 62, 63, 64, 66, 67 treatment satisfaction (28%),31, 34, 39, 42, 47, 48, 52, 54, 59, 60, 62, 64, 66 and treatment success/improvement (26%).27, 31, 32, 34, 36, 43, 49, 59, 61, 64, 67, 68 Health‐related quality of life was also the most commonly reported outcome within the core area life impact in 43% of the studies. This was followed by the outcome satisfaction within the treatment satisfaction domain, reported in 26% of the studies.31, 34, 39, 47, 48, 52, 54, 59, 60, 62, 64, 66 Table S3A lists the outcome reporting frequency in the core area life impact.
Table 4.
Reporting of outcome domains in the 47 included studies according to the OMERACT 2.0 filter core areas “life impact” and “resource use and economical impact”
| Outcome domain |
Outcome reporting in domains n studies (%) |
Outcomes reported within domain | Measurement instruments |
|---|---|---|---|
| (a) Core area life impact | |||
| Health‐related quality of life28, 31, 32, 33, 34, 42, 46, 48, 49, 50, 51, 52, 58, 59, 60, 62, 63, 64, 66, 67 | 20 (43) | • Health‐related quality of life |
• SF‐36 • GIQLI • PAC‐QOL • IBS‐QOL • Templeton score |
| Treatment satisfaction31, 34, 39, 42, 47, 48, 52, 54, 59, 60, 62, 64, 66 | 13 (28) | • Satisfaction | • 4‐ or 5‐point Likert scale |
| • Willingness to undergo treatment again | • Undefined questionnaire(s) | ||
| • Telephone interview | |||
| Success/improvement27, 31, 32, 34, 36, 43, 49, 59, 61, 64, 67, 68 | 12 (26) | • Improvement/response | • KESS score |
| • Treatment success | • CCSS | ||
| • Achievement of performance aspiration | • 4‐point Likert scale | ||
| • Perceived duration of success/improvement | • Patient diary | ||
| • (Telephone) interview | |||
| • Subjective assessment of outcome | |||
| • Undefined questionnaire(s) | |||
| Self‐care52, 58, 62 | 3 (6) | • Dietary changes | • Undefined questionnaire(s) |
| • Nutritional status | • Mini Nutritional Assessment | ||
| Domestic life31, 63 | 2 (4) | • Household work | • Undefined questionnaire(s) |
| • Patient diary | |||
| Interpersonal interactions and relationships31, 62 | 2 (4) | • Social activity | • Undefined questionnaire(s) |
| • Family relationships | |||
| • Sexual life | |||
| Well‐being26, 35 | 2 (4) | • Well‐being | • VAS |
| • Undefined questionnaire(s) | |||
| Community, social, and civic life31 | 1 (2) | • Recreation | • Undefined questionnaire |
| (b) Core area resource use and economical impact | |||
| Health‐care service use25, 26, 27, 28, 29, 32, 33, 38, 39, 42, 43, 46, 50, 51, 52, 57, 58, 59, 60, 62, 66 | 22 (47) | • Hospitalization | • Hospital records |
| • Constipation drug use | • Patient diary | ||
| • Enema use | • Undefined questionnaire(s) | ||
| • Suppository use | |||
| • Anti‐diarrheal drug use | |||
| • Further surgical intervention | |||
| Compliance23 | 1 (2) | • Compliance | • Sachet count |
CCSS, Cleveland Clinic Constipation Score System; GIQLI, Gastrointestinal Quality of Life Index; IBS‐QOL, Irritable Bowel Syndrome Quality of Life instrument; KESS, Knowles Eccersley Scott Symptom; PAC‐QOL, Patient Assessment of Constipation Quality of Life questionnaire; SF‐36, Short Form 36; VAS, Visual Analogue Scale.
Core area resource use and economical impact
The core area resource use and economical impact consists of two domains: health‐care service use and compliance. In total, 49% of the studies reported seven different types of outcomes in these domains.23, 25, 26, 27, 28, 29, 32, 33, 38, 39, 42, 43, 46, 50, 51, 52, 57, 58, 59, 60, 62, 66 Table 4b summarizes the domains, the types of outcomes, and the measurement instruments reported in the 47 included studies. The domain health‐care service use was reported in 47% of the studies,25, 26, 27, 28, 29, 32, 33, 38, 39, 42, 43, 46, 50, 51, 52, 57, 58, 59, 60, 62, 66 whereas the domain compliance was only reported in one (i.e. 2%) study.23 Within the domain health‐care service use, the outcome constipation drug use was reported in 34% of the studies,25, 26, 27, 28, 29, 32, 33, 38, 39, 43, 47, 50, 57, 59, 60, 66 followed by 9% of the studies reporting enema use.28, 43, 46, 57 Table S3B lists the outcome reporting frequency in the core area resource use and economical impact.
Core area pathophysiological manifestations
The core area pathophysiological manifestations consists of eight domains: defecation functions, digestive functions, sensation of pain, sensations associated with the digestive system, blood, ingestion functions, weight maintenance functions, and urinary functions. The domain defecation functions was divided into seven subdomains: defecation frequency, general defecation functions, elimination of feces, fecal consistency, fecal continence, flatulence, and other types of outcomes. All except two studies (i.e. 96%) reported on 35 different types of outcomes within this core area.23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 49, 50, 51, 52, 53, 54, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69 Table 5 summarizes the domains, the types of outcomes, and the measurement instruments reported in the 47 included studies.
Table 5.
Reporting of outcome domains in the 47 included studies according to the OMERACT 2.0 filter core area “pathophysiological manifestations”
| Outcome (sub)domain |
Outcome reporting in (sub)domains n studies (%) |
Outcomes reported within domain | Measurement instruments |
|---|---|---|---|
| Defecation functions23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 49, 50, 51, 52, 53, 54, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69 | 44 (94) | ||
| Defecation frequency19, 21, 22, 23, 24, 25, 27, 28, 29, 31, 32, 33, 34, 35, 36, 37, 38, 39, 41, 42, 43, 45, 46, 48, 49, 50, 52, 53, 54, 55, 56, 57, 58, 59, 60, 62, 63, 64, 65 | 39 (83) |
• Defecation frequency • Defecation frequency associated with complete evacuation |
• Patient diary • Telephone interview • Undefined questionnaire(s) |
| General defecation functions28, 31, 32, 33, 40, 41, 42, 46, 48, 49, 50, 51, 54, 55, 57, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69 | 25 (53) |
• Constipation symptoms (general) • Constipation severity |
• VAS • KESS score • Rome III criteria • SCL‐90 • CAS • PAC‐SYM • Wexner constipation score • Agachan score • CCSS • Patient diary • Undefined questionnaire(s) |
| Elimination of feces25, 26, 27, 29, 32, 33, 38, 41, 43, 47, 52, 57, 58, 59, 63, 64, 65 | 17 (36) |
• Straining • Incomplete evacuation • Need to digitate/assisted defecation • Difficulty with rectal evacuation • Anorectal motility |
• 5‐point Likert scale • Anorectal manometry • Balloon expulsion test • Stationary pull through • Patient diary • Undefined questionnaire(s) |
| Fecal consistency23, 26, 35, 38, 39, 41, 47, 52, 57, 58, 59, 60, 64, 66, 67, 68 | 16 (34) | • Stool consistency |
• Bristol stool scale • Patient diary • (Telephone) interview • Undefined questionnaire(s) |
| Fecal continence34, 39, 42, 50, 51, 52, 58, 59, 62, 63 | 10 (21) |
• Anal/fecal incontinence • Soiling |
• Holschneider score • Telephone interview • Wexner incontinence score • Vaizey incontinence score • Patient diary • Undefined questionnaire(s) |
| Other23, 26, 32, 43, 53, 63 | 6 (13) |
• Stool weight • Time spent toileting |
• Patient diary • Scale |
| Flatulence23, 64 | 2 (4) | • Flatulence |
• Interview • Patient diary • 5‐point Likert scale |
| Digestive functions23, 24, 25, 26, 27, 28, 29, 30, 33, 35, 36, 37, 40, 41, 44, 45, 47, 49, 53, 56, 64, 65, 66, 67, 68 | 25 (53) |
• Colonic/whole‐gut transit time • Mouth‐to‐cecum transit time • Gastrointestinal transit time • Geometric center • Colonic motility • Gastric emptying |
• Radiopaque marker study • Scintigraphic study • Hydrogen breath test • Manometry • Undefined questionnaire(s) |
| Sensation of pain19, 21, 22, 23, 28, 29, 30, 35, 37, 38, 43, 46, 48, 49, 52, 53, 54, 55, 56, 58, 60, 62, 65 | 23 (49) |
• Pain (general) • Abdominal pain • Pelvic and anorectal pain • Abdominal, pelvic, and anorectal pain |
• VAS • GIQLI • Patient diary • (Telephone) interview • Undefined questionnaire(s) |
| Sensations associated with digestive system23, 27, 32, 33, 41, 42, 45, 47, 56, 57, 59, 60, 62, 64, 66 | 15 (32) |
• Nausea • Abdominal bloating/fullness • Borborygmi • Urgency • Pyrosis • Belching |
• GIQLI • Interview • Patient diary • Undefined questionnaire(s) |
| Blood26, 62 | 2 (4) | • Blood levels | • Not clear |
| Ingestion functions45, 62 | 2 (4) | • Vomiting | • Undefined questionnaire(s) |
| Weight maintenance functions23 | 1 (2) | • Anorexia | • Interview |
| Urinary functions63 | 1 (2) | • Urinary complaints | • Patient diary |
CAS, Constipation Assessment Scale; CCSS, Cleveland Clinic Constipation Score System; GIQLI, Gastrointestinal Quality of Life Index; KESS, Knowles Eccersley Scott Symptom; PAC‐SYM, Patient Assessment of Constipation Symptoms; SCL‐90, Symptom Checklist 90; VAS, Visual Analogue Scale.
Domains most frequently reported were defecation functions (94%),23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 49, 50, 51, 52, 53, 54, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69 digestive functions (53%),23, 24, 25, 26, 27, 28, 29, 30, 33, 35, 36, 37, 40, 41, 44, 45, 47, 49, 53, 56, 64, 65, 66, 67, 68 sensation of pain (49%),23, 25, 26, 27, 32, 33, 34, 39, 41, 42, 47, 50, 52, 53, 56, 57, 58, 59, 60, 62, 64, 66, 69 and sensations associated with the digestive system (32%).23, 27, 32, 33, 41, 42, 45, 47, 56, 57, 59, 60, 62, 64, 66 The most commonly reported subdomains of defecation functions were defecation frequency (83%),23, 25, 26, 27, 28, 29, 31, 32, 33, 35, 36, 37, 38, 39, 40, 41, 42, 43, 45, 46, 47, 49, 50, 52, 53, 54, 56, 57, 58, 59, 60, 61, 62, 63, 64, 66, 67, 68, 69 general defecation functions (53%),28, 31, 32, 33, 40, 41, 42, 46, 48, 49, 50, 51, 54, 55, 57, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69 and elimination of feces (36%).25, 26, 27, 29, 32, 33, 38, 41, 43, 47, 52, 57, 58, 59, 63, 64, 65 Outcomes most frequently reported were defecation frequency (83%),23, 25, 26, 27, 28, 29, 31, 32, 33, 35, 36, 37, 38, 39, 40, 41, 42, 43, 45, 46, 47, 49, 50, 52, 53, 54, 56, 57, 58, 59, 60, 61, 62, 63, 64, 66, 67, 68, 69 constipation severity (43%),28, 31, 32, 33, 40, 42, 46, 48, 49, 50, 51, 54, 55, 60, 62, 63, 65, 66, 67, 68 and colonic/whole‐gut transit time (40%).25, 26, 27, 28, 33, 35, 36, 37, 40, 41, 47, 49, 53, 56, 64, 65, 66, 67, 68 Table S3C lists the outcome reporting frequency in the core area pathophysiological manifestations.
Outcome domains based on decade of publication
Reporting of the health‐related quality of life domain increased from zero studies published from inception to 1996 to 57% of the studies published after 2008. On the other hand, reporting of domains within the core area resource use and economical impact decreased from 67% of the studies published between 1997 and 2007 to 43% of the studies published after 2008. This also applies for reporting of the digestive function domain, decreasing from 89% to 43% of the studies over time. Domains of which reporting was stable over time were treatment success/improvement, treatment satisfaction, and defecation frequency. Reporting of outcomes in the domains sensation of pain and sensations associated with the digestive system ranged from 35% to 73% and 11% to 53% of the studies, respectively.
Outcome domains based on intervention studied
In studies on resectional surgical techniques and studies on stimulation therapy, outcomes in the health‐related quality of life domain were most frequently reported (77% and 50% of the studies, respectively). For resectional surgical techniques, this was followed by 69% of the studies reporting on outcomes in the treatment satisfaction domain. Reporting of the domain health‐care service use ranged from 18% in studies on pharmacological agents to 62% in studies on resectional surgical techniques. Reporting of the defecation function domain was consistent between intervention categories, ranging from 82% to 100%. The digestive function domain was reported in 91% of the studies on pharmacologic agents compared with 50% of the studies on stimulation therapy.
Outcome domains based on study type
Reporting of the core area life impact was most common in 88% of the case reports and case studies, followed by 73% of the uncontrolled trials and 21% of the (non)randomized controlled trials. Of the case reports and case studies, 65% reported on outcomes within the health‐related quality of life domain, followed by 47% reporting on the satisfaction domain. For the uncontrolled trials, domains most frequently reported were health‐related quality of life and treatment success/improvement, both in 40% of the studies. In the (non)randomized controlled trials, outcomes within the domains health‐related quality of life, treatment success/improvement, and satisfaction were reported in 14% of the studies.
The core area resource use and economical impact was reported in 65% of the case reports and case series, 53% of the uncontrolled trials, and 21% of the (non)randomized controlled trials. Within the core area pathophysiological manifestations, reporting of the domain sensation of pain ranged from 40% in uncontrolled trials to 59% in case reports and case studies. The digestive function domain was only reported in 12% of the case reports and case studies, whereas reporting in other study type categories ranged from 71% to 87%. Reporting of the domain defecation functions ranged from 86% in (non)randomized controlled trials to 100% in uncontrolled trials.
Adverse events and complications
Reporting of any adverse events or complications occurred in 43% of the studies.24, 32, 34, 39, 42, 47, 50, 51, 52, 54, 55, 58, 59, 60, 61, 62, 63, 66, 67, 68 Adverse events and complications have been increasingly reported over time, from 22% in studies published before 1996 to 57% in studies published after 2008. Whereas 77% of the studies on resectional surgical techniques reported this outcome, reporting in studies on other intervention categories ranged from 27% to 31%. Looking at study types, reporting of adverse events and complications ranged from 29% in (non)randomized controlled trials to 59% in case reports and case studies. No data were gathered on the types of adverse events and complications assessed in the included studies.
Measurement instruments
The majority of the outcomes (83%) were at least partially patient reported. However, the measurement instruments that were used differed within and between most types of outcomes. Many studies did not define the type of questionnaire that was used to assess outcomes (they only reported that “a questionnaire” was used). Therefore, these are described as undefined questionnaires. A summary of the types of outcomes and the measurement instruments used per outcome is shown in. Table S4.
All outcomes in the digestive functions domain were assessed by technical methods, as were improvement/response, anorectal motility, stool weight, and blood levels. The technical method most frequently used was a radiopaque marker study (n = 12) to assess colonic/whole‐gut transit time. Adverse events and complications, compliance, hospitalization, and constipation drug use were either clinician reported, derived from hospital records, or measured using undefined questionnaires.
Defecation frequency was measured using patient diaries, undefined questionnaires, and telephone interviews. Health‐related quality of life was assessed with various questionnaires of which the Short Form 36 was most often used (50%). Abdominal pain was assessed by seven different instruments of which a patient diary was used in 44% of the studies. Constipation severity was assessed by the Wexner constipation score questionnaire in 55% of the studies reporting this outcome.
Discussion
This systematic review categorizes the different types of outcomes and measurement instruments used to assess the efficacy and effectiveness of treatment options for STC in adult patients, based on the OMERACT 2.0 framework. We encountered heterogeneous reporting of different types of outcomes and measurement instruments in the reviewed literature. Across the 47 included studies, 59 types of outcomes were reported, classified into 18 domains within three core areas (life impact, resource use and economical impact, and pathophysiological manifestations). The core area death was not covered. The domain defecation functions was the most common domain, reported in 94% of the studies. Furthermore, many studies reported on digestive functions (53%), sensation of pain (49%), health‐care service use (49%), health‐related quality of life (43%), and adverse events and complications (43%). Other domains were reported in 40% of the studies or less. Types of outcomes most frequently reported were defecation frequency (83%), health‐related quality of life (43%), adverse events and complications (43%), constipation severity (43%), and colonic/whole‐gut transit time (40%). These results indicate a lack of consensus on criterions for assessing treatment efficacy and effectiveness, which makes it difficult to compare different treatment options for STC in adult patients.
Importantly, when zooming in on primary outcome reporting, as much as 62% of the studies (n = 29) did not define a primary outcome. In the remaining 38% of the studies (n = 18), eight different (combinations of) primary outcomes were reported. Compared with outcome reporting in all the 47 included studies, there is less heterogeneity when only taking into account the 18 studies defining a primary outcome. However, the most commonly reported primary outcome, digestive functioning, was only reported in 22% of these 18 studies.
Our results show increased reporting of health‐related quality of life over time. This aligns with previous findings showing an increased number of publications addressing (health‐related) quality of life over recent decades in gastroenterology.74 On the other hand, reporting of outcome domains within the core area resource use and economical impact decreased over the last two decades.
Next to the large variety of types of outcomes reported in the studies, there was a wide diversity in measurement instruments used within and between the reported outcomes. The majority (83%) of the outcome measures were at least partially patient reported, and many outcomes were assessed with undefined questionnaires. Not defining these questionnaires used limits the interpretability of the assessed outcomes as it is unknown whether validated measurement instruments were used. However, in some outcomes (e.g. defecation frequency), this might be of less importance compared with when more complex concepts (e.g. sensation of pain) are assessed. Technical measures were mostly used to assess colonic or oro‐cecal transit time, but the use of these objective measures for outcomes in the digestive function domain decreased over time. This might be explained by the theory that these measures are currently being used in the diagnostic phase, rather than as a treatment outcome. Next to the standardization of types of outcomes to be used, standardization is required on which measurement instruments to use to assess certain outcomes. When instruments with sound psychometric properties are unavailable, they should be developed.
Comparing the results of this systematic review with the available COS for childhood constipation shows some agreements and discrepancies.14 Defecation frequency, (health‐related) quality of life, and complications and side effects are included in the childhood constipation COS and are also reported in more than 43% of the literature on STC in adult patients. Stool consistency, however, was only reported in 34% of the studies in this literature review and is included in the childhood constipation COS. Another discrepancy with the COS is painful defecation. In the reviewed literature, painful defecation was not listed as a type of outcome in any of the studies. Assuming that this might be due to a lack of uniform definitions of the outcomes, painful defecation might be grasped in other outcomes included in this review such as straining, general constipation symptoms, difficulty with rectal evacuation, or pelvic and anorectal pain. However, the types of outcomes listed earlier are only reported in 21% of the studies or less. Furthermore, the COS for childhood constipation includes three outcomes only to be assessed when age appropriate (fecal incontinence, abdominal pain, and school attendance). One of these outcomes, abdominal pain, was reported in 38% of the studies in this literature review and might also be relevant for the adult population with STC.
To our knowledge, this is the first study providing a comprehensive overview of the types of outcomes and measurement instruments reported in studies on STC in adult patients. Our study has some limitations that need to be addressed. First, it is possible that not all relevant studies were included in the review due to the eligibility criteria, resulting in potentially missed types of outcomes. Second, one reviewer independently screened all records for eligibility, rather than the recommended two reviewers for systematic reviews. The second reviewer screened only a proportion of the titles and abstracts, and full‐text papers. This was partly due to pragmatic reasons (i.e. the search identified almost 2000 abstracts and > 200 full‐text papers), but more importantly, the aim of this review was to identify reported outcomes in clinical studies rather than synthesize effectiveness evidence. We feel that the potential risk (i.e. false exclusion or inclusion of a paper) of the screening approach used is minor, for several reasons: (i) there was full consensus between both reviewers for a proportion of the data, (ii) saturation of reported outcomes was most likely reached because the updated search resulted in six additional papers, but these revealed no new outcomes, and (iii) false inclusion of a paper in the initial screening of titles and abstracts would generally be noticed during the full‐text screening, leading to exclusion at this stage. A third limitation was that our search for types of outcomes was limited to those reported in the existing literature. This could potentially lead to missing outcomes that are relevant to patients. For example, patients often report fatigue in daily life during outpatient visits at the clinic. However, fatigue was not reported as an outcome in any of the 47 reviewed studies. This example stresses the importance of patient involvement in the development of a COS. Finally, publication and outcome reporting bias may have led to missing other additional relevant outcomes. Outcomes that are not (positively) affected by current treatments may not be reported.
Whereas the updated search did not reveal new types of outcomes, it did reveal two new treatment options and one new control treatment used in studies on adult patients with STC. We see that research on STC treatment options is continuously evolving. Heterogeneity of types of outcomes and measurement instruments reported in studies on STC, as identified in this review, limits the comparability of the research conducted on treatment options. The development of a COS for this specific patient group is therefore of great importance. To achieve standardization, consensus must be reached on the core primary and secondary outcomes for STC studies. Therefore, the next step in the development of a COS for STC is to conduct a consensus study with various stakeholders such as patients and health‐care professionals. This consensus study is best performed according to the Delphi methodology to achieve agreement on the outcomes and measurement instruments used.75
In conclusion, this systematic review reported on studies assessing the efficacy or effectiveness of treatment options for STC in adult patients. Results show a wide variety of types of outcomes reported and instruments used, with the majority of the studies not defining a primary outcome. To be able to compare future studies and synthesize evidence, developing a minimum COS for clinical research in adult patients with STC is recommended.
Supporting information
Table S1. Characteristics of the included studies for treatment options in slow‐transit constipation.
Table S2. Diagnostic methods and applied thresholds to define STC.
Table S3A–C. Frequency of outcome reporting within the three core areas and outcome domains.
Table S4. Types of outcomes and measurement instruments used in the included studies categorized per core area.
Acknowledgments
No acknowledgments.
Heemskerk, S. C. M. , Rotteveel, A. H. , Melenhorst, J. , Breukink, S. O. , Kimman, M. L. , and Dirksen, C. D. (2020) Heterogeneous outcome reporting in adult slow‐transit constipation studies: Systematic review towards a core outcome set. Journal of Gastroenterology and Hepatology, 35: 192–203. 10.1111/jgh.14818.
Declaration of conflict of interest: The authors declare that they have no conflict of interest.
References
- 1. Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults. Best Pract. Res. Clin. Gastroenterol. 2011; 25: 3–18. [DOI] [PubMed] [Google Scholar]
- 2. Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta‐analysis. Am. J. Gastroenterol. 2011; 106: 1582–1591. [DOI] [PubMed] [Google Scholar]
- 3. Basilisco G, Coletta M. Chronic constipation: a critical review. Dig. Liver Dis. 2013; 45: 886–893. [DOI] [PubMed] [Google Scholar]
- 4. Lacy BE, Mearin F, Chang L et al Bowel disorders. Gastroenterology 2016; 150: 1393–1407. [DOI] [PubMed] [Google Scholar]
- 5. Cook IJ, Talley NJ, Benninga MA, Rao SS, Scott SM. Chronic constipation: overview and challenges. Neurogastroenterol. Motil. 2009; 21: 1–8. [DOI] [PubMed] [Google Scholar]
- 6. Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology 2013; 144: 211–217. [DOI] [PubMed] [Google Scholar]
- 7. Tillou J, Poylin V. Functional disorders: slow‐transit constipation. Clin. Colon Rectal Surg. 2017; 30: 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bove A, Bellini M, Battaglia E et al Consensus statement AIGO/SICCR diagnosis and treatment of chronic constipation and obstructed defecation (part II: treatment). World J. Gastroenterol. 2012; 18: 4994–5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kamm MA. Clinical case: chronic constipation. Gastroenterology 2006; 131: 233–239. [DOI] [PubMed] [Google Scholar]
- 10. Williamson PR, Altman DG, Blazeby JM et al Developing core outcome sets for clinical trials: issues to consider. Trials 2012; 13: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boers M, Kirwan JR, Wells G et al Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0. J. Clin. Epidemiol. 2014; 67: 745–753. [DOI] [PubMed] [Google Scholar]
- 12. Clarke M. Standardising outcomes for clinical trials and systematic reviews. Trials 2007; 8: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kirkham JJ, Gargon E, Clarke M, Williamson PR. Can a core outcome set improve the quality of systematic reviews?—a survey of the Co‐ordinating Editors of Cochrane Review Groups. Trials 2013; 14: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuizenga‐Wessel S, Steutel NF, Benninga MA et al Development of a core outcome set for clinical trials in childhood constipation: a study using a Delphi technique. BMJ Paediatr. Open 2017; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int. J. Surg. 2014; 12: 1495–1499. [DOI] [PubMed] [Google Scholar]
- 16. Des Jarlais DC, Lyles C, Crepaz N. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am. J. Public Health 2004; 94: 361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moher D, Hopewell S, Schulz KF et al CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340: c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non‐randomized studies (minors): development and validation of a new instrument. ANZ J. Surg. 2003; 73: 712–716. [DOI] [PubMed] [Google Scholar]
- 19. Boers M, Brooks P, Strand V, Tugwell P. The OMERACT filter for Outcome Measures in Rheumatology. J. Rheumatol. 1998; 25: 333–336. [PubMed] [Google Scholar]
- 20. Kapadia MZ, Joachim KC, Balasingham C et al A core outcome set for children with feeding tubes and neurologic impairment: a systematic review. Pediatrics 2016; 138. [DOI] [PubMed] [Google Scholar]
- 21. Page MJ, Huang H, Verhagen AP, Gagnier JJ, Buchbinder R. Outcome reporting in randomized trials for shoulder disorders: literature review to inform the development of a core outcome set. Arthritis Care Res. 2018; 70: 252–259. [DOI] [PubMed] [Google Scholar]
- 22. van Tol RR, van Zwietering E, Kleijnen J et al Towards a core outcome set for hemorrhoidal disease‐a systematic review of outcomes reported in literature. Int. J. Colorectal Dis. 2018; 33: 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Badiali D, Corazziari E, Habib FI et al Effect of wheat bran in treatment of chronic nonorganic constipation. A double‐blind controlled trial. Dig. Dis. Sci. 1995; 40: 349–356. [DOI] [PubMed] [Google Scholar]
- 24. Bassotti G, Chiarioni G, Vantini I, Morelli A, Whitehead WE. Effect of different doses of erythromycin on colonic motility in patients with slow transit constipation. Z. Gastroenterol. 1998; 36: 209–213. [PubMed] [Google Scholar]
- 25. Battaglia E, Serra AM, Buonafede G et al Long‐term study on the effects of visual biofeedback and muscle training as a therapeutic modality in pelvic floor dyssynergia and slow‐transit constipation. Dis. Colon Rectum 2004; 47: 90–95. [DOI] [PubMed] [Google Scholar]
- 26. Celik AF, Tomlin J, Read NW. The effect of oral vancomycin on chronic idiopathic constipation. Aliment. Pharmacol. Ther. 1995; 9: 63–68. [DOI] [PubMed] [Google Scholar]
- 27. Chiarioni G, Salandini L, Whitehead WE. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology 2005; 129: 86–97. [DOI] [PubMed] [Google Scholar]
- 28. Collins B, Norton C, Maeda Y. Percutaneous tibial nerve stimulation for slow transit constipation: a pilot study. Colorectal Dis. 2012; 14: e165–e170. [DOI] [PubMed] [Google Scholar]
- 29. Dinning PG, Fuentealba SE, Kennedy ML, Lubowski DZ, Cook IJ. Sacral nerve stimulation induces pan‐colonic propagating pressure waves and increases defecation frequency in patients with slow‐transit constipation. Colorectal Dis. 2007; 9: 123–132. [DOI] [PubMed] [Google Scholar]
- 30. Dinning PG, Hunt LM, Arkwright JW et al Pancolonic motor response to subsensory and suprasensory sacral nerve stimulation in patients with slow‐Transit constipation. Br. J. Surg. 2012; 99: 1002–1010. [DOI] [PubMed] [Google Scholar]
- 31. Hassan I, Pemberton JH, Young‐Fadok TM et al Ileorectal anastomosis for slow transit constipation: long‐term functional and quality of life results. J. Gastrointest. Surg. 2006; 10: 1330–1337. [DOI] [PubMed] [Google Scholar]
- 32. Kamm MA, Dudding TC, Melenhorst J et al Sacral nerve stimulation for intractable constipation. Gut 2010; 59: 333–340. [DOI] [PubMed] [Google Scholar]
- 33. Kenefick NJ, Nicholls RJ, Cohen RG, Kamm MA. Permanent sacral nerve stimulation for treatment of idiopathic constipation. Br. J. Surg. 2002; 89: 882–888. [DOI] [PubMed] [Google Scholar]
- 34. King SK, Sutcliffe JR, Southwell BR, Chait PG, Hutson JM. The antegrade continence enema successfully treats idiopathic slow‐transit constipation. J. Pediatr. Surg. 2005; 40: 1935–1940. [DOI] [PubMed] [Google Scholar]
- 35. Klauser AG, Flaschentrager J, Gehrke A, Muller‐Lissner SA. Abdominal wall massage: effect on colonic function in healthy volunteers and in patients with chronic constipation. Z. Gastroenterol. 1992; 30: 247–251. [PubMed] [Google Scholar]
- 36. Klauser AG, Rubach A, Bertsche O, Muller‐Lissner SA. Body acupuncture: effect on colonic function in chronic constipation. Z. Gastroenterol. 1993; 31: 605–608. [PubMed] [Google Scholar]
- 37. Krevsky B, Maurer AH, Malmud LS, Fisher RS. Cisapride accelerates colonic transit in constipated patients with colonic inertia. Am. J. Gastroenterol. 1989; 84: 882–887. [PubMed] [Google Scholar]
- 38. Lee KJ, Kim JH, Cho SW. Short‐term effects of magnetic sacral dermatome stimulation for idiopathic slow transit constipation: sham‐controlled, cross‐over pilot study. J. Gastroenterol. Hepatol. 2006; 21: 47–53. [DOI] [PubMed] [Google Scholar]
- 39. Lubowski DZ, Chen FC, Kennedy ML, King DW. Results of colectomy for severe slow transit constipation. Dis. Colon Rectum 1996; 39: 23–29. [DOI] [PubMed] [Google Scholar]
- 40. Magro DO, De Oliveira LMR, Bernasconi I et al Effect of yogurt containing polydextrose, Lactobacillus acidophilus NCFM and Bifidobacterium lactis HN019: a randomized, double‐blind, controlled study in chronic constipation. Nutr. J. 2014; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Malouf AJ, Wiesel PH, Nicholls T, Nicholls RJ, Kamm MA. Short‐term effects of sacral nerve stimulation for idiopathic slow transit constipation. World J. Surg. 2002; 26: 166–170. [DOI] [PubMed] [Google Scholar]
- 42. Marchesi F, Sarli L, Percalli L et al Subtotal colectomy with antiperistaltic cecorectal anastomosis in the treatment of slow‐transit constipation: long‐term impact on quality of life. World J. Surg. 2007; 31: 1658–1664. [DOI] [PubMed] [Google Scholar]
- 43. Martellucci J, Valeri A. Colonic electrical stimulation for the treatment of slow‐transit constipation: a preliminary pilot study. Surg. Endosc. Other Interv. Tech. 2014; 28: 691–697. [DOI] [PubMed] [Google Scholar]
- 44. Marzio L, Del Bianco R, Delle Donne M, Pieramico O, Cuccurullo F. Mouth‐to‐cecum transit time in patients affected by chronic constipation: effect of glucomannan. Am. J. Gastroenterol. 1989; 84: 888–891. [PubMed] [Google Scholar]
- 45. Mollen RMHG, Hopman WPM, Oyen WJG, Kuijpers HHC, Edelbroek MAL, Jansen JBMJ. Effect of subtotal colectomy on gastric emptying of a solid meal in slow‐transit constipation. Dis. Colon Rectum 2001; 44: 1189–1195. [DOI] [PubMed] [Google Scholar]
- 46. Naldini G, Martellucci J, Moraldi L, Balestri R, Rossi M. Treatment of slow‐transit constipation with sacral nerve modulation. Colorectal Dis. off. J. Assoc. Coloproctology Great Britain Irel. 2010; 12: 1149–1152. [DOI] [PubMed] [Google Scholar]
- 47. Polymeros D, Beintaris I, Gaglia A et al Partially hydrolyzed guar gum accelerates colonic transit time and improves symptoms in adults with chronic constipation. Dig. Dis. Sci. 2014; 59: 2207–2214. [DOI] [PubMed] [Google Scholar]
- 48. Qian Q, Jiang C, Chen Y et al A modified total colonic exclusion for elderly patients with severe slow transit constipation. Tech. Coloproctol. 2014; 18: 629–634. [DOI] [PubMed] [Google Scholar]
- 49. Queralto M, Vitton V, Bouvier M, Abysique A, Portier G. Interferential therapy: a new treatment for slow transit constipation. A pilot study in adults. Colorectal Dis. 2013; 15: e35–e39. [DOI] [PubMed] [Google Scholar]
- 50. Ripetti V, Caputo D, Greco S, Alloni R, Coppola R. Is total colectomy the right choice in intractable slow‐transit constipation? Surgery 2006; 140: 435–440. [DOI] [PubMed] [Google Scholar]
- 51. Riss S, Herbst F, Birsan T, Stift A. Postoperative course and long term follow up after colectomy for slow transit constipation—is surgery an appropriate approach? Colorectal Dis. 2009; 11: 302–307. [DOI] [PubMed] [Google Scholar]
- 52. Sarli L, Costi R, Sarli D, Roncoroni L, Wexner SD. Pilot study of subtotal colectomy with antiperistaltic cecoproctostomy for the treatment of chronic slow‐transit constipation. Dis. Colon Rectum 2001; 44: 1514–1520. [DOI] [PubMed] [Google Scholar]
- 53. Soffer EE, Metcalf A, Launspach J. Misoprostol is effective treatment for patients with severe chronic constipation. Dig. Dis. Sci. 1994; 39: 929–933. [DOI] [PubMed] [Google Scholar]
- 54. Sohn G, Chang SY, Chan WK et al Surgical outcomes after total colectomy with ileorectal anastomosis in patients with medically intractable slow transit constipation. J. Kor. Soc. Coloproctology 2011; 27: 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Taghavi SA, Shabani S, Mehramiri A et al Colchicine is effective for short‐term treatment of slow transit constipation: a double‐blind placebo‐controlled clinical trial. Int. J. Colorectal Dis. 2010; 25: 389–394. [DOI] [PubMed] [Google Scholar]
- 56. Verne GN, Davis RH, Robinson ME, Gordon JM, Eaker EY, Sninksy CA. Treatment of chronic constipation with colchicine: randomized, double‐blind, placebo‐controlled, crossover trial. Am. J. Gastroenterol. 2003; 98: 1112–1116. [DOI] [PubMed] [Google Scholar]
- 57. Wang J, Luo MH, Qi QH, Dong ZL. Prospective study of biofeedback retraining in patients with chronic idiopathic functional constipation. World J. Gastroenterol. 2003; 9: 2109–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang Y, Zhai C, Niu L, Tian L, Yang J, Hu Z. Retrospective series of subtotal colonic bypass and antiperistaltic cecoproctostomy for the treatment of slow‐transit constipation. Int. J. Colorectal Dis. 2010; 25: 613–618. [DOI] [PubMed] [Google Scholar]
- 59. Zutshi M, Hull TL, Trzcinski R, Arvelakis A, Xu M. Surgery for slow transit constipation: are we helping patients? Int. J. Colorectal Dis. 2007; 22: 265–269. [DOI] [PubMed] [Google Scholar]
- 60. Dinning PG, Hunt L, Patton V et al Treatment efficacy of sacral nerve stimulation in slow transit constipation: a two‐phase, double‐blind randomized controlled crossover study. Am. J. Gastroenterol. 2015; 110: 733–740. [DOI] [PubMed] [Google Scholar]
- 61. Graf W, Sonesson AC, Lindberg B, Akerud P, Karlbom U. Results after sacral nerve stimulation for chronic constipation. Neurogastroenterol. Motil. 2015; 27: 734–739. [DOI] [PubMed] [Google Scholar]
- 62. Li F, Fu T, Tong W et al Effect of different surgical options on curative effect, nutrition, and health status of patients with slow transit constipation. Int. J. Colorectal Dis. 2014; 29: 1551–1556. [DOI] [PubMed] [Google Scholar]
- 63. Ratto C, Ganio E, Naldini G et al Long‐term results following sacral nerve stimulation for chronic constipation. Colorectal Dis. 2015; 17: 320–328. [DOI] [PubMed] [Google Scholar]
- 64. Ding C, Ge X, Zhang X et al Efficacy of synbiotics in patients with slow transit constipation: a prospective randomized trial. Nutrients 2016; 8: E605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kumar L, Liwanag J, Athanasakos E, Raeburn A, Zarate‐Lopez N, Emmanuel AV. Effectiveness of percutaneous tibial nerve stimulation in managing refractory constipation. Colorectal Dis. 2017; 19: 45–49. [DOI] [PubMed] [Google Scholar]
- 66. Patton V, Stewart P, Lubowski DZ, Cook IJ, Dinning PG. Sacral nerve stimulation fails to offer long‐term benefit in patients with slow‐transit constipation. Dis. Colon Rectum 2016; 59: 878–885. [DOI] [PubMed] [Google Scholar]
- 67. Tian H, Ding C, Gong J et al Treatment of slow transit constipation with fecal microbiota transplantation: a pilot study. J. Clin. Gastroenterol. 2016; 50: 865–870. [DOI] [PubMed] [Google Scholar]
- 68. Tian H, Ge X, Nie Y et al Fecal microbiota transplantation in patients with slow‐transit constipation: a randomized, clinical trial. PLoS ONE 2017; 12: e0171308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yang Y, Yim J, Choi W, Lee S. Improving slow‐transit constipation with transcutaneous electrical stimulation in women: a randomized, comparative study. Women Health 2017; 57: 494–507. [DOI] [PubMed] [Google Scholar]
- 70. Shawhan RR, Hatch QM, Bingham JR et al Have we progressed in the surgical literature? Thirty‐year trends in clinical studies in 3 surgical journals. Dis. Colon Rectum 2015; 58: 115–121. [DOI] [PubMed] [Google Scholar]
- 71. Solomon MJ, McLeod RS. Clinical studies in surgical journals—have we improved? Dis. Colon Rectum 1993; 36: 43–48. [DOI] [PubMed] [Google Scholar]
- 72. Gerstman BB. Chapter 9: types of epidemiologic studies In: Epidemiology Kept Simple: An Introduction to Traditional and Modern Epidemiology, 2nd edn. Hoboken, New Jersey: John Wiley & Sons, 2003. [Google Scholar]
- 73. United Nations . Provisional guidelines on standard international age classifications. In: United Nations doieasa, ed. New York 1982.
- 74. Borgaonkar MR, Irvine EJ. Quality of life measurement in gastrointestinal and liver disorders. Gut 2000; 47: 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Prinsen CA, Vohra S, Rose MR et al Core Outcome Measures in Effectiveness Trials (COMET) initiative: protocol for an international Delphi study to achieve consensus on how to select outcome measurement instruments for outcomes included in a ‘core outcome set'. Trials 2014; 15: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of the included studies for treatment options in slow‐transit constipation.
Table S2. Diagnostic methods and applied thresholds to define STC.
Table S3A–C. Frequency of outcome reporting within the three core areas and outcome domains.
Table S4. Types of outcomes and measurement instruments used in the included studies categorized per core area.


