Abstract
Abstract
Cadmium has long been recognized as an environmental contaminant that poses risks to human health. Cadmium is of concern since nearly everyone in the general population is exposed to the metal through the food supply and the ability of the element to accumulate in the body over a lifetime. In support of the United States Food and Drug Administration's (FDA) Toxic Element Working Group's efforts to reduce the risks associated with elements in food, this review sought to identify current or new mitigation efforts that have the potential to reduce exposures of cadmium throughout the food supply chain. Cadmium contamination of foods can occur at various stages, including agronomic production, processing, and consumer preparation for consumption. The presence of cadmium in food is variable and dependent on the geographical location, the bioavailability of cadmium from the soil, crop genetics, agronomic practices used, and postharvest operations. Although there are multiple points in the food supply system for foods to be contaminated and mitigations to be applied, a key step to reducing cadmium in the diet is to reduce or prevent initial uptake by plants consumed as food or feed crops. Due to complex interactions of soil chemistry, plant genetics, and agronomic practices, additional research is needed. Support for field‐based experimentation and testing is needed to inform risk modeling and to develop practical farm‐specific management strategies. This study can also assist the FDA in determining where to focus resources so that research and regulatory efforts can have the greatest impact on reducing cadmium exposures from the food supply.
Practical Application
The presence of cadmium in food is highly variable and highly dependent on the geographical location, the bioavailability of cadmium from the soil, crop genetics, and agronomic practices used. This study can assist the FDA in determining where to focus resources so that research and regulatory efforts can have the greatest impact on reducing cadmium exposures from the food supply.
Keywords: cadmium, food safety through prevention, mitigation, risk reduction
1. INTRODUCTION
1.1. Why is cadmium of concern?
Cadmium has long been recognized as an environmental contaminant that poses risks to human health. The ubiquitous nature of cadmium is of concern since nearly everyone in the general population is exposed to the heavy metal through the food supply and the element accumulates in the body over a lifetime. In support of the United States Food and Drug Administration's (FDA) Toxic Element Working Group's efforts to reduce the risks associated with toxic elements in food, this review sought to identify existing or possible new mitigation efforts throughout the food chain to determine where the FDA can have the greatest impact on reducing exposures.
1.2. Cadmium in the environment
Cadmium is a naturally occurring rare element that, in pure form, is a blue‐tinged malleable metal or grayish‐white powder that readily reacts with other substances (NTP, 2016). Cadmium is also dispersed into the environment through various anthropogenic processes, such as mining, smelting, nickel and cadmium batteries, metal plating, pigments, plastic stabilizers, sewage sludge disposal, and the use of phosphate fertilizers and manures (ATSDR, 2012; Clemens, Aarts, Thomine, & Verbruggen, 2013; Khan, Khan, Khan, & Alam, 2017; Meng et al., 2018; Nordberg et al., 2018; WHO, 1992). When dispersed into the atmosphere, cadmium compounds can be carried long distances and eventually fall to the earth. Cadmium moves easily through the soil and is taken up into the food chain through the uptake of plants (primarily leafy vegetables, root crops, cereals, and grains [10 to 150 µg/m3]) (ATSDR, 1999; EFSA, 2009; Klaassen, Casarett, & Doull, 2013; Smolders, 2001) and is also subsequently found in the livers and kidneys (>50 µg/m3) of animals from grazing on forage crops and filter feeders, such as crustaceans and mollusks (1 to 2 µg/kg) that bioaccumulate cadmium from contaminated aquatic environments (ATSDR, 1999; Klaassen et al., 2013; Nair, DeGheselle, Smeets, Kerkhove, & Cuypers, 2013). Ingestion of cadmium poses a major concern as it is a nonessential trace element that does not play a role in the growth of humans or plants, but is toxic to humans (Clemens et al., 2013; EFSA, 2009; Khan et al., 2017; Smolders, 2001).
In the late 1990s, occupational epidemiological, cohort studies found positive associations between inhalation exposure to cadmium compounds and an increased risk of death from lung cancer (NTP, 2016). As a result, policy decisions, technological advances, and process improvements have been implemented and cadmium emissions into the atmosphere have steadily declined (Clemens et al., 2013). However, cadmium does not degrade in the environment and is not easily removed from soil. Therefore, understanding and controlling cadmium contamination is imperative to the safety of the food supply.
1.3. Exposure to cadmium
Exposure to cadmium can occur through the ingestion of food, drinking water or contaminated soil and dust, and via inhalation of tobacco smoke or particulate matter from ambient air (ATSDR, 2012). The most significant source of human cadmium exposure is cigarette smoking, with smokers having elevated concentrations in the blood and kidneys (ATSDR, 2012; Bernhoft, 2013; EFSA, 2009). Inhalation exposure can also be significant in occupational settings, such as welding and soldering. Apart from individuals with high levels of exposure from tobacco use or the work environment, diet is the major source (90%) of cadmium exposure (Clemens et al., 2013; EFSA, 2009).
Exposure to cadmium in food may lead to its accumulation in the kidney (the most sensitive target for cadmium toxicity), which can cause tubular dysfunction and damage to the kidney over time (ATSDR, 2012; EFSA, 2009; WHO, 2011). Cadmium also exerts toxic effects on the skeletal system and bone demineralization may occur through direct bone damage or because of renal dysfunction (EFSA, 2009; WHO, 2011). Research also suggests that exposure to cadmium induces oxidative stress resulting in mitochondrial inflammation and damage; however, the mechanism is not fully understood (Cannino, Ferruggia, Luparello, & Rinaldi, 2009; Nair et al., 2013).
Several factors can influence cadmium body burden and the efficiency of absorption in humans. Absorption of cadmium after dietary exposure is estimated to be low in humans (3% to 5%) (ATSDR, 2012; Clemens et al., 2013; EFSA, 2009); however, some studies suggest that absorption in the intestines can be as high as 44% and further research is needed especially for children and young adults (Vesey, 2010). Understanding the bioavailable fraction of cadmium from specific foods and the factors that affect bioavailability would reduce the uncertainty about the levels of exposure associated with dietary cadmium (Chunhabundit et al., 2011).
Cadmium has similar chemical composition to essential metals, such as iron, zinc, and calcium and can be taken up by cells thru “ionic and molecular mimicry” (Cannino et al., 2009; Nair et al., 2013; Vesey, 2010). Cadmium and zinc bind to the same proteins in the blood (albumin) and tissues (metallothionein) and compete for uptake into cells (Brzoska & Moniuszko‐Jakoniuk, 2001). After absorption, cadmium is distributed throughout the body bound to proteins in the blood (that is, albumin) (Bernhoft, 2013; EFSA, 2009; Vacchi‐Suzzi, Kruse, Harrington, Levine, & Meliker, 2016). The half‐life in blood (deposition in organs) ranges from 75 to 128 days (Bernhoft, 2013). Approximately 60% of absorbed cadmium is deposited in the liver (30%) and kidney (30%), while the rest is distributed throughout the body and then excreted slowly (0.007% to 0.009% of body burden per day) through the urine and feces (ATSDR, 2012; Bernhoft, 2013). Cadmium is an accumulative toxicant with a long biological half‐life that has been estimated to be 10 to 33 years in humans, which results in increased body burden over time (Clemens et al., 2013; EFSA, 2009). Therefore, toxicity of cadmium generally results from chronic exposure.
Micronutrients have a major impact on health and play an important role in the development and protection of cadmium toxicity. Low micronutrient content in the food consumed and the percentage of cadmium absorbed in the body can be higher when iron, zinc, and calcium are low in the body and in the food commodity (ATSDR, 2012; Clemens et al., 2013, Klaassen et al., 2013; EFSA, 2009). Zinc can serve as a protective mechanism as it stimulates metallothionein that binds to cadmium preventing oxidative stress (Marreiro et al., 2017) and may prevent cadmium from disturbing bone metabolism thru the displacement of calcium (Brzoska & Moniuszko‐Jakoniuk, 2001). Animal laboratory studies (Flanagan et al., 1978; Reeves & Chaney, 2001; Reeves & Chaney, 2002; Reeves & Chaney, 2004; Reeves, Chaney, Simmons, & Cherian, 2005) suggest that even marginal deficiencies in micronutrients can enhance cadmium absorption as much as 10‐fold (Reeves & Chaney, 2008). Studies also suggest that deficiencies in calcium, protein, and vitamin D may increase susceptibility to bone effects after exposure to cadmium. Further, cadmium reduces blood flow and inhibits nutrient transport across the placenta by interfering with zinc uptake by human placental microvesicles (Gupta, 2011). Therefore, physiological status (age and gender), diet and body status of micronutrients, preexisting health conditions, and multiple pregnancies can impact cadmium bioavailability, retention, and toxicity in the body (ATSDR, 2012; Brzoska & Moniuszko‐Jakoniuk, 2001; EFSA, 2009; Vesey, 2010). Children, women of childbearing age, and diabetics are considered more vulnerable (Clemens et al., 2013; Satarug, Garrett, Sens, & Sens, 2010; Vesey, 2010).
2. CADMIUM IN THE FOOD SUPPLY
As mentioned previously, cadmium has the potential to enter the food supply through various sources (Figure 1). Cadmium naturally occurs in the environment and is also emitted through anthropogenic sources. Once emitted, cadmium is transported through the water, air, and soil where it can be taken up by plants, animals, and shellfish from the soil and water. Cadmium also enters the soil via agronomic practices through the use of phosphate fertilizers. The use of cadmium‐plated utensils and galvanized equipment, cadmium‐bearing stabilizers in plastics, and cadmium‐based pottery glazes can also contribute to cadmium in the food supply (ATSDR, 2012). Further, although minimal, the entry of cadmium into drinking‐water may also occur due to the presence of cadmium in galvanized pipes and/or cadmium‐containing solders in fittings in taps (WHO, 2011).
Figure 1.
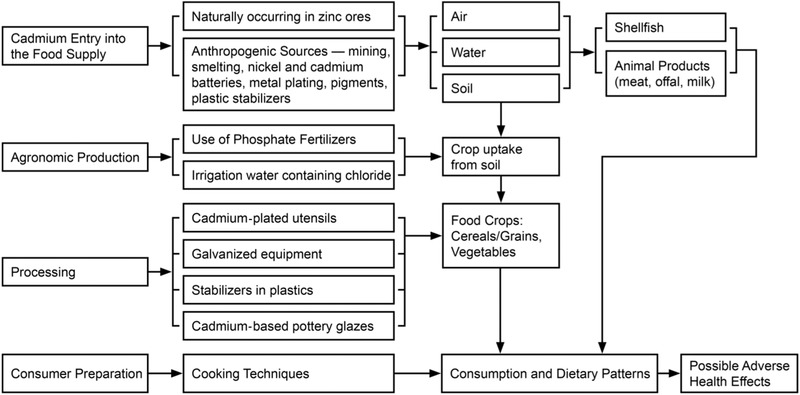
Cadmium in the food supply paradigm.
Dietary exposure and absorption of cadmium is a function of the cadmium concentration of the food and the amount of the food consumed. The European Food Safety Authority (EFSA) conducted a large‐scale analysis of cadmium in food items in 2009 and concluded that “grains and grain products,” “vegetables and vegetable products,” and “starchy roots and tubers” had the largest contribution to total cadmium intake in Europe (Clemens et al., 2013). In 2011, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) reviewed a wide range of foods distributed worldwide from Australia, Brazil, Canada, Chile, China, Ghana, Japan, Singapore, the United States, and 19 European countries (submitted via the EFSA). Mean cadmium concentrations were higher, ranging from 0.1 to 4.8 mg/kg, for crustaceans (shellfish/mollusks), organ meats (liver and kidney), vegetables, nuts and oilseeds, spices, coffee, tea, and cocoa (WHO, 2011). When consumption was considered, cereals and grains, vegetables, organ meats, and seafood contributed the most to dietary cadmium exposure (WHO, 2011). Grains, such as rice, from certain regions are known to contain higher levels of cadmium (ATSDR, 2012; EFSA, 2009; WHO, 2011).
As an ongoing effort to monitor contaminants in food, the FDA conducts The Total Diet Study (TDS), where food is purchased, prepared, and analyzed for approximately 300 foods and beverages from representative areas of the country, four times a year. Of the samples analyzed from 2014 to 2016 in the TDS, the top 10 foods with the highest mean lower bound cadmium concentrations included sunflower seeds (375 µg/kg), boiled spinach (117 µg/kg), potato chips (93 µg/kg), leaf lettuce (62 µg/kg), iceberg lettuce (54 µg/kg), peanut butter (53 µg/kg), shredded wheat cereal (51 µg/kg), dry roasted peanuts (45 µg/kg), French fries (44 µg/kg), and cooked liver (38 µg/kg) (Spungen, 2019). Studies indicate that 70% to 80% of dietary cadmium intake in humans comes from plant‐based food (Clemens et al., 2013; Khan et al., 2017; Ryan, Herbert, & Lucas, 1982). Vegetarians are estimated to a have threefold higher relative cadmium intake when compared with nonvegetarians (Clemens et al., 2013). The EFSA estimates that children, especially infants and toddlers, have a twofold higher relative cadmium intake when compared with adults (Clemens et al., 2013; EFSA, 2009) as toxic elements are more readily absorbed in the intestines of children, while renal excretion is lower than adults (Ilmiawati et al., 2015). In addition, children's dietary practices and patterns (consumption frequency and amount consumed) are often less varied than adults suggesting greater potential exposure to food borne contaminants (Vogt et al., 2012). For children in the United States (1 to 6 years old), estimated mean cadmium exposures ranged from 0.38 to 0.44 µg/kg bw/day with grains (for example, bread), mixtures (for example, brownies, chocolate cake, lasagna with meat sauce, and pizza), and vegetables (for example, lettuce, spinach, collards, and potatoes) accounting for most of the exposure based on TDS data from 2014 to 2016 (Spungen, 2019). Based on the 2011 JECFA analysis, children in Australia, Europe, and the United States (0.5 to 12 years old), estimated mean cadmium exposures ranged from 2.7 to 12.9 µg/kg bw per month; however, details regarding the contributions of specific foods were not provided in the report (WHO, 2011).
3. CADMIUM IN PLANTS
The nutrition of plants is directly dependent on how plants respond to and acquire micronutrients from the rhizosphere where soil conditions can vary in nutrients (Morrissey & Guerinot, 2009). Most often found in the environment as cadmium sulfide in zinc deposits, cadmium has similar chemical properties to zinc (Bernhoft, 2013), an essential trace element of plants (Wuana & Okieimen, 2011). Cadmium also has a similar ionic radius to the calcium cation (Ca2+) and can substitute for Ca2+ during root uptake (Meng et al., 2018). Like humans, uptake of cadmium to plant cells occurs through the same transport systems for carrying out the uptake and distribution of macro and micronutrients (magnesium, calcium, iron, zinc, and copper) throughout the plant (EFSA, 2009). The chemical similarity of cadmium to zinc allows it to exchange imperfectly for zinc in a variety of biological processes (Clemens et al., 2013; EFSA, 2009; Nordberg et al., 2018). Depending on the acidity of soil, efficient soil‐to‐plant transfer allows cadmium to travel with other micronutrients to the aerial parts of the plant leading to high accumulation of cadmium in the edible portion of plants (Khan et al., 2017, Morrissey & Gueritonot, 2009).
3.1. Factors affecting cadmium concentration of crops and controlling accumulation in agriculture
The lack of a consistent relationship between levels of cadmium in soil and the concentration of cadmium in crops presents uncertainty in defining risk factors associated with potential food contamination (Smolders, 2001). Soil characteristics, soil cadmium concentration, crop genetics, crop rotation, and fertilizer management are some of the many factors affecting the cadmium concentration in crops (Nazar et al., 2012). Total cadmium in soil is a useful predictor of cadmium uptake only if combined with knowledge of soil characteristics, crop genetics, and the agricultural management techniques used (Norvell, Wu, Hopkins, & Welch, 2000). The amount of cadmium present in agricultural soils is a function of geographical location and the agricultural practices followed; therefore, soil cadmium concentrations vary widely in the United States (Figure 2) and in different foods (ATSDR, 1999). In nonpolluted areas, cadmium concentrations in soil are usually below 1 µg/g (0.06 to 1.1 mg/kg) with an average of 0.27 mg/kg in agricultural soils (ATSDR, 2012). In polluted areas, concentrations of up to 800 µg/g have been detected (ATSDR, 2012). In addition, topsoil, where plants and organisms primarily live, is only between 5 and 10 inches thick and often had concentrations two times higher than subsoil levels (ATSDR, 2012). Knowing the distribution of the cadmium contamination (horizontally and vertically) in the soil can give clues to the origin (geogenic [weathering and volcanic] or anthropogenic [fertilizer, mines, and soil amendments]) and provides critical knowledge for developing management practices (Williams & David, 1976). Figure 2 depicts the distribution of cadmium in surface soil in the United States collected from a depth of 0 to 5 cm and in the soil A horizon (that is, topsoil) (USGS, 2014).
Figure 2.
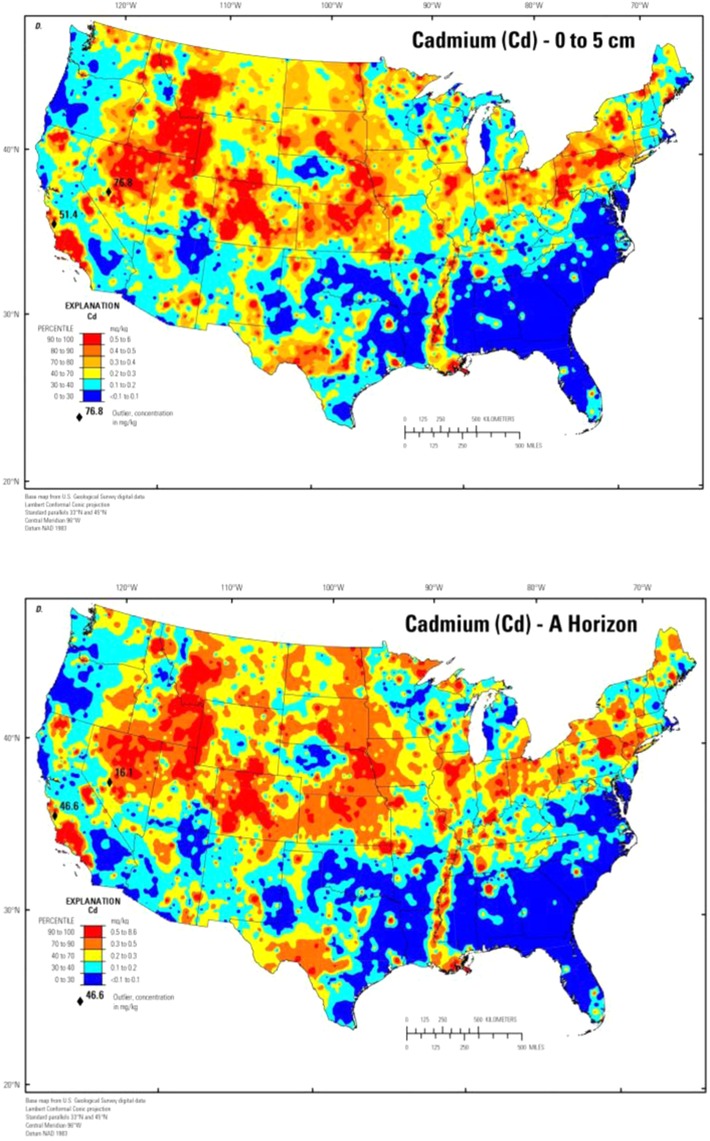
Distribution of cadmium in surface soil in the United States collected from a depth of 0 to 5 cm and in the soil A horizon. Credit: U.S. Geological Survey Dept. of the Interior/USGS.
3.1.1. Soil characteristics
Many soil factors, such as pH (Chuan, Shu, & Liu, 1996; Clemens et al., 2013; Nazar et al., 2012), availability of organic matter (Nazar et al., 2012), soil type (McLaughlin, Parker, & Clarke, 1999), redox potential (Eh) (Chuan et al., 1996; Nazar et al., 2012; Roberts, 2014; Sarwar et al., 2010; Meng et al., 2018), soil temperature (McLaughlin et al., 1999), and the application of nutrients (nitrogen, phosphorous, potassium, and zinc) (McLaughlin et al., 1999) greatly affect heavy metal solubility and uptake of cadmium from the soil (Figure 3). Soluble cadmium (Cd2+) appears to be the main form in the soil and is taken up by plants readily (Meng et al., 2018) and can bioaccumulate in all parts of the food chain (ATSDR, 1999). The solubility of cadmium in soil directly influences the bioactivity and bioavailability of cadmium and determines the accumulation, toxicity, biomodification, and transport in the environment (Meng et al., 2018).
Figure 3.
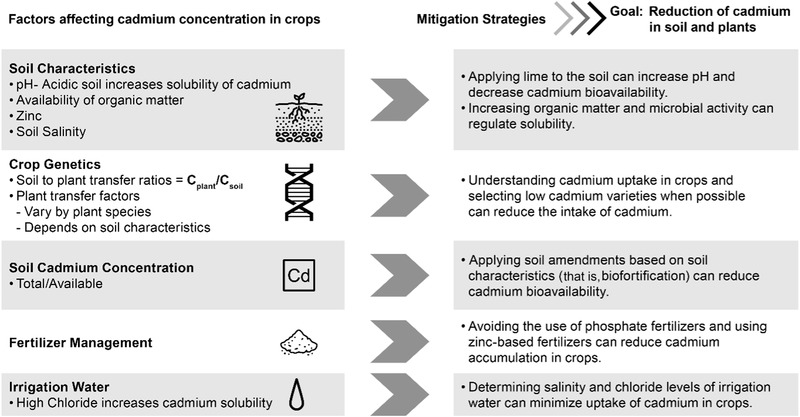
Factors affecting cadmium concentrations in crops and potential mitigation strategies to reduce cadmium in plants.
Studies have shown that pH is of prime importance since heavy metal solubilities are significantly higher when under acidic conditions (low pH = 3.0 to 5.0) (Chuan et al., 1996; McLaughlin et al., 1999; Roberts, 2014). Bioavailability is reduced when soil pH levels are greater than 6 as cadmium tends to bind with organic matter and other minerals (Roberts, 2014). Adding lime to the soil increases the pH of the soil making the soil environment less acidic (Roberts, 2014). In addition, recent studies (Bashir et al., 2018; Zheng et al., 2017) suggest that the application of rice biochar, an organic material produced from rice husks at high temperatures, to soil can increase soil pH, cation exchange capacity, and fertility thus preventing cadmium uptake and further reducing accumulation within plants.
Redox conditions also affect the solubility of cadmium in the soil and microbial communities. Changes in redox potential can affect organic matter, minerals, and the regulation of rhizosphere microbes (Meng et al., 2018; Nazar et al., 2012). The change in redox status changes the electron acceptors and the capacity for chelating with cadmium ions (Nazar et al., 2012). The ligand environment is critical as it determines the absorption rate and bioavailability of cadmium (Clemens et al., 2013). Soluble cadmium can be reduced by chemically aided microbes or fixed by microbial absorption thus affecting plant absorption of cadmium (Meng et al., 2018). The metabolism of microbes produces secretions, such as organic acids, that can dissolve cadmium and other heavy metals in soil; therefore, adding microbial species to the soil, such as bacteria and fungi, can aid in the reduction of soil cadmium (Jin, Luan, Ning, & Wang, 2018). Cadmium also binds strongly to soils with higher organic matter (higher cation exchange capacity) making cadmium less available to plant life (ATSDR, 1999; Roberts, 2014). In addition, wet and dry rotations affect microbe composition (Meng et al., 2018) and cadmium concentration in crops varies with rainfall (Eriksson, Oborn, Gunilla, & Arne, 1996). Regulating the solubility and understanding the reduction potential of soil and adding competing ions (for example, Zn – Fe) to the soil (biofortification) may contribute to lowering cadmium exposure in plants (Clemens et al., 2013; Meng et al., 2018).
3.1.2. Crop genetics
The concentration of cadmium in the soil is not the primary determinant in the human diet. Crop species and cultivars differ widely in their ability to take up and accumulate cadmium (Roberts, 2014). Crops respond differently to soil properties and naturally vary between species but also between varieties of the same species (Clemens et al., 2013; Eriksson et al., 1996). Soil to plant transfer ratios (Cplant/Csoil) not only vary by plant species, but also depend on pH, texture, organic matter, geology, soil amendments, and so on. Therefore, one option to reduce cadmium uptake is through the selection of low cadmium varieties and plant breeding (Eriksson et al., 1996). Knowledge about the key factors controlling cadmium accumulation in plants can be used to lower the background cadmium level in agricultural soils by occasionally cultivating plants that efficiently phytoextract cadmium from the soil. Furthermore, understanding cadmium uptake and translocation under different soil conditions and how cadmium interferes with iron and zinc pathways needs to be better understood (Clemens et al., 2013). For example, many plants that naturally accumulate high levels of cadmium also tend to have higher levels of iron and zinc and understanding the mechanisms involved in cadmium bioaccumulation in different plant species and soil environments is warranted.
3.1.3. Agronomic practices
Many agriculture systems contaminated with cadmium are directly related to excessive use of phosphate fertilizers (Meng et al., 2018) due to the cadmium present in phosphate rock (Roberts, 2014). Plants need some metals (for example, iron and zinc) to grow efficiently and complete the life cycle and soils are often deficient in micronutrients. These nutrients are often applied to crops through foliar spray or are added directly to the soil. Application of phosphate fertilizers often results in the addition of cadmium and other toxic elements to the soil (Wuana & Okieimen, 2011). In addition, phosphate fertilizer can restrict crop uptake of zinc thus allowing increased mobility of cadmium (Roberts, 2014). The use of potassium and nitrogen fertilizers is preferable over phosphorus fertilizers due to low cadmium content. Nitrogen fertilizers, when applied with potassium, can stimulate root growth and increase the ionic strength of the soil thus limiting cadmium mobility. Since cadmium competes with zinc and other elements in the soil for uptake and translocation by plants, the addition of zinc fertilizers can also reduce cadmium accumulation by crops (Roberts, 2014). Silicon fertilizers, such as Si‐calcium (CaSi) and rice straw, can also increase soil pH affecting the bioavailability of cadmium (Wang et al., 2015). In addition to fertilizers, irrigation water containing chloride can increase cadmium solubility and mobility in plants. Cadmium uptake by plants is greater in saline soils or soils irrigated with water containing high chloride levels (McLaughlin et al., 1999). Therefore, testing irrigation water to determine chloride levels and understanding soil salinity is crucial to minimizing cadmium uptake in crops and the food supply (Figure 3).
4. FUTURE DIRECTIONS
4.1. Mitigation strategies to reduce cadmium in the food supply
As mentioned previously, understanding the risks from exposure to cadmium in the food supply is complicated and can be difficult to predict or manage. A multifaceted approach (Table 1) can help to better understand sources of cadmium in the food supply, including the many factors leading to cadmium uptake in plants and absorption in humans (McLaughlin et al., 1999). There are also many interrelated factors to consider when considering soil remediation for cadmium, such as cost, long‐term effectiveness/permanence, commercial availability, general acceptance, applicability to high metal concentrations, and applicability to mixed wastes (heavy metals and organics). Khalid et al. (2017) provide a thorough comparison of remediation techniques for soil clean up; however, steps for the prevention and reduction of cadmium can be implemented by food producers early in the agricultural food chain (that is, understand soil delineation, apply soil amendments, understand crop genetics, and use wise agronomic practices) (Table 1). Mitigation at other steps in the food supply can also be considered. These include discontinuing the use of galvanized equipment used in postharvest processes and limiting cadmium‐containing stabilizers in plastics and cadmium‐based pottery glazes (ATSDR, 2012). Additional strategies at the consumption point can focus on reducing the rate of absorption and body retention of cadmium and may include educating consumers on the importance of eating a variety of foods and ensuring proper intake of micronutrients (zinc, iron, and calcium). In order to determine the effectiveness of mitigation strategies, a future effort could focus on a risk assessment that quantifies the potential effectiveness of mitigations throughout the food supply chain.
Table 1.
Mitigation strategies to reduce dietary exposure to cadmium
| Cadmium entry into the food supply | Terrestrial pathways/agriculture production planting, growing, and harvesting | Steps that can be taken by manufacturers | Steps that can be taken by consumers |
|---|---|---|---|
| Mitigation and prevention efforts |
|
✗ Discontinuing the use of cadmium‐plated utensils and galvanized equipment for food production can minimize excess cadmium in food | ✰Eating a variety of foods can ensure a healthy diet |
|
✗ Reducing cadmium‐bearing stabilizers in plastics can minimize cadmium exposure | ✰Getting the proper amount of micronutrients (Zn, Fe, Ca) can protect against cadmium absorption and toxicity | |
|
✗ Removing cadmium‐based pottery glazes on cookware can reduce cadmium exposure | ||
|
|||
|
|||
4.2. Commodity‐specific codes of practice and surveillance for cadmium in crops
As presented previously, the presence of cadmium in food is highly variable and highly dependent on the geographical location, the bioavailability of cadmium from the soil, crop genetics, and agronomic practices used. As EFSA noted in their Commission recommendations in 2014, these variables make an immediate reduction of the maximum levels difficult to achieve (EFSA, 2014). Results of this review of the literature indicate the need for commodity‐specific codes of practice, such as the recent development of the Codex Code of Practice (COP) for the Prevention and Reduction of Cadmium Contamination in Cocoa. The Codex Committee on Contaminants in Foods is in the process of developing a COP for cocoa and has considered setting maximum levels for cadmium in cocoa products (Abt, Fong Sam, Gray, & Robin, 2018). The COP will provide technical guidance to the cocoa production industry on the prevention and reduction of cadmium contamination in cocoa beans in geographical regions worldwide. Mitigation measures may include primary production and postharvest processing recommendations (Joint FAO/WHO Food Standards Programme, CAC 2019).
A key step in reducing cadmium in the diet is to reduce or prevent initial uptake by the plant. Due to complex interactions of soil chemistry, plant genetics, and agronomic practices, additional research is needed. Field‐based experimentation and testing is needed to inform risk modeling and to develop practical farm‐specific management strategies. The long‐term outcome will assist leaders in identifying specific needs of farmers, focusing geographic and crop‐specific efforts, educating farmers about ways to improve agronomic practices, and ultimately reducing cadmium in the food supply through preventative measures.
4.3. Absorption of cadmium
An emerging focus in the scientific literature is the importance of the amount of cadmium that can be absorbed in the body from specific commodities. For example, some studies suggest that plant fiber and phylate may play a role in protection against the absorption of cadmium (Vahter, Johansson, Akesson, & Rhanster, 1991); however, further research is warranted to increase the certainty in estimating exposures to dietary cadmium exposure. Further, as stated previously, micronutrients have a major impact on health and consumers can be educated on the importance of the role of essential elements in the prevention of risks from cadmium exposure.
4.4. Considerations in setting limits
EFSA conducted a meta‐analysis in 2009 to evaluate the dose–response and/or dose–effect relationships between urinary cadmium and urinary biomarkers. The final objective of the meta‐analysis was the derivation of a benchmark dose (BMD) and its 95% confidence lower bound (BMDL) for humans using cutoff points relevant to clinical changes in the target organ. EFSA chose the biomarker, beta2‐microglobulin (β2MG), because it was the most frequently studied (that is, the largest data set not necessarily the most sensitive/critical endpoint). For this endpoint, 165 matched pairs of urinary cadmium and β2MG levels were gathered from 35 cross‐sectional studies (75% female, 93.5% Asian, and 99.5% cross‐sectional study design). As noted in the meta‐analysis, body weight, exposure concentration, the time lag between the reported exposure to cadmium (temporality), type, and route were rarely reported. Characterization of dietary exposure from cross‐sectional studies is difficult since exposures are time‐dependent and adequate exposure records (that is, exposure concentration) are unlikely to exist at the individual level. Therefore, associations between an estimated exposure and the occurrence of disease can result from error and/or bias (Mundt, 2005). As noted in EFSA (2011), harmonizing the approach and understanding the limitations of using epidemiological data in dose–response assessment for the use in risk assessment is needed to reduce gaps in knowledge related to exposure science. Further, commodity‐specific safe‐limits and absorption factors should be further explored considering the research presented in this paper. Ongoing review of the scientific literature is important to ensure that maximum levels are based on the best available science.
5. CONCLUSIONS
This review identifies the contributing factors that are associated with the accumulation of cadmium in plants and mitigation efforts for controlling cadmium in the food supply. This study also identified important data gaps and considerations for future research:
-
➲
Support for field‐based experimentation and testing can inform risk modeling and the development of practical farm‐specific management strategies
-
➲
Develop commodity‐specific codes of practice for cadmium
-
➲
Understand cadmium uptake and translocation under different soil conditions and how cadmium interferes with iron and zinc pathways
-
➲
Develop a surveillance system for cadmium in crops
-
➲
Reduce the amount of cadmium permitted in agricultural fertilizers
-
➲
Identify cooking techniques to reduce cadmium in specific commodities
-
➲
Understand the bioavailable fraction of cadmium from specific foods and the factors that affect absorption in the body
-
➲
Gain a better understanding of the human nutrition relationship to cadmium absorption and total body burden
-
➲
Develop commodity‐specific safe limits using dose–response modeling
-
➲
Conduct a risk assessment that quantifies the potential effectiveness of mitigations throughout the food supply chain
Ultimately, the information presented in this review can also assist the FDA in determining where to focus resources so that future research and risk assessment efforts can identify regulatory efforts that will have the greatest impact on reducing dietary cadmium exposure.
AUTHOR DISCLOSURES
This work was supported by an appointment to the Research Participation Program at the Center for Food Safety and Applied Nutrition administered by the Oak Ridge Inst. for Science and Education through an interagency agreement between the US Dept. of Energy and the US FDA.
AUTHOR CONTRIBUTIONS
Heather Schaefer collected the information and drafted the manuscript. Sherri Dennis and Suzanne Fitzpatrick conceptualized the idea for the paper and provided critical feedback and recommendations during the final approval process.
REFERENCES
- Abt, E. , Fong Sam, J. , Gray, P. , & Robin, L. P. (2018). Cadmium and lead in cocoa powder and chocolate products in the US market. Food Additives & Contaminants: Part B, 112(2), 92–102. 10.1080/19393210.2017.1420700 [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) . (1999). Toxicological profile for cadmium. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) . (2012). Toxicological profile for cadmium. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. [PubMed] [Google Scholar]
- Bashir, B. , Salam, A. , Chhajro, M. , Fu, Q. , Khan, M. , Zhu, J. , … Hu, H. (2018). Comparative efficiency of rice husk‐derived biochar (RHB) and steel slag (SS) on cadmium (Cd) mobility and its uptake by Chinese cabbage in highly contaminated soil. International Journal of Phytoremediation, 20(12), 1221–1228. 10.1080/15226514.2018.1448364 [DOI] [PubMed] [Google Scholar]
- Bernhoft, R. (2013). Cadmium toxicity and treatment. Scientific World Journal, 10.1155/2013/394652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzóska, M. , & Moniuszko‐Jakoniuk, J. (2001). Interactions between cadmium and zinc in the organism. Food and Chemical Toxicology, 39(10), 967–980. 10.1016/S0278-6915(01)00048-5 [DOI] [PubMed] [Google Scholar]
- Cannino, G. , Ferruggia, E. , Luparello, C. , & Rinaldi, A. (2009). Cadmium and mitochondria. Mitochondrian, 9(6), 377–384. 10.1016/j.mito.2009.08.009 [DOI] [PubMed] [Google Scholar]
- Chuan, M. , Shu, G. , & Liu, J. (1996). Solubility of heavy metals in a contaminated soil: Effects of redox potential and pH. Water, Air, & Soil Pollution, 90(3–4), 543–556. 10.1007/BF00282668 [DOI] [Google Scholar]
- Chunhabundit, R. , Srianujata, S. , Bunyaratvej, A. , Kongkachuichai, R. , Satayavivad, J. , & Kaojarern, S. (2011). Cadmium bioavailability from vegetable and animal‐based foods assessed with in vitro digestion/caco‐2 cell model. Journal of the Medical Association of Thailand, 94(2), 164–169. [PubMed] [Google Scholar]
- Clemens, S. , Aarts, M. , Thomine, S. , & Verbruggen, M. (2013). Plant science: The key to preventing slow cadmium poisoning. Trends in Plant Science, 18(2), 92–99. [DOI] [PubMed] [Google Scholar]
- Eriksson, J. , Oborn, I. , Gunilla, J. , & Arne, A. (1996). Factors influencing Cd‐content in crops. Swedish Journal for Agricultural Research, 26(3), 125–133. [Google Scholar]
- European Safety Authority (EFSA) . (2009). Scientific opinion of the panel on contaminants in the food chain on a request from the European Commission on cadmium in food. EFSA Journal, 7(3), 1831–4732. 10.2903/j.efsa.2009.980 [DOI] [Google Scholar]
- European Safety Authority (EFSA) . (2011). Scientific opinion on statement on tolerable weekly intake for cadmium. EFSA Journal, 9(2:1975), 1–19. https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/j.efsa.2011.1975 [Google Scholar]
- European Safety Authority (EFSA) . (2014). Commission recommendation on the reduction of the presence of cadmium in foodstuffs. Official Journal of the European Union, https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014H0193&from=EN
- Flanagan, P. , McLellan, J. , Haist, J. , Cherian, M. , Chamberlain, M. , & Valberg, L. (1978). Increased dietary cadmium absorption in mice and human subjects with iron deficiency. Gastroenterology, 74(5), 841–846. [PubMed] [Google Scholar]
- Gupta, R. C. (2011). Placental toxity. In Gupta R. C. (Ed.), Reproductive and developmental toxicology (pp. 1067–1085). London: Academic Press/Elsevier. [Google Scholar]
- Ilmiawati, C. , Yoshida, T. , Itoh, T. , Nakagi, Y. , Saijo, Y. , Sugioka, Y. , … Kayama, F. (2015). Biomonitoring of mercury, cadmium, and lead exposure in Japanese children: A cross‐sectional study. Environmental Health and Preventive Medicine, 20(1), 18–27. 10.1007/s12199-014-0416-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, Y. , Luan, Y. , Ning, Y. , & Wang, L. (2018). Effects and mechanisms of microbial remediation of heavy metals in soil: A critical review. Applied Sciences, 8(8), 1336–1153. [Google Scholar]
- Joint FAO/WHO Food Standards Programme, CAC . (2019). Report of the 13th session of the Codex Committee on Contaminants in Foods . Yogyakarta, Indonesia: Joint FAO/WHO Food Standards Programme. (REP19/CF).
- Khalid, S. , Shahid, M. , Niazi, N. , Murtaza, B. , Bibi, I. , & Dumat, C. (2017). A comparison of technologies for remediation of heavy metal contaminated soils. Journal of Geochemical Exploration, 182(Part B), 247–268. 10.1016/j.gexplo.2016.11.021 [DOI] [Google Scholar]
- Khan, M. , Khan, S. , Khan, A. , & Alam, M. (2017). Soil contamination with cadmium, consequences and remediation using organic amendments. Science of the Total Environment, 601–602(1), 1591–1605. 10.1016/j.scitotenv.2017.06.030 [DOI] [PubMed] [Google Scholar]
- Klaassen, C. D. , Casarett, L. J. , & Doull, J. (2013). Casarett and Doull's toxicology: The basic science of poisons. New York: McGraw‐Hill Professional. [Google Scholar]
- Marreiro, D. D. , Cruz, K. J. , Morais, J. B. , Beserra, J. B. , Severo, J. S. , & de Oliveira, A. R. (2017). Zinc and oxidative stress: Current mechanisms. Antioxidants, 6(2), 24 10.3390/antiox6020024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, M. , Parker, D. , & Clarke, J. (1999). Metals and micronutrients and food safety issues. Field Crops Research, 60(1–2), 143–163. 10.1016/S0378-4290(98)00137-3 [DOI] [Google Scholar]
- Meng, D. , Li, J. , Liu, T. , Liu, Y. , Yan, M. , Hu, J. , … Yin, H. (2018). Effects of redox potential on soil cadmium solubility: Insight into microbial community. Journal of Environmental Sciences, 75, 224–232. 10.1016/j.jes.2018.03.032 [DOI] [PubMed] [Google Scholar]
- Morrissey, J. , & Guerinot, M. L. (2009). Trace elements: Too little or too much and how plants cope. F1000 Biology Reports, http://F1000.com/Reports/Biology/content/1/14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundt, K. (2005). Statistical challenges in evaluating dose–response using epidemiological data. Dose Response, 3(4), 453–455. 10.2203/dose-response.003.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair, R. , DeGheselle, O. , Smeets, K. , Kerkhove, E. , & Cuypers, A. (2013). Cadmium‐induced pathologies: Where is the oxidative balance lost (or not)? International Journal of Molecular Sciences, 14(3), 6116–6143. 10.3390/ijms14036116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Toxicology Program (NTP) . (2016). Report on carcinogens: Cadmium . U.S. Department of Human Services, National of Institutes of Health. https://ntp.niehs.nih.gov/ntp/roc/content/profiles/cadmium.pdf
- Nazar, R. , Iqbal, N. , Masood, A. , Khan, M. , Syeed, S. , & Khan, N. (2012). Cadmium toxicity in plants and role of mineral nutrients in its alleviation. American Journal of Plant Sciences, 3(10), 1476–1489. 10.4236/ajps.2012.310178 [DOI] [Google Scholar]
- Nordberg, G. F. , Bernard, A. , Diamond, G. L. , Duffus, J. H. , Illing, P. , Nordberg, M. , … Skerfving, S. (2018). Risk assessment of effects of cadmium on human health (IUPAC Technical Report). Pure and Applied Chemistry, 90(4), 755–808. 10.1515/pac-2016-0910 [DOI] [Google Scholar]
- Norvell, W. , Wu, J. , Hopkins, D. , & Welch, R. (2000). Association of cadmium in durum wheat grain with soil chloride and chelate‐extractable soil cadmium. Soil Science Society of America Journal, 64(6), 2162–2168. 10.2136/sssaj2000.6462162x [DOI] [Google Scholar]
- Reeves, P. G. , & Chaney, R. L. (2001). Mineral status of female rats affects the absorption and organ distribution of dietary cadmium derived from edible sunflower kernels (Helianthus annuus L.). Environmental Research, 85(3), 215–225. 10.1006/enrs.2000.4236 [DOI] [PubMed] [Google Scholar]
- Reeves, P. G. , & Chaney, R. L. (2002). Nutritional status affects the absorption and whole‐body and organ retention of cadmium in rats fed rice‐based diets. Environmental Science & Technology, 36(12), 2684–2692. 10.1021/es0158307 [DOI] [PubMed] [Google Scholar]
- Reeves, P. G. , & Chaney, R. L. (2004). Marginal nutritional status of zinc, iron, and calcium increases cadmium retention in the duodenum and other organs of rats fed rice‐based diets. Environmental Research, 96(3), 311–322. 10.1016/j.envres.2004.02.013 [DOI] [PubMed] [Google Scholar]
- Reeves, P. G. , & Chaney, R. L. (2008). Bioavailability as an issue in risk assessment and management of food cadmium: A review. Science of the Total Environment, 398(1–3), 13–19. 10.1016/j.scitotenv.2008.03.009 [DOI] [PubMed] [Google Scholar]
- Reeves, P. G. , Chaney, R. L. , Simmons, R. W. , & Cherian, M. G. (2005). Metallothionein induction is not involved in cadmium accumulation in the duodenum of mice and rats fed diets containing high‐cadmium rice or sunflower kernels and a marginal supply of zinc, iron, and calcium. Journal of Nutrition, 135(1), 9–108. 10.1093/jn/135.1.99 [DOI] [PubMed] [Google Scholar]
- Roberts, T. (2014). Cadmium and phosphorous fertilizers: The issues and the science. Procedia Engineering, 83, 52–59. 10.1016/j.proeng.2014.09.012 [DOI] [Google Scholar]
- Ryan, J. A. , Herbert, P. H. , & Lucas, J. B. (1982). Controlling cadmium in the human food chain: A review and rationale based on health effects. Environmental Research, 28(2), 251–302. 10.1016/0013-9351(82)90128-1 [DOI] [PubMed] [Google Scholar]
- Sarwar, N. , Saifullah, Malhi, S. , Zia, M. H. , Naeem, A. , Bibi, S. , & Farid, G. (2010). Role of mineral nutrition in minimizing cadmium accumulation by plants. Journal of the Science of Food and Agriculture, 90(6), 925–937. 10.1002/jsfa.3916 [DOI] [PubMed] [Google Scholar]
- Satarug, S. , Garrett, S. , Sens, M. , & Sens, D. (2010). Cadmium, environmental exposure, and health outcomes. Environmental Health Perspectives, 118(2), 182–190. 10.1590/S1413-81232011000500029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders, E. (2001). Cadmium uptake by plants. International Journal of Occupational Medicine and Environmental Health, 14(2), 177–183. [PubMed] [Google Scholar]
- Spungen, J. (2019). Children's exposures to lead and cadmium: FDA Total Diet Study 2014–16. Food Additives & Contaminants: Part A, 36(6), 893–903. 10.1080/19440049.2019.1595170 [DOI] [PubMed] [Google Scholar]
- United States Geological Survey (USGS) . (2014). Mineral resources online spatial data: Cadmium . https://mrdata.usgs.gov/soilgeochemistry/#/detail/element/48
- Vacchi‐Suzzi, C. , Kruse, D. , Harrington, J. , Levine, K. , & Meliker, J. R. (2016). Is urinary cadmium a biomarker of long‐term exposure in humans? A review. Current Environmental Health Reports, 3(4), 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter, M. , Johansson, G. , Akesson, A. , & Rhanster, B. (1991). Faecal elimination of lead and cadmium in subjects on a mixed and lactovegetarian diet. Food and Chemical Toxicology, 30(4), 281–287. 10.1016/0278-6915(92)90005-6 [DOI] [PubMed] [Google Scholar]
- Vesey, D. (2010). Transport pathways for cadmium in the intestine and kidney proximal tubule: Focus on the interaction with essential metals. Toxicology Letters, 198(1), 13–19. 10.1016/j.toxlet.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Vogt, R. , Bennett, D. , Cassady, D. , Frost, J. , Ritz, B. , & Hertz‐Picciotto, I. (2012). Cancer and non‐cancer health effects from food contaminant exposures for children and adults in California: A risk assessment. Environmental Health, 11, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Wen, S. , Chen, P. , Zhang, L. , Cen, K. , & Sun, G. (2015). Mitigation of cadmium and arsenic in rice grain by applying different silicon fertilizers in contaminated fields. Environmental Science and Pollution Research International, 23(4), 3781–3788. [DOI] [PubMed] [Google Scholar]
- Williams, C. , & David, D. (1976). The accumulation of cadmium residues from phosphate fertilizers and their effect on the cadmium content in plants. Soil Science, 121(2), 86–93. [Google Scholar]
- World Health Organization (WHO) . (1992). Cadmium. Environmental health criteria. Geneva, Switzerland: WHO. [Google Scholar]
- World Health Organization (WHO) . (2011). Cadmium. Safety evaluation of certain contaminants in food . WHO Food Additives Series, No. 64/FAO JECFA Monographs 8. WHO: Geneva, Switzerland. [Google Scholar]
- Wuana, R. , & Okieimen, F. (2011). Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecology, 10.5402/2011/402647 [DOI]
- Zheng, R. , Sun, G. , Li, C. , Reid, B. , Xie, Z. , Zhang, B. , & Wang, Q. (2017). Mitigating cadmium accumulation in greenhouse lettuce production using biochar. Environmental Science and Pollution Research, 24(7), 6532–6542. 10.1007/s11356-016-8282-9 [DOI] [PubMed] [Google Scholar]


