Abstract
Itch is a defining symptom of atopic dermatitis. Crosstalk between keratinocytes, the immune system and non‐histaminergic sensory nerves is responsible for the pathophysiology of chronic itch in atopic dermatitis. An expanding understanding of the contribution of the nervous system and its interaction with immune pathways in atopic itch are helping to identify new therapeutic strategies.
Introduction
Chronic itch that induces scratching is a defining symptom of atopic dermatitis (AD).1, 2 Inadequately controlled itch in AD significantly affects quality of life, with high levels of work impairment, loss of productivity and sleep interference.3 Pro‐inflammatory cytokines from T cells and keratinocytes play a key role in the pathogenesis of AD and atopic itch, as previously reviewed.4 The central nature of inflammatory pathways in AD is evidenced by the potent therapeutic effects of the interleukin‐4 receptor alpha (IL‐4Rα) antagonist dupilumab and the interleukin‐31 receptor alpha (IL‐31RA) antagonist nemolizumab.5, 6, 7, 8, 9, 10, 11 However, there is growing appreciation for the contribution of the nervous system in AD‐associated itch.12 Crosstalk between the nervous system, the cutaneous immune system and keratinocyte populations is central to the development and persistence of atopic itch.13 While immunosuppressants and corticosteroids reduce inflammatory components of AD, as well as itch, most of these treatments fail to target the substantial neural component of itch pathophysiology and are associated with suboptimal risk–benefit profiles.14 Alternative therapeutic strategies may directly target the nervous system, or target points of intersection between nerves, immune cells and keratinocytes. Here, we review the pathways that link keratinocytes, the immune system and the nervous system in the pathophysiology of chronic itch in AD and outline possible therapeutic strategies to target these circuits.
Neural pathways that mediate pruritus in AD
Itch occurs when sensory nerves are exposed to exogenous and endogenous stimuli (pruritogens) including allergens, amines, proteases, neuropeptides and cytokines.4, 15, 16 In the peripheral nervous system, the first event is binding of pruritogens to a subset of primary afferent C‐fibre somatosensory neurons (pruritoceptors) that innervate skin. Pruritoceptor cell bodies are located in the dorsal root ganglia (DRG); they synapse to interneurons in the dorsal horn of the spinal cord. After pruritogens activate pruritogen receptors on the cutaneous nerve endings of pruritoceptors, calcium influx and activation of intracellular signalling pathways result in the transmission of an electrical impulse from the skin to the DRG and the spinal cord. This impulse is subsequently conveyed to the brain via the spinothalamic tract neurons.17, 18, 19 The brain processes the itch signal, and motor activity (scratching) is induced.20
Individual pruritoceptors are defined by their signalling response to specific pruritogens. One system for functionally classifying groups of pruritoceptors is by sensitivity to histamine, a common pruritogen. Histamine‐responsive (histaminergic) and non‐histaminergic pruritoceptors use largely distinct receptors and distinct cutaneous nerve fibres that follow separate spinothalamic tracts to connect with different neural pathways in the central nervous system (CNS).4, 21 Figure 1 depicts the neuroanatomy of both pathways from the periphery to the CNS. This review focuses on non‐histaminergic pathways, as histamine‐dependent pathways do not contribute substantially to chronic itch in AD.18, 22
Figure 1.
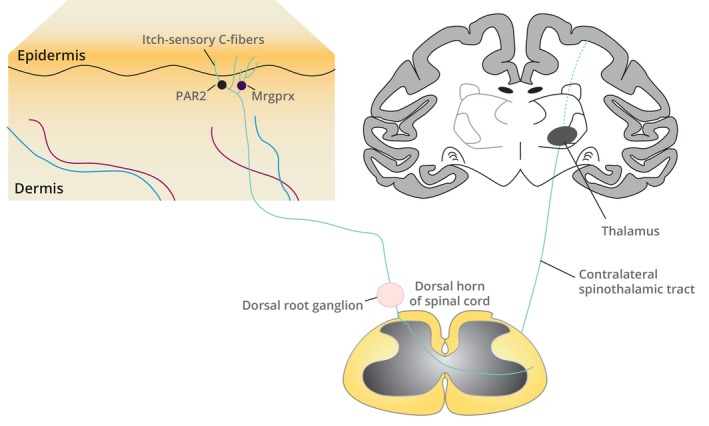
The neuroanatomy of itch pathways from the skin to the CNS. Itch is mediated by pruritogen binding to pruritogen receptors, such as PAR2 and Mrgprx, located on a subset of itch‐sensitive primary afferent somatosensory neurons whose nerve endings innervate the dermis and epidermis. Itch‐sensory neurons are C fibres; their cell bodies reside in the dorsal root ganglia of the spinal cord. Itch is perceived after signals initiated in cutaneous C‐fibre neurons are transmitted by relay through the dorsal root ganglia to interneurons in the dorsal horn of the spinal cord and then via contralateral spinothalamic tracts to the brain. CNS, central nervous system; Mrgprx, Mas‐related G protein‐coupled receptors, in particular the subfamily X; PAR2, proteinase‐associated receptor 2.
Activation of many different pruritogen receptors can trigger non‐histaminergic pathways relevant to AD. Pruritogens that activate these receptors include keratinocyte‐derived proteins, mast cell factors, environmental chemicals, pathogen‐derived molecules and cytokines (discussed below; also reviewed in Voisin et al. 2017, Dong and Dong 201823, 24). A few notable examples of pruritogen receptor–pruritogen pairs relevant to AD are as follows: (i) proteinase‐associated receptor 2 (PAR2), which binds a pro‐peptide released by mast cell proteases or house dust mite extract proteases4, 25; and (ii) several members of the Mas‐related G protein‐coupled receptor (Mrgprx) family, in particular Mrgprx2, which can be activated by the neuropeptide substance P.16, 26, 27, 28
Many non‐histaminergic pruritoceptors require the calcium ion channels TRPA1 and TRPV1 for itch signalling to the spinal cord.26, 29 Within the spinal cord, itch signals are transmitted through the spinothalamic tract via gastrin‐releasing peptide receptors (GRPR)+ neurons.4, 30 Transmission of pruritoceptive signals via GPRP+ spinal cord neurons is regulated by inhibitory gamma‐aminobutyric acid (GABA)ergic interneurons. Several studies have demonstrated that loss of GABAergic interneurons or downregulation of a GABA receptor subunit is essential for chronic itch in mice, suggesting GABA agonists could effectively treat itch in AD patients as well.31, 32, 33
Crosstalk between immune cells, keratinocytes and peripheral nerves mediates atopic itch
Pruritus in AD results from orchestrated interactions between histamine‐independent C fibres in the skin, keratinocytes and immune cells. Figure 2 illustrates lines of communication between these key populations in chronic itch in AD.
Figure 2.
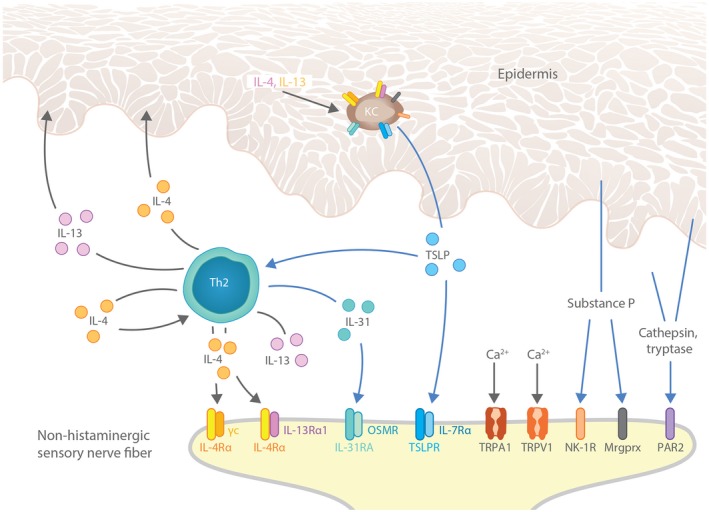
Crosstalk between nerves, immune cells and keratinocytes fuel pruritus in AD lesional skin. Immune cells and keratinocytes release pruritogens (IL‐31, TSLP, substance P) and other factors that alter sensitivity to pruritus in AD, including the type 2 cytokine IL‐4. These factors promote pruritus by interacting with their receptors (IL‐31RA, TSLPR, NK‐1R, IL‐4Rα, respectively) expressed on histamine‐independent C fibres innervating skin. Blue arrows designate direct pruritogenic signalling pathways. Keratinocyte‐derived factors (TSLP, substance P) can also promote itch indirectly by activating Th2 immune cells, leading to further production of IL‐31, IL‐4 and IL‐13. Finally, feedback loops involving IL‐4 and IL‐13 contribute to pruritus by inducing more TSLP expression in keratinocytes. The widespread expression of pruritogen and cytokine receptors on both keratinocytes and cutaneous C fibres illustrates the potential for crosstalk between keratinocytes, immune cells and the peripheral nervous system to drive itch in AD. AD, atopic dermatitis; CNS, central nervous system; KC, keratinocyte; Mrgprx, Mas‐related G protein‐coupled receptors, in particular the subfamily X; OSMR, oncostatin M receptor; PAR2, proteinase‐associated receptor 2; TRPA1, transient receptor potential cation channel subfamily A member 1; TRPV1, transient receptor potential cation channel subfamily V member 1; TSLP, thymic stromal lymphopoietin.
Immune cell‐derived factors
T helper cell 2 (Th2) lymphocytes, eosinophils, neutrophils and mast cells amplify inflammatory and pruritoceptive pathways in AD by releasing cytokines and neurogenic peptides.4, 34, 35 Some AD‐associated cytokines, IL‐31 and thymic stromal lymphopoietin (TSLP), can directly promote itch via activation of pruritoceptive TRPV1+ TRPA1+ neurons that express their receptors.36, 37 In addition, IL‐4 may potentiate itch by sensitizing itch‐sensory neurons to direct pruritogens, such as histamine and IL‐31.38 Cytokine‐to‐neuron signalling by IL‐31, TSLP and IL‐4 – all present in skin during AD flares – may explain the rapid benefit of JAK1/2 and IL‐4Rα inhibition vs. chronic pruritus and pruritus in AD.36, 37, 38, 39, 40, 41 Pruritoceptors bearing receptors for IL‐31 and TSLP express high levels of JAK1.42
In addition to communicating with neurons, IL‐31 binds to its receptor IL‐31RA on keratinocytes. A role for the IL‐31:IL‐31RA pathway in atopic itch is supported by the genetic association between IL‐31 and lichen amyloidosis, an itchy skin condition associated with mutations that result in increased epidermal expression of IL‐31RA.43
Contributions from keratinocytes
Keratinocytes promote itch by releasing additional pruritogens, including the alarmin TSLP, to directly activate pruritoceptive neurons.25, 37, 44 Interestingly, keratinocytes express some of the same receptors that mediate pruritus sensation when expressed on neurons; this shared gene expression program may facilitate feed‐forward loops in AD‐associated itch. For example, mast cell proteases not only trigger pruritus via activation of PAR2+ sensory afferent neurons,25 but also stimulate release of TSLP from keratinocytes.37 In addition to its direct role as a pruritogen, TSLP from keratinocytes promotes atopic skin inflammation and pruritus pathways indirectly by activating immune cells. TSLP binds to TSLPR on Th2 cells and type 2 innate lymphoid cells, leading to production of pruritogenic type 2 cytokines.45, 46, 47 Additional keratinocyte alarmins (e.g. IL‐33) function similarly.4 IL‐4 and IL‐13 can also synergize to induce TSLP expression in keratinocytes,48 suggesting that additional pathogenic signalling networks may sustain the inflammatory response and associated pruritus pathways in AD.46
Neuronal inputs
Neuropeptides released by activated cutaneous neurons can also stimulate keratinocyte release of pro‐inflammatory mediators, including substance P and calcitonin gene‐related peptide (CGRP)49; this feed‐forward mechanism could fuel further neurogenic inflammation, keratinocyte proliferation and epidermal thickening – processes implicated in AD lesion formation.25, 50 Both substance P and its receptor, neurokinin‐1 (NK‐1R), are overexpressed in pruritic AD lesional skin, as are TRPV2, TRPA1, Mrgprs, PAR2 and PAR4.27 Substance P is a neuropeptide, i.e. factor secreted by peptidergic neurons, but it is also produced by keratinocytes.51 In fact, as keratinocytes largely outnumber all other cell types in skin and therefore dominate whole‐tissue transcriptional profiling, upregulation of TRP and PAR gene expression in AD lesional skin biopsy tissue probably also reflects gene expression in keratinocytes as well as neurons. These molecules represent potential new therapeutic targets to address the neuroimmune pathophysiology of itch in AD.
Itch–scratch phenomenon in AD
Scratching itchy AD lesional skin injures epithelial keratinocytes, the consequences of which include release of inflammatory alarmins, direct and indirect activation of type 2 immune cells and release of pruritogenic cytokines from both keratinocytes and immune cells.25 Binding of these pruritogenic factors to pruritoceptive nerves triggers the desire to continue scratching. These feed‐forward loops form the cellular and molecular basis of the ‘itch–scratch cycle’ in AD.52, 53
Neural sensitization of pruritus in AD
Sensitization describes the phenomenon that occurs with chronic itch (or pain) wherein a minimal stimulus leads to an enhanced neural response. In the setting of sensitization, the perception of itch (or pain) is enhanced, triggered by a lower threshold stimulus and may persist after the stimulus is removed. Many AD patients with chronic pruritus exhibit sensitization.
Neural sensitization of pruritus can occur peripherally and centrally.12 Peripheral sensitization, defined by a decreased activation threshold of nociceptors on neurons, is induced by inflammatory mediators54 and results in increased nerve fibre responsiveness and release of neurotransmitters such as glutamate and brain‐derived neurotrophic factor.54, 55 In central sensitization, which can also be induced by decreased inhibitory synaptic transmission via GABA and glycine receptors,54 CNS pathways couple non‐pruritic stimuli to pruritic sensation (alloknesis) and overreact to pruritic stimuli (hyperknesis).56 Within the spinal cord, both interneurons and astrocytes contribute to central sensitization.57, 58 In the skin of patients with AD, the threshold for electrically evoked itch is lower than in healthy controls,18, 59, 60 and the sensitivity to pruritogens is increased.18 Furthermore, a recent study found increased susceptibility to both cowhage and mechanically evoked itch, particularly intra‐lesionally, in AD patients, a finding suggesting involvement of sensitization of the non‐histaminergic pathway as well as mechanosensitive circuitry not normally associated with itch.61 NK‐1R‐ and GRPR‐expressing dorsal spinal neurons were shown to play a key role in central sensitization, with involvement of NK‐1R in both alloknesis and hyperknesis, and GRPR in hyperknesis alone.15
Inflammation plays a key role in neural sensitization, as peripheral sensitization induces activation of glial cells in the spinal cord via the release of adenosine triphosphate, chemokines and proteases.54, 62 Importantly, due to sensitization, alloknesis and hyperknesis persist after dermal inflammation has subsided, as illustrated by the intense itching experienced by patients with relatively mild eczema.63, 64
Keratinocyte‐derived nerve growth factor (NGF) has been linked to peripheral sensitization through induction of hyperinnervation.65, 66 It has also been associated with enhanced membrane current via upregulation of TRPV1 expression on cutaneous nerve endings.4 In addition, NGF upregulates the release of substance P and CGRP by nerve fibres, which contribute to hyperknesis and neurogenic inflammation.18 Direct communication between substance P and mast cells plays a role in the sensitization of nociceptors on nerve terminals by enhancing their responsiveness.
Current therapeutic targets and treatments for atopic itch
Studies of neuroimmune pathways have provided novel approaches for reducing itch in AD. New and emerging treatments for atopic itch are discussed here and summarized in the Table 1. We included agents that have been tested in AD, and also those which may be of benefit based upon current scientific understanding of the pathogenesis of itch in AD.
Table 1.
Products approved or in development that have potential to manage itch in AD
| Target | Compound | Status | Key clinical data | Magnitude of effect on itch | Reference |
|---|---|---|---|---|---|
| Small molecule products | |||||
| JAK1 | Upadacitinib | Phase 2b | Significantly reduced itch in moderate‐to‐severe AD by Week 1 throughout Week 16 and further to Week 32 | Mean percent change from baseline in pruritus Numerical Rating Scale (NRS):
|
AbbVie 201867; AbbVie 201869; ClinicalTrials.gov identifier: NCT02925117 |
| JAK1, JAK3 | Tofacitinib | Phase 2a | Significantly reduced itch in mild‐to‐moderate AD by Day 2 throughout Week 4 | Least squares (LS) mean percent change from baseline in Itch Severity Item:
|
Bissonnette 201640 |
| JTE‐052 | Phase 2 | Significantly reduced itch in moderate‐to‐severe AD by Day 1 (night‐time) and Week 1 | Mean percent change from baseline in pruritus NRS:
|
Nakagawa 201870 | |
| PDE4 | Crisaborole | Approved (mild‐to‐moderate AD) | Significantly improved itch in mild‐to‐moderate AD as early as Day 2 through Day 29 | Proportions of patients achieving improvement in pruritus score 0 or 1 and ≥1‐grade reduction (scale 0–4) from baseline:
|
Yosipovitch 201868 |
| Roflumilast |
Approved (COPD) Phase 2a |
Significant improvement in patient assessment of itch in moderate AD by Day 15 | Mean difference in pruritus score between the roflumilast‐ and placebo‐treated groups:
|
ClinicalTrials.gov Identifier: NCT01856764 | |
| Apremilast | Approved (psoriasis and PsA); Phase 2 in AD | Significant improvement in itch in AD in an open‐label trial by Week 2 | Reduction in pruritus visual analog scale (VAS) by Week 2:
|
Samrao 201271 | |
| Non‐significant improvement in itch in moderate‐to‐severe AD by Week 12 | Mean percent change from baseline in pruritus VAS at Week 12:
|
Simpson 201872 | |||
| E6005/RVT‐501 | Phase 2 | Non‐significant reduction in itch in AD by Week 12 | Mean percent difference (95% confidence interval [CI]) in Itch Behavioral Rating Scale score between the E6005 group and placebo:
|
Furue 201473 | |
| NGF (TrkA) | Pegcantratinib (CT327†) | Phase 2b | Significantly reduced itch in adults with mild‐to‐moderate psoriasis | Significant reductions from baseline at Week 8 in pruritus VAS and Psoriasis Area Severity Index with 0.05% and 0.1% CT327 vs. placebo, respectively | Roblin 201574 |
| α2δ‐1 subunit of spinal N‐type Ca2+ channels | Gabapentin†, Pregabalin† | Approved (pain) | Significantly reduced itch of neuropathic origin | Commencement of pregabalin 75 mg twice daily and increased to 150 mg twice daily resulted in a more than 70% reduction in itch 5–8 weeks after treatment initiation | Ehrchen 200875 |
| NK‐1 receptor (antagonist) | Aprepitant† (oral) | Phase 1 | Anti‐pruritic effect observed as early as Day 2 in severe refractory chronic pruritus associated with several non‐malignant conditions, including atopic diathesis and prurigo nodularis | 16 out of 20 patients (80%) with chronic pruritus responded to short‐term aprepitant monotherapy
|
Ständer 201076 |
| Serlopitant (oral)† | Phase 2 | Significantly reduced itch in patients with severe refractory chronic pruritus of various aetiologies | Mean percent changes from baseline in pruritus NRS at Week 6:
|
Yosipovitch 201877 | |
| Non‐significant reduction of itch in a phase 2 trial in adolescents and adults with a history of AD | Mean absolute change from baseline in pruritus NRS at Week 6:
|
Menlo Therapeutics 201878; ClinicalTrials.gov Identifier: NCT02975206 | |||
| Significantly reduced several measures of itch in patients with prurigo nodularis |
LS mean difference (95% CI) in average itch VAS scores between the serlopitant and placebo group:
Mean difference (95% CI) in average itch NRS scores between the serlopitant and placebo group:
|
Ständer 201979 | |||
| Tradipitant (oral) | Phase 2 | Significant and clinically meaningful improvements in several measures of itch in AD |
Improvements were observed in the measurement of Worst Itch VAS (P = 0.019) with tradipitant vs. placebo More tradipitant‐treated vs. placebo‐treated patients achieved ≥40 points improvement from baseline in Worst Itch VAS scores (P = 0.037) or ≥30 points (P = 0.049) More tradipitant‐treated patients showed improvement in Average Itch VAS over placebo‐treated patients, but this improvement was not significant |
Vanda Pharmaceuticals 201780; ClinicalTrials.gov Identifier: NCT02651714 | |
| Serotonin norepinephrine reuptake inhibitor | Mirtazapine† | Case series | Significant reduction in chronic nocturnal pruritus | Two of three cases had underlying AD, both reported itch symptom alleviation within 1 week on mirtazapine | Hundley 200481 |
| μ‐opioid receptor (antagonist) | Naltrexone | Placebo‐controlled case series | Non‐significant decrease in allokinesis and duration of acetylcholine‐induced acute itch in AD patients | Heyer 200282 | |
| Nalmefene (SRD174; topical) | Phase 2 | Non‐significant reduction in itch after two 7‐day periods of treatment in adults with persistent moderate‐to‐severe pruritus associated with AD |
The LS mean difference (95% CI) in sum of pruritus intensity difference from 0 to 4 h (SPID0–4) between the SRD174 cream and placebo group was −1.3 (−25.9 to 23.3); P = 0.914 The LS mean difference (95% CI) in average daily pruritus score between the SRD174 cream and placebo group was −0.1 (−0.2 to 0.0); P = 0.095 |
Herzog 201183 | |
| κ‐opioid receptor (agonist) | Nalfurafine (oral)† | Approved in Japan for uraemic pruritus | Significant reduction in pruritus in patients with liver disease in a phase 3 randomized, placebo‐controlled trial |
The changes in pruritus scores at Week 4 were:
The difference between the 2.5 μg group vs. placebo was 0.35 (0.13 to 0.56, P = 0.0007), and between 5 μg vs. placebo, 0.26 (0.05 to 0.47, P = 0.0071) |
Kumada 201784 |
| κ‐opioid receptor (full agonist) and μ‐opioid receptor (partial agonist) | Nalbuphine/Nubain† (extended‐release tablet formulation) | Phase 2/3 | Significant reduction in severe chronic uraemic pruritus in haemodialysis patients | The mean NRS (±SE) declined by 3.5 (2.4) and 2.8 (2.2) in the 120 mg nalbuphine and placebo groups, respectively (P = 0.017 vs. placebo) from a baseline NRS of 6.9 (1.5) | Mathur 201785; ClinicalTrials.gov Identifier: NCT02373215 |
| Successfully completed a phase 2 trial for pruritus in patients with prurigo nodularis | Among the 12 of 18 enrolled patients who completed the 10‐week study, the proportion who reported > 50% reduction in 7‐day worst itch NRS vs. baseline achieved significance (P = 0.028) | Trevi Therapeutics 201686; ClinicalTrials.gov Identifier: NCT02174419 | |||
| κ‐opioid receptor (agonist) and μ‐opioid receptor (antagonist) | Butorphanol† | Significantly reduced itch in 5 patients with severe, chronic intractable pruritus | No specific data are reported (anecdotal only) | Dawn 200687 | |
| Spinal cannabinoid 1 receptor (agonist) | WIN 55,212‐2† | Preclinical | Dose‐dependently decreased serotonin‐induced scratching in an animal study | Bilir 201888 | |
| Spinal cannabinoid 2 receptor (agonist) | N‐palmitoyl ethanolamine (PEA)† | Open application observation | Reduced itch by 86.4% in 14/22 patients with prurigo, lichen simplex, and pruritus | The average reduction in itch was 86.4% | Ständer 200689 |
| S‐777469† | Preclinical | Significantly reduced scratching behaviour induced by histamine or substance P in animal studies | Haruna 201590 | ||
| Histamine 4 receptor (H4R) | ZPL‐3893787 | Phase 2 | Non‐significant decrease in pruritus in both treatment and control groups at Week 8; clinical implications unclear | Worst pruritus NRS mean change (SD) from baseline to Week 8 was −3.03 (2.186) with ZPL‐3893787 and −2.66 (2.057) with placebo (P = 0.249); TCS permitted as rescue (75.4% ZPL‐3893787 and 84.8% placebo patients received rescue) | Werfel 201991 |
| Biologics | |||||
| TSLP | Tezepelumab (monoclonal antibody) | Phase 2a | Marginal reduction in itch in moderate‐to‐severe AD |
Adjusted mean percentage improvement (±SE) in pruritus NRS from baseline to Week 12 with tezepelumab + TCS vs. placebo + TCS was 35.53 (5.9) vs. 21.05 (5.9); P = 0.050 vs. placebo Adjusted mean percentage improvement (±SE) in peak pruritus NRS from baseline to Week 12 with tezepelumab + TCS vs. placebo + TCS was 33.54 (6.0) vs. 25.41 (6.1); P = 0.258 vs. placebo |
Simpson 201992 |
| IL‐33 (ST2) | IL‐33 mouse antibody† | Preclinical | Scratching behaviour was reduced in an animal model | Peng 201893 | |
| IL‐4Rα (IL‐4 and IL‐13) | Dupilumab (monoclonal antibody) | Approved (moderate‐to‐severe AD) | Significantly reduced itch in moderate‐to‐severe AD for up to 52 weeks in phase 3 studies; reduction in itch was reported as early as Day 2 in a post‐hoc analysis |
LS mean percent change from baseline (±SE) in peak pruritus NRS score:
LS mean percent change from baseline (±SE) in peak pruritus NRS score:
Proportions of patients achieving peak pruritus NRS ≥3‐point improvement from baseline:
|
Silverberg 2017 (pooled SOLO 1 and 2)41; Blauvelt 2017 (CHRONOS)8; Simpson 20167 |
| IL‐22 | Fezakinumab (monoclonal antibody) | Phase 2a | Non‐significant difference in itch in moderate‐to‐severe AD by Week 12 | No significant differences in SCORing Atopic Dermatitis VAS pruritus score, but a sustained treatment effect was observed among patients with baseline pruritus > 5 after Week 12 vs. placebo | Guttman‐Yassky 201894 |
| IL‐13 | Lebrikizumab (monoclonal antibody) | Phase 2; phase 2b | Numerical reduction in itch in moderate‐to‐severe AD by Week 12 (phase 2); no itch data available yet from phase 2b study | Adjusted mean percent reductions from baseline pruritus VAS at Week 12 were:
|
Simpson 201895; ClinicalTrials.gov Identifier: NCT03443024 (phase 2b) |
| Tralokinumab (monoclonal antibody) | Phase 2b | Significant reduction in itch in moderate‐to‐severe AD by Week 12 | Improvements (95% CI) with tralokinumab‐treated patients vs. placebo for pruritus NRS at Week 12:
|
Wollenberg 201996 | |
| IL‐17A | Secukinumab (monoclonal antibody) | Phase 2; approved for psoriasis | A placebo‐controlled trial assessing the efficacy and safety of secukinumab in moderate‐to‐severe AD is currently in recruitment | No data available yet | Clinicaltrials.gov Identifier: NCT02594098 |
| IL‐17A | Ixekizumab (monoclonal antibody) | Phase 3 | Significant, rapid reduction in itch in moderate‐to‐severe psoriasis by Week 12 |
Greater differences in time to pruritus NRS ≥4‐point improvement for patients treated with ixekizumab every 2 weeks or every 4 weeks vs. placebo (P < 0.001) The median time for 50% of patients to achieve a ≥4‐point reduction in pruritus NRS was shorter for ixekizumab‐treated patients (2 weeks, with both 80 mg ixekizumab every 4 weeks and every 2 weeks) compared with placebo‐treated patients (> 12 weeks) |
Leonardi 201797 |
| IL‐31RA | Nemolizumab (monoclonal antibody) | Phase 2; phase 2 long‐term extension | Significantly decreased itch in moderate‐to‐severe AD by Week 12 (phase 2 randomized trial) and Week 64 (open‐label extension) | Changes on the pruritus VAS were:
|
Ruzicka 201710; Kabashima 201811 |
†Agents effective in itch but not tested in AD.
AD, atopic dermatitis; CI, confidence interval; COPD, chronic obstructive pulmonary disease; GABA, gamma‐aminobutyric acid; LS, least squares; NGF, nerve growth factor; NRS, numerical rating scale; PsA, psoriatic arthritis; q2w, every 2 weeks; q4w, every 4 weeks; qw, every week; SD, standard deviation; SE, standard error; VAS, visual analog scale.
Several monoclonal antibodies targeting IL‐4, IL‐13, IL‐22 or IL‐31 have been investigated in AD clinical trials (Table 1). Nemolizumab (anti‐IL‐31RA), tralokinumab (anti‐IL‐13) and dupilumab (anti‐IL‐4Rα) all significantly reduced itch in AD, whereas lebrikizumab (anti‐IL‐13) only numerically reduced AD‐related itch (Table 1). Dupilumab is a human monoclonal antibody that blocks the shared receptor subunit for IL‐4 and IL‐13 (IL‐4Rα), thus inhibiting signalling of both IL‐4 and IL‐13. As IL‐4Rα‐mediated type 2 cytokine signalling via Janus kinase‐signal transducers and activators of transcriptions (JAK‐STAT) in sensory neurons promotes itch,38 dupilumab may also directly attenuate itch symptoms by inhibiting neuronal IL‐4Rα and JAK signalling in addition to reducing type 2 inflammation. A novel human monoclonal anti‐TSLP antibody (MEDI9929), given concomitantly with topical corticosteroids, only marginally reduced itch (compared with placebo) in a recently completed study in adults with moderate‐to‐severe AD (Table 1), a finding suggesting that not all compounds targeting specific itch mediators have an anti‐pruritic effect in AD. Antibody‐mediated inhibition of IL‐33, on the other hand, has been effective in an AD animal model and safe and tolerable in a phase 1 clinical trial (Table 1).
As a result of increasing appreciation for the neural contribution to AD‐associated itch pathophysiology, neurally acting agents may serve as new therapeutic alternatives to immunomodulatory agents for AD. Treatments targeting GABA, TRPA1, NK‐1R, opioid and cannabinoid receptors are yet to be investigated in AD but have been effective in chronic itch of other aetiologies. Based upon their mechanisms of action, they have potential to alleviate AD‐associated itch. Selective JAK inhibitors and phosphodiesterase 4 (PDE4) inhibitors have shown efficacy in AD. Oral upadacitinib, a JAK1‐selective inhibitor, was recently granted Breakthrough Therapy designation for AD by the US Food and Drug Administration,67 and crisaborole, a topical treatment that inhibits PDE4 signalling, was approved in Europe and the US for the treatment of mild‐to‐moderate AD in patients aged ≥2 years.17, 68 Both agents were effective in reducing itch (Table 1).
Conclusions
Recent studies provide strong support for crosstalk between the nervous and immune systems in chronic atopic itch. Further elucidating the roles of peripheral and central sensitization and hypersensitization is key to understanding the chronicity and severity of itch in AD. Novel approaches, such as the use of agents that target neural or neuroimmune pathways, may provide additional treatment options for AD, thereby improving outcomes for AD patients and potentially other chronic pruritic diseases.
Conflicts of interest
Dr. Yosipovitch is a consultant and advisory board member for AbbVie, Bayer, Cervae, Eli Lilly, Galderma, Menlo Therapeutics, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Sanofi, Sienna and Trevi Therapeutics and is an investigator funded by Kiniksa Pharmaceuticals, LEO Pharma, Menlo Therapeutics, Pfizer, Regeneron Pharmaceuticals, Inc., Sun Pharma. Dr. Berger is a consultant and advisory board member for Sanofi and Menlo Therapeutics. Dr. Fassett is a consultant for Regeneron Pharmaceuticals, Inc.
Funding sources
Medical writing/editorial assistance was provided by Jamie Lim, PhD, of Excerpta Medica and funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
References
- 1. Kabashima K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci 2013; 70: 3–11. [DOI] [PubMed] [Google Scholar]
- 2. Eichenfield LF, Tom WL, Chamlin SL et al Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol 2014; 70: 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wei W, Anderson P, Gadkari A et al Extent and consequences of inadequate disease control among adults with a history of moderate to severe atopic dermatitis. J Dermatol 2018; 45: 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mollanazar NK, Smith PK, Yosipovitch G. Mediators of chronic pruritus in atopic dermatitis: getting the itch out? Clin Rev Allergy Immunol 2016; 51: 263–292. [DOI] [PubMed] [Google Scholar]
- 5. Beck LA, Thaci D, Hamilton JD et al Dupilumab treatment in adults with moderate‐to‐severe atopic dermatitis. N Engl J Med 2014; 371: 130–139. [DOI] [PubMed] [Google Scholar]
- 6. Thaçi D, Simpson EL, Beck LA et al Efficacy and safety of dupilumab in adults with moderate‐to‐severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo‐controlled, dose‐ranging phase 2b trial. Lancet 2016; 387: 40–52. [DOI] [PubMed] [Google Scholar]
- 7. Simpson EL, Bieber T, Guttman‐Yassky E et al Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016; 375: 2335–2348. [DOI] [PubMed] [Google Scholar]
- 8. Blauvelt A, de Bruin‐Weller M, Gooderham M et al Long‐term management of moderate‐to‐severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1‐year, randomised, double‐blinded, placebo‐controlled, phase 3 trial. Lancet 2017; 389: 2287–2303. [DOI] [PubMed] [Google Scholar]
- 9. de Bruin‐Weller M, Thaçi D, Smith CH et al Dupilumab with concomitant topical corticosteroids in adult patients with atopic dermatitis who are not adequately controlled with or are intolerant to ciclosporin A, or when this treatment is medically inadvisable: a placebo‐controlled, randomized phase 3 clinical trial (LIBERTY AD CAFÉ). Br J Dermatol 2018; 178: 1083–1101. [DOI] [PubMed] [Google Scholar]
- 10. Ruzicka T, Hanifin JM, Furue M et al Anti‐interleukin‐31 receptor A antibody for atopic dermatitis. N Engl J Med 2017; 376: 826–835. [DOI] [PubMed] [Google Scholar]
- 11. Kabashima K, Furue M, Hanifin JM et al Nemolizumab in patients with moderate‐to‐severe atopic dermatitis: randomized, phase II, long‐term extension study. J Allergy Clin Immunol 2018; 142: 1121–1130. [DOI] [PubMed] [Google Scholar]
- 12. Yosipovitch G, Rosen JD, Hashimoto T. Itch: from mechanism to (novel) therapeutic approaches. J Allergy Clin Immunol 2018; 142: 1375–1390. [DOI] [PubMed] [Google Scholar]
- 13. Yosipovitch G, Papoiu AD. What causes itch in atopic dermatitis? Curr Allergy Asthma Rep 2008; 8: 306–311. [DOI] [PubMed] [Google Scholar]
- 14. He A, Feldman SR, Fleischer AB. Trends in atopic dermatitis management: comparison of 1990‐1997 to 2003‐2012. J Drugs Dermatol 2018; 17: 135–140. [PubMed] [Google Scholar]
- 15. Akiyama T, Carstens E. Neural processing of itch. Neuroscience 2013; 250: 697–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoeck EA, Marker JB, Gazerani P et al Preclinical and human surrogate models of itch. Exp Dermatol 2016; 25: 750–757. [DOI] [PubMed] [Google Scholar]
- 17. Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought? J Allergy Clin Immunol 2017; 140: 633–643. [DOI] [PubMed] [Google Scholar]
- 18. Ikoma A, Steinhoff M, Ständer S et al The neurobiology of itch. Nat Rev Neurosci 2006; 7: 535–547. [DOI] [PubMed] [Google Scholar]
- 19. Ross SE. Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Curr Opin Neurobiol 2011; 21: 880–887. [DOI] [PubMed] [Google Scholar]
- 20. Murota H, Katayama I. Exacerbating factors of itch in atopic dermatitis. Allergol Int 2017; 66: 8–13. [DOI] [PubMed] [Google Scholar]
- 21. Davidson S, Zhang X, Yoon CH et al The itch‐producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci 2007; 27: 10007–10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paus R, Schmelz M, Biro T et al Frontiers in pruritus research: scratching the brain for more effective itch therapy. J Clin Invest 2006; 116: 1174–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Voisin T, Bouvier A, Chiu IM. Neuro‐immune interactions in allergic diseases: novel targets for therapeutics. Int Immunol 2017; 29: 247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dong X, Dong X. Peripheral and central mechanisms of itch. Neuron 2018; 98: 482–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schwendinger‐Schreck J, Wilson SR, Bautista DM. Interactions between keratinocytes and somatosensory neurons in itch. Handb Exp Pharmacol 2015; 226: 177–190. [DOI] [PubMed] [Google Scholar]
- 26. Andersen HH, Elberling J, Arendt‐Nielsen L. Human surrogate models of histaminergic and non‐histaminergic itch. Acta Derm Venereol 2015; 95: 771–777. [DOI] [PubMed] [Google Scholar]
- 27. Nattkemper LA, Tey HL, Valdes‐Rodriguez R et al The genetics of chronic itch: gene expression in the skin of atopic dermatitis and psoriasis patients with severe itch. J Invest Dermatol 2018; 138: 1311–1317. [DOI] [PubMed] [Google Scholar]
- 28. Meixiong J, Dong X. Mas‐related G protein‐coupled receptors and the biology of itch sensation. Annu Rev Genet 2017; 51: 103–121. [DOI] [PubMed] [Google Scholar]
- 29. Wilson SR, Bautista DM. Role of transient receptor potential channels in acute and chronic itch In: Carstens E, Akiyama T, eds. Itch: Mechanisms and Treatment. Frontiers in Neuroscience. CRC Press/Taylor & Francis, Boca Raton, FL, 2014: 281–292. [PubMed] [Google Scholar]
- 30. Sun YG, Chen ZF. A gastrin‐releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 2007; 448: 700–703. [DOI] [PubMed] [Google Scholar]
- 31. Ross SE, Mardinly AR, McCord AE et al Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron 2010; 65: 886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Braz JM, Juarez‐Salinas D, Ross SE et al Transplant restoration of spinal cord inhibitory controls ameliorates neuropathic itch. J Clin Invest 2014; 124: 3612–3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cevikbas F, Braz JM, Wang X et al Synergistic antipruritic effects of gamma aminobutyric acid A and B agonists in a mouse model of atopic dermatitis. J Allergy Clin Immunol 2017; 140: 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Steinhoff M, Buddenkotte J, Lerner EA. Role of mast cells and basophils in pruritus. Immunol Rev 2018; 282: 248–264. [DOI] [PubMed] [Google Scholar]
- 35. Hashimoto T, Rosen J, Sanders K, Yosipovitch G. Possible role of neutrophils in itch. Itch 2018; 3: e17. [Google Scholar]
- 36. Cevikbas F, Wang X, Aklyama T et al A sensory neuron‐expressed IL‐31 receptor mediates T helper cell‐dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol 2014; 133: 448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilson SR, Thé L, Batia LM et al The epithelial cell‐derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013; 155: 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oetjen L, Mack M, Feng J et al Sensory neurons co‐opt classical immune signaling pathways to mediate chronic itch. Cell 2017; 171: 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol 2015; 73: 395–399. [DOI] [PubMed] [Google Scholar]
- 40. Bissonnette R, Papp KA, Poulin Y et al Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. Br J Dermatol 2016; 175: 902–911. [DOI] [PubMed] [Google Scholar]
- 41. Silverberg JI, Chao JD, Eckert L et al Dupilumab treatment rapidly improves itch in patients with moderate‐to‐severe atopic dermatitis. Abstract presented at the 75th Annual Scientific Meeting of the American College of Asthma, Allergy and Immunology (ACAAI), Boston, MA, 2017. [Google Scholar]
- 42. Usoskin D, Furlan A, Islam S et al Unbiased classification of sensory neuron types by large‐scale single‐cell RNA sequencing. Nat Neurosci 2015; 18: 145–153. [DOI] [PubMed] [Google Scholar]
- 43. Tey HL, Cao T, Nattkemper LA et al Pathophysiology of pruritus in primary localized cutaneous amyloidosis. Br J Dermatol 2016; 174: 1345–1350. [DOI] [PubMed] [Google Scholar]
- 44. Ziegler SF, Roan F, Bell BD et al The biology of thymic stromal lymphopoietin (TSLP). Adv Pharmacol 2013; 66: 129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Soumelis V, Reche PA, Kanzler H et al Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 2002; 3: 673–680. [DOI] [PubMed] [Google Scholar]
- 46. Indra AK. Epidermal TSLP: a trigger factor for pathogenesis of atopic dermatitis (AD). Expert Rev Proteomics 2013; 10: 309–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ochiai S, Jagot F, Kyle RL et al Thymic stromal lymphopoietin drives the development of IL‐13+ Th2 cells. Proc Natl Acad Sci USA 2018; 115: 1033–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bogiatzi SI, Fernandez I, Bichet JC et al Cutting edge: proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J Immunol 2007; 178: 3373–3377. [DOI] [PubMed] [Google Scholar]
- 49. Shi X, Wang L, Clark JD et al Keratinocytes express cytokines and nerve growth factor in response to neuropeptide activation of the ERK1/2 and JNK MAPK transcription pathways. Regul Pept 2013; 186: 92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Roggenkamp D, Kopnick S, Stab F et al Epidermal nerve fibers modulate keratinocyte growth via neuropeptide signaling in an innervated skin model. J Invest Dermatol 2013; 133: 1620–1628. [DOI] [PubMed] [Google Scholar]
- 51. Bae S, Matsunaga Y, Tanaka Y et al Autocrine induction of substance P mRNA and peptide in cultured normal human keratinocytes. Biochem Biophys Res Commun 1999; 263: 327–333. [DOI] [PubMed] [Google Scholar]
- 52. Verhoeven EW, de Klerk S, Kraaimaat FW et al Biopsychosocial mechanisms of chronic itch in patients with skin diseases: a review. Acta Derm Venereol 2008; 88: 211–218. [DOI] [PubMed] [Google Scholar]
- 53. Oyoshi MK, Larson RP, Ziegler SF et al Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol 2010; 126: 976–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ji RR. Neuroimmune interactions in itch: do chronic itch, chronic pain, and chronic cough share similar mechanisms? Pulm Pharmacol Ther 2015; 35: 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Potenzieri C, Undem BJ. Basic mechanisms of itch. Clin Exp Allergy 2012; 42: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mochizuki H, Schut C, Nattkemper LA et al Brain mechanism of itch in atopic dermatitis and its possible alteration through non‐invasive treatments. Allergol Int 2017; 66: 14–21. [DOI] [PubMed] [Google Scholar]
- 57. Shiratori‐Hayashi M, Koga K, Tozaki‐Saitoh H et al STAT3‐dependent reactive astrogliosis in the spinal dorsal horn underlies chronic itch. Nat Med 2015; 21: 927–931. [DOI] [PubMed] [Google Scholar]
- 58. Ross SE, Hachisuka J, Todd AJ. Spinal microcircuits and the regulation of itch In: Carstens E, Akiyama T, eds. Itch: Mechanisms and Treatment. Frontiers in Neuroscience. CRC Press/Taylor & Francis, Boca Raton (FL), 2014: 339–358. [PubMed] [Google Scholar]
- 59. Ikoma A, Handwerker H, Miyachi Y et al Electrically evoked itch in humans. Pain 2005; 113: 148–154. [DOI] [PubMed] [Google Scholar]
- 60. Ozawa M, Tsuchiyama K, Gomi R et al Neuroselective transcutaneous electrical stimulation reveals neuronal sensitization in atopic dermatitis. J Am Acad Dermatol 2009; 60: 609–614. [DOI] [PubMed] [Google Scholar]
- 61. Andersen HH, Elberling J, Sølvsten H et al Nonhistaminergic and mechanical itch sensitization in atopic dermatitis. Pain 2017; 158: 1780–1791. [DOI] [PubMed] [Google Scholar]
- 62. Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain 2013; 154(Suppl 1): S10–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yosipovitch G, Eckert L, Chen Z et al Correlations of itch with quality of life and signs of atopic dermatitis across dupilumab trials. Ann Allergy Asthma Immunol 2017; 119: S95. Abstract P480. [Google Scholar]
- 64. Andersen HH, Akiyama T, Nattkemper LA et al Alloknesis and hyperknesis‐mechanisms, assessment methodology, and clinical implications of itch sensitization. Pain 2018; 159: 1185–1197. [DOI] [PubMed] [Google Scholar]
- 65. Lewin GR, Mendell LM. Nerve growth factor and nociception. Trends Neurosci 1993; 16: 353–359. [DOI] [PubMed] [Google Scholar]
- 66. Chen PS, Chen LS, Cao JM et al Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc Res 2001; 50: 409–416. [DOI] [PubMed] [Google Scholar]
- 67. AbbVie press release, February 17, 2018. AbbVie Presents New Late‐Breaking Phase 2b Data on Upadacitinib in Atopic Dermatitis at the 2018 American Academy of Dermatology Annual Meeting. URL https://news.abbvie.com/news/abbvie-presents-new-late-breaking-phase-2b-data-on-upadacitinib-in-atopic-dermatitis-at-2018-american-academy-dermatology-annual-meeting.htm (last accessed: 20 August 2019).
- 68. Yosipovitch G, Gold LF, Lebwohl MG et al Early relief of pruritus in atopic dermatitis with crisaborole ointment, a non‐steroidal, phosphodiesterase 4 inhibitor. Acta Derm Venereol 2018; 98: 484–489. [DOI] [PubMed] [Google Scholar]
- 69. AbbVie press release, September 13, 2018. AbbVie Presents Upadacitinib Longer‐Term (32‐Week) and Patient‐Reported Outcomes Data from Phase 2b Atopic Dermatitis Study at 27th European Academy of Dermatology and Venereology (EADV) Congress. URL https://news.abbvie.com/news/abbvie-presents-upadacitinib-longer-term-32-week-and-patient-reported-outcomes-data-from-phase-2b-atopic-dermatitis-study-at-27th-european-academy-dermatology-and-venereology-eadv-congress.htm (last accessed: 20 August 2019).
- 70. Nakagawa H, Nemoto O, Igarashi A et al Efficacy and safety of topical JTE‐052, a Janus kinase inhibitor, in Japanese adult patients with moderate‐to‐severe atopic dermatitis: a phase II, multicentre, randomized, vehicle‐controlled clinical study. Br J Dermatol 2018; 178: 424–432. [DOI] [PubMed] [Google Scholar]
- 71. Samrao A, Berry TM, Goreshi R et al A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol 2012; 148: 890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Simpson EL, Imafuku S, Poulin Y et al A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol 2019; 139: 1063–1072. [DOI] [PubMed] [Google Scholar]
- 73. Furue M, Kitahara Y, Akama H et al Safety and efficacy of topical E6005, a phosphodiesterase 4 inhibitor, in Japanese adult patients with atopic dermatitis: results of a randomized, vehicle‐controlled, multicenter clinical trial. J Dermatol 2014; 41: 577–585. [DOI] [PubMed] [Google Scholar]
- 74. Roblin D, Yosipovitch G, Boyce B et al Topical TrkA kinase inhibitor CT327 is an effective, novel therapy for the treatment of pruritus due to psoriasis: results from experimental studies, and efficacy and safety of CT327 in a phase 2b clinical trial in patients with psoriasis. Acta Derm Venereol 2015; 95: 542–548. [DOI] [PubMed] [Google Scholar]
- 75. Ehrchen J, Ständer S. Pregabalin in the treatment of chronic pruritus. J Am Acad Dermatol 2008; 58(Suppl 2): S36–S37. [DOI] [PubMed] [Google Scholar]
- 76. Ständer S, Siepmann D, Herrgott I et al Targeting the neurokinin receptor 1 with aprepitant: a novel antipruritic strategy. PLoS ONE 2010; 5: e10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yosipovitch G, Ständer S, Kerby MB et al Serlopitant for the treatment of chronic pruritus: results of a randomized, multicenter, placebo‐controlled phase 2 clinical trial. J Am Acad Dermatol 2018; 78: 882–891. [DOI] [PubMed] [Google Scholar]
- 78. Menlo Therapeutics press release, April 8, 2018. Menlo Therapeutics Announces Results from a Phase 2 Trial of Serlopitant for Pruritus Associated with Atopic Dermatitis. URL http://www.menlotherapeutics.com/uncategorized/menlo-therapeutics-announces-results-from-a-phase-2-trial-of-serlopitant-for-pruritus-associated-with-atopic-dermatitis (last accessed: 20 August 2019). [Google Scholar]
- 79. Ständer S, Kwon P, Hirman J et al Serlopitant reduced pruritus in patients with prurigo nodularis in a phase 2, randomized, placebo‐controlled trial. J Am Acad Dermatol 2019; 80: 1395–1402. [DOI] [PubMed] [Google Scholar]
- 80. Vanda Pharmaceuticals Inc. press release, September 13, 2017. Vanda's Tradipitant Improves Itch and Disease Severity in Patients with Atopic Dermatitis. URL https://www.prnewswire.com/news-releases/vandas-tradipitant-improves-itch-and-disease-severity-in-patients-with-atopic-dermatitis-300519177.html (last accessed: 20 August 2019).
- 81. Hundley JL, Yosipovitch G. Mirtazapine for reducing nocturnal itch in patients with chronic pruritus: a pilot study. J Am Acad Dermatol 2004; 50: 889–891. [DOI] [PubMed] [Google Scholar]
- 82. Heyer G, Groene D, Martus P. Efficacy of naltrexone on acetylcholine‐induced allokinesis in atopic eczema. Exp Dermatol 2002; 11: 448–455. [DOI] [PubMed] [Google Scholar]
- 83. Herzog JL, Solomon JA, Draelos Z et al A randomized, double‐blind, vehicle‐controlled crossover study to determine the anti‐pruritic efficacy, safety and local dermal tolerability of a topical formulation (srd174cream) of the long‐acting opioid antagonist nalmefene in subjects with atopic dermatitis. J Drugs Dermatol 2011; 10: 853–860. [PubMed] [Google Scholar]
- 84. Kumada H, Miyakawa H, Muramatsu T et al Efficacy of nalfurafine hydrochloride in patients with chronic liver disease with refractory pruritus: a randomized, double‐blind trial. Hepatol Res 2017; 47: 972–982. [DOI] [PubMed] [Google Scholar]
- 85. Mathur VS, Kumar J, Crawford PW et al A multicenter, randomized, double‐blind, placebo‐controlled trial of nalbuphine ER tablets for uremic pruritus. Am J Nephrol 2017; 46: 450–458. [DOI] [PubMed] [Google Scholar]
- 86. Trevi Therapeutics press release, October 13, 2016. Trevi Therapeutics Announces Positive Results from Phase 2 Trial in Prurigo Nodularis. URL https://www.trevitherapeutics.com/wp-content/uploads/2019/01/TreviPNToplineDataPR.10_.13_.16_.FINAL_.pdf (last accessed: 20 August 2019).
- 87. Dawn AG, Yosipovitch G. Butorphanol for treatment of intractable pruritus. J Am Acad Dermatol 2006; 54: 527–531. [DOI] [PubMed] [Google Scholar]
- 88. Bilir KA, Anli G, Ozkan E et al Involvement of spinal cannabinoid receptors in the antipruritic effects of WIN 55,212‐2, a cannabinoid receptor agonist. Clin Exp Dermatol 2018; 43: 553–558. [DOI] [PubMed] [Google Scholar]
- 89. Ständer S, Reinhardt HW, Luger TA. Topical cannabinoid agonists. An effective new possibility for treating chronic pruritus. Hautarzt 2006; 57: 801–807. [DOI] [PubMed] [Google Scholar]
- 90. Haruna T, Soga M, Morioka Y et al S‐777469, a novel cannabinoid type 2 receptor agonist, suppresses itch‐associated scratching behavior in rodents through inhibition of itch signal transmission. Pharmacology 2015; 95: 95–103. [DOI] [PubMed] [Google Scholar]
- 91. Werfel T, Layton G, Yeadon M et al Efficacy and safety of the histamine H4 receptor antagonist ZPL‐3893787 in patients with atopic dermatitis. J Allergy Clin Immunol 2019; 143: 1830–37.e4. [DOI] [PubMed] [Google Scholar]
- 92. Simpson EL, Parnes JR, She D et al Tezepelumab, an anti‐thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: a randomized phase 2a clinical trial. J Am Acad Dermatol 2019; 80: 1013–1021. [DOI] [PubMed] [Google Scholar]
- 93. Peng G, Mu Z, Cui L et al Anti‐IL‐33 antibody has a therapeutic effect in an atopic dermatitis murine model induced by 2, 4‐dinitrochlorobenzene. Inflammation 2018; 41: 154–163. [DOI] [PubMed] [Google Scholar]
- 94. Guttman‐Yassky E, Brunner PM, Neumann AU et al Efficacy and safety of fezakinumab (an IL‐22 monoclonal antibody) in adults with moderate‐to‐severe atopic dermatitis inadequately controlled by conventional treatments: a randomized, double‐blind, phase 2a trial. J Am Acad Dermatol 2018; 78: 872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Simpson EL, Flohr C, Eichenfield LF et al Efficacy and safety of lebrikizumab (an anti‐IL‐13 monoclonal antibody) in adults with moderate‐to‐severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo‐controlled phase II trial (TREBLE). J Am Acad Dermatol 2018; 78: 863–871. [DOI] [PubMed] [Google Scholar]
- 96. Wollenberg A, Howell MD, Guttman‐Yassky E et al Treatment of atopic dermatitis with tralokinumab, an anti‐IL‐13 mAb. J Allergy Clin Immunol 2019; 143: 135–141. [DOI] [PubMed] [Google Scholar]
- 97. Leonardi CL, Blauvelt A, Sofen HL et al Rapid improvements in health‐related quality of life and itch with ixekizumab treatment in randomized phase 3 trials: results from UNCOVER‐2 and UNCOVER‐3. J Eur Acad Dermatol Venereol 2017; 31: 1483–1490. [DOI] [PubMed] [Google Scholar]


