ABSTRACT
Introduction: In post‐dilution online hemodiafiltration, a very thin balance subsists in preventing coagulation of the extracorporeal circuit (ECC) during treatment and bleeding in the patient, concerning dialyzer status and anticoagulation dose.
The aim of this study was to assess whether there are clinical outcome differences between the visual aspect of the dialyzer's status in terms of clotted fibers at end of dialysis treatments, single‐pool urea kinetic modeling (spKt/V) and substitution volume (SubsVol).
Methods: It is a multicenter, descriptive‐correlational study, involving 2829 patients during April 2016. Previous training was given to the Nursing staff to evaluate and classify both the dialyzer's and the venous chamber's appearance of the ECC venous line. Registration was performed at bedside immediately after the patient disconnection.
Findings and discussion: Mean age was 68.96 years (SD = 13.75), 60.8% were men. The average hematocrit was 33.91% (SD = 3.45%). The average dry weight was 68.53 kg (SD = 13.27 kg). Mean unfractioned heparin (UFH) dose was 58.13 IU/kg. Only 32.4% of the patients had a clean dialyzer at the end of treatment. 19.4% of patients finished the treatment with more than 10% of clotted fibers. Patients with no residual blood (clean, 32.4%) presented a higher UFH dose (66.32 IU/kg) compared to overall average dose. UFH dose had a significant effect on dialyzer status. There were significant differences in average of spKt/V and SubsVol between the category clean and the other categories of dialyzer's status.
Evaluating the dialyzer status represents an excellent opportunity to help the physicians to establish an ideal heparin dose. Only the category clean is significant to achieve the target. The nursing staff, by classifying the ECC appearance at patient's bedside and recording it in a centralized database, can be a major contributor to achieve an individualized and optimal UFH dose and subsequently better patient outcomes.
Keywords: anticoagulation, hemodiafiltration, heparin, extracorporeal circuits, dialysis adequacy
INTRODUCTION
On a routine basis, a hemodialysis (HD) treatment is only possible by counteracting the propensity for blood to coagulate when in contact with a foreign surface. In fact, for a proper anticoagulation (AC) in HD patients, a very delicate equilibrium is necessary and ideally should be targeted to prevent coagulation in the extracorporeal blood circuit (ECC) during treatment and at the same time to prevent bleeding in the patient.
In many patients this balance remains a major challenge as the patient's blood is exposed to procoagulant substances such as ECC synthetic components and dialyzer membranes that have the tendency to activate coagulation cascade.1, 2 There are also mechanical factors such as shear stress, particularly in the venous and arterial lines, due to blood‐air interface and the possibility of low blood flow that will favor clotting. However, it is important to recognize that many uremic patients are prone to bleeding diathesis.3 This pathogenesis is considered multifactorial and is related to anemia, uremic toxins, and other comorbidities.4 Additionally, patients may also be taking medication that interferes with hemostasis. If no anticoagulation agent is used during treatment, approximately 7% to 20% of the dialyzers can coagulate,5, 6 resulting in a reduction of the effective dialysis dose delivered or even loss of 100 to 150 mL of blood,7 if no rinse back is possible. There are other anticoagulant alternative agents, although the British Renal Association recommends unfractionated heparin (UFH) as the standard anticoagulant and low‐molecular‐weight heparin (LMWH) as an alternative agent in patients with lower bleeding risks.8 The regional citrate infusion is considered a complex technique1, 8 that is unsuitable for routine use or should be limited to intensive care units. Currently, UFH is the most common anticoagulant being used in patients receiving dialysis because it is easy to administer, has a short half‐life and is low cost.9, 10 According to the European Best Practice Guidelines (EBPG),11 the initial loading dose of UFH should be 50 IU/kg followed by a maintenance dose of 800–1500 IU/h, given via constant infusion into arterial line, in patients without hemorrhagic complications. Nonetheless, there is no clinical consensus about the ideal UFH dose for low bleeding risk in patients on HD, and the use of clinical and biological monitoring of anticoagulation during HD sessions is currently not clearly defined in routine clinical practice.12, 13 So, because of the individual patient profiles regarding optimization of anticoagulation therapy in HD patients, there is a need for a standardized approach regarding anticoagulation, with an individualized prescription considering the patient's profile. Maintaining full patency in the ECC during HD sessions is essential for providing safe and effective dialysis1 minimizing morbidity and mortality in the population. Often, ECC clotting leads to blood loss, increased nursing workload, disposables’ consumption and thus increased treatment costs.
Nowadays, several studies are considering that online hemodiafiltration (OL‐HDF) is the most advanced treatment modality14, 15, 16 and that the true benefits derive from a proper convection volume. This therapy combines diffusive and convective volume of at least 20% of the total blood volume processed during the treatment session.17 In OL‐HDF post‐dilution mode, effective convection volume is the combination of the substitution volume (SubsVol) and the volume of fluid removed for weight loss.
However, in OL‐HDF, a very thin balance occurs when maximizing substitution rate and filtration factor vs. dialyzer status, especially concerning anticoagulation dose.18 Post‐dilution OL‐HDF is associated with a higher convective mass transfer compared to HD caused by a greater ultrafiltration volume,18 which can result in the deposition of plasma proteins on the membranes’ surface, clogging the membrane pores and occluding the blood fibers of the dialyzer.17 At the same time there is an increase in transmembrane pressure (TMP), causing alarms, reducing clearance, and possibly resulting in clotting of the ECC. Thus, it seems to us that poor heparinization and the consequent decrease in the dialyzer's area by clotting fibers can be a determining factor for the convection volume, as well as treatment time and blood flow rate.19
The aim is to assess the association between the classification of the dialyzer's status and the prescribed anticoagulation's dose, dry weight, SubsVol, spKt/V and assess whether there are clinical outcome differences between the different categories of the dialyzer's aspect.
MATERIALS AND METHODS
A multicenter, retrospective, descriptive‐correlational study was conducted, involving all active patients who underwent HDF with on‐line‐prepared substitution fluid for 1 month, in 37 Clinics of Fresenius Medical Care (FMC), NephroCare Portugal, S.A.
Patients were dialyzed thrice‐weekly, with a 4‐hour schedule, using a Fresenius 5008 CorDiax HDF machine (FMC – Bad Homburg, Germany) with high‐flux polysulfone dialyzers with 1.6 m2 (FX CorDiax 600 – FMC). The dialysis machines were all equipped with a dialysis fluid ultrafiltration system (Diasafe®, FMC). The convection volume was driven automatically using the “AutoSubplus” function of the 5008 machines,20 which prevents excessive hemoconcentration within the dialyzer by continuously adapting the substitution flow rate according to changes in blood viscosity within the dialyzer. The dialysate flow in all treatments was automatically set by the “AutoFlow” function, preset to 1.0. Dialysis dose (spKt/V) was calculated in every treatment based on ionic dialyzance obtained by the integrated module Online Clearance Monitor (OCM®, FMC) from the 5008 CorDiax machine, and “V” was derived from total body composition assessment with a bioimpedance device (Body Composition Monitor—FMC).21 The exact blood flow of each dialysis was maintained within safety shunt pressures, according to dialysis prescription, routine physical examination performed by the dialysis nurse, ease of cannulation and progress of the dialysis session per se, taking into account individual patient's needs and dynamic shunt characteristics. Both arterial and venous pressures were kept above −220 mmHg and under 250 mmHg, respectively.22, 23, 24
All treatments were performed using a personal patient card that recalls all necessary treatment parameters from EuCliD® (European Clinical Data Base—FMC) to the hemodialysis machines, including medication. At the end of each treatment, data are sent back to EuCliD®, attesting liability of all outputs.25, 26
The study consisted of two phases. The first was a comprehensive training of all the clinics’ head nurses for them to subsequently replicate their training to the nursing teams, which began in October 2015 till December of the same year. Concomitantly a dedicated e‐learning training course was created for nurses in which one of the topics was the necessity for the nurses to classify and register at the end of each treatment, both the dialyzer and venous drip chamber aspects. Also, in order to minimize observation's variability, a schematic with pictures was made available to all units displaying the categorization for both the dialyzer and venous drip chamber. The dialyzer was classified by visual inspection into five categories (clean = 1, light pink = 2, some coagulated fibers = 3, many coagulated fibers = 4, and totally coagulated = 5) (Figure 1) and the venous drip chamber into three (clean, presence of clots, and totally coagulated) (Figure 2). Immediately after the rinse back procedure, the nurses classified both the dialyzer and the venous drip chamber's aspect category at the patient's bedside.
Figure 1.

Categorization of the dialyzer status. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 2.
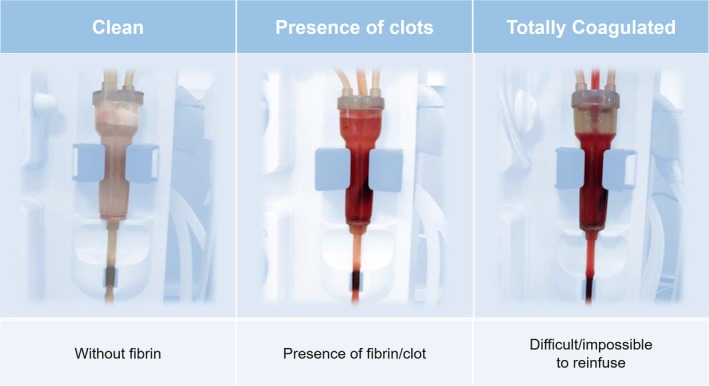
Categorization of the venous drip chamber status. [Color figure can be viewed at http://wileyonlinelibrary.com]
A dedicated report with several treatment parameters was created for all the dialysis units, including the aspect for the dialyzer and venous drip chamber. Apart from individual medical judgment and decisions, there are standards on good dialysis care in our network (“NephroCare Standard Good Dialyses Care”) which are periodically audited, thus maintaining a minimal standard of care. As part for routine lab monitoring, blood samples were collected before start of treatment through the cannulated shunt venous needle to determine Hematocrit (Hct) concentration of all patients during follow up.
UFH regime was by an initial loading bolus and maintenance infusion dose according to the dialysis prescription by the dialysis machine and stopped 30–60 minutes before the end of the treatment. Each nephrologist prescribed the UHF dose for each patient according to their clinical criteria and personal judgment considering treatment time, patient weight, and comorbidities, but as initial standard prescription, prescribed loading dose was between 40 and 50 IU/Kg and as for maintenance dose was 10 IU/kg/h.
Data collection
After the first phase, 4520 patients (Figure 3) from EuCliD® database fulfilling sufficient inclusion and exclusion criteria were initially enrolled, obtaining approximately 58,760 observations per each of the clinical variables. Nine hundred and eighty one patients were excluded due to lack of data in at least 3 variables under study. At the second phase the remaining 3539 patients were subject to the following inclusion criteria: Patients had to be on post‐dilution On‐Line HDF with arteriovenous fistula (AVF) or arteriovenous graft (AVG) as vascular access and effective treatment time between 230 and 250 minutes. Patients also had to have UFH as the anticoagulant agent, being administered in the standard regime of initial bolus followed by subsequent maintenance dose in continuous infusion. Patients with central venous catheter as vascular access, without prescribed anticoagulant or with low molecular weight heparin and patients with a prescription other than 4 hours or who did not comply with the prescription were excluded.
Figure 3.
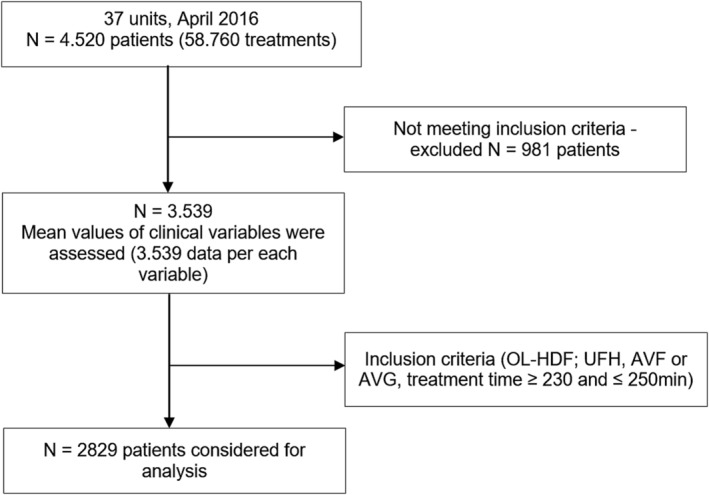
Study flow diagram.
Patients were characterized by gender, age, body weight, time in HD; type of vascular access (AV); Charlson's comorbidities index and hematocrit (Hct) (standard monthly single measurement profile). For each patient 8 to 13 consecutive treatments were considered for 1 month follow up to minimize observations variability. For the characterization of the treatments the following variables were selected: duration of treatment; total ultrafiltration (UF) removed; dose of UFH and categorization of the venous drip chamber and dialyzer status. From these variables we took the average scores. The venous chamber and dialyzer appearance were classified according the stage ranges of the average scores. For this last variable, only 4 categories were considered because there were 4 observations in the fifth category “Totally coagulated” (impossible to reinfuse), hence the communion between the fourth and fifth categories.
Data analysis strategy
First, a descriptive analysis was conducted. The continuous variables were reported as proportions. For continuous variables mean, SD, and range were assessed. Monthly average values for each variable per patient were calculated. The mean UFH dose was calculated for the different groups of dialyzer status and dry weight.
The analysis focused on the evaluation of correlation between dialyzer status, venous drip chamber, and UFH dose (IU/Kg) and between dry weight and UFH dose (IU/Kg) using Spearman's rho coefficient. One‐way analysis of variance (ANOVA) between dialyzer status and dry weight, OCM spKt/V, and SubsVol was also performed. Linear regression was assessed to estimate the magnitude of SubsVol and spKt/V variation explained by UFH dose variation. The relationship between Hct and dialyzer status was also analyzed.
Results were considered significant when P < 0.05 and Bonferroni adjustment27 was used for multiple comparisons (α ′ = α/m). All the statistical analysis was performed using the support of SPSS (version 23; IBM, Armonk, NY).
Compliance with ethical standards
This study was approved by the institution's ethical committee in accordance with legal national and international requirements. Because of its retrospective nature, noninterventional, registry‐based with large sample data anonymously retrieved from a central database (EuCliD® ‐ FMC), the anonymity of the studied patients was always maintained. Moreover, individual patient consent was waived to all patients, according to FMC Portugal internal policy, consenting confidentiality data analysis from this central database, licensed by the Portuguese National Data Protection Commission. All anonymized data used for this study are part of routine treatment clinical care data and no patient identifiable data were used.
RESULTS
Patients’ characteristics and treatment data
After applying the exclusion criteria, the final sample included 2829 patients, mostly male (N = 1720, 60.8%). The characteristics of the cohort are shown in Table 1. Mean age was 68.96 years old (SD = 13.75) and ranged from 25 to 98 years old. Three age groups were created (≤ 65, 66–80, and ≥ 81 years old) according to Portuguese Society of Nephrology (SPN) groups.28 The most frequent was the group whose age ranged from 66 to 80 years old (N = 1253, 44.3%) and 21.9% of patients were over 81 years old. Mean treatment time was 241.54 minutes (SD = 1.67; ranged from 230.23 to 249.64 minutes). Most of the patients had an AVF (N = 2327, 82.3%) and the remaining patients had AVG. Mean dry weight was 68.53 Kg (SD = 13.27; range = 34.4–118.5). It should be noted that approximately 10% of the patients weighed more than 86 kg.
Table 1.
Baseline characteristics of the 2829 eligible patients
| Mean (± SD) | % | |
|---|---|---|
| Demographic data | ||
| Gender malea | 1720 | 60.8% |
| Age (years) | 68.96 (13.75) | |
| Clinical characteristics | ||
| Vascular access | ||
| Fistulaa | 2327 | 82.3% |
| Grafta | 502 | 17.7% |
| Dry weight (kg) | 68.53 (13.27) | |
| UF removed (L) | 2.56 (0.65) | |
| Overhydration status (L) | 1.53 (1.35) | |
| Charlson comorbidity index | 3.75 (1.70) | |
| Dialysis vintage (mo) | 73.21 (69.75) | |
| Extracorporeal blood flow (mL/min) | 427.13 (35.60) | |
| Dialysis treatment time (min) | 241.54 (1.67) | |
| Average blood volume treated (L) | 100.64 (11.72) | |
| Anticoagulation (IU/kg) | 58.13 (19.34) | |
| Substitution volume (L) | 24.13 (2.60) | |
| spKt/V | 2.04 (0.38) | |
| Laboratory parameters | ||
| Hematocrit (%) | 33.91 (3.45) | |
N was reported.
Four dry weight groups were created (≤ 58, 59–68, 69–78, and ≥ 79 kg). Average UFH dose in the full cohort was 58.13 IU/kg (SD = 19.34) (the equivalence of the product between the average dry weight and UFH = ± 3984 IU). The initial loading dose was 1767 IU (SD = 672) (25.78 IU/kg) administered immediately after treatment start, followed by a continuous infusion of an average 2295 IU (SD = 951) (average 571 IU/h) during the dialysis treatment. In these patients with multiple measurements the coefficient of variation of dialyzer and venous chamber scores was 34.2% and 21.5%, respectively.
Relation between dialyzer status and unfractioned heparin dose
The highest proportion of the assessment of the dialyzer status was 48.1% (N = 1362) (Table 2) in the “light pink” category and less frequent with only 1.7% in “many coagulated fibers.”
Table 2.
Cross analysis between dialyzer status and unfractioned heparin dose, spKt/V, SubsVol, Hct, body dry weight
| Dialyzer status | N | % | UFH (IU/Kg) | spKt/V | SubsVol | Hct | Body dry weight | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (±SD) | Mean (±SD) | Mean (±SD) | Mean (±SD) | Mean (±SD) | ||||||||
| Clean | 918 | 32.5 | 66.32 ± 20.81 | 2.11 ± .38 | 24.47 ± 2.56 | 33.84 ± 3.39 | 66.31 ± 12.75 | |||||
| Light pink | 1362 | 48.1 | 56.00 ± 17.47 | 2.04 ± .39 | 24.04 ± 2.58 | 33.95 ± 3.44 | 68.99 ± 13.43 | |||||
| Some coagulated fibers | 501 | 17.7 | 50.18 ± 15.77 | 1.98 ± .37 | 23.81 ± 2.70 | 33.93 ± 3.53 | 70.90 ± 13.27 | |||||
| Many coagulated fibers | 48 | 1.7 | 44.74 ± 17.12 | 1.88 ± .31 | 23.41 ± 2.68 | 33.23 ± 4.17 | 73.16 ± 12.35 | |||||
| Total | 2829 | 100 | ||||||||||
Only 32.5% of the patients had a clean dialyzer at the end of treatment, which also presented the higher UFH dose (66.32 IU/kg). These data also reveal that 19.4% of the patients finished the treatment with some “coagulated fibers.” The correlation between dialyzer status and UFH dose is significant negative (r S = −.330, P < 0.001), with a medium effect size.29 Thus, when the mean dose of UFH decreases, dialyzer status increases. Identical results were obtained with the correlation between the venous chamber and UFH (r S = −.257, P < 0.001).
A linear regression analysis was used to regress dialyzer status (in its quantitative version) on UFH dose. The results showed that 10.9% (R 2 = .109) of dialyzer status variation was significantly explained by UFH dose (F (1, 2827) = 345.573, P < 0.001). The effect is negative, thus as the UFH dose increases, the dialyzer status decreases (B = −.011, t = −18.590, 95% CI = −.12, −.10).
Relation between dry weight and unfractioned heparin dose
The correlation between patient dry weight and UFH dose was negative and significant (r S = −.237, P < 0.001). Thus, when the dry weight increases the dose of UFH decreases. A deeper analysis showed that patients with dry weight exceeding 69 kg (48.2%) presented an average UFH dose 53.92 IU/kg or 4280 IU, when compared to overall average dose (Table 3). Additionally, as dry weight increases, it also increases the possibility of having more clotted dialyzer fibers (r S = .146, P < 0.001).
Table 3.
Cross analysis between dry weight and unfractioned heparin dose
| Dry weight (kg) by groups | N | % | UFH (IU/kg) | Initial loading dose (IU/kg) | Maintenance dose (IU/kg) |
|---|---|---|---|---|---|
| Mean (±SD) | Mean (±SD) | Mean (±SD) | |||
| ≤ 58 | 623 | 22.0 | 66.05 ± 21.87 | 29.95 ± 10.79 | 37.01 ± 15.28 |
| 59–68 | 843 | 29.8 | 59.08 ± 18.71 | 26.78 ± 9.62 | 32.77 ± 13.34 |
| 69–78 | 747 | 26.4 | 54.91 ± 17.41 | 23.94 ± 8.54 | 31.01 ± 12.61 |
| ≥ 79 | 616 | 21.8 | 52.72 ± 16.85 | 22.96 ± 8.37 | 29.85 ± 14.40 |
| Total | 2829 | 100 |
Relation between dry weight and dialyzer status
As can be seen in Table 2 as the dry weight of the individuals increases the dialyzer status also increases (more clotted fibers in the dialyzer). From one‐way ANOVA using dialyzer status in its original scoring, the conclusion was that dry weight had a significant effect on dialyzer status (F (3, 2825) = 19.650, P < 0.001). Post hoc multiple comparisons showed that patients with lower dry weight (≤ 58 kg) exhibited significant differences from patients with higher dry weight (≥ 69 kg, P < 0.001). Patients with 59–68 kg also had a significant difference from patients with the highest dry weight (≥ 79 kg, P < 0.001).
We also assessed the influence of dry weight as a predictor of dialysis efficacy (spKt/V). The results showed that the dry weight explained 36.9% (R 2 = .369) of dialysis dose variation. The correlation between both as variables is significant and negative (r = −.608, P < 0.001). As expected as the dry weight increases, inversely the dialysis efficacy decreases.
Relation between dialyzer status and dialytic efficacy
The next analysis was performed to compare the 4 categories of the dialyzer status in relation to the dialytic efficacy, through the obtained result of spKt/V and SubsVol.
Table 2 shows that patients with clean dialyzer have on average better results (M = 2.11 spKt/V) than patients with “light pink” (M = 2.04 spKt/V) and the other 2 categories. The difference between the maximum and the minimum value obtained shows a reduction of 11% in the effectiveness of the dialysis dose. The mean dialysis dose was 2.04 (SD = 0.39) of spKt/V (range = 0.96–3.70). Linear regression was used and the analysis showed that dialyzer status (in its quantitative version) significantly influences the value of spKt/V and explained 2% (R 2 = .020), P < 0.001 of dialysis dose variation.
From ANOVA test, the conclusion was that the dialyzer status significantly influences the value of spKt/V (F (3, 2825) = 18.100, P = 0.019). Pairwise comparisons of means of dialyzer status according to spKt/V categories were performed by Post hoc tests. The results from Scheffe test (Table 4) showed that there were significant mean differences between the first category (clean) and the other three categories (P < 0.001), as well as between the second (light pink) and the other categories (P = 0.005). Between the remaining categories there were no significant differences from each other.
Table 4.
One‐way ANOVA test and pairwise comparisons of means of dialyzer status according to spKt/V
| Light pink | Some coagulated fibers | Many coagulated fibers | |
|---|---|---|---|
| Clean | 0.078** | 0.134** | 0.234** |
| Light pink | 0.056* | 0.156* |
Note: The results displayed correspond to the difference between the mean of dialyzer status in pairwise comparisons. Only the significant mean differences were reported.
*P < 0.05, **P < 0.001.
The relationship between dialyzer status and SubsVol was analyzed and the results showed that patients with a clean dialyzer had on average, better results (24.47 L) than patients in the other categories (Table 2). Dialyzer status also had a significant effect on SubsVol (F (3, 2825) = 9.605, P = 0.010). The results from Scheffe test showed that there were only significant mean differences in SubsVol between clean and light pink (P = 0.002), and some coagulated fibers (P < 0.001).
A weak and negative correlation was observed between the venous drip chamber status and the results obtained from spKt/V. This means that as the venous camera coagulates, it decreases the dialytic efficiency (r S = −.126, P < 0.001). However, the correlation between the venous chamber status and SubsVol was positive with a medium effect size r S = .301 P < 0.001, probably due to increased venous pressure. It was also observed a strong and positive correlation between the dialyzer status and venous chamber (r S = .778, P < 0.001).
Relation between Hct and dialyzer status
No relationship was observed between Hct, anticoagulation, extracorporeal blood flow, UF removed, overhydration status, and dialyzer status. Instead, only a negative and no significant relationship was observed between Hct and the following dependent variables: spKt/V (r = −155) and SV (r = −131). The objective was to test the mediating effect of the dialyzer on the relationship between Hct with spKt/V and SubsVol. As Hct had no significant effect on the mediator dialyzer it is no longer possible to find the mediation effect.
Multiple linear regression model was evaluated via the predictors of Hct and dialyzer status. The model was statistically significant (F (2, 2826) = 64.274, P < 0.001) and explains 4.3% of the spKt/V variation (R 2 = 0.043). For each unit of Hct, the dialysis dose decreases in B = − 0.153. A new analysis was performed to study the effect of the same predictors on SubsVol. The model was statistically significant (F (2, 2826) = 36.804, P < 0.001) and explains 2.5% of SubsVol variation (R 2 = 0.025). For each unit of Hct, the SubsVol decreases in B = − 0.129.
DISCUSSION
During a hemodialysis treatment, the patient's blood is exposed to many substances and surfaces including the dialyzer membrane and blood tubing, and the venous pressure chambers that contribute to the risk of thrombogenesis. In convective dialysis therapies, especially on post‐dilution OL‐HDF, there is high likelihood of the circuit to clot due to hemoconcentration caused by excessive ultrafiltration rate. Concomitantly, clinicians tend to expose the patient as little as possible to high doses of UFH and induced collateral complications. Effectively, these patients are exposed to some complications including thrombocytopenia,11, 30 platelet dysfunction,31 and an increased risk of hemorrhage. However, complications associated with prolonged administration to UFH were not considered for this study.
The results show that only 32% of the patients presented a “clean” dialyzer and indicate that an UFH dose of greater than 66 IU/kg (approximately 4500 IU per treatment) is required. This dosage is however much lower compared to the value presented in the investigation of B. Canaud et al.,14 in which UFH was used for anticoagulation as an intravenous bolus dose of 50 ± 15 IU/kg followed by a continuous infusion of 1000 IU/h. Comparing with our results it represents approximately twice the dosage in both the initial bolus and maintenance dose prescriptions.
Heparin dosage was also not adjusted as the average patient weight increased and as we can see in Table 2 the group of heavier patients on averaged had less UFH (IU/kg). From the results, dry weight had a very large impact on the variation of dialytic efficacy, but we cannot ignore the negative effect of the dialyzer status that explains 2% of the spKt/V′ variation.
We consider that body weight is a factor that should be considered for adjusting the dose of UFH and ought to be associated with coagulation of the dialyzer/bloodlines, bleeding after disconnection and the poor results obtained with the dialyzer status, consequence of the anticoagulation regime. Time for a proper hemostasis greater than 15 minutes can also be a signal of a high dose of UFH.1, 31 The results of another study32 demonstrated that body weight was the most frequently used indicator for adjusting the UFH dosage. Suboptimal UFH doses (most especially in overweighed patients) can lead to thrombus formation and cause occlusion and clotting of the ECC, or even to an early treatment disconnection, leading to less than ideal outcomes that several papers have demonstrated that can have implications on patient survival.33, 34 The variation of the spKt/V in relation to dialyzer status, although significance is very low probably because small molecules clearance is higher on the first half of the treatment. The efficacy of heparinization may also be measured by the activated partial thromboplastin time or the whole‐blood clotting time.11, 35
No relationship was observed between Hct and dialyzer status. Our results are not in accordance with the literature36; however, we believe this reflects the good performance of the 5008 CorDiax dialysis machine with “AutoSubplus,” which maximizes substitution volumes for high convective removal of middle molecules and on the other hand preventing excessive hemoconcentration within the dialyzer by continuously adapting the substitution flow rate according to changes in blood viscosity.
In conclusion it was noticed that the changes occurred in the dialyzer status represent an excellent indicator of deficient heparinization with significant implications in dialysis outcomes. It is important to evaluate the individual needs of each patient13 and to take into consideration the dose of UFH in relation to the dry weight32 and also to the dialyzer aspect or the presence of fibrin on the walls of the venous drip chamber35, 37, 38, 39 that may indicate clotting. It is the responsibility of the physician to prescribe, but it is also the responsibility of the nurses to administer and constantly evaluate these indicators. The study results also show that spKt/V and SubsVol decreased significantly when comparing “clean” status and the remaining dialyzer classifications. Only the “clean” group is the most significant to achieve the target. On the other hand, about 11% of the variation in the dialyzer's appearance is explained by anticoagulation and this limitation has significant consequences on both the outcomes of spKt/V and the SubsVol. A correct heparinization and maintenance of ECC permeability is indeed a prerequisite for optimal HD quality2 and a higher survival rate of these patients and the convective effects of convective dialysis may reduce cardiovascular mortality.40 Also, the nursing staff, by classifying the ECC appearance at patient's bedside on a centralized database, can be a major contributor to achieve an individualized and optimal UFH dose and subsequent better patient outcomes.
Limitations of the study: As this study was intended to assess on real‐world heparin dose vs. dialyzer status at end of dialysis, clotting tests, and other lab results like platelet count, total proteins, hemoglobin and albumin are available, but were not considered for this study. Also, patient comorbidities or other medications that may have interfered with clotting factors were not considered in this study and thus may have interfered with some results. However, we consider that the large sample size and concise data collection were important strengths.
AUTHOR CONTRIBUTIONS
J. F. M., R. P., and B. P. carried out the design. R. P., H. C., and J. F. M. analyzed data. R. P. and B. P. wrote the manuscript. J. F. M. and C. F. revision the manuscript. J. F. M. and P. P. were responsible for final version of the manuscript.
Conflict of Interest: J. F. M., B. P., C. F., P. P., and R. P. are employees of Fresenius Medical Care and may hold shares in the company.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
REFERENCES
- 1. Suranyi M, Chow JSF. Anticoagulation for haemodialysis. Nephrol Ther. 2010;15:4 386–4 392. [DOI] [PubMed] [Google Scholar]
- 2. Fischer K‐G. Essentials of anticoagulation in hemodialysis. Hemodial Int. 2007;11:178–189. [DOI] [PubMed] [Google Scholar]
- 3. Özkan G, Ulusoy Ş. Bleeding diathesis in hemodialysis patients. INTECH Open Access. 2013;4:59–80. [Google Scholar]
- 4. Jalal DI, Chonchol M, Targher G. Disorders of hemostasis associated with chronic kidney disease. Semin Thromb Hemost. 2010;31:034–040. [DOI] [PubMed] [Google Scholar]
- 5. Schwab SJ, Onorato JJ, Sharar LR, Dennis PA. Hemodialysis without anticoagulation. One‐year prospective trial in hospitalized patients at risk for bleeding. Am J Med. 1987;83:405–410. [DOI] [PubMed] [Google Scholar]
- 6. Caruana RJ, Raja RM, Bush JV, Kramer MS, Goldstein SJ. Heparin free dialysis: Comparative data and results in high risk patients. Kidney Int. 1987;31:1351–1355. 10.1038/ki.1987.149. [DOI] [PubMed] [Google Scholar]
- 7. Roy A, Kalra V. Anticoagulation in haemodialysis. JIMSA. 2012;25:107–109. [Google Scholar]
- 8. Mactier R, Hoenich N, Breen C. Renal association clinical practice guideline on haemodialysis. Nephron Clin Pract. 2011;118:c241–c286. [DOI] [PubMed] [Google Scholar]
- 9. Golper TA, Fissell R, Fissell WH, Hartle PM, Sanders ML, Schulman G. Hemodialysis: Core curriculum 2014. Am J Kidney Dis. 2014;63:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cronin RE, Reilly RF. Unfractionated heparin for hemodialysis: Still the best option. Semin Dial. 2010;23:510–515. 10.1111/j.1525-139X.2010.00770.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. European Best Practice Guidelines Expert Group . Section V: Chronic intermittent haemodialysis and prevention of clotting in the extracorporeal system. Nephrol Dial Transplant. 2002;17:63–71. [DOI] [PubMed] [Google Scholar]
- 12. Shen JI, Montez‐Rath ME, Mitani AA, Erickson KF, Winkelmayer WC. Correlates and variance decomposition analysis of heparin dosing for maintenance hemodialysis in older US patients. Pharmacoepidemiol Drug Saf. 2014;23:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kessler M, Moureau F, Nguyen P. Anticoagulation in chronic hemodialysis: Progress toward an optimal approach. Semin Dial. 2015;28:474–489. [DOI] [PubMed] [Google Scholar]
- 14. Canaud B, Bosc JY, Leray‐Moragues H, et al. On‐line haemodiafiltration. Safety and efficacy in long‐term clinical practice. Nephrol Dial Transplant. 2000;15:60–67. [DOI] [PubMed] [Google Scholar]
- 15. Ok E, Asci G, Toz H, et al. Turkish online Haemodiafiltration study. Mortality and cardiovascular events in online haemodiafiltration (OL‐HDF) compared with high‐flux dialysis: Results from the Turkish OL‐HDF study. Nephrol Dial Transplant. 2013;28:192–202. [DOI] [PubMed] [Google Scholar]
- 16. Den Hoedt CH, Bots ML, Grooteman MP, et al. Online hemodiafiltration reduces systemic inflammation compared to low‐flux hemodialysis. Kidney Int. 2014;86:423–432. [DOI] [PubMed] [Google Scholar]
- 17. Tattersall JE, Ward RA. Online haemodiafiltration: Definition, dose quantification and safety revisited. Nephrol Dial Transplant. 2013;28:542–550. 10.1093/ndt/gfs530. [DOI] [PubMed] [Google Scholar]
- 18. Klingel R, Schaefer M, Schwarting A, et al. Comparative analysis of procoagulatory activity of haemodialysis, haemofiltration and haemodiafiltration with a polysulfone membrane (APS) and with different modes of enoxaparin anticoagulation. Nephrol Dial Transplant. 2004;19:164–170. 10.1093/ndt/gfg459. [DOI] [PubMed] [Google Scholar]
- 19. Chapdelaine I, Mostovaya IM, Blankestijn PJ, et al. Treatment policy rather than patient characteristics determines convection volume in online post‐dilution hemodiafiltration. Blood Purif. 2014;37:229–237. 10.1159/000362108. [DOI] [PubMed] [Google Scholar]
- 20. Maduell F, Rodríguez N, Sahdala L, et al. Impact of the 5008 monitor software update on total convective volume. Nefrología (English Edition). 2014;34:599–604. [DOI] [PubMed] [Google Scholar]
- 21. Wizemann V, Rode C, Wabel P. Whole‐body spectroscopy (BCM) in the assessment of normovolemia in hemodialysis patients. Contributions to Nephrology. 2008;161:115–118. 10.1159/000130423. [DOI] [PubMed] [Google Scholar]
- 22. Chambers SD, Ceccio SL, Annich GA, Bartlett RH. Extreme negative pressure does not cause erythrocyte damage in flowing blood. ASAIO J. 1999;45:431–435. [DOI] [PubMed] [Google Scholar]
- 23. Twardowski ZJ, Haynie JD, Moore HL. Blood flow, negative pressure, and hemolysis during hemodialysis. Hemodial Int. 1999;3:45–50. [DOI] [PubMed] [Google Scholar]
- 24. Parisotto MT, Schoder VU, Miriunis C, et al. Cannulation technique influences arteriovenous fistula and graft survival. Kidney Int. 2014;86:790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marcelli D, Kirchgessner J, Amato C, et al. EuCliD (European clinical database): A database comparing different realities. J Nephrol. 2001;14:S94–S100. [PubMed] [Google Scholar]
- 26. Stopper A, Amato C, Gioberge S, Giordana G, Marcelli D, Gatti E. Managing complexity at dialysis service centers across Europe. Blood Purif. 2007;25:77–89. [DOI] [PubMed] [Google Scholar]
- 27. Chen SY, Feng Z, Yi X. A general introduction to adjustment for multiple comparisons. J Thorac Dis. 2017;9:1725–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Macário, F. Sociedade Portuguesa de Nefrologia. Relatório Gabinete de Nefrologia da SPN Lisboa. 2017. http://www.bbg01.com/cdn/clientes/spnefro/noticias/130/REGISTO_DRCV2016.pdf (March 4, 2018).
- 29. Cohen J. A power primer. Psychol Bull. 1992;112:155–159. [DOI] [PubMed] [Google Scholar]
- 30. Syed S, Reilly RF. Heparin‐induced thrombocytopenia: A renal perspective. Nat Rev Nephrol. 2009;5:501–511. [DOI] [PubMed] [Google Scholar]
- 31. Gawaz MP, Dobos G, Späth M, Schollmeyer P, Gurland HJ, Mujais SK. Impaired function of platelet membrane glycoprotein IIb‐IIIa in end‐stage renal disease. J Am Soc Nephrol. 1994;5:36–46. [DOI] [PubMed] [Google Scholar]
- 32. Herrero‐Calvo JA, Gonzales‐Parra E, Perez‐Garcia R, Tornero‐Molina F. Spanish study of anticoagulation in haemodialysis. Nefrologia. 2012;32:143–152. [DOI] [PubMed] [Google Scholar]
- 33. Maduell F, Moreso F, Pons M, et al. ESHOL study group. High‐efficiency postdilution online hemodiafiltration reduces all‐cause mortality in hemodialysis patients. J Am Soc Nephrol. 2013;24:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Allon M, Depner TA, Radeva M, Bailey J, et al. Impact of dialysis dose and membrane on infection‐related hospitalization and death: Results of the HEMO study. J Am Soc Nephrol. 2003;14:1863–1870. [DOI] [PubMed] [Google Scholar]
- 35. Vareesangthip K, Thitiarchakul S, Kanjanakul I, et al. Efficacy and safety of enoxaparin during hemodialysis: Results from the HENOX study. J Med Assoc Thai. 2011;1:94. [PubMed] [Google Scholar]
- 36. Bowry SK, Canaud B. Achieving high convective volumes in on‐line Hemodiafiltration. Blood Purif. 2013;35:23–28. 10.1159/000346379. [DOI] [PubMed] [Google Scholar]
- 37. Sabry A, Taha M, Nada M, Al Fawzan F, Alsaran K. Anticoagulation therapy during haemodialysis: A comparative study between two heparin regimens. Blood Coagul Fibrinolysis. 2009;20:57–62. [DOI] [PubMed] [Google Scholar]
- 38. Beijering RJ, ten Cate H, Stevens P, et al. Randomised long‐term comparison of tinzaparin and dalteparin in haemodialysis. Clin Drug Investig. 2003;23:85–97. [Google Scholar]
- 39. Davenport A. Optimization of heparin anticoagulation for hemodialysis. Hemodial Int. 2011;15:S1. [DOI] [PubMed] [Google Scholar]
- 40. Nistor I, Palmer SC, Craig JC, et al. Convective versus diffusive dialysis therapies for chronic kidney failure: An updated systematic review of randomized controlled trials. Am J Kidney Dis. 2014;63:954–967. [DOI] [PubMed] [Google Scholar]


