Abstract
The tripartite‐motif (TRIM) family of proteins represents one of the largest classes of putative single protein RING‐finger E3 ubiquitin ligases. The members of this family are characterized by an N‐terminal TRIM motif containing one RING‐finger domain, one or two zinc‐finger domains called B boxes (B1 box and B2 box), and a coiled‐coil region. The TRIM motif can be found in isolation or in combination with a variety of C‐terminal domains, and based on C‐terminus, TRIM proteins are classified into 11 distinct groups. Because of the complex nature of TRIM proteins, they are implicated in a variety of cellular functions and biological processes, including regulation of cell proliferation, cell division and developmental processes, cancer transformation, regulation of cell metabolism, autophagocytosis, modification of chromatin status, regulation of gene transcription, post‐translational modifications, and interactions with pathogens. Here, we demonstrate the specific activities of TRIM family proteins that contribute to the cancer stem cell phenotype. A growing body of evidence demonstrates that several TRIM members guarantee the acquisition of stem cell properties and the ability to sustain stem‐like phenotype by cancer cells using distinct mechanisms. For other members, further work is needed to understand their full contribution to stem cell self‐renewal. Identification of TRIM proteins that possess the potential to serve as therapeutic targets may result in the development of new therapeutic strategies. Finally, these strategies may result in the disruption of the machinery of stemness acquisition, which may prevent tumor growth, progression, and overcome the resistance to anticancer therapies.
Keywords: cancer, pluripotency, RING, self‐renewal, stem cells, TRIM
The tripartite‐motif (TRIM) family of proteins is involved in a variety of cellular functions and biological processes that facilitate acquisition or maintenance of stem cell self‐renewal.

Significance statement.
Tripartite‐motif (TRIM) proteins are involved in a variety of cellular functions and biological processes, and recent data revealed their significant role in the acquisition and maintenance of the stem cell phenotype. The present article describes those TRIM family members that facilitate cell stemness and discusses others for which further studies are needed to understand their full contribution to stem cell self‐renewal.
1. INTRODUCTION
The members of the tripartite‐motif (TRIM) family of proteins are involved in many different cellular processes while sharing some functional properties such as: (a) acting as scaffold proteins for larger multiprotein complexes and (b) possessing RING‐mediated E3 ubiquitin ligase activity that is directed toward distinct proteins.1 In humans, there are about 80 members of the TRIM family, which is characterized by an N‐terminal TRIM motif containing one RING‐finger domain, one or two zinc‐finger domains called B boxes (B1 box and B2 box), and its associated coiled‐coil region.2 The main structural differences within the family of TRIM proteins are determined by diverse C‐terminal domains. Based on the C‐terminus, TRIM proteins are classified into 11 groups (from C‐I to C‐XI). Some of the TRIM proteins do not possess a RING‐finger domain within the TRIM motif and constitute the 12th group of unclassified (UC) TRIM proteins.3
Mammalian TRIM gene expression is generally constitutive and ubiquitous during embryonic development and in adult tissues (with some exceptions).4 Within the same cell, several different TRIM transcripts (originating from alternative splicing events) may emerge, leading to diverse predicted protein products. These TRIM isoforms can possess different biochemical properties and play various roles in cell biology.5, 6 To date, TRIM proteins were demonstrated to regulate cell proliferation,7, 8 cell division, and developmental processes9, 10; facilitate or suppress cell transformation to cancer11; control cell metabolism12; regulate autophagocytosis13; mediate chromatin modifications8; positively or negatively regulate gene transcription14, 15, 16; add post‐translational modifications to target proteins2, 17; directly interact with pathogens having a predominant role in innate immunity2; and many others. In this review, the relationship between TRIM family members and cell stemness was highlighted (with tumorigenesis as a background).
2. MECHANISMS PROVIDING STEM CELL SELF‐RENEWAL
The last two decades of research have harnessed enormous forces to define and deeply characterize the specific population of cancer cells termed cancer stem cells (CSCs). Originally, CSCs were defined as a population with the capability to self‐renew and differentiate, and they are highly responsible for tumor growth and progression.18, 19 CSCs are endowed with intrinsic resistance to chemo‐ and radiotherapy, possessing a high metastatic potential, and providing tumor relapse after treatment. Usually considered to be a small population of cells, CSCs appeared to be heterogeneous and sometimes numerous within specific types of cancer. Functional and phenotypical properties of these cells are substantially influenced by genetic, epigenetic, and microenvironmental factors.18, 20, 21 Because of their high plasticity, CSCs may experience phases of transition between stem‐like and non‐stem‐like states.18, 20, 21 Therefore, the concept of CSCs has to be redefined. A growing body of evidence demonstrates that bulk tumor cells can acquire stem cell properties in response to exogenous stimuli. This suggests that the process of cancer cell differentiation can be reversed and further adds to the complexity of cancer stemness.19, 20
In this review, we have considered the stemness of cancer cells as a specific state a cancer cell can achieve and maintain and characterized the mechanisms that regulate this phenomenon. Among others, the activation of specific signaling pathways and the induction of several transcription factors play a fundamental role18, 22 in the regulation of stem cell pluripotency.
Signaling pathways that provide stem cell self‐renewal are frequently harnessed by cancer cells, leading to increased aggressiveness and the acquisition of the stem cell phenotype.22 Specifically, the JAK/STAT, Hedgehog, Wnt/β‐catenin, Notch, PI3K/Akt, TGF‐β, and NF‐κB signaling pathways have all been shown to mediate various stem cell properties. The over‐activation or abnormal signaling within these pathways contributes to the survival of CSCs.18, 22 Many of these pathways are not linear, but rather interwoven networks of signaling mediators that feed into one another, facilitating inter‐pathway cross talk. Furthermore, both extrinsic and intrinsic molecular signals, as well as several regulatory elements, escalate the complexity of these pathways, making it difficult to identify the central stemness determinant.
The activation of pluripotency‐facilitating signaling pathways leads ultimately to the induction of stem cell‐specific core transcription factor machinery, including Oct‐3/4, Sox2, and Nanog transcription factors.23 These factors regulate the expression of two distinct groups of genes: those engaged in self‐renewal (activated) and those involved in cell differentiation (silenced). Additionally, numerous studies have demonstrated their role in tumorigenesis and revealed a significant correlation with worse patient outcome, tumor dedifferentiation status, and stemness acquisition.21
Besides core pluripotency transcription factor machinery, there are other mediators that regulate the expression of pluripotency or differentiating genes. These include non‐coding RNAs (such as miRNAs) and chromatin regulators, which stabilize stem cell expression patterns through post‐transcriptional modulations and epigenetic mechanisms (including DNA methylation and histone modifications), respectively.24 The role of epigenetic mechanisms in defining tumor heterogeneity and acquisition or maintenance of the CSC phenotype is now being extensively studied.25, 26
Previous studies demonstrated that normal stem cell and cancer cell metabolism are overtly similar, using mainly glycolysis. However, a closer look at the metabolic requirements of the CSC population revealed a distinct metabolic phenotype for normal stem cells and for bulk tumor cells.27, 28 Studies have shown that mitochondrial function is crucial for the maintenance of the stem cell phenotype.29 Additionally, the “metabolic switch” from glycolysis to OXPHOS (oxidative phosphorylation) is believed to play a critical role in the stemness acquisition rather than being the consequence of metabolic reprogramming to pluripotency.27, 28
Recent studies have also highlighted the indisputable role of epithelial‐to‐mesenchymal transition (EMT) in the acquisition and maintenance of stem cell‐like properties. As demonstrated previously, EMT is sufficient to endow cancer cells with stem cell characteristics, facilitating cancer resistance to therapeutic agents and resulting in cancer recurrence and progression.30, 31
In summary, numerous mechanisms enable cancer cells to acquire and maintain the stem cell phenotype. Most of them are interrelated and influence each other, considerably increasing the complexity of CSC regulation. An ever‐growing number of studies demonstrate significant engagement of distinct TRIM family members in each of the abovementioned biological processes.
3. THE ENGAGEMENT OF SPECIFIC TRIMS IN STEM CELL MAINTENANCE
Because of the large number of TRIM family members and the complex nature of the mechanisms governing stem cell maintenance, in this review, we focused on TRIM proteins, which play a role in the machinery that provides stem cell self‐renewal and that was directly confirmed experimentally. However, the involvement of the remaining TRIM family members in the cell stemness acquisition or maintenance should not be excluded or negated because of the high relativity of TRIMs to each other.
Among the members of classes IV to VII and among the 12th group of UC TRIMs, there are proteins that were previously reported to provide or to suppress the stem‐like phenotype acquisition or maintenance (Table 1).
Table 1.
The engagement of tripartite‐motif (TRIM) family members in stem cell phenotype acquisition or maintenance
| Gene name | Synonyms | Class | C‐terminal domains | Stem cell‐associated function | Stem cell regulator | References |
|---|---|---|---|---|---|---|
| TRIM6 | IV | PRY, SPRY | TRIM6 regulates c‐Myc expression | Positive | 32 | |
| TRIM8 | RNF27, GERP | V | None | TRIM8 activates STAT3 signaling | Positive | 33, 34 |
| TRIM8 inhibits translocation of STAT3 into the nucleus | Negative | 35 | ||||
| TRIM11 | IV | PRY, SPRY | TRIM11 activates EGFR signaling | Positive | 36 | |
| TRIM14 | UC | PRY, SPRY | TRIM14 activates Akt signaling | Positive | 37, 38, 39 | |
| TRIM16 | UC | PRY, SPRY | TRIM16 mediates Gli‐1 degradation | Negative | 40 | |
| TRIM19 | PML | V | None | PML regulates Oct‐3/4, STAT3, c‐Myc expression | Positive | 14, 41 |
| TRIM21 | IV | PRY, SPRY | TRIM21 enhances Oct‐1 ubiquitination | Negative | 42 | |
| TRIM24 | TIF1A | VI | PHD, BROMO | TRIM24 activates STAT3 signaling | Positive | 7 |
| TRIM24 ubiquitinates p53 protein | Positive | 43, 44 | ||||
| TRIM24 suppresses pro‐differentiation genes | Positive | 45 | ||||
| TRIM25 | EFP | IV | PRY, SPRY | TRIM25 upregulates POU5F1, NANOG, and SOX2 expression | Positive | 46, 47 |
| TRIM27 | IV | PRY, SPRY | TRIM27 promotes EMT and activates Akt signaling | Positive | 48 | |
| TRIM28 | TIF1B, KAP1 | VI | PHD, BROMO | TRIM28 represses pro‐differentiation genes | Positive | 15, 16 |
| TRIM28 forms a unique module in the self‐renewal transcription network distinct from the module of Nanog‐Sox2‐Oct‐4 | Positive | 49, 50 | ||||
| Trim28 (phosphorylated at Ser824) promotes Nanog, Sox2, and Oct‐4 expression | Positive | 51 | ||||
| TRIM28 regulates AMPK level | Positive | 52 | ||||
| TRIM28 induces EMT | Positive | 53 | ||||
| TRIM32 | HT2A | VII | NHL | TRIM32 degrades c‐Myc | Negative | 54, 55 |
| TRIM71 | LIN‐41 | VII | Filamin, NHL | Trim71 facilitates the G1‐S transition | Positive | 56 |
TRIM proteins regulate stem cell characteristics mostly positively by enhancing the activity of core transcription factors, induction of specific signaling pathways, epigenetic silencing of pro‐differentiation genes, metabolic reprogramming, and activation of the EMT program (Figure 1). Several members negatively impact stem cell self‐renewal presumably by ubiquitin‐mediated degradation of stem cell transcription factors or inhibition of specific signaling pathways. For some members, the role in stem cell regulation is multilayered and involves engagement at several distinct levels of stemness machinery (ie, TRIM24 and TRIM28), whereas others control the stem cell phenotype both positively and negatively (TRIM8). For better clarity, specific TRIM members and the mechanisms that they use to regulate cell stemness are individually characterized below.
Figure 1.
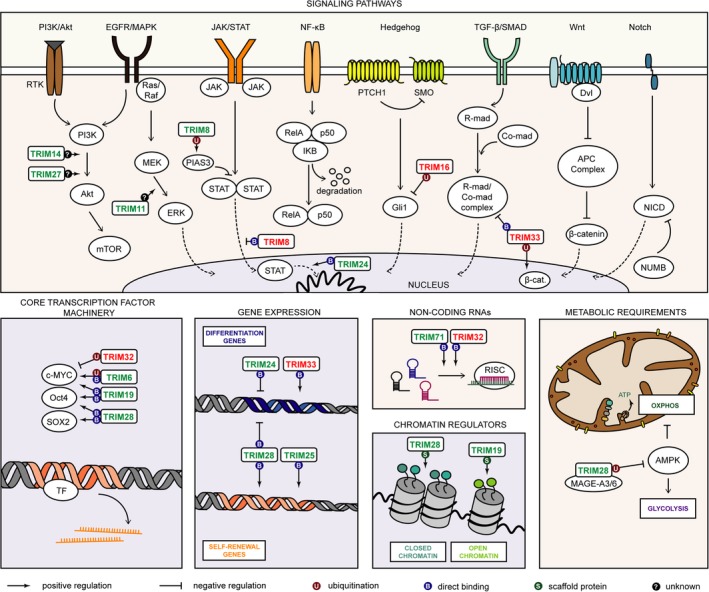
Several distinct mechanisms or biological processes confer the unlimited self‐renewal potential and the pluripotency of stem cells. A growing number of tripartite‐motif (TRIM) proteins are being recognized as important modifiers of stemness machinery. Positive regulators of stem cell self‐renewal are marked in green and negative regulators are marked in red. TRIM proteins are able to ubiquitinate (U, brown dot), directly bind target protein (B, dark blue dot), or serve as a scaffold (S, dark green dot) to exert their stem cell‐associated function. Unfortunately, the exact molecular mode of action remains unknown for some members (question mark, black dot)
3.1. TRIM family members with C‐terminal PRY and/or SPRY domains (class IV and UC proteins)
TRIM6 (member of class IV of TRIM proteins) is selectively expressed in embryonic stem cells and it directly interacts with a c‐Myc transcription factor, leading to attenuation of c‐Myc activity. As reported by Sato et al,32 downregulation of TRIM6 expression in mouse ES cells enhanced the transcriptional activity of c‐Myc and suppressed the expression of NANOG, and thereby promoted stem cell differentiation. The detailed analysis demonstrated that TRIM6 functions as an ubiquitin ligase for c‐Myc in embryonic stem cells that keeps c‐Myc at the appropriate level to maintain the pluripotency.
Another member of class IV of TRIM proteins, TRIM21, also uses the ubiquitin‐ligase activity to regulate stem cell properties, but it is a negative regulator of self‐renewal. As recently reported by Du et al,42 TRIM21 enhances Oct‐1 ubiquitination and, consequently, reduces Oct‐1 stability, leading to a loss of self‐renewal of colorectal CSCs.
TRIM11, TRIM25, and TRIM27, which are also class IV members, act as positive regulators of stem cell self‐renewal, but they use distinct mechanisms of action. TRIM11 exerts its effect through activation of the EGFR signaling pathway, thereby promoting a stem‐like phenotype in vitro and enhancing tumor growth in vivo. As presented by Di et al,36 downregulation of TRIM11 led to decreased EGFR expression and reduced c‐Raf, MEK1/2, and p44/p42MAPK phosphorylation. Conversely, overexpressing TRIM11 increased EGFR expression and enhanced the MAPK pathway activity. However, further studies will be needed to identify the molecular target for TRIM11 in the EGFR/MAPK pathway. TRIM25 regulates the core genes that are involved in maintaining quiescent CSC‐like phenotypes (POU5F1, SOX2, and NANOG) through transcriptional and/or post‐transcriptional mechanisms, but E3 ubiquitin ligase activity is not involved.46, 47 Moreover, TRIM25 promotes metastasis through increasing the stemness and tumorigenicity of breast cancer cells, thereby enhancing their capacity for colonization, survival, and outgrowth at the secondary site, as recently demonstrated by Walsh et al.47 TRIM27 promotes EMT and activates Akt signaling pathway in colorectal cancer cells, leading to the acquisition of a very invasive and metastatic phenotype reflecting the CSC‐like phenotype.48 Although Zhang et al48 observed significant Akt signaling activation upon TRIM27 overexpression, they did not unravel the molecular mode of action for TRIM27.
Additionally, other TRIM proteins that possess PRY and SPRY C‐terminal domains (but are not classified as class IV members) were previously demonstrated to take part in the regulation of stem cell properties. Wang et al37 had reported that TRIM14 overexpression induced EMT and resulted in the formation of cancer‐initiating cells and acquisition of a chemoresistant phenotype by oral tongue squamous cell cancer. TRIM14 was further reported to enhance migration and invasion of osteosarcoma cells38 and gastric cancer cells39 by regulating EMT transition via activation of the Akt signaling pathway, which may correspond to the attainment of CSC‐like phenotype. Similar to TRIM27, the exact molecular mode of TRIM14 action in Akt signaling promotion requires further investigation, because neither TRIM14 nor TRIM27 can phosphorylate Akt and other downstream targets.
However, TRIM16, a member of an UC group that contains PRY and SPRY C‐terminal domains but lacks an N‐terminal RING domain, was reported to negatively regulate CSC self‐renewal. As demonstrated by Yao et al,40 TRIM16 inhibits the emergence of CSC‐like cells in breast cancer cell lines through degradation of Gli‐1—the effector of the Hedgehog (Hh) signaling pathway. TRIM16 has no classic RING domain. However, using three‐dimensional modeling, Bell et al57 demonstrated that the TRIM16 B‐box domains adopt RING‐like folds, facilitating ubiquitin ligase activity. Additionally, TRIM16 can heterodimerize with other TRIM family members and it, therefore, targets proteins for proteasomal degradation.57
3.2. TRIM family members without specific C‐terminal domain (class V)
Among the class V of TRIM family members (which do not possess C‐terminal functional domains), only two proteins were reported to regulate stem cell biology to date—TRIM8 and TRIM19. TRIM8 seems to control stem cell properties both positively and negatively. In 2011, Okumura et al35 demonstrated that TRIM8 selectively downregulated transcription of Nanog in embryonic stem cells through inhibition of Hsp90β/STAT3 complex nuclear translocation. The upregulation of TRIM8 expression leads to spontaneous differentiation of ES cells. However, Okumura et al33 showed that TRIM8 enhanced the STAT3‐dependent signaling pathway by inhibiting the function of PIAS3 (a protein inhibitor of activated STAT3). Similar results were also reported by Zhang et al,34 who demonstrated that TRIM8 suppresses the expression of PIAS3 most likely through E3‐mediated ubiquitination and proteasomal degradation, consequently maintaining the stemness and self‐renewing capabilities of glioma stem cells. Therefore, TRIM8 is considered to be a simultaneous positive and negative regulator of stem cell properties, and TRIM8 expression needs to be tightly regulated at an appropriate level to maintain the pluripotency of stem cells.
TRIM19, better known as the PML (promyelocytic leukemia) gene, which is the main constituent of PML nuclear bodies, contributes to stem cell self‐renewal by controlling the cell‐cycle progression and sustaining the expression of crucial pluripotency factors. Furthermore, TRIM19 recruits specific transcription factors and the chromatin‐remodeling complex to maintain the open chromatin conformation of the Oct‐3/4 promoter, thereby activating Oct‐3/4 transcription.41 Additionally, Hadjimichael et al14 reported that TRIM19 associates with Oct‐3/4, STAT3, c‐MYC, and NR0B1, which are four essential regulators of the naive pluripotent state. TRIM19 ablation resulted in significant changes in ES cell morphology, alteration of the global gene expression profile, and prolonged G1 phase, which resembles that of differentiated cells.
3.3. TRIM family members with C‐terminal PHD and BROMO domains (class VI)
The sixth class of TRIM family proteins consists of three proteins: TRIM24, TRIM28, and TRIM33, all of which possess PHD and BROMO C‐terminal domains and were recently reported to regulate stem cell self‐renewal and differentiation. This subfamily is also known as the transcriptional intermediary factor 1 (TIF1) family of chromatin‐associated/related proteins.9 TRIM24 (also known as TIF1α) functions as a transcriptional coactivator that, in response to EGFR, recruits STAT3, leading to stabilized STAT3‐chromatin interactions and subsequent activation of a downstream signaling pathway. Therefore, TRIM24 is essential to mediate EGFR‐driven glioma stem cell proliferation and self‐renewal.7 Additionally, TRIM24, as a ubiquitin E3 ligase, keeps the p53 protein at a low level in normal pluripotent stem cells, which facilitates their self‐renewal.43 Downregulation of TRIM24 expression in human ES cells leads to spontaneous differentiation, which is in contrast to human breast cancer cells where TRIM24 depletion induces spontaneous apoptosis.43, 44 Moreover, TRIM24 converges with Oct‐3/4, Sox2, and NANOG on multiple enhancers and suppresses the expression of developmental genes to support the pluripotent state of stem cells.45
TRIM28, also known as TIF1β or KAP1 (Krüppel‐Associated Box [KRAB]‐Associated Protein 1) protein, was first described as a universal cofactor for a huge family of KRAB Zinc Finger Protein transcription factors.8 TRIM28 is strictly associated with the maintenance of stem cell self‐renewal and contributes to the stemness machinery on several distinct levels. It was previously reported that TRIM28 cooccupies many putative gene promoters and, together with other pluripotency markers such as Cnot3, Zfx, and c‐Myc, forms a unique module in the self‐renewal transcription network that is distinct from the Nanog‐Sox2‐Oct‐3/4 module.49, 50 Furthermore, Trim28 efficiently controls self‐renewal of pluripotent stem cells in a phosphorylation‐dependent manner. Using mouse embryonic stem cells, Seki et al51 demonstrated that Trim28 protein (specifically phosphorylated at Ser824) formed a complex with Oct‐3/4 on the promoters of pluripotency‐specific genes, and that it promotes the expression of Nanog, Sox2, and Oct‐3/4 and the expression of various chromatin remodeling proteins. Therefore, phospho‐Trim28 directly induces ES cell‐specific genes and enables prolonged maintenance of an undifferentiated state. Besides inducing the expression of pluripotency markers, TRIM28 has the opposite effect on differentiation genes. TRIM28 uses KRAB‐ZNFs to cause epigenetic silencing of its target differentiation genes via H3K9me3 and DNA methylation, sustaining the self‐renewal of human pluripotent stem cells.15, 16 Additionally, TRIM28 is essential to maintain stem cell properties of cancer cells. Together with MAGE‐A3/6, TRIM28 forms a cancer‐specific ubiquitinase that regulates the AMPK level in cancer cells, enhancing oxidative phosphorylation and maintaining stem cell traits.52 Moreover, TRIM28 regulates specific mediators of EMT at both transcriptional and posttranscriptional levels and it was previously reported to directly induce EMT program,53 which may further result in the acquisition of stem cell‐like phenotype.
TRIM33 (TIF1γ) is known to be a transcriptional co‐repressor.58 In contrast to TRIM24 and TRIM28, TRIM33 seems to be a positive regulator of stem cell differentiation (TRIM33 loss does not affect stem cell self‐renewal, but it impairs differentiation process) and a growing amount of data suggests that TRIM33 directly mediates TGF‐β signaling and plays a role in Wnt/β‐catenin signaling to provide proper differentiation during early mouse embryonic development.59
3.4. TRIM family members with C‐terminal NHL and FIL domains (class VII)
TRIM32 is a member of class VII with C‐terminal NHL domain. The molecular role of TRIM32 in stem cell fate specification is based on two mechanisms: the induction of specific microRNAs activity (through NHL‐mediated association with Argonaute 1 protein)54 and RING‐mediated ubiquitination and proteasomal degradation of c‐Myc.54, 55, 60 Another class VII member, TRIM71, has a C‐terminal NHL domain preceded by Filamin domain, and was reported to positively regulate stem cell self‐renewal. TRIM71, also known as LIN41, facilitates the G1‐S transition (through CDKN1A repression) to promote rapid embryonic stem cell self‐renewal.56 TRIM71 associates with and catalytically activates Argonaute 2 through the NHL domain. This interaction potentiates specific microRNA functions and consequently leads to suppression of CDKN1A expression. Additionally, TRIM71 promotes reprogramming of somatic cells to induce pluripotent stem cells by suppression of a broad array of differentiation genes (as a consequence of direct inhibition of EGR1 mRNA translation).61 Altogether, TRIM71 is critical for the regulation of stem cell self‐renewal.
3.5. Other TRIM family members potentially involved in the regulation of stem cell properties
For several TRIMs, the exact mechanism of stem cell regulation remains unknown, although their engagement seems essential. Low et al62 have demonstrated spontaneous differentiation of glioma CSCs upon shRNA‐mediated downregulation of several TRIMs (TRIM3, TRIM13, TRIM41, and TRIM52), implicating their role in CSC self‐renewal. However, the authors did not verify the exact mechanism of TRIM‐mediated stemness regulation.
Several other TRIM family members were reported to play a role in cancer progression through induction of cancer cell phenotype alterations that frequently resemble acquisition of CSC properties or through the use of mechanisms that were previously demonstrated to facilitate stem cell pluripotency. For example, TRIM59 (class XI member with C‐terminal TM domain) promotes tumor growth and migration in various cancer types, including lung cancer or osteosarcoma.63, 64 It was recently reported to be a critical mediator of cell metastasis in medulloblastoma, engaging the PI3K/Akt signaling pathway to induce EMT and to promote cell migration and invasion,65 which are characteristics of the CSC population. TRIM37 (a class VIII member with C‐terminal MATH domain) also promotes EMT in cancer cells by activation of Wnt/β‐catenin signaling. Colorectal cancer cells that overexpress TRIM37 are more aggressive, with higher tumorigenicity and increased migratory and invasive capabilities,66 suggesting a possible role for TRIM37 in stemness acquisition. However, some of the TRIM members are known tumor suppressors. TRIM3 (class VII member with C‐terminal NHL domain) suppresses the tumorigenicity of liver cancer cells by inhibiting proliferation, colony formation, migration, and invasion and by inducing cell cycle arrest of tumor cells,67 suggesting that TRIM3 may act as a negative regulator for stem cell phenotype acquisition.
However, to unequivocally define those TRIMs as the regulators of stem cell phenotype, more detailed data are needed that clearly demonstrate that these proteins are required and/or sufficient for regulation of stem cell self‐renewal.
4. MOLECULAR MODES OF TRIM ACTIONS
TRIM proteins are very heterogeneous and are involved in stem cell regulation on several distinct levels using different molecular modes of actions. However, a closer look at specific TRIM‐mediated mechanisms of stemness acquisition or maintenance suggests an indisputable role for the TRIM motif.
In cancer cells, the regulation of stem cell‐associated signaling pathways by TRIM proteins occurs either via the ubiquitination and proteasomal degradation of specific pathway components33, 57, 59 or by direct binding and stabilization of downstream mediators34, 35 (further recruited to chromatin to regulate target gene expression). However, for several TRIMs, the molecular mode of action is yet not fully understood and the data suggest the participation of additional factors.36, 38, 39, 48 Similarly, TRIM‐mediated regulation of core pluripotency transcription factor machinery is based on the E3 ubiquitin ligase activity (mediated by the RING domain)32, 55, 60 or the ability to form a complex and stabilize specific transcription factors14, 50, 51 (unfortunately, it has not been precisely defined whether the TRIM motif is always required for protein‐protein interactions).
Several TRIMs also play the role of transcription cofactors that are involved activating the expression of other pluripotency genes or in suppressing differentiating markers. All members of the sixth class of TRIM proteins (TRIM24, TRIM28, and TRIM33) that possess C‐terminal PHD and BROMO domains are engaged in the stemness regulation at this level (either as activators of pluripotency genes or suppressors of differentiating markers). Besides using the TRIM motif, these proteins engage both PHD and BROMO domains to exert their specific functions.15, 16, 45, 58 Distinct TRIM members were also shown to participate in epigenetic mechanisms underlying the acquisition or maintenance of CSC phenotype. Among the factors that recruit chromatin‐remodeling complexes, we can find both mediators of chromatin condensation (TRIM28) and open chromatin maintainers (TRIM19). In both cases, the TRIM motif plays an essential role in forming a supramolecular assembly.8, 15, 41, 68
The E3 ubiquitin ligase activity mediated by the RING domain is commonly used by specific TRIM proteins to exert the stem cell‐associated function. Besides the abovementioned targets for ubiquitination and proteasomal degradation, the level of several other proteins is tightly regulated by TRIM members to maintain the pluripotent state (ie, components of the cell cycle regulatory circuit [p53] or proteins associated with cell metabolism [AMPK]).8, 43, 52 Therefore, the presence of a catalytically active RING domain that could be blocked by chemical compounds might make TRIM family members attractive targets for the manipulation of stem cell homeostasis.
5. CONCLUSION
The role of TRIM family members in cancer development and progression has been studied for years, determining both oncogenic and tumor suppressive members. A growing number of TRIM proteins are engaged in regulating the CSC population, which is mostly responsible for tumor growth and progression, metastases, and resistance to anticancer therapies. Acquisition of stem cell properties and the ability to sustain a stem‐like phenotype by cancer cells is guaranteed by several TRIM members, but via the use of distinct mechanisms. For other members, further work using biochemical approaches as well as genetic knock‐in and knockout experiments in mice is needed to understand the full contribution of these TRIMs to stem cell self‐renewal. Additionally, it is important to identify the TRIMs that potentially serve as therapeutic targets. Targeting those TRIMs might result in disruption of the machinery of stemness acquisition and consequently, might prevent tumor relapse.
CONFLICT OF INTEREST
The authors indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
A.M.J., N.A.W., P.C.: conception and design, manuscript writing; A.M., P.C.: final approval of manuscript; P.C.: financial support.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
ACKNOWLEDGMENT
The work was supported by the Polish National Science Centre grant No. UMO‐2017/26/D/NZ3/00848 to P.C.
Jaworska AM, Wlodarczyk NA, Mackiewicz A, Czerwinska P. The role of TRIM family proteins in the regulation of cancer stem cell self‐renewal. Stem Cells. 2020;38:165–173. 10.1002/stem.3109
Funding information Narodowe Centrum Nauki, Grant/Award Number: UMO‐2017/26/D/NZ3/00848
REFERENCES
- 1. Short KM, Cox TC. Subclassification of the RBCC/TRIM superfamily reveals a novel motif necessary for microtubule binding. J Biol Chem. 2006;281(13):8970‐8980. [DOI] [PubMed] [Google Scholar]
- 2. Hatakeyama S. TRIM family proteins: roles in autophagy, immunity, and carcinogenesis. Trends Biochem Sci. 2017;42(4):297‐311. [DOI] [PubMed] [Google Scholar]
- 3. Carthagena L, Bergamaschi A, Luna JM, et al. Human TRIM gene expression in response to interferons. PLoS One. 2009;4(3):e4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reymond A, Meroni G, Fantozzi A, et al. The tripartite motif family identifies cell compartments. EMBO J. 2001;20(9):2140‐2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jensen K, Shiels C, Freemont PS. PML protein isoforms and the RBCC/TRIM motif. Oncogene. 2001;20(49):7223‐7233. [DOI] [PubMed] [Google Scholar]
- 6. Battivelli E, Migraine J, Lecossier D, et al. Modulation of TRIM5alpha activity in human cells by alternatively spliced TRIM5 isoforms. J Virol. 2011;85(15):7828‐7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lv D, Li Y, Zhang W, et al. TRIM24 is an oncogenic transcriptional co‐activator of STAT3 in glioblastoma. Nat Commun. 2017;8(1):1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Czerwinska P, Mazurek S, Wiznerowicz M. The complexity of TRIM28 contribution to cancer. J Biomed Sci. 2017;24(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cammas F, Mark M, Dollé P, et al. Mice lacking the transcriptional corepressor TIF1beta are defective in early postimplantation development. Development. 2000;127(13):2955‐2963. [DOI] [PubMed] [Google Scholar]
- 10. Napolitano LM, Meroni G. TRIM family: pleiotropy and diversification through homomultimer and heteromultimer formation. IUBMB Life. 2012;64(1):64‐71. [DOI] [PubMed] [Google Scholar]
- 11. Bhatnagar S, Gazin C, Chamberlain L, et al. TRIM37 is a new histone H2A ubiquitin ligase and breast cancer oncoprotein. Nature. 2014;516:116‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cambiaghi V, Giuliani V, Lombardi S, et al. TRIM proteins in cancer. Exp Med Biol. 2012;770:77‐91. [DOI] [PubMed] [Google Scholar]
- 13. Mandell MA, Jain A, Arko‐Mensah J, et al. TRIM proteins regulate autophagy and can target autophagic substrates by direct recognition. Dev Cell. 2014;30:394‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hadjimichael C, Chanoumidou K, Nikolaou C, et al. Promyelocytic leukemia protein is an essential regulator of stem cell pluripotency and somatic cell reprogramming. Stem Cell Reports. 2017;8(5):1366‐1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng B, Ren X, Kerppola TK. KAP1 represses differentiation‐inducible genes in embryonic stem cells through cooperative binding with PRC1 and derepresses pluripotency‐associated genes. Mol Cell Biol. 2014;34(11):2075‐2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oleksiewicz U, Gladych M, Raman AT, et al. TRIM28 and interacting KRAB‐ZNFs control self‐renewal of human pluripotent stem cells through epigenetic repression of pro‐differentiation genes. Stem Cell Reports. 2017;9(6):2065‐2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Y, Wu H, Wu W, et al. Structural insights into the TRIM family of ubiquitin E3 ligases. Cell Res. 2014;24(6):762‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Czerwinska P, Kaminska B. Regulation of breast cancer stem cell features. Contemp Oncol. 2015;19(1A):A7‐A15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malta TM, Sokolov A, Gentles AJ, et al. Machine learning identifies stemness features associated with oncogenic dedifferentiation. Cell. 2018;173(2):338‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee G, Hall RG, Ahmed AU. Cancer stem cells: cellular plasticity, niche, and its clinical relevance. J Stem Cell Res Ther. 2016;6(10):363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Czerwińska P, Mazurek S, Wiznerowicz M. Application of induced pluripotency in cancer studies. Rep Pract Oncol Radiother. 2018;23(3):207‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matsui WH. Cancer stem cell signaling pathways. Medicine. 2016;95(1):S8‐S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hadjimichael C, Chanoumidou K, Papadopoulou N, et al. Common stemness regulators of embryonic and cancer stem cells. World J Stem Cells. 2015;7(9):1150‐1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Srinageshwar B, Maiti P, Dunbar GL, et al. Role of epigenetics in stem cell proliferation and differentiation: implications for treating neurodegenerative diseases. Int J Mol Sci. 2016;17(2):199‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Young RA. Control of embryonic stem cell state. Cell. 2011;144(6):940‐954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bradner JE, Hnisz D, Young RA. Transcriptional addiction in cancer. Cell. 2017;168(4):629‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sancho P, Barneda D, Heeschen C. Hallmarks of cancer stem cell metabolism. Br J Cancer. 2016;114(12):1305‐1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chae YC, Kim JH. Cancer stem cell metabolism: target for cancer therapy. BMB Rep. 2018;51(7):319‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wanet A, Arnould T, Najimi M, et al. Connecting mitochondria, metabolism, and stem cell fate. Stem Cells Dev. 2015;24(17):1957‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sarrio D, Franklin CK, Mackay A, et al. Epithelial and mesenchymal subpopulations within normal basal breast cell lines exhibit distinct stem cell/progenitor properties. Stem Cells. 2012;30(2):292‐303. [DOI] [PubMed] [Google Scholar]
- 31. Chang YW, Su YJ, Hsiao M, et al. Diverse targets of beta‐catenin during the epithelial‐mesenchymal transition define Cancer stem cells and predict disease relapse. Cancer Res. 2015;75(16):3398‐3410. [DOI] [PubMed] [Google Scholar]
- 32. Sato T, Okumura F, Ariga T, et al. TRIM6 interacts with Myc and maintains the pluripotency of mouse embryonic stem cells. J Cell Sci. 2012;125(6):1544‐1555. [DOI] [PubMed] [Google Scholar]
- 33. Okumura F, Matsunaga Y, Katayama Y, et al. TRIM8 modulates STAT3 activity through negative regulation of PIAS3. J Cell Sci. 2010;123(13):2238‐2245. [DOI] [PubMed] [Google Scholar]
- 34. Zhang C, Mukherjee S, Tucker‐Burden C, et al. TRIM8 regulates stemness in glioblastoma through PIAS3‐STAT3. Mol Oncol. 2017;11:280‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Okumura F, Okumura AJ, Matsumoto M, et al. TRIM8 regulates Nanog via Hsp90 β ‐mediated nuclear translocation of STAT3 in embryonic stem cells. Biochim Biophys Acta. 2011;1813(10):1784‐1792. [DOI] [PubMed] [Google Scholar]
- 36. Di K, Linskey ME, Bota DA. TRIM11 is over‐expressed in high‐grade gliomas and promotes proliferation, invasion, migration and glial tumor growth. Oncogene. 2013;32(42):5038‐5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang X, Guo H, Yao B, et al. miR‐15b inhibits cancer‐initiating cell phenotypes and chemoresistance of cisplatin by targeting TRIM14 in oral tongue squamous cell cancer. Oncol Rep. 2017;37(5):2720‐2726. [DOI] [PubMed] [Google Scholar]
- 38. Xu G, Guo Y, Xu D, et al. TRIM14 regulates cell proliferation and invasion in osteosarcoma via promotion of the AKT signaling pathway. Sci Rep. 2017;7:42411‐42420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang F, Ruan L, Yang J, et al. TRIM14 promotes the migration and invasion of gastric cancer by regulating epithelialtomesenchymal transition via activation of AKT signaling regulated by miR1955p. Oncol Rep. 2018;40(6):3273‐3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yao J, Xu T, Tian T, et al. Tripartite motif 16 suppresses breast cancer stem cell properties through regulation of Gli‐1 degradation via the ubiquitin‐proteasome pathway. Oncol Rep. 2016;35(2):1204‐1212. [DOI] [PubMed] [Google Scholar]
- 41. Chuang Y‐S, Huang W‐H, Park SW, et al. Promyelocytic leukemia protein in retinoic acid‐induced chromatin remodeling of Oct4 gene promoter. Stem Cells. 2011;29(4):660‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Du L, Li Y, Fakih M, et al. Role of SUMO activating enzyme in cancer stem cell maintenance and self‐renewal. Nat Commun. 2016;7:12326‐12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Allton K, Jain AK, Herz H‐M, et al. Trim24 targets endogenous p53 for degradation. Proc Natl Acad Sci USA. 2009;106(28):11612‐11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jain AK, Allton K, Iacovino M, et al. p53 regulates cell cycle and microRNAs to promote differentiation of human embryonic stem cells. PLoS Biol. 2012;10(2):1001268‐1001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rafiee M‐R, Girardot C, Sigismondo G, et al. Expanding the circuitry of pluripotency by selective isolation of chromatin‐associated proteins. Mol Cell. 2016;64(3):624‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kwon SC, Yi H, Eichelbaum K, et al. The RNA‐binding protein repertoire of embryonic stem cells. Nat Struct Mol Biol. 2013;20(9):1122‐1130. [DOI] [PubMed] [Google Scholar]
- 47. Walsh LA, Alvarez MJ, Sabio EY, et al. An integrated systems biology approach identifies TRIM25 as a key determinant of breast cancer metastasis. Cell Rep. 2017;20(7):1623‐1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang Y, Feng Y, Ji D, et al. TRIM27 functions as an oncogene by activating epithelial‐mesenchymal transition and p‐AKT in colorectal cancer. Int J Oncol. 2018;53(2):620‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hu G, Kim J, Xu Q, et al. A genome‐wide RNAi screen identifies a new transcriptional module required for self‐renewal. Genes Dev. 2009;23(7):837‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Samudyata APP, Engström PG, et al. Interaction of Sox2 with RNA binding proteins in mouse embryonic stem cells. Exp Cell Res. 2019;381(1):129‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Seki Y, Kurisaki A, Watanabe‐Susaki K, et al. TIF1beta regulates the pluripotency of embryonic stem cells in a phosphorylation‐dependent manner. Proc Natl Acad Sci USA. 2010;107(24):10926‐10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Czerwinska P, Shah P, Tomczak K, et al. TRIM28 multi‐domain protein regulates cancer stem cell population in breast tumor development. Oncotarget. 2017;8(1):863‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Venkov CD, Link AJ, Jennings JL, et al. A proximal activator of transcription in epithelial‐mesenchymal transition. J Clin Invest. 2007;117(2):482‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schwamborn JC, Berezikov E, Knoblich JA. The TRIM‐NHL protein TRIM32 activates microRNAs and prevents self‐renewal in mouse neural progenitors. Cell. 2009;136(5):913‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nicklas S, Otto A, Wu X, et al. TRIM32 regulates skeletal muscle stem cell differentiation and is necessary for normal adult muscle regeneration. PLoS One. 2012;7(1):30445‐30458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chang HM, Martinez NJ, Thornton JE, et al. Trim71 cooperates with microRNAs to repress Cdkn1a expression and promote embryonic stem cell proliferation. Nat Commun. 2012;3:923‐933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bell JL, Malyukova A, Holien JK, et al. TRIM16 acts as an E3 ubiquitin ligase and can heterodimerize with other TRIM family members. PLoS One. 2012;7(5):37470‐37479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Herquel B, Ouararhni K, Khetchoumian K, et al. Transcription cofactors TRIM24, TRIM28, and TRIM33 associate to form regulatory complexes that suppress murine hepatocellular carcinoma. Proc Natl Acad Sci USA. 2011;108(20):8212‐8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Massagué J, Xi Q. TGF‐ b control of stem cell differentiation genes. FEBS Lett. 2012;586:1953‐1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nicklas S, Hillje AL, Okawa S, et al. A complex of the ubiquitin ligase TRIM32 and the deubiquitinase USP7 balances the level of c‐Myc ubiquitination and thereby determines neural stem cell fate specification. Cell Death Differ. 2019;26(4):728‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Worringer KA, Rand TA, Hayashi Y, et al. The let‐7/LIN‐41 pathway regulates reprogramming to human induced pluripotent stem cells by controlling expression of prodifferentiation genes. Cell Stem Cell. 2014;14(1):40‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Low J, Blosser W, Dowless M, et al. Knockdown of ubiquitin ligases in glioblastoma cancer stem cells leads to cell death and differentiation. J Biomol Screen. 2012;17(2):152‐162. [DOI] [PubMed] [Google Scholar]
- 63. Zhan W, Han T, Zhang C, et al. TRIM59 promotes the proliferation and migration of non‐small cell lung cancer cells by upregulating cell cycle related proteins. PLoS One. 2015;10(11):e0142596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liang J, Xing D, Li Z, et al. TRIM59 is upregulated and promotes cell proliferation and migration in human osteosarcoma. Mol Med Rep. 2016;13(6):5200‐5206. [DOI] [PubMed] [Google Scholar]
- 65. Gao RAN, Lv G, Zhang C, et al. TRIM59 induces epithelial‐to‐mesenchymal transition and promotes migration and invasion by PI3K/AKT signaling pathway in medulloblastoma. Oncol Lett. 2018;15(6):8253‐8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hu C‐E, Gan J. TRIM37 promotes epithelialmesenchymal transition in colorectal cancer. Mol Med Rep. 2017;15(3):1057‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Huang XQ, Zhang XF, Xia JH, et al. Tripartite motif‐containing 3 (TRIM3) inhibits tumor growth and metastasis of liver cancer. Chin J Cancer. 2017;36(1):77‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Eskiw CH, Dellaire G, Bazett‐Jones DP. Chromatin contributes to structural integrity of promyelocytic leukemia bodies through a SUMO‐1‐independent mechanism. J Biol Chem. 2004;279(10):9577‐9585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


