Abstract
Aim
To evaluate efficacy and safety of combination of tadalafil + mirabegron for overactive bladder/benign prostatic hyperplasia (OAB/BPH).
Methods
Male patients with lower urinary tract symptoms (50 to 89 years), with remaining OAB symptoms even after administering tadalafil for more than 8 weeks were randomly assigned to either tadalafil monotherapy group (5 mg/day) or tadalafil/mirabegron combination therapy group (5 mg/50 mg/day). The primary endpoint was change from baseline in total OAB symptom score (OABSS) at week 12. The secondary endpoints were changes in International Prostate Symptom Score (IPSS), NIH‐chronic prostatitis symptom index (NIH‐CPSI), and micturition chart parameters at weeks 4 and 12.
Results
A total of 176 patients were randomized to either monotherapy (87 patients) or combination therapy (89 patients). The baseline characteristics of patients in the two groups were similar. The total OABSS (95% confidence interval) of combination therapy was significantly decreased by 1.78 (1.05‐2.50) points compared with that of monotherapy (P < .001). Changes from baseline in OABSS nighttime voiding score, urgency score, urgency incontinence score, IPSS storage subscores, NIH‐CPSI total score, and numbers of voids, nighttime‐voids, and urgency episodes/day in micturition chart were significantly reduced in combination therapy (all P < .001). Patient‐reported outcome was significantly more satisfactory in combination therapy than in monotherapy (P < .001). One moderate adverse event (pain in hip joint) with hardly presumed causal relationship with therapy and seven mild adverse events were noted in monotherapy and combination therapy group, respectively.
Conclusions
The effect of tadalafil/mirabegron combination therapy on relieving OAB symptoms appeared to be greater than that of tadalafil monotherapy and can be safely used.
Keywords: benign prostatic hyperplasia, mirabegron, overactive, tadalafil, urinary bladder
1. INTRODUCTION
α1‐Blockers have been used for the first‐line treatment of lower urinary tract symptoms suggestive of benign prostatic hyperplasia (“LUTS/BPH”). 1 , 2 , 3 , 4 These drugs may relieve voiding symptoms as well as storage symptoms. 1 , 2 , 3 , 4 However, patients may still have persistent storage symptoms despite α1‐blocker treatment. Anticholinergics in combination with α1‐blockers have been reported to be effective in these cases. 5 , 6 , 7 , 8 , 9 Recently, it has been reported that mirabegron, a β3‐adrenoceptor (AR) agonist, was equally effective to anticholinergics for the treatment of overactive bladder (OAB), but has less side effects including dry mouth and constipation, and was more tolerable and cost‐effective than anticholinergics. 10 , 11 , 12 The open‐label add‐on study of mirabegron with α1‐blocker (tamsulosin) was reported to be more effective than monotherapy with tamsulosin for the treatment of OAB induced by benign prostatic obstruction. 13
A phosphodiesterase 5 inhibitor (tadalafil) has recently been used for the treatment of “LUTS/BPH” 14 , 15 However, there have been no studies on combination or add‐on therapies of tadalafil and β3‐AR agonist for the treatment of overactive bladder associated with benign prostatic hyperplasia (“BPH/OAB”).
The objective of this study is to evaluate whether the combination of tadalafil and mirabegron have additive effect on OAB symptoms as compared to tadalafil monotherapy in patients who had remaining OAB symptoms after monotherapy with tadalafil.
2. MATERIALS AND METHODS
2.1. Patient inclusion criteria and study design
The present prospective, multicenter, randomized, parallel‐group, open‐label study was carried out (see acknowledgments). The study was carried out in accordance with the Declaration of Helsinki and the Ethical Guidelines for Clinical Studies (revision of July 2008). eClinical Base (Translational Research Informatics Center, Kobe, Japan), a web‐based system, was used for the assignment of patients following data input by investigators for patient enrollment and confirmation of eligibility. This study was registered (UMIN000025282) and was reviewed and approved by the ethics committee of each medical institution, and written informed consent was obtained from each patient.
Male patients with LUTS, aged from 50 to 89 years, who visited during a period from December 2016 to April 2018, and who had remaining OAB symptoms despite at least 8 weeks of treatment with tadalafil 5 mg/day were included in this study.
Exclusion criteria included patients who were taking anticholinergics, cholinergics, β‐agonists or antagonists, α‐blockers within the previous 8 weeks, 5α‐reductase inhibitors, and antiandrogen drugs within the previous 6 months, who had a history of urinary retention, maximum flow rate (Qmax) less than 5 mL/s, postvoid residual (PVR) more than 150 mL, with prostate or bladder cancer, neurogenic bladder, urethral stenosis, active urinary tract infection, urinary calculus, interstitial cystitis, serious cardiac disorder, serious cerebrovascular disease, serious liver or kidney dysfunction, and those who were deemed inappropriate by the investigator for any reason.
Eligible patients were randomly assigned to either tadalafil monotherapy group (TG: 5 mg) or tadalafil/mirabegron combination therapy group (TMG: 5 mg/50 mg) at a 1:1 ratio, and were followed for 12 weeks.
Self‐administered questionnaires overactive bladder symptom score (OABSS), International Prostate Symptom Score (IPSS), and NIH Chronic Prostatitis Symptom Index (NIH‐CPSI) were each administered at weeks 0, 4, and 12. The micturition chart was completed for 3 days before, and at 12 weeks of treatment. Uroflowmetry and PVR were calculated before, and at 12 weeks of treatment. PVR was determined by transabdominal ultrasonography, and bladder voiding efficiency (BVE) (%) was calculated using the equation BVE = voided volume (VV)/(VV + PVR) * 100(%).
Adverse events were assessed during the study period. As increase in blood pressure and pulse rate were reported to be the potential side effects, we recorded them at each visit. The safety was judged by a safety review committee, which was independent from this study.
The primary endpoint was change from baseline to end of treatment at 12 weeks in total OABSS. The secondary endpoints were changes in each question of OABSS, IPSS, total and individual IPSS score, NIH‐CPSI total score, and each domain of NIH‐CPSI, micturition chart variables, uroflowmetry parameters, and PVR and BVE. These efficacies and the safety were evaluated at weeks 4 and 12 of treatment.
Patient‐reported outcome was assessed at week 12 of treatment using a 4‐grade scale as follows: 1 = extremely satisfied, 2 = very satisfied, 3 = satisfied, and 4 = not satisfied.
2.2. Statistical analysis
The previous report comparing tamsulosin monotherapy and add‐on of tamsulosin/mirabegron for “BPH/OAB” showed that the treatment difference in OABSS total score at the 8th week of the treatment between the two groups was estimated to be 1.34. 13 The standard deviation of the change from the baseline value ranged from 2.2 to 2.5 in the ASSIST study. 6 Based on these results, the mean difference in the changes in OABSS score from the baseline to the 12th week of the treatments was set to be 1.3 with the standard deviation of 2.5. The number of patients needed to detect the between‐group mean score difference of 1.3 with the significance level of two‐sided α = .05 with t test was 68 patients per group to achieve a statistical power of 85%. Assuming a dropout rate of around 20%, we planned to enroll 170 patients among the two groups. The differences in patient characteristics between the two treatment groups were tested with the Wilcoxon rank‐sum test for continuous variables and χ 2 test for categorical variables.
The primary endpoint was analyzed in the full analysis set (FAS), defined as all patients excluding those who received no study treatment after randomization, those for whom there were no postbaseline data, and those who were later found to be ineligible for the study. Patients who prematurely discontinued study treatment or whose data at week 12 were missing were handled as censored cases.
In the analysis of the primary endpoint, the change in OABSS total score from baseline to week 12 was compared between two groups by the analysis of covariance (ANCOVA) with 12 weeks total OABSS as a dependent variable, OABSS total score at baseline, and a dummy variable for the treatment group as covariates. No imputation or substitution was made for missing values. As the secondary analyses, point estimates of means and proportions and their 95% confidence interval (CI) for the primary and secondary endpoints were estimated and compared between the groups or within each group with the longitudinal data consisting of observations at weeks 0, 4, and 12. On the OABSS total score, accordingly, ANCOVA was performed as the primary statistical analysis and a mixed‐effect linear model for the additional analysis. On the assumption that the variables could be approximated as continuous variables, a linear mixed‐effect model or ANCOVA, depending on the times of observations, was used to estimate the difference at weeks 4 and 12, and other models, if possible, were applied to validate the approximation to continuous variable models. A two‐sided P value of less than .05 was considered statistically significant. At the additional analyses, multiplicity in statistical tests was not considered because of their exploratory nature. All statistical analyses were performed with Stata version 14.2 software (Stata, College Station, TX).
3. RESULTS
3.1. Patient population
A total of 176 patients were randomized to either TG (87 patients) or TMG (89 patients). The safety analysis set comprised 87 and 87 patients (excluding two patients who did not take medication), respectively. Seven patients in TG and six patients in TMG were excluded from FAS due to allocation violation (n = 3), violation of tadalafil treatment period at enrollment (n = 3), concomitant medication (n = 6), and no efficacy data after drug administration (n = 1). Therefore, the number of patients in the FAS was 80 and 81 in TG and TMG, respectively (Figure 1).
Figure 1.
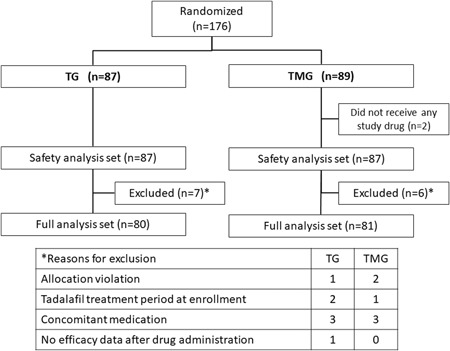
Study disposition
Patient baseline characteristics including patient demographics, micturition scores, micturition chart parameters were similar between the groups (Table 1).
Table 1.
Baseline characteristics of patients (FAS): mean (SD)
| TG | TMG | Total | ||||
|---|---|---|---|---|---|---|
| N | N | N | ||||
| Age, y | 72.3 (8.0) | 80 | 72.4 (7.4) | 81 | 72.3 (7.7) | 161 |
| Prostate volume, mL | 30.5 (12.8) | 75 | 32.1 (13.1) | 77 | 31.3 (12.9) | 152 |
| OABSS | ||||||
| Total score | 8.0 (2.7) | 80 | 8.5 (2.5) | 81 | 8.3 (2.6) | 161 |
| Daytime frequency score | 1.0 (0.5) | 80 | 1.1 (0.5) | 81 | 1.1 (0.5) | 161 |
| Nighttime frequency score | 2.1 (1.0) | 80 | 2.2 (0.8) | 81 | 2.2 (0.9) | 161 |
| Urgency score | 3.2 (1.0) | 80 | 3.3 (1.0) | 81 | 3.2 (1.0) | 161 |
| Urgency incontinence score | 1.8 (1.5) | 80 | 1.9 (1.4) | 81 | 1.8 (1.4) | 161 |
| IPSS | ||||||
| Total score | 13.6 (6.8) | 80 | 13.5 (5.9) | 81 | 13.6 (6.3) | 161 |
| Voiding symptom score | 4.6 (4.4) | 80 | 4.5 (4.1) | 81 | 4.5 (4.2) | 161 |
| Storage symptom score | 7.7 (2.9) | 80 | 7.8 (2.7) | 81 | 7.7 (2.8) | 161 |
| Postmicturition symptom score | 1.4 (1.5) | 80 | 1.3 (1.3) | 81 | 1.3 (1.4) | 161 |
| QOL index | 4.5 (1.0) | 80 | 4.4 (1.0) | 81 | 4.4 (1.0) | 161 |
| NIH‐CPSI | ||||||
| Total score | 12.4 (5.7) | 80 | 12.3 (5.4) | 81 | 12.3 (5.6) | 161 |
| Pain or discomfort score | 1.7 (3.1) | 80 | 1.4 (2.7) | 81 | 1.5 (2.9) | 161 |
| Impact of symptoms score | 3.8 (2.3) | 80 | 3.7 (2.3) | 81 | 3.7 (2.3) | 161 |
| QOL score | 6.9 (2.5) | 80 | 7.2 (2.3) | 81 | 7.1 (2.4) | 161 |
| Micturition chart | ||||||
| Number of voids per 24 h | 11.8 (4.0) | 79 | 11.5 (3.3) | 79 | 11.7 (3.7) | 158 |
| Number of nighttime voids | 2.4 (1.4) | 79 | 2.4 (1.3) | 79 | 2.4 (1.3) | 158 |
| Number of urgency episodes | 2.8 (3.4) | 79 | 2.6 (2.9) | 78 | 2.7 (3.1) | 157 |
| Number of urgency incontinence episodes | 1.1 (2.3) | 79 | 0.9 (1.8) | 79 | 1.0 (2.1) | 158 |
| Uroflowmetry | ||||||
| Voided volume, mL | 177.4 (84.2) | 80 | 171.3 (89.8) | 81 | 174.4 (86.9) | 161 |
| Qmax, mL/s | 13.2 (5.7) | 80 | 12.7 (5.8) | 81 | 13.0 (5.7) | 161 |
| Qave, mL/s | 7.7 (3.7) | 79 | 7.0 (3.0) | 81 | 7.3 (3.3) | 160 |
| PVR, mL | 28.9 (20.1) | 80 | 32.0 (28.1) | 81 | 30.4 (2.6) | 161 |
| VE (%) | 84.9 (11.5) | 80 | 82.8 (14.2) | 81 | 83.9 (12.9) | 161 |
Abbreviations: FAS, full analysis set; IPSS, international prostate symptom score; NIH‐CPSI, NIH‐chronic prostatitis symptom index; OABSS, overactive bladder symptom score; PVR, postvoid residual.
3.2. Primary endpoint
The total OABSS (95% CI) of TMG at 12 weeks was significantly decreased by 1.78 (1.05‐2.50) points compared with that of TG (P < .001) (Figure 2A).
Figure 2.
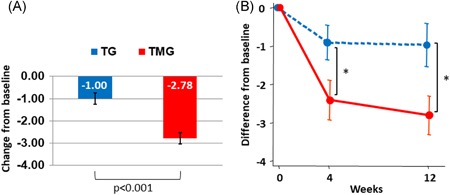
A, Primary endpoint: change from baseline to 12 weeks in OABSS total score. P value was calculated using ANCOVA, with group and baseline OABSS as covariates. Mean ± SE. B, Mean difference of OABSS total score (mean ± 95% CI) before (week 0), and at weeks 4 and 12 after the therapy: dotted line represents TG and straight line represents TMG. *P < .001, between groups. ANCOVA, analysis of covariance; CI, confidence interval; OABSS, OAB symptom score; TG, tadalafil monotherapy group; TMG, mirabegron combination therapy group
The mean (SD) total OABSS significantly (P < .001) decreased from baseline by 8.175 (2.709) to 7.262 (2.666) and 7.263 (3.091) points at weeks 4 and 12 in TG, and that in TMG significantly (P < .001) decreased from baseline by 8.469 (2.460) to 6.025 (2.595) and 5.649 (2.851) at weeks 4 and 12, respectively (Figure 2B and Table S1).
The mean change in OABSS total score from baseline to 12 weeks (95% CI) was −0.974 (−1.540 to −0.408) in the TG and −2.805 (−3.309 to −2.302) in the TMG, showing a statistically significant improvement in the TMG as compared with the TG according to the mixed‐effect linear model [between‐group difference in change: −1.849 (−2.565 to −1.133), P < .001] (Figure 2B, Table 2, and Table S1).
Table 2.
Mean difference in symptom scores from baseline to 4 and 12 wk after the treatment between the two groups
| Mean difference | (95% CI) | P | ||
|---|---|---|---|---|
| OABSS | ||||
| Total score | W4 | −1.505 | (−2.212 to −0.799) | <.001 |
| W12 | −1.849 | (−2.565 to −1.133) | <.001 | |
| Daytime frequency score | W4 | −0.087 | (−0.244 to 0.070) | .28 |
| W12 | −0.076 | (−0.235 to 0.083) | .35 | |
| Nighttime frequency score | W4 | −0.212 | (−0.410 to −0.014) | .036 |
| W12 | −0.399 | (−0.599 to −0.198) | <.001 | |
| Urgency score | W4 | −0.677 | (−1.074 to −0.280) | <.001 |
| W12 | −0.672 | (−1.074 to −0.270) | .001 | |
| Urgency incontinence score | W4 | −0.532 | (−0.895 to −0.169) | .004 |
| W12 | −0.710 | (−1.078 to −0.343) | <.001 | |
| IPSS | ||||
| Total score | W4 | −0.657 | (−2.051 to 0.737) | .36 |
| W12 | −1.864 | (−3.276 to −0.451) | .010 | |
| Voiding symptom subscore | W4 | 0.312 | (−0.546 to 1.169) | .48 |
| W12 | −0.019 | (−0.888 to 0.850) | .97 | |
| Storage symptom subscore | W4 | −1.006 | (−1.713 to −0.299) | .005 |
| W12 | −1.646 | (−2.362 to −0.930) | <.001 | |
| Postmicturition symptom score | W4 | 0.041 | (−0.281 to 0.363) | .80 |
| W12 | −0.191 | (−0.516 to 0.135) | .25 | |
| QOL index | W4 | −0.224 | (−0.558 to 0.109) | .19 |
| W12 | −0.484 | (−0.822 to −0.146) | .005 | |
| NIH‐CPSI | ||||
| Total score | W4 | −1.170 | (−2.440 to 0.099) | .07 |
| W12 | −2.968 | (−4.256 to −1.681) | <.001 | |
| Pain or discomfort score | W4 | −0.184 | (−0.830 to 0.461) | .58 |
| W12 | −0.255 | (−0.910 to 0.399) | .44 | |
| Impact of symptoms score | W4 | 0.047 | (−0.487 to 0.580) | .86 |
| W12 | −0.859 | (−1.399 to −0.318) | .002 | |
| QOL score | W4 | −1.032 | (−1.731 to −0.333) | .004 |
| W12 | −1.838 | (−2.546 to −1.129) | <.001 | |
Abbreviations: CI, confidence interval; IPSS, international prostate symptom score; NIH‐CPSI, NIH‐chronic prostatitis symptom index; OABSS, overactive bladder symptom score.
3.3. Secondary endpoints
The mean changes from baseline to 4 and 12 weeks in OABSS individual scores, IPSS total score, IPSS storage and voiding subscores, IPSS‐QOL index, NIH‐CPSI total score, and NIH‐CPSI individual score domains are summarized in Table S1. The changes from baseline in IPSS total score to 4 and 12 weeks after the therapy were significantly decreased in both groups, but the change in IPSS total score from the baseline to 12 weeks was significantly greater in TMG compared with TG (Table 2 and Table S1).
The mean difference in NIH‐CPSI total score from baseline to 12 weeks was −0.974 in TG and −3.987 in TMG, showing a statistically significant improvement in the TMG as compared with the TG (P < .001, Table S1). However, NIH‐CPSI pain and discomfort score did not show significant intergroup difference, while significant decreases were noted only in the TMG group.
The mean differences between the groups in symptom subscores from baseline to 4 and 12 weeks are shown in Table 2. The mean differences in OABSS nighttime frequency score, OABSS urgency score, OABSS urgency incontinence score, IPSS storage symptom subscore, IPSS QOL index, NIH‐CPSI impact of symptoms score, and NIH‐CPSI QOL score at weeks 12 in TMG were significantly greater than those in TG (Table 2).
Changes in micturition chart parameters, and uroflowmetry parameters and PVR at 12 weeks after the treatment are summarized in Tables 3 and 4, respectively. The change in the numbers of voids per 24 hours, numbers of voids/nighttime, and number of urgency episodes per 24 hours were significantly reduced in the TMG compared with TG (Table 3).
Table 3.
Mean changes in micturition diary parameters from baseline to 12 wk after the treatment, and mean difference between the two groups
| N | Mean | SD | a α | (95% CI) | P | b β | (95% CI) | P | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of voids (times/day) | TG | W0 | 79 | 11.835 | 4.045 | 0 | −1.243 | (−2.113 to −0.372) | .005 | ||
| W12 | 74 | 12.090 | 5.091 | 0.194 | (−0.594 to 0.981) | .63 | |||||
| TMG | W0 | 79 | 11.492 | 3.303 | 0 | ||||||
| W12 | 76 | 10.379 | 3.689 | −1.044 | (−1.527 to −0.562) | <.001 | |||||
| Number of nighttime voids | TG | W0 | 79 | 2.390 | 1.365 | 0 | −0.645 | (−0.958 to −0.331) | <.001 | ||
| W12 | 74 | 2.484 | 1.727 | 0.099 | (−0.158 to 0.356) | .44 | |||||
| TMG | W0 | 79 | 2.367 | 1.333 | 0 | ||||||
| W12 | 76 | 1.849 | 1.130 | −0.549 | (−0.767 to −0.331) | <.001 | |||||
| Urgency episodes (times/day) | TG | W0 | 79 | 2.785 | 3.372 | 0 | −0.904 | (−1.754 to −0.054) | .037 | ||
| W12 | 74 | 2.313 | 3.530 | −0.511 | (−1.302 to 0.279) | .20 | |||||
| TMG | W0 | 78 | 2.615 | 2.917 | 0 | ||||||
| W12 | 76 | 1.200 | 1.895 | −1.412 | (−2.002 to −0.823) | <.001 | |||||
| Urgency incontinence episodes (times/day) | TG | W0 | 79 | 1.080 | 2.270 | 0 | −0.728 | (−1.516 to 0.060) | .07 | ||
| W12 | 74 | 1.198 | 4.210 | 0.086 | (−0.705 to 0.876) | .83 | |||||
| TMG | W0 | 79 | 0.914 | 1.815 | 0 | ||||||
| W12 | 76 | 0.298 | 0.936 | −0.642 | (−0.926 to −0.358) | <.001 |
Abbreviations: CI, confidence interval; SD, standard deviation.
Mean difference from baseline.
Estimated difference at W12 from a mixed model.
Table 4.
Mean changes in urinary flow rate from baseline to 12 wk after the treatment, and mean difference between the two groups
| N | Mean | SD | a α | (95% CI) | P | b β | (95% CI) | P | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Voided volume, mL | ||||||||||
| TG | W0 | 80 | 177.447 | 84.225 | 0 | 20.456 | (0.144 to 40.768) | .048 | ||
| W12 | 74 | 161.142 | 94.051 | −13.072 | (−27.824 to 1.681) | .08 | ||||
| TMG | W0 | 81 | 171.305 | 89.819 | 0 | |||||
| W12 | 77 | 179.303 | 100.098 | 7.017 | (−9.170 to 23.204) | .39 | ||||
| Qave, mL/s | ||||||||||
| TG | W0 | 79 | 7.653 | 3.675 | 0 | 0.555 | (−0.290 to 1.400) | .20 | ||
| W12 | 73 | 6.967 | 3.773 | −0.666 | (−1.384 to 0.053) | .07 | ||||
| TMG | W0 | 81 | 7.014 | 2.961 | 0 | |||||
| W12 | 77 | 6.771 | 3.039 | −0.092 | (−0.675 to 0.490) | .75 | ||||
| Qmax, mL/s | ||||||||||
| TG | W0 | 80 | 13.105 | 5.945 | 0 | 1.301 | (−0.190 to 2.791) | .09 | ||
| W12 | 74 | 11.985 | 6.283 | −1.109 | (−2.380 to 0.162) | .09 | ||||
| TMG | W0 | 81 | 12.378 | 4.939 | 0 | |||||
| W12 | 77 | 12.440 | 5.485 | 0.213 | (−0.807 to 1.233) | .68 | ||||
| PVR, mL | ||||||||||
| TG | W0 | 80 | 29.099 | 20.140 | 0 | 4.293 | (−3.050 to 11.636) | .25 | ||
| W12 | 75 | 20.908 | 24.742 | −7.411 | (−12.911 to −1.911) | .009 | ||||
| TMG | W0 | 81 | 33.719 | 26.562 | 0 | |||||
| W12 | 77 | 31.643 | 28.523 | −3.568 | (−9.402 to 2.267) | .23 | ||||
| VE (%) | ||||||||||
| TG | W0 | 80 | 84.881 | 11.460 | 0 | −1.811 | (−5.766 to 2.143) | .37 | ||
| W12 | 74 | 88.276 | 13.427 | 3.526 | (0.418 to 6.634) | .027 | ||||
| TMG | W0 | 81 | 82.834 | 14.201 | 0 | |||||
| W12 | 77 | 83.988 | 15.445 | 1.823 | (−1.227 to 4.873) | .24 |
Abbreviations: CI, confidence interval; PVR, postvoid residual; SD, standard deviation; TG, tadalafil monotherapy group; TMG, mirabegron combination therapy group; VE, voiding efficiency.
Mean difference from baseline.
Estimated difference at W12 from a mixed model.
In uroflowmetry, VV, average flow rate (Qave), and Qmax did not significantly change from baseline in either group, and the change in PVR from baseline significantly decreased and the change in VE significantly increased in TG. Regarding treatment group comparisons, only change in VV was statistically significant (P < .048), but between‐group difference in change was only 20.456 mL (0.144 to 40.768) (Table 4).
3.4. Patient‐reported outcome
Patient‐reported outcomes were extremely satisfied in 2 patients (2.7%) and 8 patients (11.4%); very satisfied in 3 patients (4.1%) and 14 patients (20.0%); satisfied in 20 patients (27.0%) and 39 patients (55.7%); and not satisfied in 49 patients (66.2%) and 9 patients (12.9%) in TG and TMG, respectively. Patient‐reported outcome was significantly better in TMG than in TG (the Wilcoxon rank sum test, P < .001).
3.5. Safety analysis
The systolic blood pressure in TG and TMG, respectively, was 135.6 mm Hg and 137.6 mm Hg before the therapy and 135.0 mm Hg and 133.7 mm Hg at 12 weeks after the therapy; diastolic pressure was 77.5 mm Hg and 78.2 mm Hg before the therapy and 75.4 mm Hg and 75.4 mm Hg at 12 weeks after the therapy; pulse rate was 74.4 beat per minute (bpm) and 74.8 bpm before the therapy and 74.8 bpm and 75.4 bpm at 12 weeks after the therapy. The changes from baseline to 12 weeks in the systolic blood pressure, the diastolic pressure, and pulse rate were −0.8 mm Hg and −4.7 mm Hg, −2.1 mm Hg and −3.2 mm Hg, and 1.1 bpm and 0.3 bpm in TG and TMG, respectively. There were no statistically significant differences between groups for any vital signs.
One moderate adverse event (1.1%; 1/87) was noted in the TG, and seven mild adverse events (8.0%; 7/87) in the TMG. The adverse event in the TG was pain in the left hip joint due to pseudogout, presumably hardly related to the study treatment. The seven adverse events in the TMG were not serious, and consisted of discomfort of bladder, pharyngitis, diarrhea, lightheadedness, lower limb edema, voiding difficulty, and drug rash. A total of two events led to discontinuation of study treatment for reasons of safety: one case of discomfort of bladder and one case of voiding difficulty.
4. DISCUSSION
BPH is the histological terminology, and in itself does not require treatment and is not the target of therapeutic intervention. Although we are not sure that the term, “BPH/LUTS” is scientifically correct or not, this term is accepted terminology for the treatment of male LUTS by AUA. For the treatment of “LUTS/BPH,” α‐blockers and/or PDE5‐inhibitors have been used as the first line of treatment. These drugs may improve storage symptoms, including OAB, as well as voiding symptoms. 1 , 2 , 3 , 4 , 14 , 15 However, OAB symptoms may remain despite these drugs. 5 In these patients, several combinations or add‐on therapies have been reported to be effective. 5 , 6 , 7 , 8 , 9 , 13
The most frequently used combination was α‐blocker and anticholinergic drugs, and these combinations can be effective for the treatment of “BPH/OAB” symptoms. 5 , 6 , 7 , 8 , 9 However, addition of anticholinergics may cause several side effects, such as dry mouth, constipation, blurred vision, and voiding difficulty. Although rarely, these combinations may cause increase in PVR, and urinary retention may occur in about 1%‐3% of patients. 1 , 5 , 6
For the treatment of “LUTS/BPH” with large prostate (prostate volume >30 mL), the combination of an α‐blocker and a 5α‐reductase inhibitor (dutasteride) has been reported to be more effective, even in the storage symptoms, compared to each monotherapy. 16 , 17 However, the combination of α‐blocker, dutasteride, and anticholinergic drug has been reported to be more effective than the combination of α‐blocker plus dutasteride for the treatment of “BPH/OAB” with large prostate (DIrecT study). 18 , 19
The purpose our study is to evaluate whether the combination of tadalafil and mirabegron is more effective than monotherapy with tadalafil in men with remaining OAB symptoms despite monotherapy with tadalafil. Thus, we recruited patients who had been taking tadalafil monotherapy but had remaining OAB symptoms. We could not provide data before the monotherapy with tadalafil.
In our present study, OABSS total score and urgency score were decreased significantly in the two groups at weeks 4 and 12, but the difference was significantly greater in the TMG than TG. The same results were obtained in IPSS total, storage subscore, and QOL index. We used the IPSS as the standard LUTS questionnaire in this study. The results showed that IPSS storage symptoms were significantly improved but IPSS voiding symptoms were not improved in the TMG compared with TG. Total NIH‐CPSI score improved significantly, but pain or discomfort score in NIH‐CPSI did not change significantly between the two groups. We measured NIH‐CPSI score because we were interested in the anti‐inflammatory effects of tadalafil, and the combination of mirabegron did not show add‐on effects for pain symptoms. Consequently, in our subgroup of patients complaining persistence of OAB symptoms after tadalafil 5 mg/day during 8 weeks, the combination of tadalafil and mirabegron appeared to be more effective than tadalafil monotherapy up to 3 months. OABSS daytime frequency score decreased significantly only in TMG, but the intergroup difference did not reach significance (Table S1). However, decrease in number of voids per 24 hours and nighttime voids, and number of urgency episodes in the micturition chart were significantly greater in the TMG than in the TG.
In uroflowmetry, VV, Qave, and Qmax did not change significantly from baseline in either group, but the change in PVR from baseline significantly decreased and the change in BVE significantly increased only in TG. The change in VV was statistically significant between the groups at week 12, but the mean difference was only 22 mL.
The mean differences in PVR at 12 weeks from baseline was −7.4 mL and those in BE was 3.5% in the TG group. These differences appeared to be statistically significant, but not clinically significant. The intergroup difference was not found between the two groups. Therefore, the addition of mirabegron may not affect urinary flow, PVR, nor BVE.
One moderate adverse event (1.1%) was noted in TG (pain in hip joint due to pseudogout) and seven mild adverse events (8.0%) in TMG. The side effect of pseudogoat had not been reported previously. Therefore, the adverse event in TG was presumably hardly related to the study treatment. A total of two events led to discontinuation of study treatment for reasons of safety: one case of discomfort of bladder and one case of voiding difficulty. The systolic blood pressure, diastolic blood pressure, and pulse rate did not change after the therapy in the two groups, and no significant difference between the groups was observed.
A limitation of this study was that placebo was not used because of ethical reasons (it was difficult to obtain the IRB approval in the postmarket study in our country). Recent study may recommend the use of placebo in the randomized controlled study. However, most of the studies using placebo are organized by pharmaceutical companies. And there are many good quality RCTs without placebo. 3 , 5 , 7 , 16 , 17 , 18 , 19 Although this study was not double‐blinded using placebo, the quality of this study seemed considerable because randomization and analyses of data were strictly centrally organized and computer‐based.
5. CONCLUSIONS
The present study is the first report on treatment with PDE5 inhibitor and β3 agonist combination regimen. It was suggested that the effect of tadalafil/mirabegron combination therapy on relieving OAB symptoms appeared to be greater than that of tadalafil monotherapy, and that this combination therapy can be safely used.
CONFLICT OF INTERESTS
Tomonori Yamanishi received a grant from Astellas. All other authors declare that there are no conflict of interests.
Supporting information
Supporting information
ACKNOWLEDGMENTS
This study was funded by Astellas Pharma Inc. (Grant No. BE‐ICL‐006) Sekido N, Naya Y, and Asakura H comprised the protocol committee. Nakano S, Kakizaki H, and Nakamura T were comprised the committee responsible for the safety review. We would like to thank all CONTACT study investigators: Yasuhiro Shigeta, Nishifunabashi Urological Clinic; Hitoshi Ohoka, NHO Kobe Medical Center; Kosaku Yasuda, Yasuda Urological Hospital; Atsushi Sone, Miyazu Takeda Hospital; Yukio Naya, Teikyo University Chiba Medical Center; Yasuharu Takeuchi, Toho University Omori Medical Center; Naoki Nihei, Mihama Hospital; Koichiro Akakura, JCHO Tokyo Shinjuku Medical Center; Minoru Kobayashi, Utsunomiya Memorial Hospital; and Hiroomi Nakatsu, Asahi General Hospital. The supporting organization of the study, Clinical Research Support Center Kyushu, was responsible for support of individual study centers, study progress management, and data management. The supporting organization of the study, Clinical Research Support Center Kyushu, was responsible for support of individual study sites, study progress management, and data management.
Yamanishi T, Kaga K, Sakata K, et al. A randomized controlled study of the efficacy of tadalafil monotherapy versus combination of tadalafil and mirabegron for the treatment of persistent overactive bladder symptoms in men presenting with lower urinary tract symptoms (CONTACT Study). Neurourology and Urodynamics. 2020;39:804–812. 10.1002/nau.24285
REFERENCES
- 1. Homma Y, Gotoh M, Kawauchi A, et al. Clinical guidelines for male lower urinary tract symptoms and benign prostatic hyperplasia. Int J Urol. 2017;24:716‐729. [DOI] [PubMed] [Google Scholar]
- 2. Yamanishi T, Mizuno T, Tatsumiya K, Watanabe M, Kamai T, Yoshida K. Urodynamic effects of silodosin, a new alpha1A‐adrenoceptor selective antagonist, for the treatment of benign prostatic hyperplasia. Neurourol Urodyn. 2010;29:558‐562. [DOI] [PubMed] [Google Scholar]
- 3. Watanabe M, Yamanishi T, Mizuno T, et al. Effects of silodosin on lower urinary tract symptoms in patients with benign prostatic hyperplasia: evaluation by frequency/volume chart. Low Urin Tract Symptoms. 2010;2:31‐36. [DOI] [PubMed] [Google Scholar]
- 4. Yamanishi T, Kaga K, Fuse M, Shibata C, Kamai T, Uchiyama T. A six‐year followup of silodosin monotherapy for the treatment of LUTS/BPH: what are the factors for continuation or withdrawal? Int J Urol. 2015;22:1143‐1148. [DOI] [PubMed] [Google Scholar]
- 5. Nishizawa O, Yamaguchi O, Takeda M, Yokoyama O TAABO Study Group . Randomized controlled trial to treat benign prostatic hyperplasia with overactive bladder using an alpha‐blocker combined with anticholinergics. Low Urin Tract Symptoms. 2011;3:29‐35. [DOI] [PubMed] [Google Scholar]
- 6. Yamaguchi O, Kakizaki H, Homma Y, et al. Solifenacin as add on therapy for overactive bladder symptoms in men treated for lower urinary tract symptoms‐ASSIST, randomized controlled study. Urology. 2011;78:126‐133. [DOI] [PubMed] [Google Scholar]
- 7. Takeda M, Nishizawa O, Gotoh M, Yoshida M, Takahashi S, Masumori N. Clinical efficacy and safety of imidafenacin as add‐on treatment for persistent overactive bladder symptoms despite α‐blocker treatment in patients with BPH: the ADDITION study. Urology. 2013;82:887‐893. [DOI] [PubMed] [Google Scholar]
- 8. Chapple C, Herschorn S, Abrams P, Sun F, Brodsky M, Guan Z. Tolterodine treatment improves storage symptoms suggestive of overactive bladder in men treated with alpha‐blockers. Eur Urol. 2009;56:534‐541. [DOI] [PubMed] [Google Scholar]
- 9. Kaplan SA, McCammon K, Fincher R, Fakhoury A, He W. Safety and tolerability of solifenacin add‐on therapy to alpha‐blocker treated men with residual urgency and frequency. J Urol. 2009;182:2825‐2830. [DOI] [PubMed] [Google Scholar]
- 10. Yamaguchi O, Ikeda Y, Ohkawa S. Phase III study to assess long‐term (52‐week) safety and efficacy of mirabegron, a β3‐Adrenoceptor agonist, in japanese patients with overactive bladder. Low Urin Tract Symptoms. 2017;9:38‐45. [DOI] [PubMed] [Google Scholar]
- 11. Kelleher C, Hakimi Z, Zur R, et al. Efficacy and tolerability of mirabegron compared with antimuscarinic monotherapy or combination therapies for overactive bladder: a systematic review and network meta‐analysis. Eur Urol. 2018;74:324‐333. [DOI] [PubMed] [Google Scholar]
- 12. Yamanishi Y, Yamanishi T, Tajima H, Ikeda S. Mirabegron or tolterodine for the treatment of overactive bladder in Japan: which drug is more cost‐effective as the first‐line treatment? Int J Urol. 2018;25:863‐870. [DOI] [PubMed] [Google Scholar]
- 13. Ichihara K, Masumori N, Fukuta F, Tsukamoto T, Iwasawa A, Tanaka Y. A randomized controlled study of the efficacy of tamsulosin monotherapy and its combination with mirabegron for overactive bladder induced by benign prostatic obstruction. J Urol. 2015;193:921‐926. [DOI] [PubMed] [Google Scholar]
- 14. Yokoyama O, Yoshida M, Kim SC, et al. Tadalafil once daily for lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a randomized placebo‐ and tamsulosin‐controlled 12‐week study in Asian men. Int J Urol. 2013;20:193‐201. [DOI] [PubMed] [Google Scholar]
- 15. Gacci M, Andersson KE, Chapple C, et al. Latest evidence on the use of phosphodiesterase type 5 inhibitors for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur Urol. 2016;70:124‐133. [DOI] [PubMed] [Google Scholar]
- 16. Montorsi F, Roehrborn C, Garcia‐Penit J, et al. The effects of dutasteride or tamsulosin alone and in combination on storage and voiding symptoms in men with lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH): 4‐year data from the Combination of Avodart and Tamsulosin (CombAT) study. BJU Int. 2011;107:1426‐1431. [DOI] [PubMed] [Google Scholar]
- 17. Haque N, Masumori N, Sakamoto S, et al. Superiority of dutasteride 0.5 mg and tamsulosin 0.2 mg for the treatment of moderate‐to‐severe benign prostatic hyperplasia in Asian men. Int J Urol. 2018;25:944‐951. [DOI] [PubMed] [Google Scholar]
- 18. Yamanishi T, Asakura H, Seki N, Tokunaga S. Efficacy and safety of combination therapy with tamsulosin, dutasteride and imidafenacin for the management of overactive bladder symptoms associated with benign prostatic hyperplasia: a multicenter, randomized, open‐label, controlled trial (DIrecT Study). Int J Urol. 2017;24:525‐531. [DOI] [PubMed] [Google Scholar]
- 19. Yamanishi T, Asakura H, Seki N, Tokunaga S. A 52‐week multicenter randomized controlled study of the efficacy and safety of add‐on dutasteride and imidafenacin to tamsulosin in patients with benign prostatic hyperplasia with remaining overactive bladder symptoms (DIrecT study). Low Urin Tract Symptoms. 2019;11:115‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


